The Overlooked Decomposers: Effects of Composting Materials and Duration on the Mesofauna Mediating Humification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Site and Treatments
2.2. Evaluation of the Physical–Chemical Evolution of Compost
2.3. Sample Collection and Total Compost DNA Extraction
2.4. Compost Mesofauna Microbiome Amplification
2.5. Bioinformatics and Sequence Data Statistical Analyses
3. Results
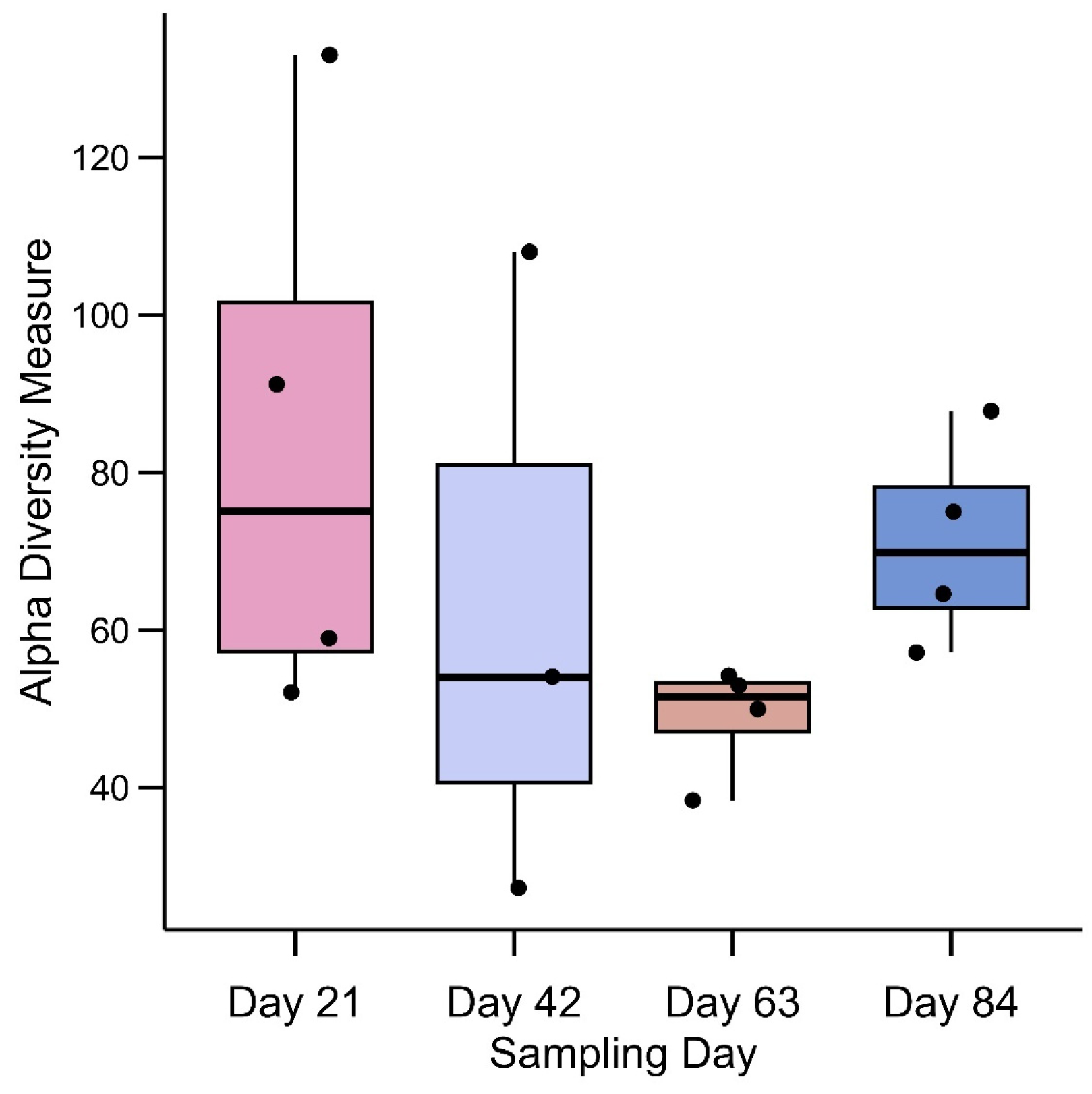
3.1. Alpha Diversity of Mesofauna Community under the Influence of Composting Materials and Duration
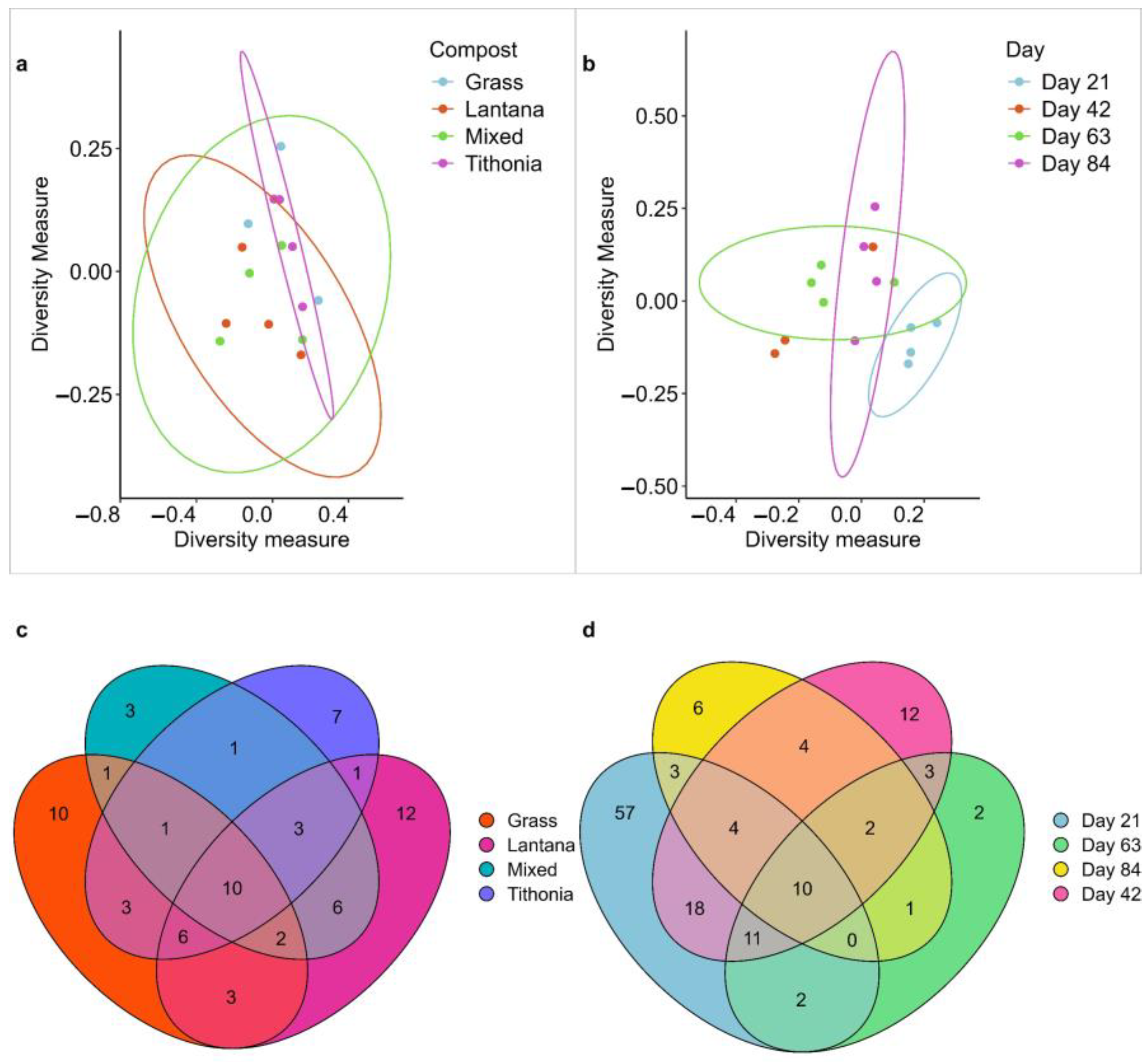
3.2. PERMANOVA and Species Composition of Compost Mesofauna
3.3. Distribution and Uniqueness of Compost Mesofauna Communities
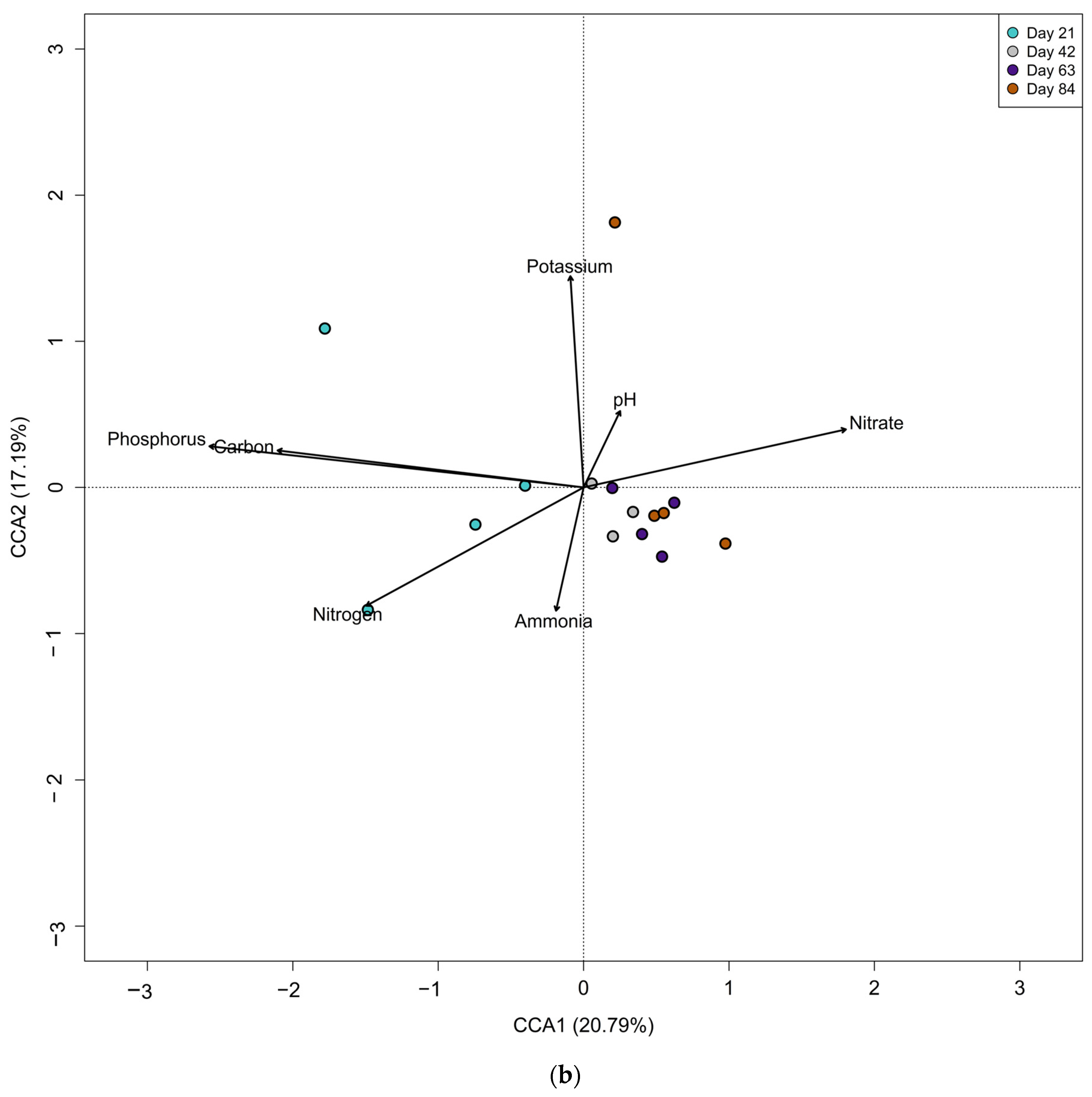
3.4. Influence of Environmental Factors on Compost Mesofauna Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wezel, A.; Herren, B.G.; Kerr, R.B.; Barrios, E.; Gonçalves, A.L.; Sinclair, F. Agroecological principles and elements and their implications for transitioning to sustainable food systems. A review. Agron. Sustain. Dev. 2020, 40, 40. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Jhariya, M.K. Resources Use Efficiency in Agriculture. Resour. Use Effic. Agric. 2020, 1, 1–760. [Google Scholar]
- Rahman, M.M.; Alam, M.S.; Kamal, M.Z.U.; Rahman, G.K.M.M. Organic Sources and Tillage Practices for Soil Management. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 283–328. Available online: https://link.springer.com/chapter/10.1007/978-981-15-6953-1_9 (accessed on 10 April 2024).
- Pérez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
- Masters-Clark, E.; Shone, E.; Paradelo, M.; Hirsch, P.R.; Clark, I.M.; Otten, W. Development of a defined compost system for the study of plant-microbe interactions. Sci. Rep. 2020, 10, 7521. [Google Scholar] [CrossRef] [PubMed]
- Lepesteur, M. Human and livestock pathogens and their control during composting. Crit. Rev. Env. Sci. Technol. 2022, 52, 1639–1683. [Google Scholar] [CrossRef]
- Shelly, P.; Neemisha; Sharma, S. Modification in the Composting Environment through Additives. Commun. Soil Sci. Plant Anal. 2022, 53, 2141–2155. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Koyama, M.; Nakasaki, K. Effects of oxygen supply rate on organic matter decomposition and microbial commu-nities during composting in a controlled lab-scale composting system. Waste Manag. 2022, 153, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, K.B. Soil macrofauna: Study problems and perspectives. Soil Biol. Biochem. 2021, 159, 108281. [Google Scholar] [CrossRef]
- Pant, M.; Negi, G.C.S.; Kumar, P. Macrofauna contributes to organic matter decomposition and soil quality in Himalayan agroe-cosystems. India. Appl. Soil Ecol. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Miller, J.J.; Battigelli, J.P.; Beasley, B.W.; Drury, C.F. Response of Soil Mesofauna to Long-Term Application of Feedlot Manure on Irrigated Cropland. J. Environ. Qual. 2017, 46, 185–192. [Google Scholar] [CrossRef]
- Geisen, S.; Briones, M.J.; Gan, H.; Behan-Pelletier, V.M.; Friman, V.P.; de Groot, G.A.; Hannula, S.E.; Lindo, Z.; Philippot, L.; Tiunov, A.V.; et al. A methodological framework to embrace soil biodiversity. Soil Biol. Biochem. 2019, 136, 107536. [Google Scholar] [CrossRef]
- Steel, H.; Bert, W. Biodiversity of compost mesofauna and its potential as an indicator of the composting process status. Dyn. Soil Dyn. Plant 2012, 5, 45–50. [Google Scholar]
- Dervash, M.; Bhat, R.; Mushtaq, N.; Singh, V. Dynamics and importance of soil mesofauna. Int. J. Adv. Res. Sci. Eng. 2018, 7, 2010–2019. [Google Scholar]
- Schröder, P. Mesofauna. In Perspectives for Agroecosystem Management: Balancing Environmental and Socio-Economic Demands; Elsevier: Amsterdam, The Netherlands, 2011; pp. 293–306. [Google Scholar]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Tang, X.; Xu, D.; Liu, B.; Mei, J. The contributions of soil mesofauna to leaf and root litter decomposition of dominant plant species in grassland. Appl. Soil Ecol. 2020, 155, 103651. [Google Scholar] [CrossRef]
- Gonçalves, F.; Carlos, C.; Crespo, L.; Zina, V.; Oliveira, A.; Salvação, J. Soil arthropods in the douro demarcated region vine-yards: General characteristics and ecosystem services provided. Sustainability 2021, 13, 7837. [Google Scholar] [CrossRef]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef]
- Coleman, D.C.; Geisen, S.; Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology Ecology and Biochemistry, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 131–159. [Google Scholar]
- Sun, M.; Chao, H.; Zheng, X.; Deng, S.; Ye, M.; Hu, F. Ecological role of earthworm intestinal bacteria in terrestrial environments: A review. Sci. Total Environ. 2020, 740, 140008. [Google Scholar] [CrossRef]
- Taguiling, L.G. Quality improvement of organic compost using green biomass. Eur. Sci. J. 2013, 9, 319–341. [Google Scholar]
- Matheri, F.; Kambura, A.K.; Mwangi, M.; Ongeso, N.; Karanja, E.; Adamtey, N. Composition, structure, and functional shifts of prokaryotic communities in response to co-composting of various nitrogenous green feedstocks. BMC Microbiol. 2023, 23, 50. [Google Scholar] [CrossRef]
- Adamtey, N. Evaluation of Agricultural and Agro-industrial Residues for Composting for Agricultural Use in Ghana (A Case Study in the Kwaebibirem District). Master’s Thesis, University of Ghana, Accra, Ghana, 2005. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; Sacred Africa: Nairobi, Kenya, 2002; Volume 21, pp. 25–26. [Google Scholar]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Reeder, J.; Knight, R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 2010, 7, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Matheri, F.; Kambura, A.K.; Mwangi, M.; Karanja, E.; Adamtey, N.; Wanjau, K. Evolution of fungal and non-fungal eukary-otic communities in response to thermophilic co-composting of various nitrogen-rich green feedstocks. PLoS ONE 2023, 18, e0286320. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, L.; Wu, B.; Liu, Y.; Li, J.; Xu, L. Improving mesophilic anaerobic digestion of food waste by side-stream thermo-philic reactor: Activation of methanogenic, key enzymes and metabolism. Water Res. 2023, 241, 120167. [Google Scholar] [CrossRef] [PubMed]
- Barus, J.; Meithasari, D.; Lumbanraja, J.; Sudarsono, H.; Hidayat, K.F.; Dermiyati. Soil mesofauna amount and diversity by return-ing fresh and compost of crops biomass waste in ultisols in-situ. Biodiversitas 2021, 22, 92–98. [Google Scholar]
- Dombos, M. Collembola of loess grassland: Effects of grazing and landscape on community composition. Soil Biol. Biochem. 2001, 33, 2037–2045. [Google Scholar] [CrossRef]
- Nitzu, E.; Nae, A.; Băncilă, R.; Popa, I.; Giurginca, A.; Plăiaşu, R. Scree habitats: Ecological function, species conservation and spatial-temporal variation in the arthropod community. Syst. Biodivers. 2014, 12, 65–75. [Google Scholar] [CrossRef]
- Bondarenko-Borisova, I.V.; Sandul, N.G. The fauna of springtails (Collembola) from the forest ecosystems of south-east Ukraine. In Bulletin of Zoology; National Academy of Sciences of Ukraine: Kyiv, Ukraine, 2002. [Google Scholar]
- Grisez, C.; Perrin, W.; Begou, M.; Jay-Robert, P.; Jacquiet, P. An initial investigation of the predatory activity of the phoretic mites of dung beetles, Macrocheles sp. (Mesostigmata: Macrochelidae), on the gastrointestinal nematode of sheep Haemonchus contortus (Strongylida: Trichostrongylidae). Biol. Control 2023, 185, 105301. [Google Scholar] [CrossRef]
- Ondrejková, K.; Fenďa, P. First records of three Pergamasinae species (Acari, Mesostig-mata, Parasitidae) from Slovakia. Check List 2022, 18, 725–731. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Onete, M. Effect of Grazing Management on Predator Soil Mite Communities (Acari: Mesotigmata) in Some Subalpine Grasslands from the Făgăraş Mountains—Romania. Insects 2023, 14, 626. [Google Scholar] [CrossRef] [PubMed]
- Peschel, K.; Norton, R.A.; Scheu, S.; Maraun, M. Do oribatid mites live in enemy-free space? Evidence from feeding experiments with the predatory mite Pergamasus septentrionalis. Soil Biol. Biochem. 2006, 38, 2985–2989. [Google Scholar] [CrossRef]
- Harta, I.; Simon, B.; Vinogradov, S.; Winkler, D. Collembola communities and soil conditions in forest plantations established in an intensively managed agricultural area. J. For. Res. 2021, 32, 1819–1832.42. [Google Scholar] [CrossRef]
- Soto-Adames, F.N. Molecular phylogeny of the Puerto Rican Lepidocyrtus and Pseudosinella (Hexapoda: Collembola), a validation of Yoshii’s “color pattern species”. Mol. Phylogenetics Evol. 2002, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Santeshwari, X.; Raghuraman, M.; Singh, J. Collembola (Insecta: Collembola) community from Varanasi and nearby regions of Uttar Pradesh, India. J. Exp. Zool. India 2015, 18, 571–577. [Google Scholar]
- Leo, C.; Nardi, F.; Cucini, C.; Frati, F.; Convey, P.; Weedon, J.T. Evidence for strong environmental control on bacterial microbiomes of Antarctic springtails. Sci. Rep. 2021, 11, 2973. Available online: https://www.nature.com/articles/s41598-021-82379-x (accessed on 26 July 2024). [CrossRef]
- Villani, M.G.; Allee, L.; Diaz, A.; Robbins, P.S. Adaptive strategies of edaphic arthropods. Annu. Rev. Entomol. 1999, 44, 233–256. [Google Scholar] [CrossRef]
- Wehner, K.; Scheu, S.; Maraun, M. Resource availability as driving factor of the reproductive mode in soil microarthropods (Acari, Oribatida). PLoS ONE 2014, 9, e104243. [Google Scholar] [CrossRef]
- Walter, D.E.; Proctory, H.C.; Walter, D.E.; Proctor, H.C. Mites in soil and litter systems. In Mites: Ecology, Evolution & Behaviour; Springer: Dordrecht, The Netherlands, 2013; pp. 161–228. [Google Scholar] [CrossRef]
- Hawes, T.C.; Bale, J.S.; Worland, M.R.; Convey, P. Trade-offs between microhabitat selection and physiological plasticity in the Antarctic springtail, Cryptopygus antarcticus (Willem). Polar Biol. 2008, 31, 681–689. [Google Scholar] [CrossRef]
- Everatt, M.J.; Convey, P.; Worland, M.R.; Bale, J.S.; Hayward, S.A.L. Heat tolerance and physiological plasticity in the Antarctic collembolan, Cryptopygus antarcticus, and mite, Alaskozetes antarcticus. J. Therm. Biol. 2013, 38, 264–271. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Worland, M.R.; Convey, P.; Bale, J.S. Habitat moisture availability and the local distribution of the Antarctic Collembola Cryptopygus antarcticus and Friesea grisea. Soil Biol Biochem. 2004, 36, 927–934. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Strauss, S.L.; Park, J.H.; Krieg, M.L.; Krna, M.A. Response of plants and the dominant microarthro-pod, Cryptopygus antarcticus, to warming and contrasting precipitation regimes in Antarctic tundra. Glob Chang. Biol. 2009, 15, 1640–1651. [Google Scholar] [CrossRef]
- Enríquez, N.; Tejedo, P.; Benayas, J.; Albertos, B.; Luciáñez, M.J. Collembola of Barrientos Island.; Antarctica: First census and as-sessment of environmental factors determining springtail distribution. Polar Biol. 2018, 41, 713–725. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Liu, X.; Jeannotte, R.; Reese, J.C.; Harris, M. Hessian Fly (Mayetiola destructor) Attack Causes a Dramatic Shift in Carbon and Nitrogen Metabolism in Wheat. MPMI 2007, 21, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, D.; Ganjewala, D. Effect of Leaf Positions on Total Phenolics.; Flavonoids and Proantho-cyanidins Content and Anti-oxidant Activities in Lantana Camara (L). J. Sci. Res. 2009, 1, 363–369. [Google Scholar] [CrossRef]
- Cortet, J.; Joffre, R.; Elmholt, S.; Krogh, P.H. Increasing species and trophic diversity of mesofauna affects fungal biomass.; mesofauna community structure and organic matter decomposition processes. Biol. Fertil. Soils 2003, 37, 302–312. [Google Scholar] [CrossRef]
- Maraun, M.; Alphei, J.; Beste, P.; Bonkowski, M.; Buryn, R.; Migge, S. Indirect effects of carbon and nutrient amendments on the soil meso- and microfauna of a beechwood. Biol. Fertil. Soils 2001, 34, 222–229. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef]
- Payne, R.J.; Thompson, A.M.; Standen, V.; Field, C.D.; Caporn, S.J.M. Impact of simulated nitrogen pollution on heathland micro-fauna, mesofauna and plants. Eur. J. Soil Biol. 2012, 49, 73–79. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.H.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Tao, Y.; Wang, S.; Wu, Z.; Wu, H. Soil mesofauna community composition predicts ecosystem multifunctionality along a coastal-inland gradient of the Bohai Bay. Land Degrad. Dev. 2021, 32, 4574–4582. [Google Scholar] [CrossRef]
- de Oliveira Filho, L.C.I.; Zeppelini, D.; Sousa, J.P.; Baretta, D.; Klauberg-Filho, O. Collembola community structure under different land management in subtropical Brazil. Ann. Appl. Biol. 2020, 177, 294–307. [Google Scholar] [CrossRef]


| Treatment | Main Composting Materials |
|---|---|
| Lantana-based compost | Fresh cow dung manure, chopped dry maize stalks, chopped lantana twigs (4:2:1 w/w) |
| Tithonia-based compost | Fresh cow dung manure, chopped dry maize stalks, chopped tithonia twigs (4:2:1 w/w) |
| Grass-based compost | Fresh cow dung manure, chopped dry maize stalks, chopped grass clippings (4:2:1 w/w) |
| Lantana + tithonia + grass-based compost [Mixed] | Fresh cow dung manure, chopped dry maize stalks, chopped * (lantana + tithonia + grass) (4:2:1 w/w) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matheri, F.; Ongeso, N.; Bautze, D.; Runo, S.; Mwangi, M.; Kambura, A.; Karanja, E.; Tanga, C.; Kiboi, M. The Overlooked Decomposers: Effects of Composting Materials and Duration on the Mesofauna Mediating Humification. Sustainability 2024, 16, 6534. https://doi.org/10.3390/su16156534
Matheri F, Ongeso N, Bautze D, Runo S, Mwangi M, Kambura A, Karanja E, Tanga C, Kiboi M. The Overlooked Decomposers: Effects of Composting Materials and Duration on the Mesofauna Mediating Humification. Sustainability. 2024; 16(15):6534. https://doi.org/10.3390/su16156534
Chicago/Turabian StyleMatheri, Felix, Nehemiah Ongeso, David Bautze, Steven Runo, Maina Mwangi, AnneKelly Kambura, Edward Karanja, Chrysantus Tanga, and Milka Kiboi. 2024. "The Overlooked Decomposers: Effects of Composting Materials and Duration on the Mesofauna Mediating Humification" Sustainability 16, no. 15: 6534. https://doi.org/10.3390/su16156534





