Temporal Variations in Urban Air Pollution during a 2021 Field Campaign: A Case Study of Ethylene, Benzene, Toluene, and Ozone Levels in Southern Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
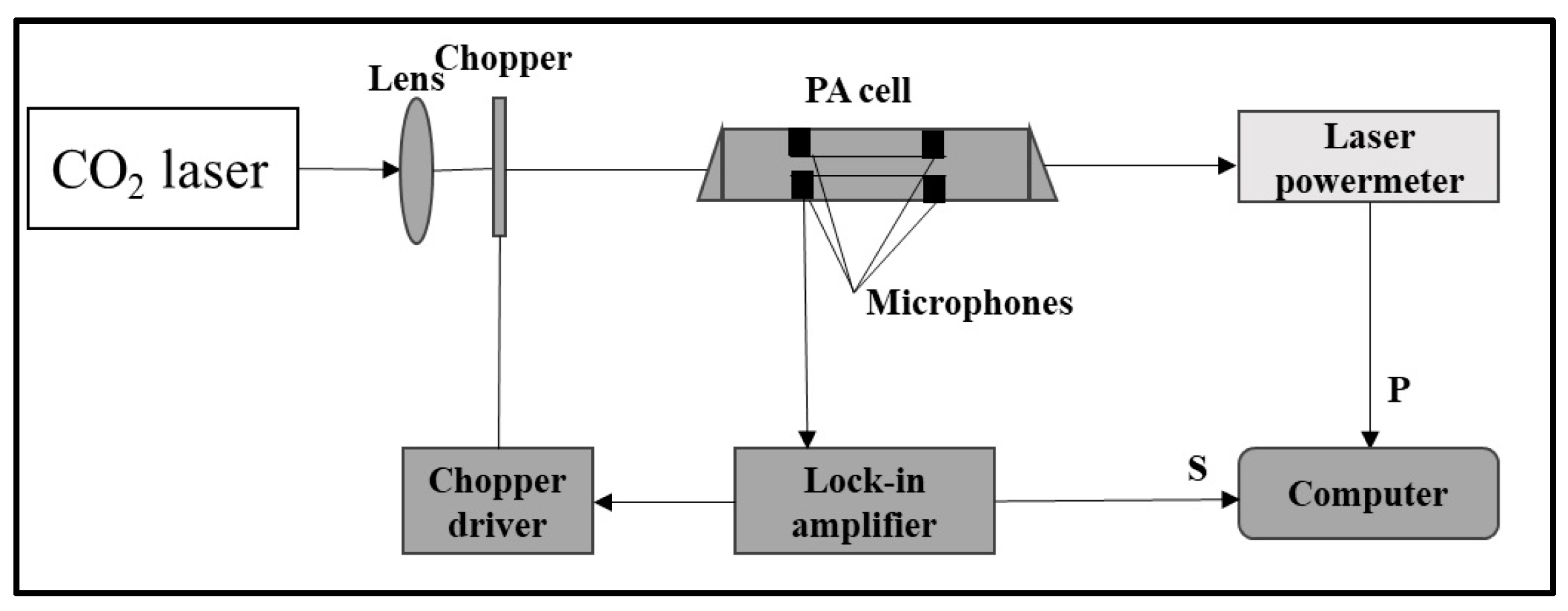
2.2. Laser Photoacoustic Spectroscopy Method and Passive Air Sampling
- -
- c [atm] represents the trace gas concentration;
- -
- V [V] denotes the PA signal (peak-to-peak value);
- -
- α [cm−1atm−1] is the gas absorption coefficient at a specified wavelength;
- -
- PL [W] signifies the continuous-wave laser power (unchopped value; twice the measured average value);
- -
- SM [V Pa] is the microphone responsivity.
2.3. Ambient Meteorological Variables
3. Results
3.1. Atmospheric VOCs Measurements
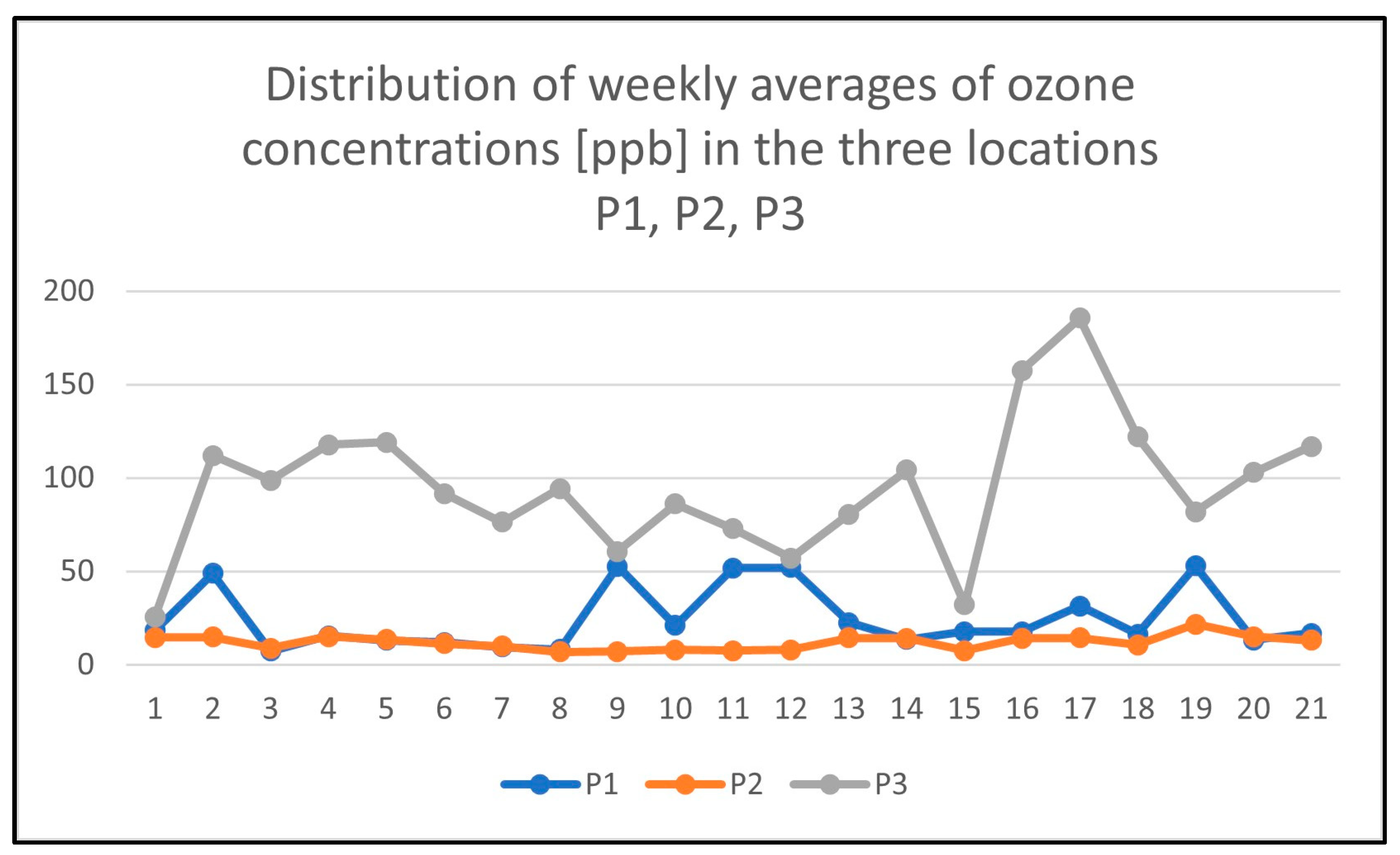
3.2. Atmospheric Ozone Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Zeng, L.; Zhou, S.; Wang, X.; Hua, J.; Zhang, X.; Gu, Z.; He, L. Combined Effects of Photochemical Processes, Pollutant Sources and Urban Configuration on Photochemical Pollutant Concentrations. Sustainability 2023, 15, 3281. [Google Scholar] [CrossRef]
- Srivastava, D.; Vu, T.V.; Tong, S.; Shi, Z.; Harrison, R.M. Formation of secondary organic aerosols from anthropogenic precursors in laboratory studies. NPJ Clim. Atmos. Sci. 2022, 5, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Liu, X.; Zhang, Y.; Hui, L.; Kong, L.; Zhang, Y.; Zhang, C.; Qu, Y.; An, J.; et al. Characterization and sources of volatile organic compounds (VOCs) and their related changes during ozone pollution days in 2016 in Beijing, China. Environ. Pollut. 2019, 257, 113599. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shon, Z.; Kang, Y.; Kim, K.; Han, S.; Kang, M.; Bang, J.; Oh, I. Source apportionment of VOCs and their impact on air quality and health in the megacity of Seoul. Environ. Pollut. 2019, 247, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Murtadah, I.; Al-Sharify, Z.T.; Hasan, M.B. Atmospheric Concentration Saturated and Aromatic Hydrocarbons Around Dura Refinery. IOP Conf. Ser. Mater. Sci. Eng. 2020, 870, 012033. [Google Scholar] [CrossRef]
- Pilia, I.; Campagna, M.; Marcias, G.; Fabbri, D.; Meloni, F.; Spatari, G.; Cottica, D.; Cocheo, C.; Grignani, E.; De-Giorgio, F.; et al. Biomarkers of Low-Level Environmental Exposure to Benzene and Oxidative DNA Damage in Primary School Children in Sardinia, Italy. Int. J. Environ. Res. Public Health 2021, 18, 4644. [Google Scholar] [CrossRef]
- Cruz, L.P.S.; Santos, D.F.; dos Santos, I.F.; Gomes, I.V.S.; Santos, A.V.S.; Souza, K.S.P.P. Exploratory analysis of the atmospheric levels of BTEX, criteria air pollutants and meteorological parameters in a tropical urban area in Northeastern Brazil. Microchem. J. 2020, 152, 104265. [Google Scholar] [CrossRef]
- Malakootian, M.; Maleki, S.; Rajabi, S.; Hasanzadeh, F.; Nasiri, A.; Mohammdi, A.; Faraji, M. Source identification, spatial distribution and ozone formation potential of benzene, toluene, ethylbenzene, and xylene (BTEX) emissions in Zarand, an industrial city of southeastern Ira. JAPH 2022, 7, 217–232. [Google Scholar] [CrossRef]
- Rodriguez Valido, M.; Gomez-Cardenes, O.; Magdaleno, E. Monitoring Vehicle Pollution and Fuel Consumption Based on AI Camera System and Gas Emission Estimator Model. Sensors 2023, 23, 312. [Google Scholar] [CrossRef]
- Royer, S.-J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of methane and ethylene from plastic in the environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Billard, G. The challenges of measuring plastic pollution. Field Actions Sci. Rep. 2018, 19, 68–75. [Google Scholar]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Natividade Vieira, M. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Han, Y.J.; Beck, W.; Mewis, I.; Förster, N.; Ulrichs, C. Effect of Ozone Stresses on Growth and Secondary Plant Metabolism of Brassica campestris L. ssp. chinensis. Horticulturae 2023, 9, 966. [Google Scholar] [CrossRef]
- Soares, A.R.; Neto, D.; Avelino, T.; Silva, C. Ground Level Ozone Formation Near a Traffic Intersection: Lisbon “Rotunda De Entrecampos” Case Study. Energies 2020, 13, 1562. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Poulopoulos, S.G. Chapter 2—Atmospheric Environment. Environment and Development. In Basic Principles, Human Activities, and Environmental Implications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 45–136. ISBN 9780444627339. [Google Scholar] [CrossRef]
- Ma, M.; Liu, M.; Liu, M.; Xing, H.; Wang, Y.; Meng, F. Spatiotemporal Patterns and Quantitative Analysis of Factors Influencing Surface Ozone over East China. Sustainability 2024, 16, 123. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, J.; Yu, H.; Shang, H. Effects of Elevated Ozone Levels on Photosynthesis, Biomass and Non-structural Carbohydrates of Phoebe bournei and Phoebe zhennan in Subtropical China. Front. Plant Sci. 2018, 9, 1764. [Google Scholar] [CrossRef]
- Iordache, A.; Iordache, M.; Sandru, C.; Voica, C.; Stegarus, D.; Zgavarogea, R.; Ionete, R.E.; Ticu, S.C.; Miricioiu, M.G.A. Fugacity Based Model for the Assessment of Pollutant Dynamic Evolution of VOCS and BTEX in the Olt River Basin (Romania). Rev. Chim. 2019, 70, 3456–3463. [Google Scholar] [CrossRef]
- Popitanu, C.; Cioca, G.; Copolovici, L.; Iosif, D.; Munteanu, F.-D.; Copolovici, D. The Seasonality Impact of the BTEX Pollution on the Atmosphere of Arad City, Romania. Int. J. Environ. Res. Public Health 2021, 18, 4858. [Google Scholar] [CrossRef]
- Petrus, M.; Popa, C.; Bratu, A.-M. Determination of Ozone Concentration Levels in Urban Environments Using a Laser Spectroscopy System. Environments 2024, 11, 9. [Google Scholar] [CrossRef]
- Henderson, B.; Khodabakhsh, A.; Metsälä, M.; Ventrillard, I.; Schmidt, F.M.; Romanini, D.; Ritchie, G.A.D.; te Lintel Hekkert, S.; Briot, R.; Risby, T.; et al. Laser spectroscopy for breath analysis: Towards clinical implementation. Appl. Phys. B 2018, 124, 161. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T. Photoacoustic spectroscopy for process analysis. Anal. Bioanal. Chem. 2006, 384, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Palzer, S. Photoacoustic-Based Gas Sensing: A Review. Sensors 2020, 20, 2745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Y.; Adamu, A.I.; Dasa, M.K.; Antoni-Lopez, J.E.; Amezcua-Correa, R.; Markos, C. Mid-infrared photoacoustic gas monitoring driven by a gas-filled hollow-core fiber laser. Sci. Rep. 2021, 11, 3512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, T.; Wang, G.; He, S. A Dual-Gas Sensor Using Photoacoustic Spectroscopy Based on a Single Acoustic Resonator. Appl. Sci. 2021, 11, 5224. [Google Scholar] [CrossRef]
- Roba, C.; Rosu, C.; Stefanie, H.; Török, Z.; Kovacs, M.; Ozunu, A. Determination of volatile organic compounds and particulate matter levels in an urban area from Romania. Environ. Eng. Manag. J. 2014, 13, 2261–2268. [Google Scholar] [CrossRef]
- Petrus, M.; Popa, C.; Bratu, A.-M. Ammonia Concentration in Ambient Air in a Peri-Urban Area Using a Laser Photoacoustic Spectroscopy Detector. Materials 2022, 15, 3182. [Google Scholar] [CrossRef] [PubMed]
- Dumitras, D.C.; Banita, S.; Bratu, A.M.; Cernat, R.; Dutu, D.C.A.; Matei, C.; Patachia, M.; Petrus, M.; Popa, C. Ultrasensitive CO2 laser photoacoustic system. Infrared Phys. Technol. 2010, 53, 308–314. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Magureanu, A.; Petrus, M.; Popa, C. Laser photoacoustic spectroscopy: Principles, instrumentation, and characterization. J. Optoelectron. Adv. Mater. 2007, 9, 3655. [Google Scholar]
- Bratu, A.M.; Popa, C.; Matei, C.; Banita, S.; Dutu, D.C.A.; Dumitras, D.C. Removal of interfering gases in breath biomarker measurements. J. Optoelectron. Adv. Mater. 2011, 13, 1045–1050. [Google Scholar]
- Mayer, A.; Comera, J.; Charpentier, H.; Jaussaud, C. Absorption coefficients of various pollutant gases at CO2 laser wavelengths; application to the remote sensing of those pollutants. Appl. Opt. 1978, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Persson, U. Absorption coefficients at CO2 laser wavelengths for toluene, m-xylene, o-xylene, and p-xylene. Appl. Opt. 1984, 23, 1302–1303. [Google Scholar] [CrossRef]
- Codnia, J.; Azcárate, M.L. Absorption coefficients of O3 at CO2 laser wavelengths. Opt. Lasers Eng. 2003, 39, 619–627. [Google Scholar] [CrossRef]
- Cao, X.; Yi, J.; Li, Y.; Zhao, M.; Duan, Y.; Zhang, F.; Duan, L. Characteristics and Source Apportionment of Volatile Organic Compounds in an Industrial Area at the Zhejiang–Shanghai Boundary, China. Atmosphere 2024, 15, 237. [Google Scholar] [CrossRef]
- Deng, C.X.; Jin, Y.J.; Zhang, M.; Liu, X.W.; Yu, Z.M. Emission Characteristics of VOCs from On-Road Vehicles in an Urban Tunnel in Eastern China and Predictions for 2017–2026. Aerosol Air Qual. Res. 2018, 18, 3025–3034. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Mo, Z.; Zhang, Z.; Wang, X.; Yin, S.; Peng, K.; Yang, Y.; Fen, X.; Cai, H. Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Deng, H.; Bai, Z.; Kong, S.; Wang, X.; Hao, J.; Han, X.; Ning, P. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515, 101–108. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S. Compilation of a source profile database for hydrocarbon and OVOC emissions in China. Atmos. Environ. 2016, 143, 209–217. [Google Scholar] [CrossRef]
- Wang, M.; Hu, K.; Chen, W.; Shen, X.; Li, W.; Lu, X. Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation. Atmosphere 2020, 11, 1205. [Google Scholar] [CrossRef]
- Song, M.; Li, X.; Yang, S.; Yu, X.; Zhou, S.; Yang, Y.; Chen, S.; Dong, H.; Liao, K.; Chen, Q.; et al. Spatiotemporal variation, sources, and secondary transformation potential of volatile organic compounds in Xi’an, China. Atmos. Chem. Phys. 2021, 21, 4939–4958. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Liu, Z.; Sun, Y.; Wang, C.; Xiao, Y.; Lu, X.; Yan, G.; Xu, C. Characteristics, Secondary Transformation Potential and Health Risks of Atmospheric Volatile Organic Compounds in an Industrial Area in Zibo, East China. Atmosphere 2023, 14, 158. [Google Scholar] [CrossRef]
- Khattab, A.; Levetin, E. Effect of sampling height on the concentration of airborne fungal spores. Ann. Allergy Asthma Immunol. 2008, 101, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, S.; Zhao, W.; Wang, Y.; Zhang, Q.; Qu, C.; Liu, X.; Wen, X. Impacts of Meteorological Factors, VOCs Emissions and Inter-Regional Transport on Summer Ozone Pollution in Yuncheng. Atmosphere 2021, 12, 1661. [Google Scholar] [CrossRef]
- Maji, S.; Beig, G.; Yadav, R. Winter VOCs and OVOCs measured with PTR-MS at an urban site of India: Role of emissions, meteorology and photochemical sources. Environ. Pollut. 2020, 258, 113651. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, F.J.; Alonso-Blanco, E.; Díaz, E.; Coz, E.; Molero, F.; Nunez, L.; Palacios, M.; Barreiro, M.; Fernández, J.; Salvador, P. On the influence of VOCs on new particle growth in a Continental-Mediterranean region. Environ. Res. Commun. 2022, 4, 125010. [Google Scholar] [CrossRef]
- Marando, F.; Salvatori, E.; Fusaro, L.; Manes, F. Removal of PM10 by forests as a nature-based solution for air quality improvement in the metropolitan city of Rome. Forests 2016, 7, 150. [Google Scholar] [CrossRef]
- Tallis, M.; Taylor, G.; Sinnett, D.; Freer-Smith, P. Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landsc. Urban Plan. 2011, 103, 129–138. [Google Scholar] [CrossRef]
- Žlender, V.; Thompson, C.W. Accessibility and use of peri-urban green space for inner-city dwellers: A comparative study. Landsc. Urban Plan. 2017, 165, 193–205. [Google Scholar] [CrossRef]
- Hans-Örjan, N. Natural formation of ethylene in forest soils and methods to correct results given by the acetylene-reduction assay. Soil Biol. Biochem. 1983, 15, 281–286. [Google Scholar] [CrossRef]
- Cai, M.; Ren, Y.; Gibilisco, R.G.; Grosselin, B.; McGillen, M.R.; Xue, C.; Mellouki, A.; Daële, V. Ambient BTEX Concentrations during the COVID-19 Lockdown in a Peri-Urban Environment (Orléans, France). Atmosphere 2022, 13, 10. [Google Scholar] [CrossRef]
- Garg, A.; Gupta, N.; Tyagi, S. Study of seasonal and spatial variability among Benzene, Toluene, and p-Xylene (BTp-X) in ambient air of Delhi, India. Pollution 2019, 5, 135–146. [Google Scholar] [CrossRef]
- Salvador, C.M.; Chou, C.C.K.; Ho, T.T.; Ku, I.T.; Tsai, C.Y.; Tsao, T.M.; Tsai, M.J.; Su, T.C. Extensive urban air pollution footprint evidenced by submicron organic aerosols molecular composition. NPJ Clim. Atmos. Sci. 2022, 5, 96. [Google Scholar] [CrossRef]
- Norris, C.L.; Edwards, R.; Ghoroi, C.; Schauer, J.J.; Black, M.; Bergin, M.H. A Pilot Study to Quantify Volatile Organic Compounds and Their Sources Inside and Outside Homes in Urban India in Summer and Winter during Normal Daily Activities. Environments 2022, 9, 75. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef]
- Menchaca-Torre, H.L.; Mercado-Hernández, R.; Mendoza-Domínguez, A. Diurnal and seasonal variation of volatile organic compounds in the atmosphere of Monterrey, Mexico. Atmos. Pollut. Res. 2015, 6, 1073–1081. [Google Scholar] [CrossRef]
- Kong, L.; Luo, T.; Jiang, X.; Zhou, S.; Huang, G.; Chen, D.; Lan, Y.; Yang, F. Seasonal Variation Characteristics of VOCs and Their Influences on Secondary Pollutants in Yibin, Southwest China. Atmosphere 2022, 13, 1389. [Google Scholar] [CrossRef]
- Pateraki, S.; Asimakopoulos, D.N.; Flocas, H.A.; Maggos, T.; Vasilakos, C. The role of meteorology on different-sized aerosol fractions (PM10, PM2.5, PM2.5e10). Sci. Total Environ. 2012, 419, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, G.; Yu, Y.; Ji, Y.; An, T. Atmospheric diffusion profiles and health risks of typical VOC: Numerical modelling study. J. Clean. Prod. 2020, 275, 122982. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.-B.; Woo, D.; Bae, G.N. Influence of wind direction and speed on the transport of particle-bound PAHs in a roadway environment. Atmos. Pollut. Res. 2015, 6, 1024–1034. [Google Scholar] [CrossRef]
- Huang, Y.D.; Hou, R.W.; Liu, Z.Y.; Song, Y.; Cui, P.Y.; Kim, C.N. Effects of Wind Direction on the Airflow and Pollutant Dispersion inside a Long Street Canyon. Aerosol Air Qual. Res. 2019, 19, 1152–1171. [Google Scholar] [CrossRef]
- Yan, L.; Hu, W.; Yin, M. An Investigation of the Correlation between Pollutant Dispersion and Wind Environment: Evaluation of Static Wind Speed. Pol. J. Environ. Stud. 2021, 30, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gao, H. Study of Wind Flow Patterns and Heavy Gas Pollutants Dispersion Under Isolated Building Terrain. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Cichowicz, R.; Wielgosiński, G.; Fetter, W. Effect of wind speed on the level of particulate matter PM10 concentration in atmospheric air during winter season in vicinity of large combustion plant. J. Atmos. Chem. 2020, 77, 35–48. [Google Scholar] [CrossRef]
- Kim, C.; Henneman, L.R.F.; Choirat, C.; Zigler, C.M. Health Effects of Power Plant Emissions Through Ambient Air Quality. J. R. Stat. Soc. Ser. A Stat. Soc. 2020, 183, 1677–1703. [Google Scholar] [CrossRef]
- Ulpiani, G. On the linkage between urban heat island and urban pollution island: Three-decade literature review towards a conceptual framework. Sci. Total Environ. 2021, 751, 141727. [Google Scholar] [CrossRef] [PubMed]
- Shikwambana, L.; Kganyago, M.; Mhangara, P. Temporal Analysis of Changes in Anthropogenic Emissions and Urban Heat Islands during COVID-19 Restrictions in Gauteng Province, South Africa. Aerosol Air Qual. 2021, 21, 200437. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Wang, L.; Liu, X.; Wu, Z. Influencing Factors on Airflow and Pollutant Dispersion around Buildings under the Combined Effect of Wind and Buoyancy—A Review. Int. J. Environ. Res. Public Health 2022, 19, 12895. [Google Scholar] [CrossRef] [PubMed]
- Pinthong, N.; Thepanondh, S.; Kultan, V.; Keawboonchu, J. Characteristics and Impact of VOCs on Ozone Formation Potential in a Petrochemical Industrial Area, Thailand. Atmosphere 2022, 13, 732. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Ying, F.; White, S.J.; Jang, C.; Wu, X.; Gao, X.; Hong, S.; Shen, J.; Azzi, M.; et al. Meteorological and chemical impacts on ozone formation: A case study in Hangzhou, China. Atmos. Res. 2017, 196, 40–52. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhao, F.; Jiang, L.; Li, N. Can Green Finance Mitigate China’s Carbon Emissions and Air Pollution? An Analysis of Spatial Spillover and Mediation Pathways. Sustainability 2024, 16, 1377. [Google Scholar] [CrossRef]
- Huang, D.; Li, Q.; Wang, X.; Li, G.; Sun, L.; He, B.; Zhang, L.; Zhang, C. Characteristics and Trends of Ambient Ozone and Nitrogen Oxides at Urban, Suburban, and Rural Sites from 2011 to 2017 in Shenzhen, China. Sustainability 2018, 10, 4530. [Google Scholar] [CrossRef]
- Pyrgou, A.; Hadjinicolaou, P.; Santamouris, M. Enhanced near-surface ozone under heatwave conditions in a Mediterranean island. Sci. Rep. 2018, 8, 9191. [Google Scholar] [CrossRef] [PubMed]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Stevenson, D.S.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. 2015, 15, 8889–8973. [Google Scholar] [CrossRef]
- Khoder, M.I. Diurnal, seasonal and weekdays-weekends variations of ground level ozone concentrations in an urban area in greater Cairo. Environ. Monit. Assess. 2009, 149, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Samaei, M.R.; Shahsavani, A.; Azizi, K.; Mohammadpour, A.; Derakhshan, Z.; Giannakis, S.; Rodriguez-Chueca, J.; Bilal, M. Investigation of the Presence Volatile Organic Compounds (BTEX) in the Ambient Air and Biogases Produced by a Shiraz Landfill in Southern Iran. Sustainability 2022, 14, 1040. [Google Scholar] [CrossRef]
- Song, X.; Hao, Y. Analysis of Ozone Pollution Characteristics and Transport Paths in Xi’an City. Sustainability 2022, 14, 16146. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, S.T.; Daggupaty, S.M. Meteorological Processes and Ozone Exceedances in the Northeastern United States during the 12-16 July 1995 Episode. J. Appl. Meteorol. 1998, 37, 776–789. [Google Scholar] [CrossRef]
- Ren, S.; Ji, X.; Zhang, X.; Huang, M.; Li, H.; Wang, H. Characteristics and Meteorological Effects of Ozone Pollution in Spring Season at Coastal City, Southeast China. Atmosphere 2022, 13, 2000. [Google Scholar] [CrossRef]
- Sicard, P.; Agathokleous, E.; De Marco, A.; Paoletti, E.; Calatayud, V. Urban population exposure to air pollution in Europe over the last decades. Environ. Sci. Eur. 2021, 33, 28. [Google Scholar] [CrossRef]
- Sicard, P.; Paoletti, E.; Agathokleous, E.; Araminien, V.; Proietti, C.; Coulibaly, F.; De Marco, A. Ozone weekend effect in cities: Deep insights for urban air pollution control. Environ. Res. 2020, 191, 110193. [Google Scholar] [CrossRef] [PubMed]

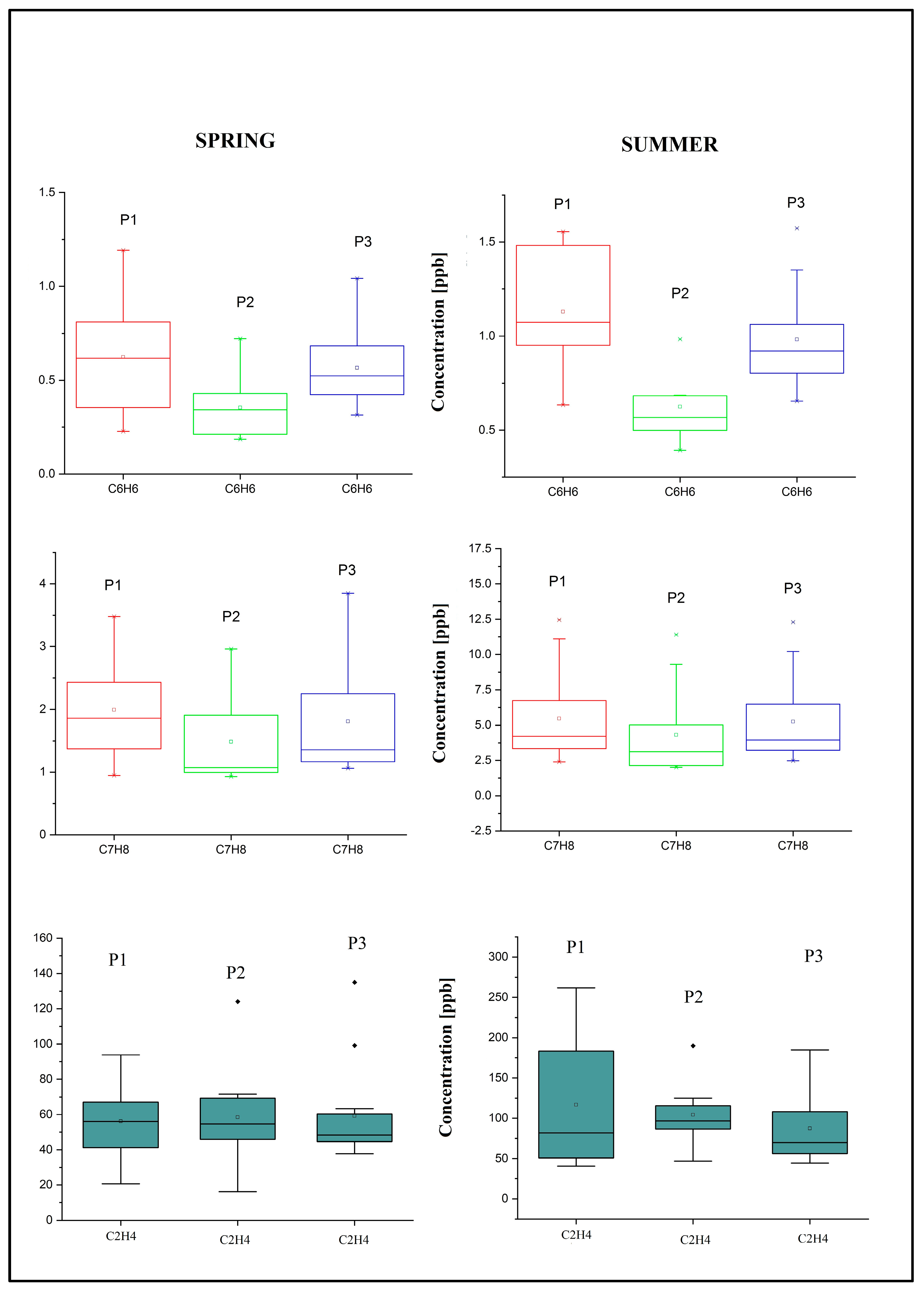
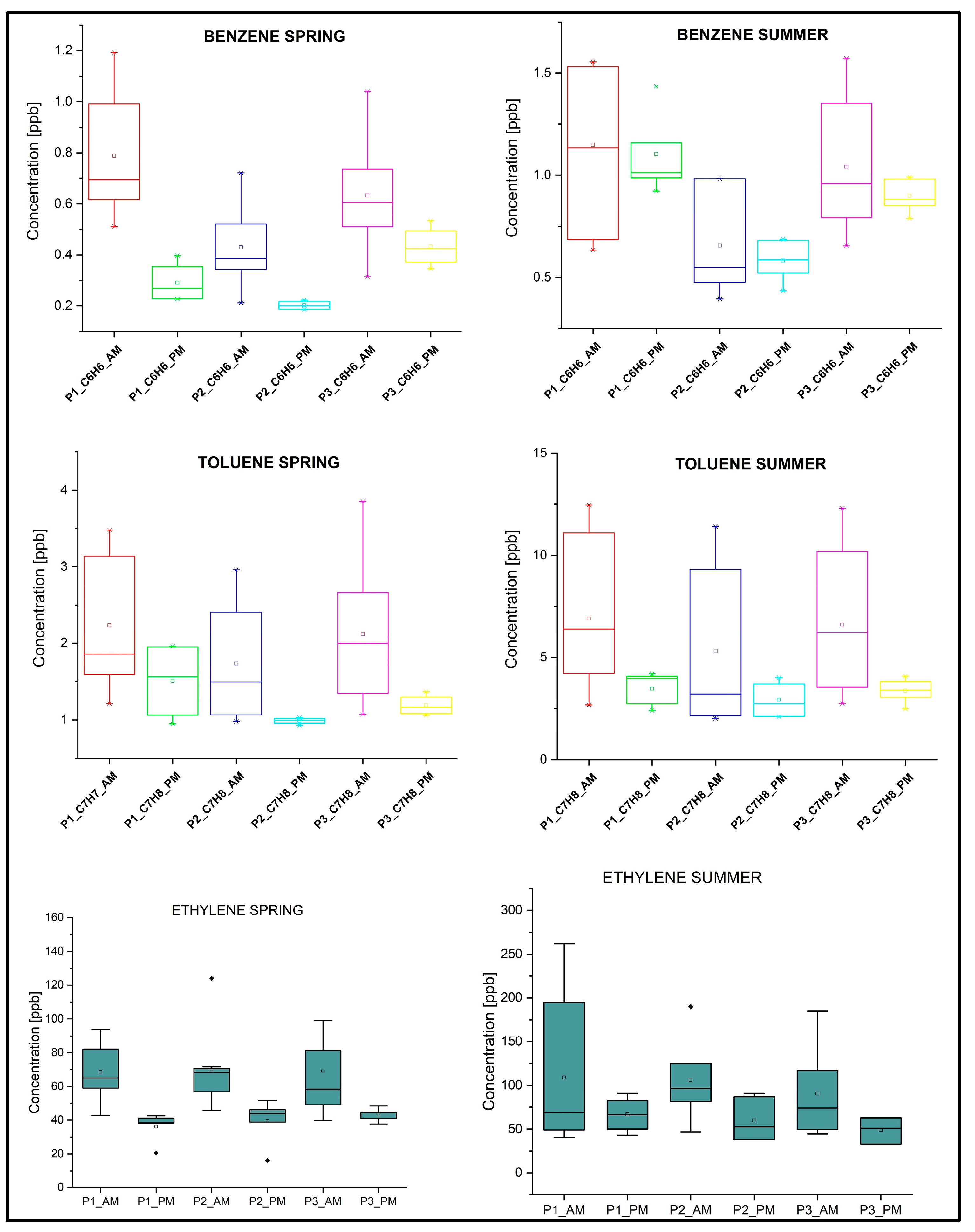
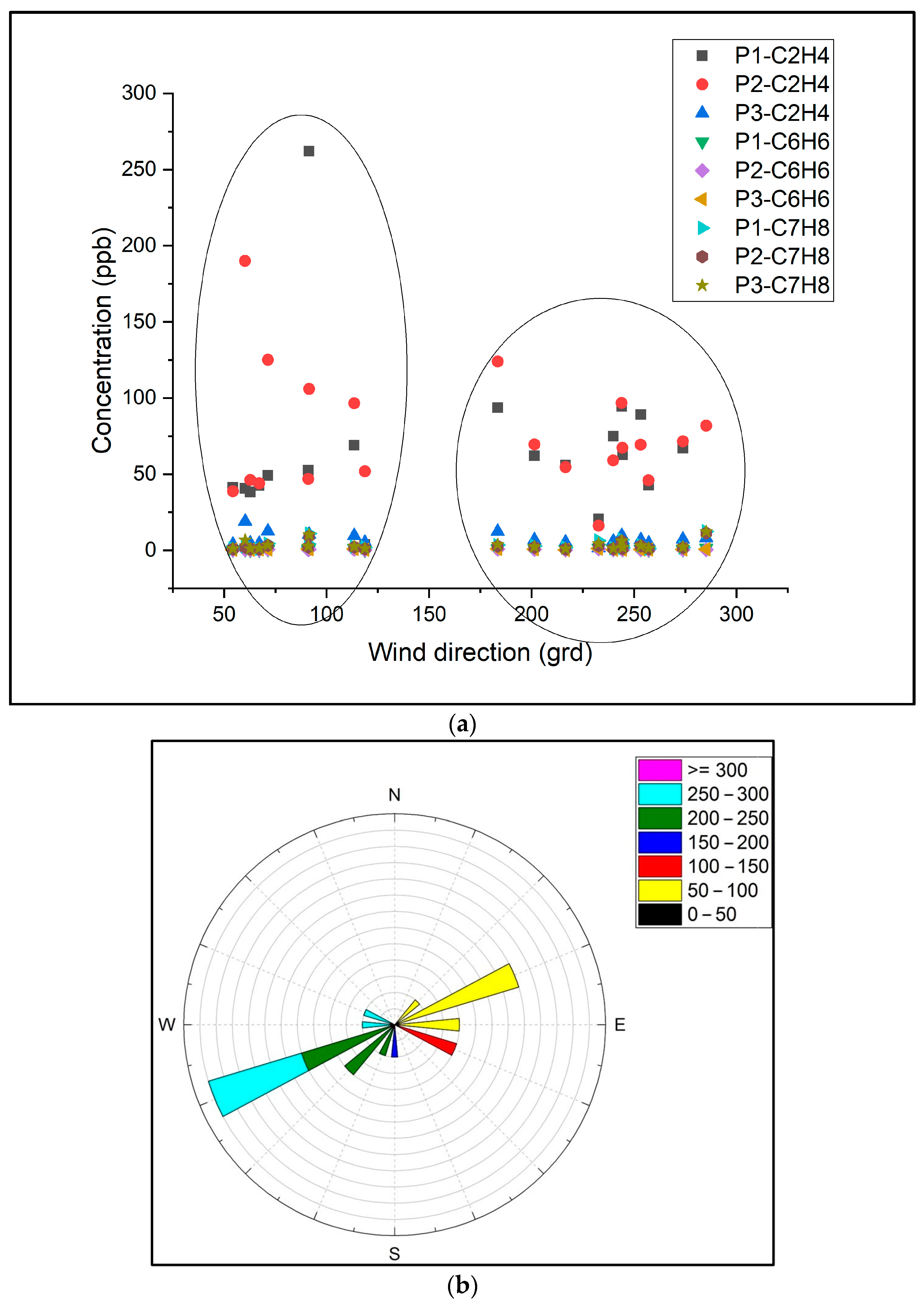
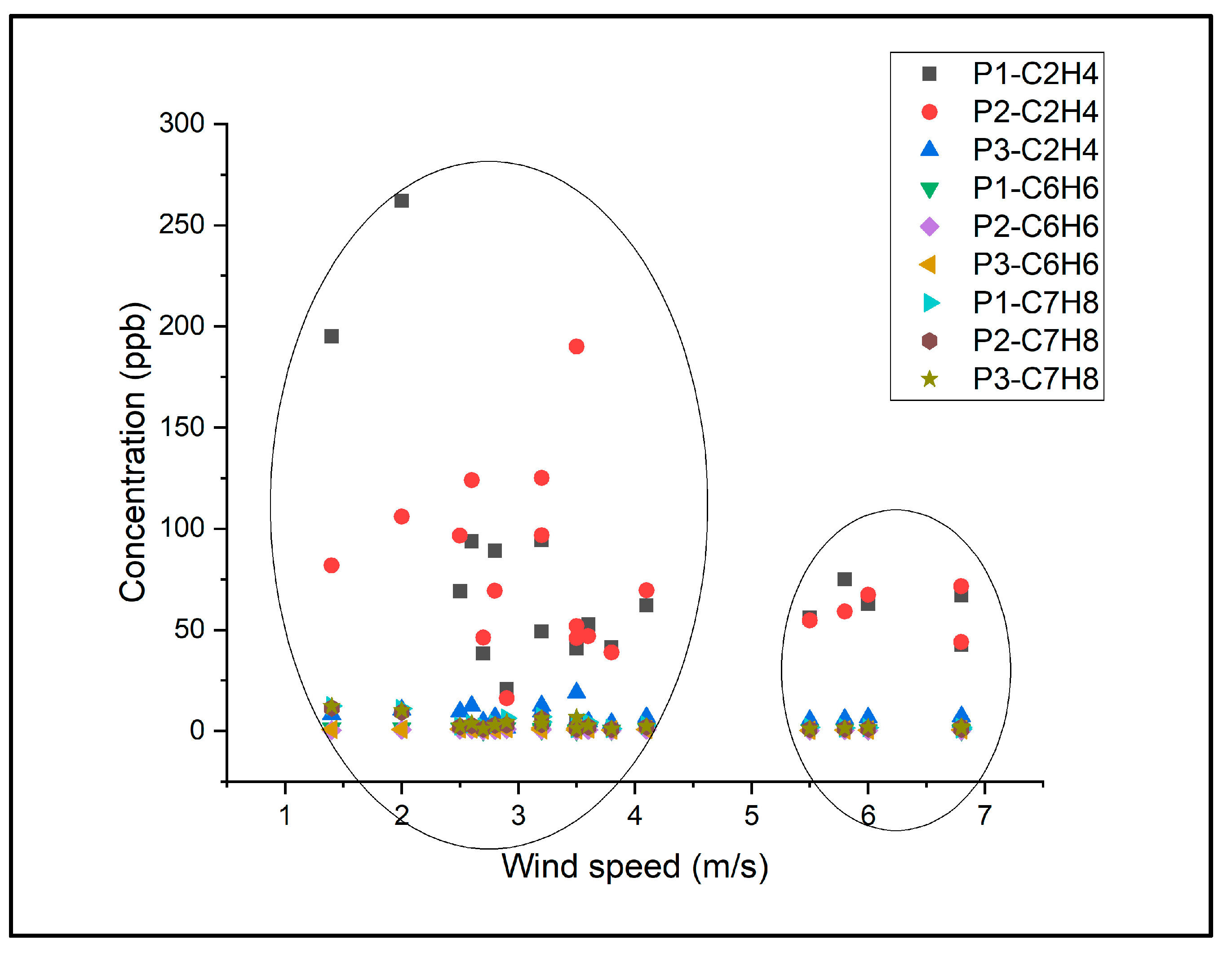


| VOCs | Spring Concentration ± SD ppb | Summer Concentration ± SD ppb | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| ethylene | 56.14 ± 21.49 | 58.34 ± 25.06 | 55.76 ± 31.61 | 116.86 ± 82.37 | 104.28 ± 41.17 | 87.23 ± 46.43 |
| benzene | 0.62 ± 0.32 | 0.35± 0.17 | 0.57± 0.21 | 1.13 ± 0.32 | 0.624 ± 0.19 | 0.98 ± 0.26 |
| toluene | 1.99 ± 0.84 | 1.49 ± 0.73 | 1.80 ± 0.88 | 5.48 ± 3.27 | 4.32 ± 3.07 | 5.26 ± 3.11 |
| Location | Spring | Summer | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P1 | P2 | P3 | |
| T/B ratio | 3.21 | 4.26 | 3.16 | 4.85 | 6.92 | 5.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrus, M.; Popa, C.; Bratu, A.-M. Temporal Variations in Urban Air Pollution during a 2021 Field Campaign: A Case Study of Ethylene, Benzene, Toluene, and Ozone Levels in Southern Romania. Sustainability 2024, 16, 3219. https://doi.org/10.3390/su16083219
Petrus M, Popa C, Bratu A-M. Temporal Variations in Urban Air Pollution during a 2021 Field Campaign: A Case Study of Ethylene, Benzene, Toluene, and Ozone Levels in Southern Romania. Sustainability. 2024; 16(8):3219. https://doi.org/10.3390/su16083219
Chicago/Turabian StylePetrus, Mioara, Cristina Popa, and Ana-Maria Bratu. 2024. "Temporal Variations in Urban Air Pollution during a 2021 Field Campaign: A Case Study of Ethylene, Benzene, Toluene, and Ozone Levels in Southern Romania" Sustainability 16, no. 8: 3219. https://doi.org/10.3390/su16083219
APA StylePetrus, M., Popa, C., & Bratu, A.-M. (2024). Temporal Variations in Urban Air Pollution during a 2021 Field Campaign: A Case Study of Ethylene, Benzene, Toluene, and Ozone Levels in Southern Romania. Sustainability, 16(8), 3219. https://doi.org/10.3390/su16083219



.jpg)

.jpg)


