Management of Spartina alterniflora: Assessing the Efficacy of Plant Growth Regulators on Ecological and Microbial Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Setup and Samples Collection
2.3. DNA Extraction and Sequencing
2.4. Bioinformatic Analyses
2.5. Statistical Analyses
3. Results
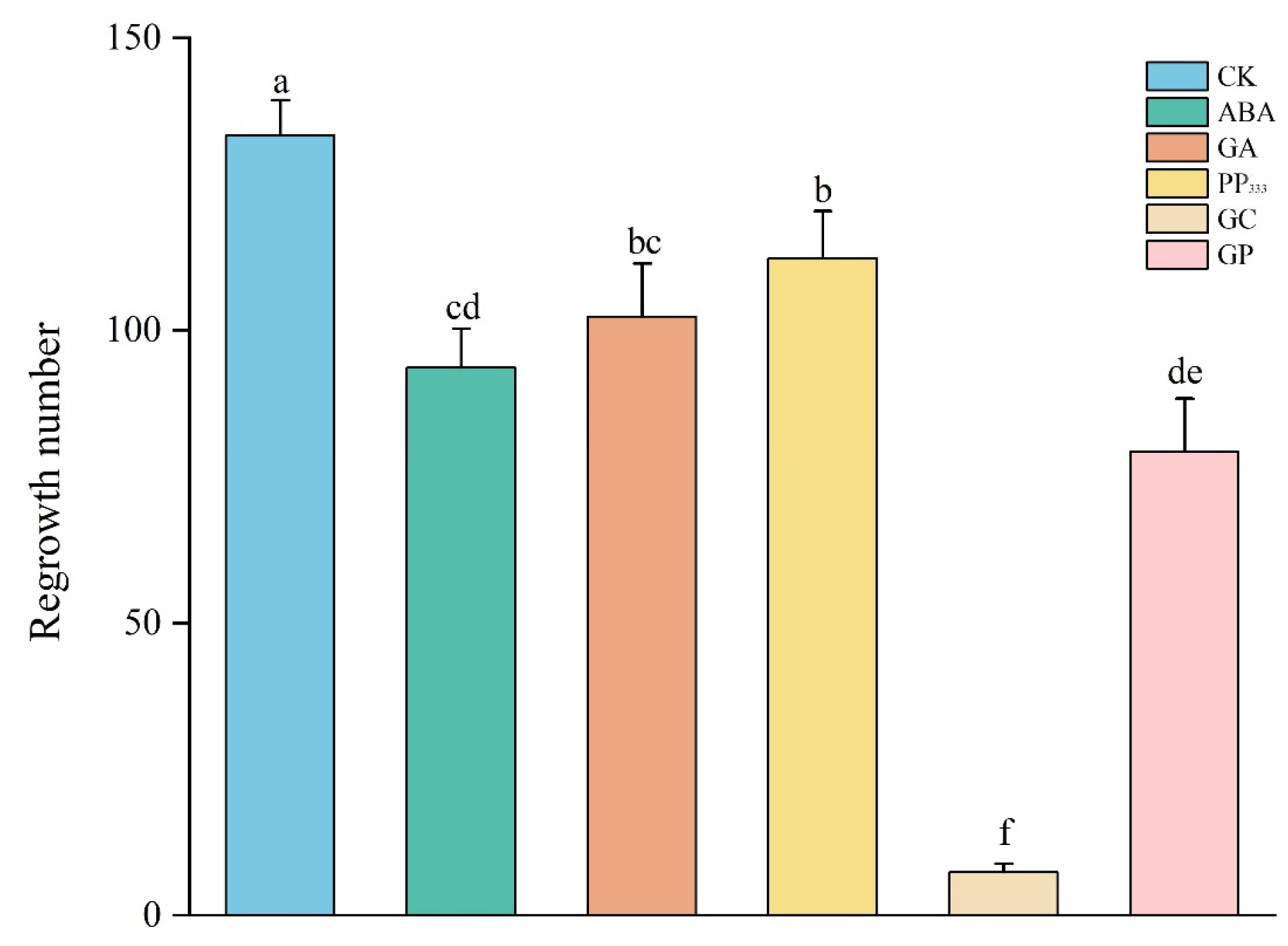
3.1. Regeneration Dynamics of S. alterniflora Following Treatment
3.2. Impacts of Treatment on Microbial Diversity
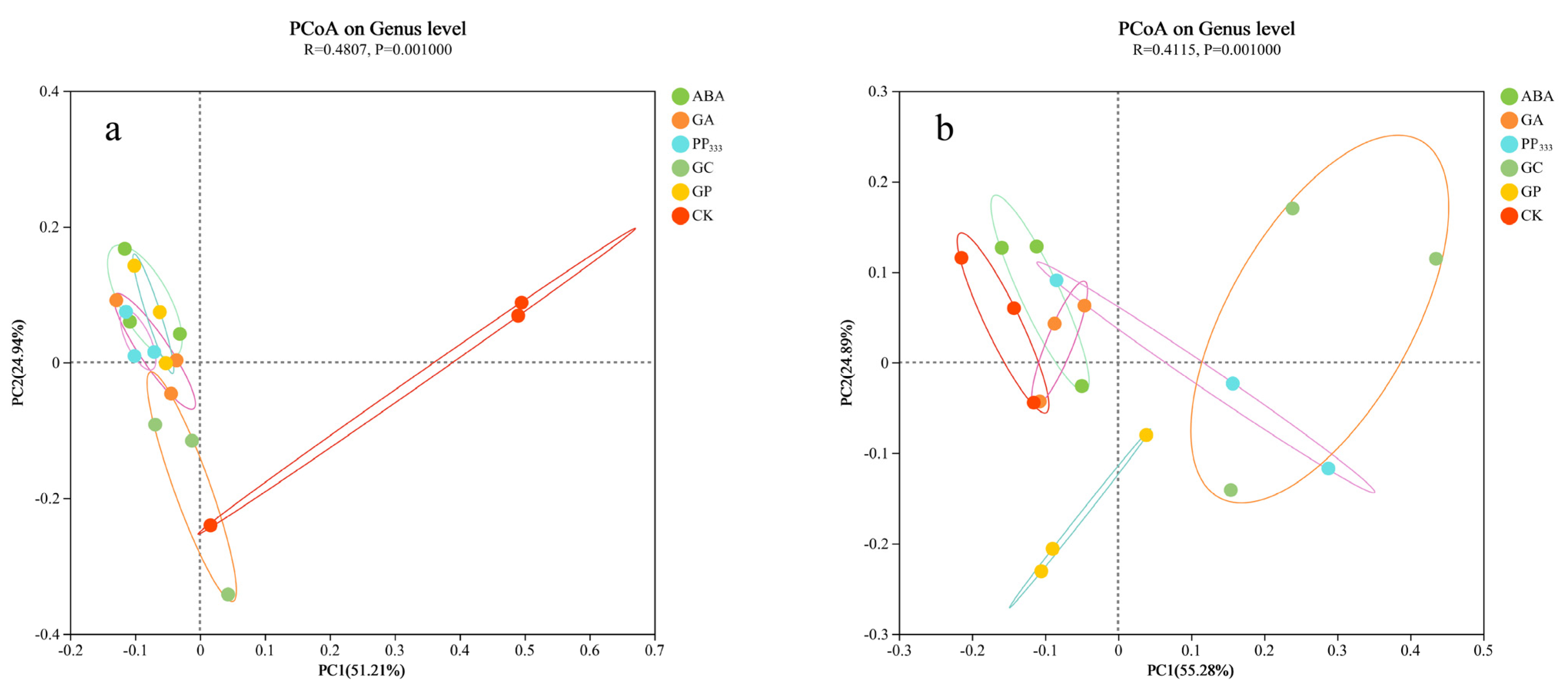
3.3. Diverse Treatment Impacts on Microbial Communities
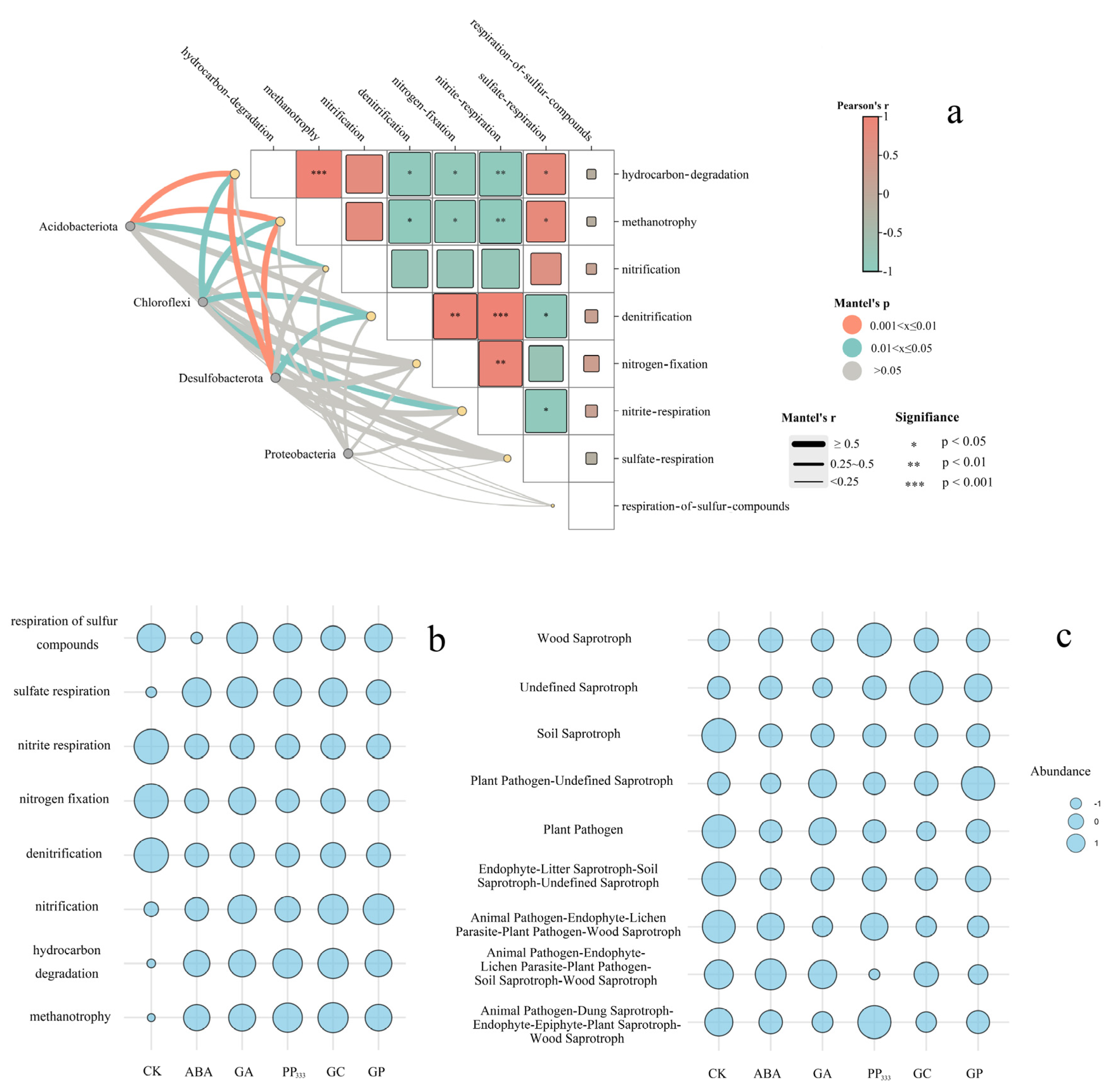
3.4. Diverse Treatment Impacts on Microbial Functional Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, G.; He, Y.; Lu, J.; Chen, H.; Feng, J. Seasonal variations in soil physicochemical properties and microbial community structure influenced by Spartina alterniflora invasion and Kandelia obovata restoration. Sci. Total Environ. 2021, 797, 149213. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Li, L.; Zhang, Z.; Zang, Y.; Chen, J.; Wang, J.; Wu, T.; Xia, J.; Tang, X. The Effects of Secondary Growth of Spartina alterniflora after Treatment on Sediment Microorganisms in the Yellow River Delta. Microorganisms 2022, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.N.; Diagne, C.; Hudgins, E.J.; Turbelin, A.; Ahmed, D.A.; Albert, C.; Bodey, T.W.; Briski, E.; Essl, F.; Haubrock, P.J.; et al. Biological invasion costs reveal insufficient proactive management worldwide. Sci. Total Environ. 2022, 819, 153404. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissiere, A.-C.; Gozlan, R.E.; Roiz, D.; Jaric, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Britton, J.R.; Lynch, A.J.; Bardal, H.; Bradbeer, S.J.; Coetzee, J.A.; Coughlan, N.E.; Dalu, T.; Tricarico, E.; Gallardo, B.; Lintermans, M.; et al. Preventing and controlling nonnative species invasions to bend the curve of global freshwater biodiversity loss. Environ. Rev. 2023, 31, 310–326. [Google Scholar] [CrossRef]
- Pysek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Skein, L.; Alexander, M.; Robinson, T. Contrasting invasion patterns in intertidal and subtidal mussel communities. Afr. Zool. 2018, 53, 47–52. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Zhang, Y.; Zhang, X.; He, Q.; Wan, N.-F.; Liu, H.; Guo, H.; Ma, J.; Zhang, Q.; et al. Reducing nitrogen inputs mitigates Spartina invasion in the Yangtze estuary. J. Appl. Ecol. 2024, 61, 588–598. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Hu, W.; Lu, C.; Chen, P. The native reed-specific bird, reed parrotbill, has been detected in exotic smooth cordgrass. Ecol. Evol. 2023, 13, e10417. [Google Scholar] [CrossRef]
- Nie, M.; Liu, W.; Pennings, S.C.; Li, B. Lessons from the invasion of Spartina alterniflora in coastal China. Ecology 2023, 104, e3874. [Google Scholar] [CrossRef]
- Ning, Z.; Chen, C.; Xie, T.; Li, S.; Zhu, Z.; Wang, Q.; Cai, Y.; Bai, J.; Cui, B. Invasive plant indirectly affects its self-expansion and native species via bio-geomorphic feedbacks: Implications for salt marsh restoration. Catena 2023, 226, 107056. [Google Scholar] [CrossRef]
- Peng, H.-B.; Shi, J.; Gan, X.; Zhang, J.; Ma, C.; Piersma, T.; Melville, D.S. Efficient removal of Spartina alterniflora with low negative environmental impacts using imazapyr. Front. Mar. Sci. 2022, 9, 1054402. [Google Scholar] [CrossRef]
- Liu, J.; Duan, X.; Li, G.; Cai, Z.; Wei, S.; Song, Q.; Zheng, Z. Changes in Bacterial Communities and Their Effects on Soil Carbon Storage in Spartina alterniflora Invasion Areas, Coastal Wetland Bare Flats, and Sueada salsa Areas. Int. J. Environ. Res. Public Health 2023, 20, 4308. [Google Scholar] [CrossRef] [PubMed]
- Simura, J.; Antoniadi, I.; Siroka, J.; Tarkowska, D.; Strnad, M.; Ljung, K.; Novak, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sanchez, C.; Vidal, N.; Vielba, J.M. Interactions of Gibberellins with Phytohormones and Their Role in Stress Responses. Horticulturae 2022, 8, 241. [Google Scholar] [CrossRef]
- Williams, D.R.; Ross, J.J.; Reid, J.B.; Potts, B.M. Response of Eucalyptus nitens seedlings to gibberellin biosynthesis inhibitors. Plant Growth Regul. 1999, 27, 125–129. [Google Scholar] [CrossRef]
- Baddar, Z.E.; Xu, X. Evaluation of changes in the microbial community structure in the sediments of a constructed wetland over the years. Arch. Microbiol. 2022, 204, 552. [Google Scholar] [CrossRef]
- Gao, J.; Liu, M.; Shi, S.; Liu, Y.; Duan, Y.; Lv, X.; Bohu, T.; Li, Y.; Hu, Y.; Wang, N.; et al. Disentangling Responses of the Subsurface Microbiome to Wetland Status and Implications for Indicating Ecosystem Functions. Microorganisms 2021, 9, 211. [Google Scholar] [CrossRef]
- Ward, N.D.; Morrison, E.S.; Liu, Y.; Rivas-Ubach, A.; Osborne, T.Z.; Ogram, A.V.; Bianchi, T.S. Marine microbial community responses related to wetland carbon mobilization in the coastal zone. Limnol. Oceanogr. Lett. 2019, 4, 25–33. [Google Scholar] [CrossRef]
- Xie, B.; Han, G.; Qiao, P.; Mei, B.; Wang, Q.; Zhou, Y.; Zhang, A.; Song, W.; Guan, B. Effects of mechanical and chemical control on invasive Spartina alterniflora in the Yellow River Delta, China. Peerj 2019, 7, e7655. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cai, A.; Wang, J.; Luo, Y.; Cheng, X.; An, S. Exotic Spartina alterniflora Loisel. Invasion significantly shifts soil bacterial communities with the successional gradient of saltmarsh in eastern China. Plant Soil 2020, 449, 97–115. [Google Scholar] [CrossRef]
- Song, Z.; Huang, Y.; Liu, Q.; Hu, X. Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area. J. Mar. Sci. Eng. 2022, 10, 1981. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, J.; Zhao, Q.; Wang, W.; Wang, D.; Bai, J. Seasonality and assembly of soil microbial communities in coastal salt marshes invaded by a perennial grass. J. Environ. Manag. 2023, 331, 117247. [Google Scholar] [CrossRef]
- He, C.; Cheng, L.; Wang, D.; Zhao, Z.; Wang, Z.; Wang, F.; Wang, X.; Zhang, P.; Chen, X.; Liu, X. Spartina alterniflora raised soil sulfide content by regulating sulfur cycle-associated bacteria in the Jiuduansha Wetland of China. Plant Soil 2021, 469, 107–121. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Zhou, Q.; Chen, J.; Zang, Y.; Zhang, Z.; Gao, C.; Tang, X.; Shang, S. Responses of Soil Microbiota to Different Control Methods of the Spartina alterniflora in the Yellow River Delta. Microorganisms 2022, 10, 1122. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Qiao, P.; Mei, B.; Wang, X.; Han, G.; Xie, B. Control effects of different herbicides on Spartina alterniflora. Ecol. Indic. 2023, 154, 110824. [Google Scholar] [CrossRef]
- Wang, X.X.; Hao, W.D. Reproductive and developmental toxicity of plant growth regulators in humans and animals. Pestic. Biochem. Physiol. 2023, 196, 105640. [Google Scholar] [CrossRef]
- Jan, S.D.; Bhardwaj, R.; Sharma, N.R.; Singh, R. Unraveling the Role of Plant Growth Regulators and Plant Growth Promoting Rhizobacteria in Phytoremediation. J. Plant Growth Regul. 2024, 43, 2471–2487. [Google Scholar] [CrossRef]
- Tang, X.; Fei, X.T.; Sun, Y.N.; Shao, H.H.; Zhu, J.Y.; He, X.Y.; Wang, X.Y.; Yong, B.; Tao, X. Abscisic acid-polyacrylamide (ABA-PAM) treatment enhances forage grass growth and soil microbial diversity under drought stress. Front. Plant Sci. 2022, 13, 973665. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Murugesan, K.; Thayanithi, S.; Palanisamy, S.B. A review of the impact of herbicides and insecticides on the microbial communities. Environ. Res. 2024, 245, 118020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Li, Q.L.; Jin, X.T.; Li, D.; Zhu, Z.Q.; Li, Q.X. Chiral enantiomers of the plant growth regulator paclobutrazol selectively affect community structure and diversity of soil microorganisms. Sci. Total Environ. 2021, 797, 148942. [Google Scholar] [CrossRef]
- Bhardwaj, L.; Kumar, D.; Singh, U.P.; Joshi, C.G.; Dubey, S.K. Herbicide application impacted soil microbial community composition and biochemical properties in a flooded rice field. Sci. Total Environ. 2024, 914, 169911. [Google Scholar] [CrossRef]
- Wang, S.; Han, Y.Y.; Wu, X.Y.; Sun, H.G. Metagenomics reveals the effects of glyphosate on soil microbial communities and functional profiles of C and P cycling in the competitive vegetation control process of Chinese fir plantation. Environ. Res. 2023, 238, 117162. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, D.E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants. Sustainability 2021, 13, 5802. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbo, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, S.; Gu, X.; Gao, A.; Liu, L.; Wu, X.; Pan, H.; Zhang, H. Pantoea jilinensis D25 enhances tomato salt tolerance via altering antioxidant responses and soil microbial community structure. Environ. Res. 2024, 243, 117846. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Drigo, B.; Doolette, C.L.; Vasileiadis, S.; Donner, E.; Karpouzas, D.G.; Lombi, E. Repeated applications of fipronil, propyzamide and flutriafol affect soil microbial functions and community composition: A laboratory-to-field assessment. Chemosphere 2023, 331, 138850. [Google Scholar] [CrossRef]
- Chávez-Ortiz, P.; Tapia-Torres, Y.; Larsen, J.; García-Oliva, F. Glyphosate-based herbicides alter soil carbon and phosphorus dynamics and microbial activity. Appl. Soil Ecol. 2022, 169, 104256. [Google Scholar] [CrossRef]
- Liang, Q.Y.; Yan, Z.Z.; Li, X.Z. Influence of the herbicide haloxyfop-R-methyl on bacterial diversity in rhizosphere soil of Spartina alterniflora. Ecotoxicol. Environ. Saf. 2020, 194, 110366. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Wang, Y.W.; Chen, M.; Fuh, G.; Lin, C.H. The effect of paclobutrazol on soil bacterial composition across three consecutive flowering stages of mung bean. Folia Microbiol. 2019, 64, 197–205. [Google Scholar] [CrossRef]
- Kepler, R.M.; Schmidt, D.J.E.; Yarwood, S.A.; Cavigelli, M.A.; Reddy, K.N.; Duke, S.O.; Bradley, C.A.; Williams, M.M.; Buyer, J.S.; Maul, J.E. Soil Microbial Communities in Diverse Agroecosystems Exposed to the Herbicide Glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef] [PubMed]
- Phookamsak, R.; Liu, J.-K.; McKenzie, E.H.C.; Manamgoda, D.S.; Ariyawansa, H.; Thambugala, K.M.; Dai, D.-Q.; Camporesi, E.; Chukeatirote, E.; Wijayawardene, N.N.; et al. Revision of Phaeosphaeriaceae. Fungal Divers. 2014, 68, 159–238. [Google Scholar] [CrossRef]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Davidson, E.A.; Semrau, J.D.; Nguyen, N.K.; Microbes, A.A.C.R. Improved scientific knowledge of methanogenesis and methanotrophy needed to slow climate change during the next 30 years. mBio 2023, 14, e0205923. [Google Scholar] [CrossRef]
- Zhang, Y.; Naafs, B.D.A.; Huang, X.; Song, Q.; Xue, J.; Wang, R.; Zhao, M.; Evershed, R.P.; Pancost, R.D.; Xie, S. Variations in wetland hydrology drive rapid changes in the microbial community, carbon metabolic activity, and greenhouse gas fluxes. Geochim. Et Cosmochim. Acta 2022, 317, 269–285. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E.; Schindlbacher, A.; Siciliano, S.; Breulmann, M.; Yannarell, A.; Bemans, J.M.; Abell, G.; Philippot, L.; Prosser, J.; et al. Microbes as Engines of Ecosystem Function: When Does Community Structure Enhance Predictions of Ecosystem Processes? Front. Microbiol. 2016, 7, 214. [Google Scholar] [CrossRef]
- Li, Z.L.; Tang, Z.; Song, Z.P.; Chen, W.N.; Tian, D.S.; Tang, S.M.; Wang, X.Y.; Wang, J.S.; Liu, W.J.; Wang, Y.; et al. Variations and controlling factors of soil denitrification rate. Glob. Change Biol. 2022, 28, 2133–2145. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.F.; Fang, W.W.; Yang, T.; Chen, G.H.; He, Z.L.; Wang, S.Q. Microbial sulfur metabolism and environmental implications. Sci. Total Environ. 2021, 778, 146085. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, X.T.; Huang, X.M.; Chen, Z.Y.; Marietou, A.; Holmkvist, L.; Qu, L.Y.; Finster, K.; Gong, X.Z. Genomic insight of sulfate reducing bacterial genus Desulfofaba reveals their metabolic versatility in biogeochemical cycling. BMC Genom. 2023, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meckenstock, R.U.; Weng, S.; Wei, G.; Hubert, C.R.J.; Wang, J.H.; Dong, X. Marine sediments harbor diverse archaea and bacteria with the potential for anaerobic hydrocarbon degradation via fumarate addition. FEMS Microbiol. Ecol. 2021, 97, fiab045. [Google Scholar] [CrossRef] [PubMed]
 to ensure precise location identification for the study.
to ensure precise location identification for the study.
 to ensure precise location identification for the study.
to ensure precise location identification for the study.

| Group | Sobs | Chao | Shannon | Simpson | |
|---|---|---|---|---|---|
| Bacteria | CK | 3198.00 ± 356.58 a | 4257.02 ± 635.15 a | 6.76 ± 0.11 a | 0.0036 ± 0.0012 a |
| ABA | 4005.00 ± 268.3 bc | 5222.95 ± 396.95 ab | 7.10 ± 0.12 b | 0.0024 ± 0.0004 a | |
| GA | 4428.33 ± 419.12 bc | 5939.44 ± 633.85 b | 7.20 ± 0.20 b | 0.0024 ± 0.0006 a | |
| PP333 | 4136.33 ± 181.95 bc | 5418.03 ± 333.38 b | 7.12 ± 0.02 b | 0.0025 ± 0.0001 a | |
| GC | 3915.00 ± 442.2 b | 5147.30 ± 772.64 ab | 7.04 ± 0.2 b | 0.0027 ± 0.0006 a | |
| GP | 4546.67 ± 100.53 c | 6023.13 ± 132.62 b | 7.27 ± 0.01 b | 0.0022 ± 0.0002 a | |
| Fungi | CK | 141.67 ± 70.19 a | 166.13 ± 67.52 a | 1.12 ± 0.25 a | 0.55 ± 0.12 a |
| ABA | 198.33 ± 37.58 ab | 268.58 ± 69.32 b | 1.67 ± 0.14 b | 0.44 ± 0.09 ab | |
| GA | 232.00 ± 14.00 b | 289.94 ± 27.61 b | 1.41 ± 0.14 ab | 0.45 ± 0.01 ab | |
| PP333 | 176.33 ± 39.21 ab | 234.01 ± 47.05 ab | 1.66 ± 0.38 b | 0.37 ± 0.14 bc | |
| GC | 223.67 ± 23.01 b | 284.27 ± 31.54 b | 2.21 ± 0.16 c | 0.26 ± 0.05 c | |
| GP | 186.00 ± 35.76 ab | 251.27 ± 35.23 ab | 1.72 ± 0.36 b | 0.33 ± 0.07 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, C.; Wang, Z.; Cao, J.; Chen, J.; Shen, C.; Zhang, J.; Wang, Q.; Wang, M. Management of Spartina alterniflora: Assessing the Efficacy of Plant Growth Regulators on Ecological and Microbial Dynamics. Sustainability 2024, 16, 7848. https://doi.org/10.3390/su16177848
Sha C, Wang Z, Cao J, Chen J, Shen C, Zhang J, Wang Q, Wang M. Management of Spartina alterniflora: Assessing the Efficacy of Plant Growth Regulators on Ecological and Microbial Dynamics. Sustainability. 2024; 16(17):7848. https://doi.org/10.3390/su16177848
Chicago/Turabian StyleSha, Chenyan, Zhixiong Wang, Jiajie Cao, Jing Chen, Cheng Shen, Jing Zhang, Qiang Wang, and Min Wang. 2024. "Management of Spartina alterniflora: Assessing the Efficacy of Plant Growth Regulators on Ecological and Microbial Dynamics" Sustainability 16, no. 17: 7848. https://doi.org/10.3390/su16177848





