Ecotoxicity of Tar from Coffee Grounds and Pine Pellet Gasification Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organic Waste for Energy Production
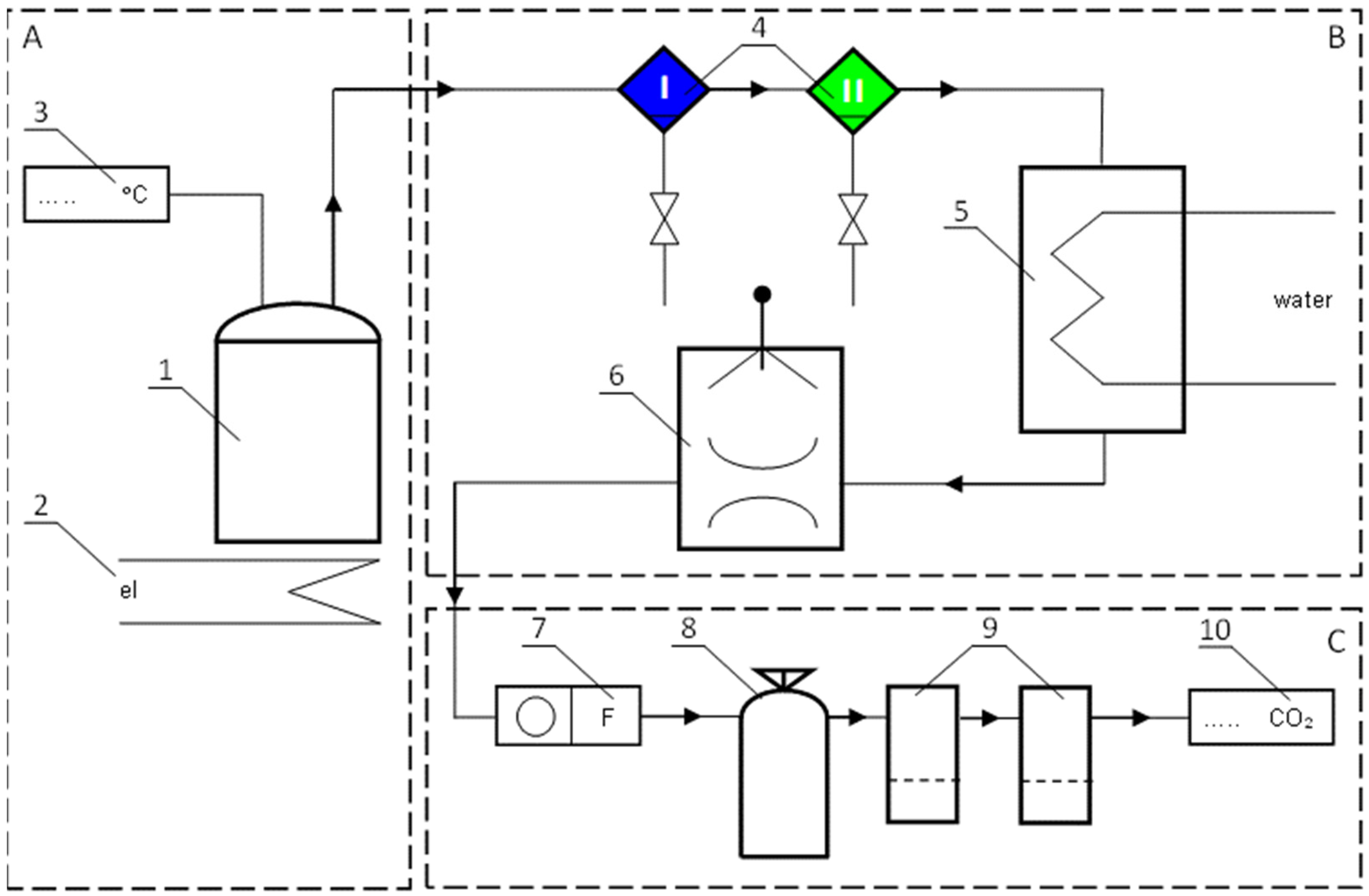
2.2. Experimental Setup
3. Discussion of Results
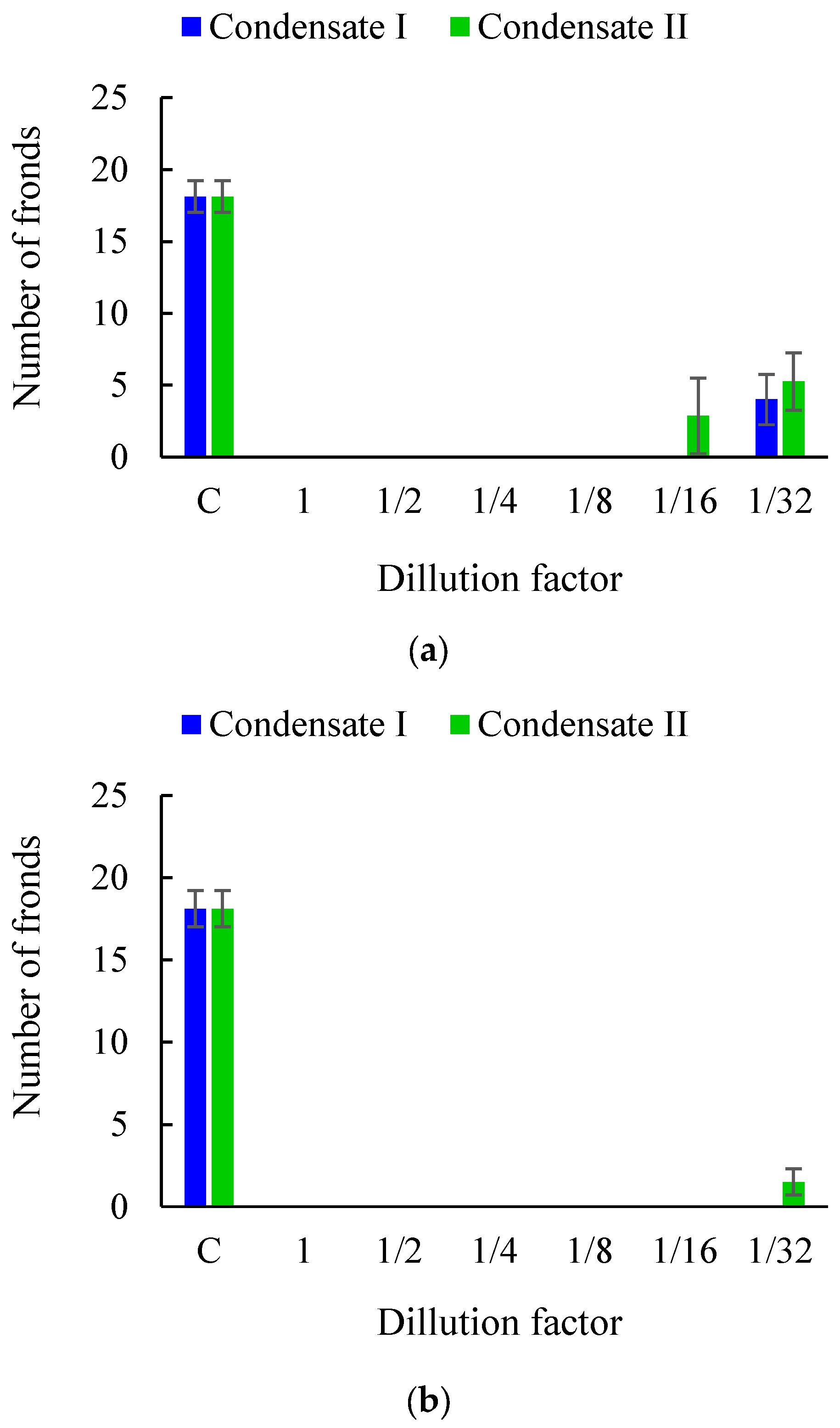
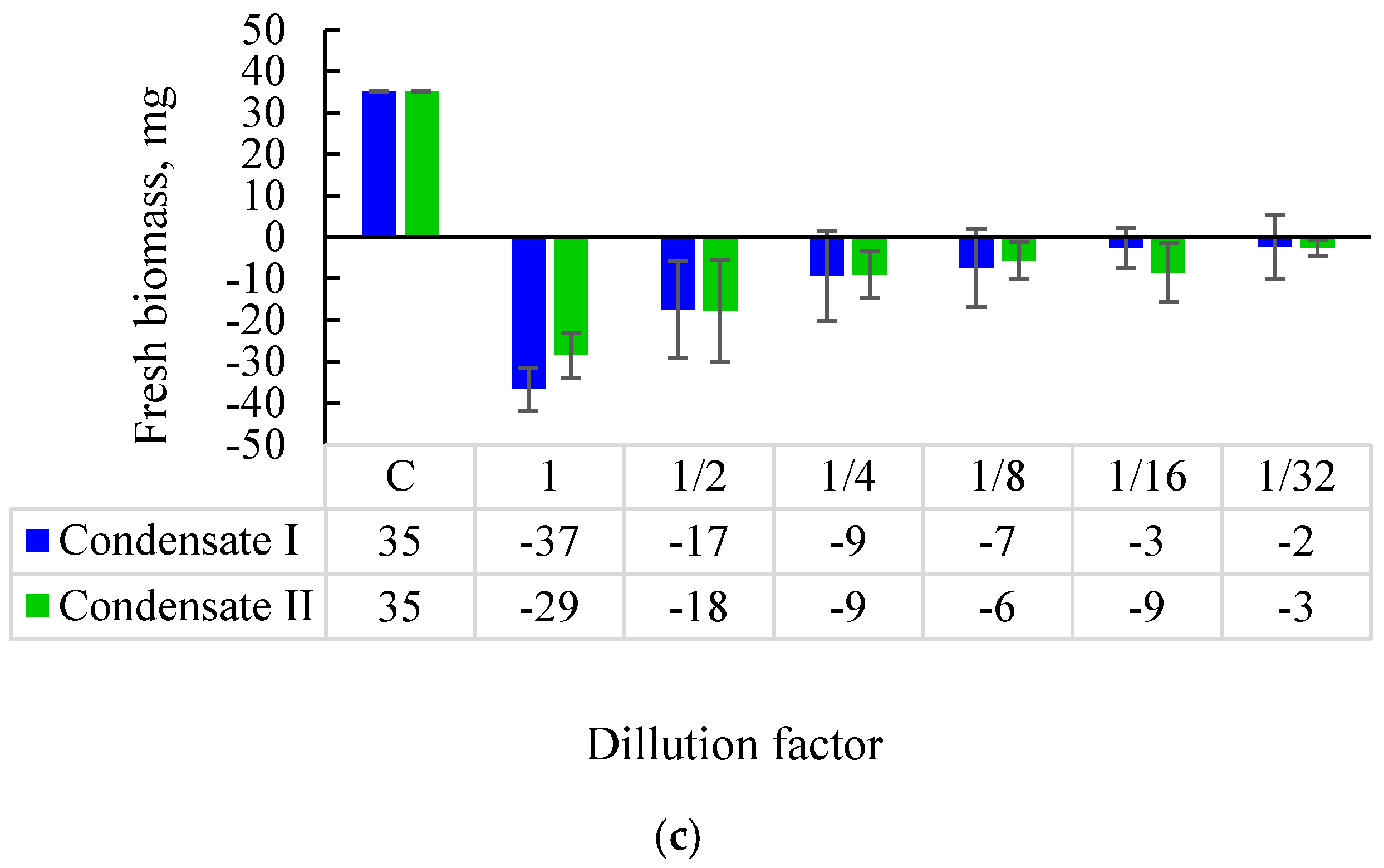
3.1. Effects of Soil Tar Contamination on Germination and Growth of Terrestrial Plants and Aquatic Organisms
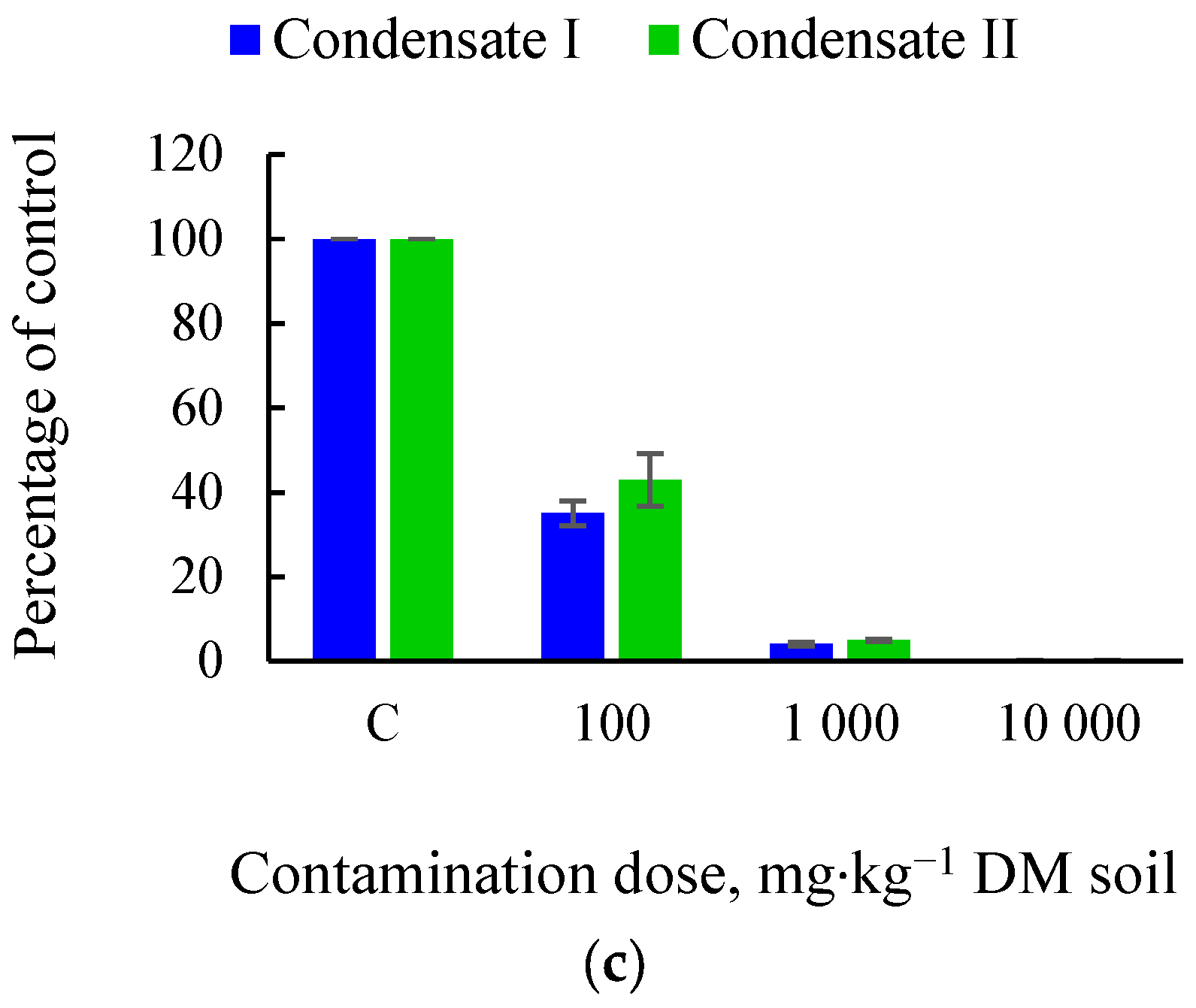
3.2. Impact of Tar on the Soil Microbiome and Soil Fertility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, A.K.M.S.; Ahiduzzaman, M. Biomass Energy: Sustainable Solution for Greenhouse Gas Emission. The 4th International Meeting of Advances in Thermofluids (IMAT 2011). AIP Conf. Proc. 2012, 1440, 23–32. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Potential contribution of biomass to the sustainable energy development. Energy Convers. Manag. 2009, 50, 1746–1760. [Google Scholar] [CrossRef]
- Harrison, S.P.; Bartlein, P.J.; Brovkin, V.; Houweling, S.; Kloster, S.; Prentice, I.C. The biomass burning contribution to climate-carbon-cycle feedback. Earth Syst. Dyn. 2018, 9, 663–677. [Google Scholar] [CrossRef]
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotechnol. 2016, 9, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tazikeh, S.; Zendehboudi, S.; Ghafoori, S.; Lohi, A.; Mahinpey, N. Algal bioenergy production and utilization: Technologies, challenges, and prospects. J. Environ. Chem. Eng. 2022, 10, 107863. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Subudhi, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Shadangi, K.P.; Srivastava, R.K.; Pattnaik, B.; Arya, R.K. Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ. Sci. Pollut. Res. 2023, 30, 8526–8539. [Google Scholar] [CrossRef]
- Greinert, A.; Mrówczyńska, M.; Szefner, W. The use of waste biomass from the wood industry and municipal sources for energy production. Sustainability 2019, 11, 3083. [Google Scholar] [CrossRef]
- Alizadeh, P.; Tabil, L.G.; Mupondwa, E.; Li, X.; Cree, D. Technoeconomic Feasibility of Bioenergy Production from Wood Sawdust. Energies 2023, 16, 1914. [Google Scholar] [CrossRef]
- Omer, A.M. Organic waste treatment for power production and energy supply. J. Cell Anim. Biol. 2007, 1, 034–047. Available online: http://www.academicjournals.org/JCAB (accessed on 23 January 2018).
- Ju, X.; Zhang, F.; Bao, X.; Römheld, V.; Roelcke, M. Utilization and management of organic wastes in Chinese agriculture: Past, present and perspectives. Sci. China Ser. C Life Sci. 2005, 48, 965–979. [Google Scholar] [CrossRef]
- Yılmaz, S.; Selim, H. A review on the methods for biomass to energy conversion systems design. Renew. Sustain. Energy Rev. 2013, 25, 420–430. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Chapter 8—Biomass Conversion Technologies. In Greenhouse Gas Balances of Bioenergy Systems; Thornley, P., Adams, P., Eds.; Academic Press: New York, NY, USA, 2018; pp. 107–139. [Google Scholar]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.B. Biomass-Fired Steam Power Cogeneration System: A Theoretical Study. Energy Convers. Manag. 1995, 1, 65–77. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Biorefin. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Gruber, H.; Groß, P.; Rauch, R.; Reichhold, A.; Zweiler, R.; Aichernig, C.; Müller, S.; Ataimisch, N.; Hofbauer, H. Fischer-Tropsch products from biomass-derived syngas and renewable hydrogen. Biomass Convers. Biorefin. 2021, 11, 2281–2292. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Wang, Y.; Yoshikawa, K.; Namioka, T.; Hashimoto, Y. Performance optimization of two-staged gasification system for woody biomass. Fuel Process. Technol. 2007, 88, 243–250. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; van Paasen, S.V.B.; Bergman, P.C.A.; Kiel, J.H.A. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Simell, P.; Hannula, I.; Tuomi, S.; Nieminen, M.; Kurkela, E.; Hiltunen, I.; Kaisalo, N.; Kihlman, J. Clean syngas from biomass-process development and concept assessment. Biomass Convers. Biorefin. 2014, 4, 357–370. [Google Scholar] [CrossRef]
- Menya, E.; Olwa, J.; Hagström, P.; Okure, M. Assessment of pollution levels resulting from biomass gasification. J. Environ. Chem. Eng. 2014, 2, 1228–1235. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Mikiciuk, M.; Izwikow, M.; Stawicki, T.; Sędłak, P. Analysis of ecotoxic influence of waste from the biomass gasification process. Environ. Sci. Pollut. Res. Int. 2017, 24, 15022–15030. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Zmuda, A.; Koniuszy, A. Thermochemical biomass conversion processes and their impact on the environment. Przem. Chem. 2019, 98, 1126–1129. [Google Scholar]
- Operating Instruction. Available online: http://www.mru-instruments.pl/produkty/analizatory-przemyslowe/varioplus-iv (accessed on 23 January 2018). (In Polish).
- PN-EN 50379-1:2013-03; Requirements for Portable Electrical Instruments for Measuring Exhaust Gas Parameters of Heating Devices. Part 1. Basic Requirements and Test Methods/Requirements for Portable Electrical Instruments for Measuring Exhaust Gas Parameters of Heating Devices. Th. 1. Basic Requirements and Test Methods. Available online: https://sklep.pkn.pl/catalogsearch/advanced (accessed on 21 September 2020). (In Polish).
- OECD. Test No. 221: Lemna sp. Growth Inhibition Test; OECD: Paris, France, 2006. [Google Scholar]
- Steinberg, R.A. Mineral requirements of Lemna minor. Plant Physiol. 1946, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Cyganov, V.A.; Žukov, R.A. Morfologobiohimičiskie osobennosti novovo vida actionomiceta. Mikrobiologiâ 1964, 33, 863–869. [Google Scholar]
- Martin, J.P. Use of acid rose bengale and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 6, 215–233. [Google Scholar] [CrossRef]
- Myśków, W. Attempts to use indicators of microbial activity to assess soil fertility. Post. Microbiol. 1981, 3–4, 173–192. (In Polish) [Google Scholar]
- Siddiqui, S.; Adams, W.A. The fate of diesel hydrocarbons in soils and their effect on the germination of perennial ryegrass. Environ. Toxicol. 2002, 17, 49–62. [Google Scholar] [CrossRef]
- Maila, M.P.; Cleote, T.E. Germination of Lepidium sativum as a method to evaluate polycyclic aromatic hydrocarbons (PAHs) removal from contaminated soil. Int. Biodeterior. Biodegrad. 2002, 50, 107–113. [Google Scholar] [CrossRef]
- Serrano, A.; Tejada, M.; Gallego, M.; Gonzalez, J.L. Evaluation of soil biological activity after a diesel fuel spill. Sci. Total Environ. 2009, 407, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Ekanem, A.N.; Osabor, V.N.; Ekpo, B.O. Polycyclic aromatic hydrocarbons (PAHs) contamination of soils and water around automobile repair workshops in Eket metropolis, Akwa Ibom State, Nigeria. SN Appl. Sci. 2019, 1, 447. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Duncan, H.J.; Alder, J. Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 2006, 141, 519–525. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Z.; Liu, Y.; Xie, X.; Huang, L.; Zhang, R.; Dong, B. Effect of pyrene-induced changes in root activity on growth of Chinese cabbage (Brassica campestris L.), and the health risks caused by pyrene in Chinese cabbage at different growth stages. Chem. Biol. Technol. Agric. 2022, 9, 7. [Google Scholar] [CrossRef]
- Koniuszy, A.; Hawrot-Paw, M.; Podsiadło, C.; Sędłak, P.; Możdżer, E. Gasification of Cup Plant (Silphium perfoliatum L.) Biomass-Energy Recovery and Environmental Impacts. Energies 2020, 13, 4960. [Google Scholar] [CrossRef]
- Henner, P.; Schiavon, M.; Druelle, V.; Lichtfouse, E. Phytotoxicity of Ancient Gaswork Soils. Effect of Polycyclic Aromatic Hydrocarbons (PAHs) on Plant Germination. Org. Geochem. 1999, 30, 963–969. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Liu, S.; Liu, N.; Lu, H.; Zhu, L. Disturbed phospholipid metabolism by three polycyclic aromatic hydrocarbons in Oryza sativa. Environ. Pollut. 2021, 283, 117073. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Li, N.; Możdżeń, K.; Zhang, Z.; Liu, C.; Zandi, P.; Sardar, M.F.; Zhu, C. Rhizosphere effect on removal and bioavailability of PAHs in contaminated agricultural soil. Biologia 2021, 76, 841–851. [Google Scholar] [CrossRef]
- Ailijiang, N.; Zhong, N.; Zhou, X.; Mamat, A.; Chang, J.; Cao, S.; Hua, Z.; Li, N. Levels, sources, and risk assessment of PAHs residues in soil and plants in urban parks of Northwest China. Sci. Rep. 2022, 12, 21448. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, A.; Krzyszczak, J. Effects of Polycyclic Aromatic Hydrocarbons on Germination and Initial Growth of Selected Lawn Grass Species in Soil Polluted with PAHs. J. Ecol. Eng. 2024, 25, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, A.; Shrestha, D.K.; Dijkwel, P.P. Primary metabolic processes as drivers of leaf ageing. Cell. Mol. Life Sci. 2021, 78, 6351–6364. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Thingujam, D.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Duckweed: Beyond an Efficient Plant Model System. Biomolecules 2024, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Zezulka, S.; Kummerová, M.; Babula, P.; Váňová, L. Lemna minor exposed to fluoranthene: Growth, biochemical, physiological and histochemical changes. Aquat. Toxicol. 2013, 140–141, 37–47. [Google Scholar] [CrossRef]
- Park, J.; Yoo, E.-J.; Shin, K.; Depuydt, S.; Li, W.; Appenroth, K.-J.; Lillicrap, A.D.; Xie, L.; Lee, H.; Kim, G.; et al. Interlaboratory Validation of Toxicity Testing Using the Duckweed Lemna minor Root-Regrowth Test. Biology 2022, 11, 37. [Google Scholar] [CrossRef]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Detecting the effects of hydrocarbon pollution in the Amazon forest using hyperspectral satellite images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef]
- Li, J.; Cao, X.; Jia, X.; Liu, L.; Cao, H.; Qin, W.; Li, M. Iron Deficiency Leads to Chlorosis through Impacting Chlorophyll Synthesis and Nitrogen Metabolism in Areca catechu L. Front. Plant Sci. 2021, 12, 710093. [Google Scholar] [CrossRef]
- Chidikofan, G.; Benoist, A.; Sawadogo, M.; Volle, G.; Valette, J.; Coulibaly, Y.; Pailhes, J.; Pinta, F. Assessment of environmental impacts of tar releases from a biomass gasifier power plant for decentralized electricity generation. Energy Procedia 2017, 118, 158–163. [Google Scholar] [CrossRef]
- Paolacci, S.; Harrison, S.; Jansen, M.A.K. A comparative study of the nutrient responses of the invasive duckweed Lemna minuta, and the native, co-generic species Lemna minor. Aquat. Bot. 2016, 134, 47–53. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef]
- Haynes, R.J. Soil organic matter quality and the size and activity of the microbial biomass: Their significance to the quality of agricultural soils. In Soil Mineral-Microbe Organic Interactions: Theories and Applications; Huang, Q., Huang, P.M., Violante, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 201–230. [Google Scholar]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A meta-analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Pacholak, E.; Zydlik, Z.; Rutkowski, K. Effect of cherry nitrogen fertilization on the content of minerals in the leaves and soil. Acta Sci. Pol. Hortorum Cultus 2011, 10, 105–112. [Google Scholar]
- Moral, F.J.; Rebollo, F.J. Characterization of soil fertility using the Rasch model. J. Soil Sci. Plant Nutr. 2017, 17, 486–498. [Google Scholar] [CrossRef]
- Myśków, W. Methodological remarks on microbiological studies of cultivated soils diversified under the influence of agrotechnical treatments. Post. Microbiol. 1986, 35, 319–331. (In Polish) [Google Scholar]
- Hawrot-Paw, M.; Martynus, M.; Koniuszy, A. Microbial Metabolic Activity of Soils Contaminated with Diesel Oil and Biodiesel. Ochr. Srodowiska 2017, 39, 25–30. [Google Scholar]
- Alrumman, S.A.; Standing, D.B.; Paton, G.I. Effects of hydrocarbon contamination on soil microbial community and enzyme activity. J. King Saud Univ. Sci. 2015, 27, 31–41. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Frazier, R.S.; Kumar, A. Comparative Life Cycle Assessment of Gasification and Landfilling for Disposal of Municipal Solid Wastes. Energies 2021, 14, 7032. [Google Scholar] [CrossRef]





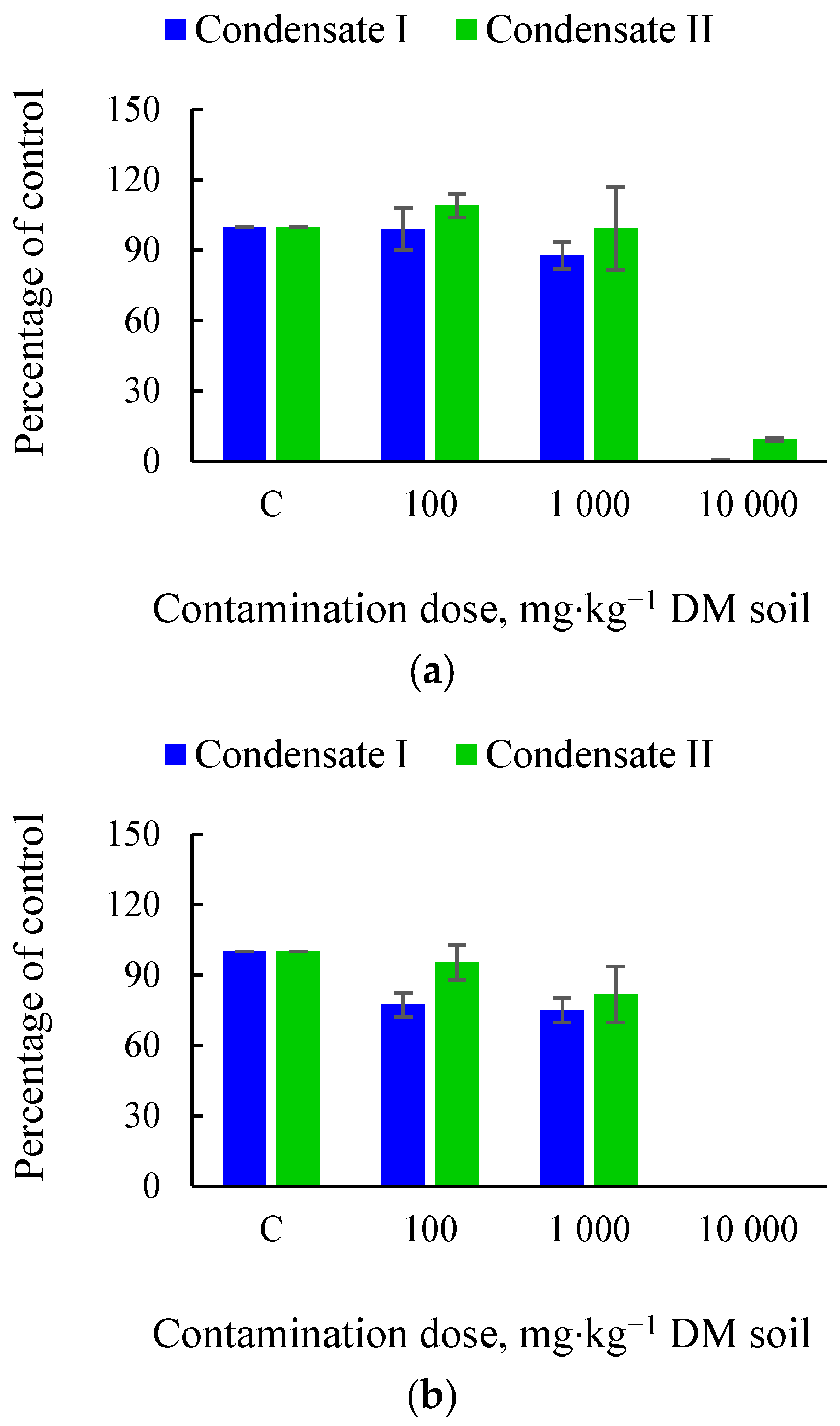
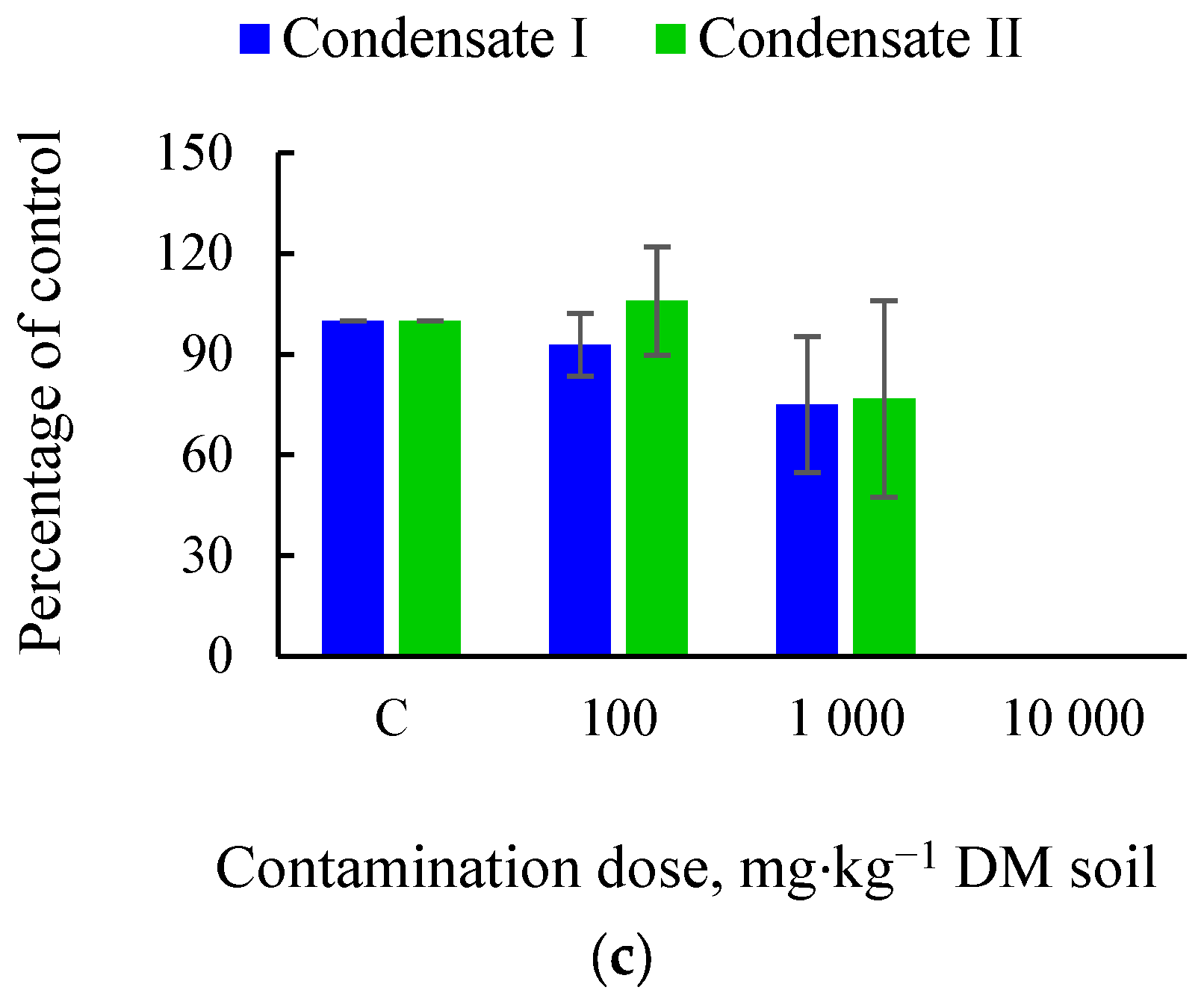
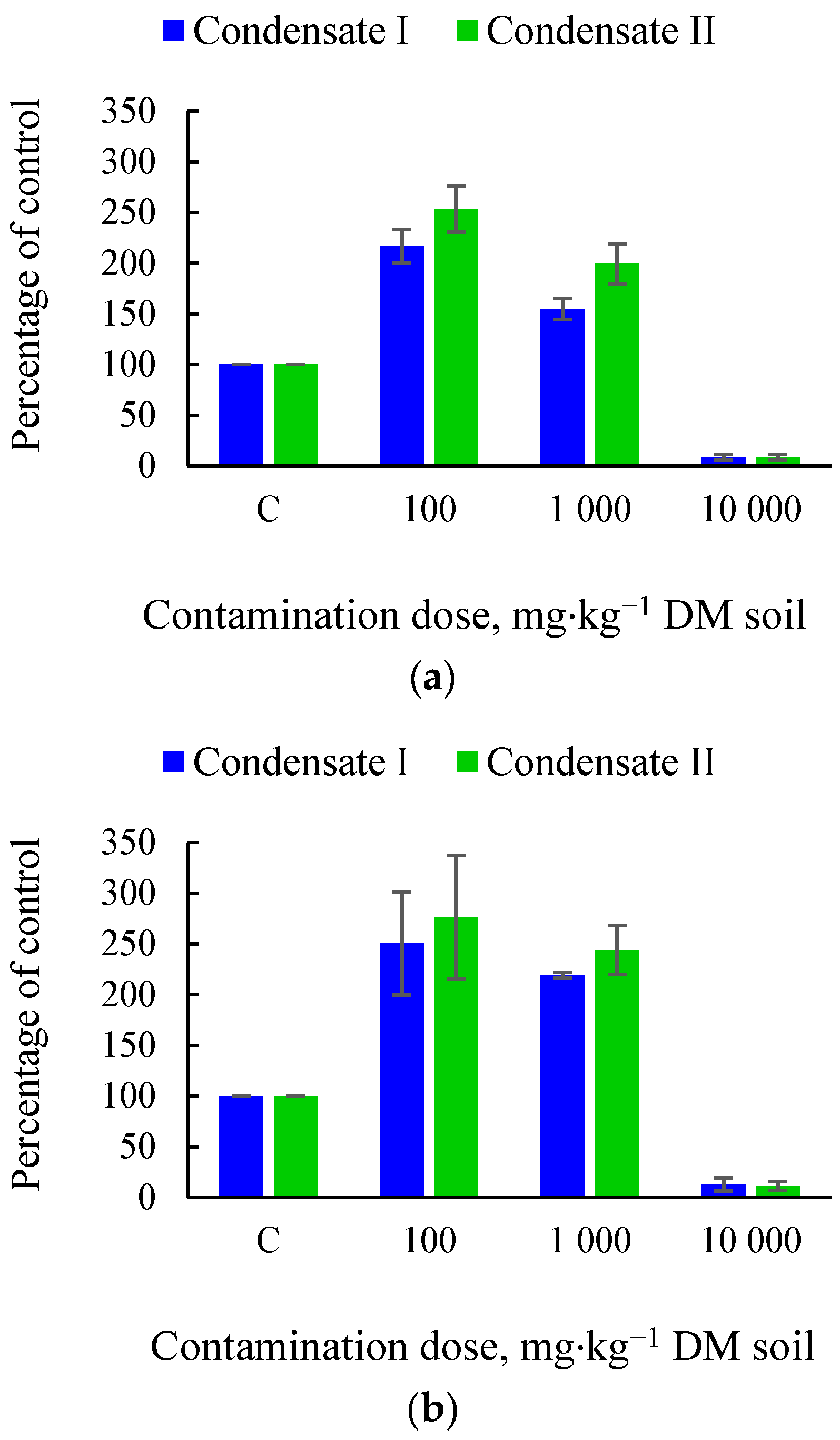
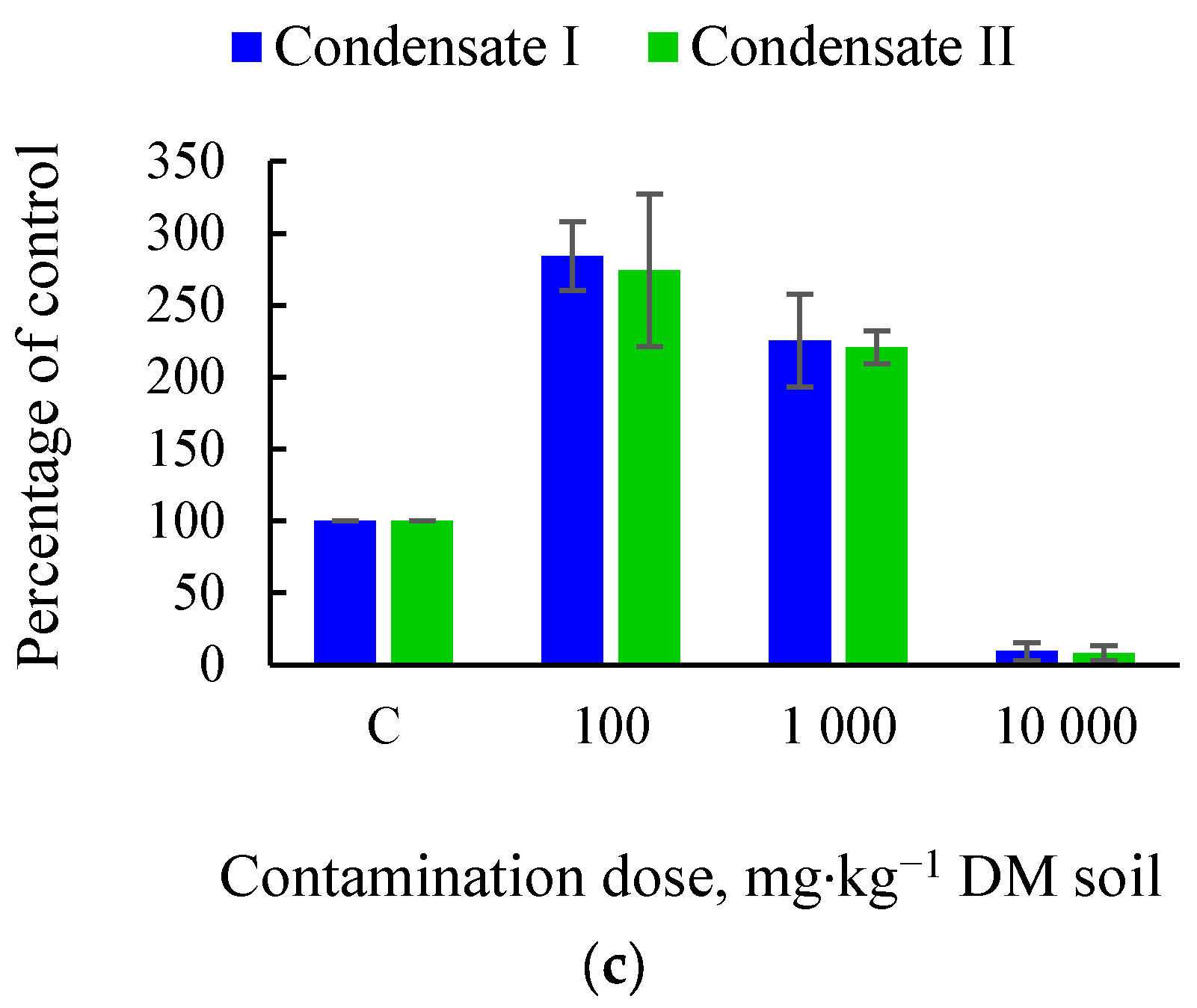
| Objects | Condensate | Contamination Dose, mg·kg−1 DM Soil | |||
|---|---|---|---|---|---|
| 0 | 100 | 1000 | 10,000 | ||
| CR | I | 45 | 20 | 3 | 1 |
| II | 45 | 18 | 2 | 3 | |
| CF:PP | I | 45 | 10 | 2 | 3 |
| II | 45 | 10 | 2 | 3 | |
| PP | I | 45 | 6 | 1 | 1 |
| II | 45 | 7 | 2 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Koniuszy, A.; Borusiewicz, A.; Skibko, Z.; Romaniuk, W.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of Tar from Coffee Grounds and Pine Pellet Gasification Process. Sustainability 2024, 16, 6291. https://doi.org/10.3390/su16156291
Hawrot-Paw M, Koniuszy A, Borusiewicz A, Skibko Z, Romaniuk W, Zając G, Szyszlak-Bargłowicz J. Ecotoxicity of Tar from Coffee Grounds and Pine Pellet Gasification Process. Sustainability. 2024; 16(15):6291. https://doi.org/10.3390/su16156291
Chicago/Turabian StyleHawrot-Paw, Małgorzata, Adam Koniuszy, Andrzej Borusiewicz, Zbigniew Skibko, Wacław Romaniuk, Grzegorz Zając, and Joanna Szyszlak-Bargłowicz. 2024. "Ecotoxicity of Tar from Coffee Grounds and Pine Pellet Gasification Process" Sustainability 16, no. 15: 6291. https://doi.org/10.3390/su16156291






