PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment
Abstract
:1. Introduction
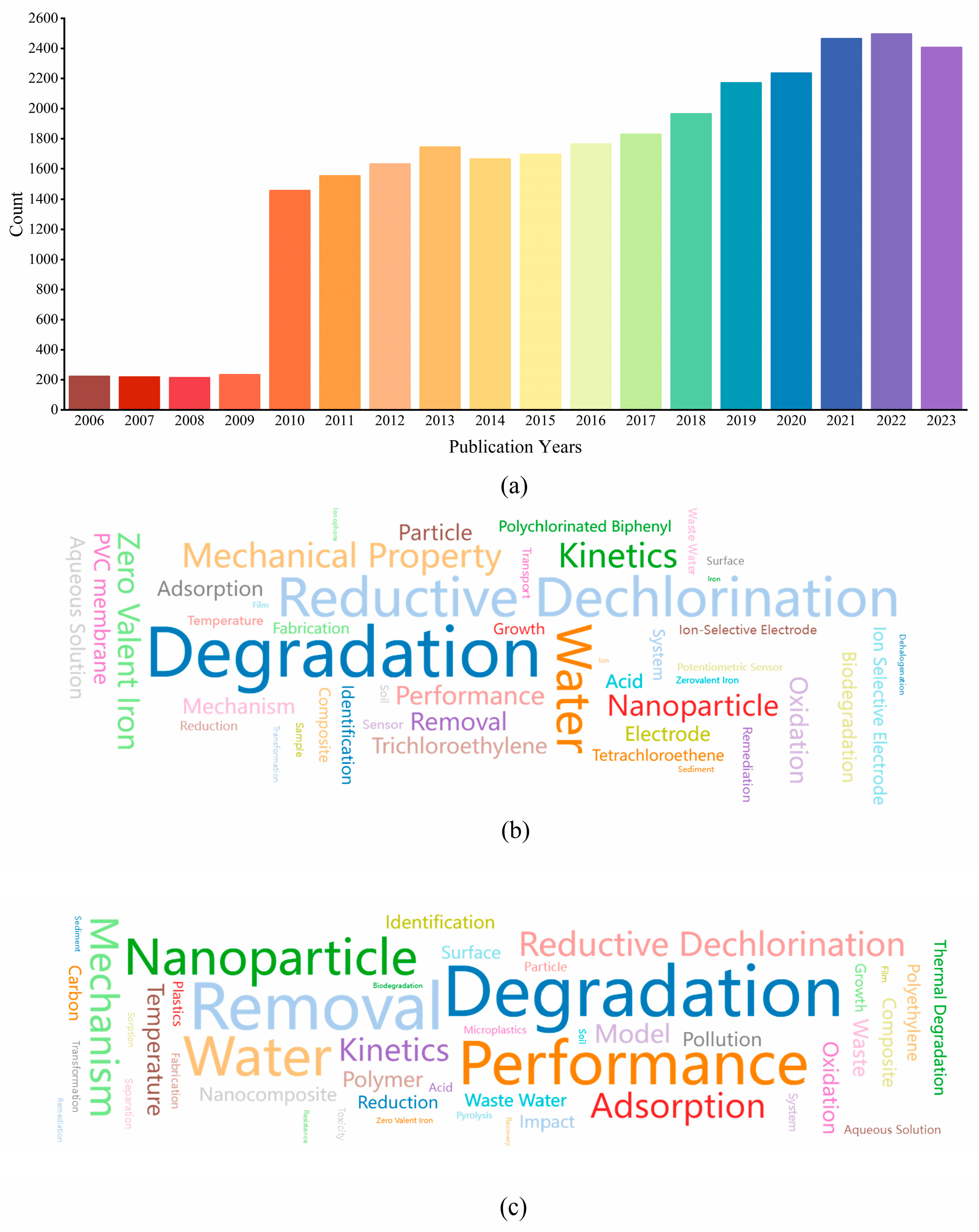
2. Summary of Literature Review
3. Methodology
4. Recent Advances of Dechlorination Technology Development
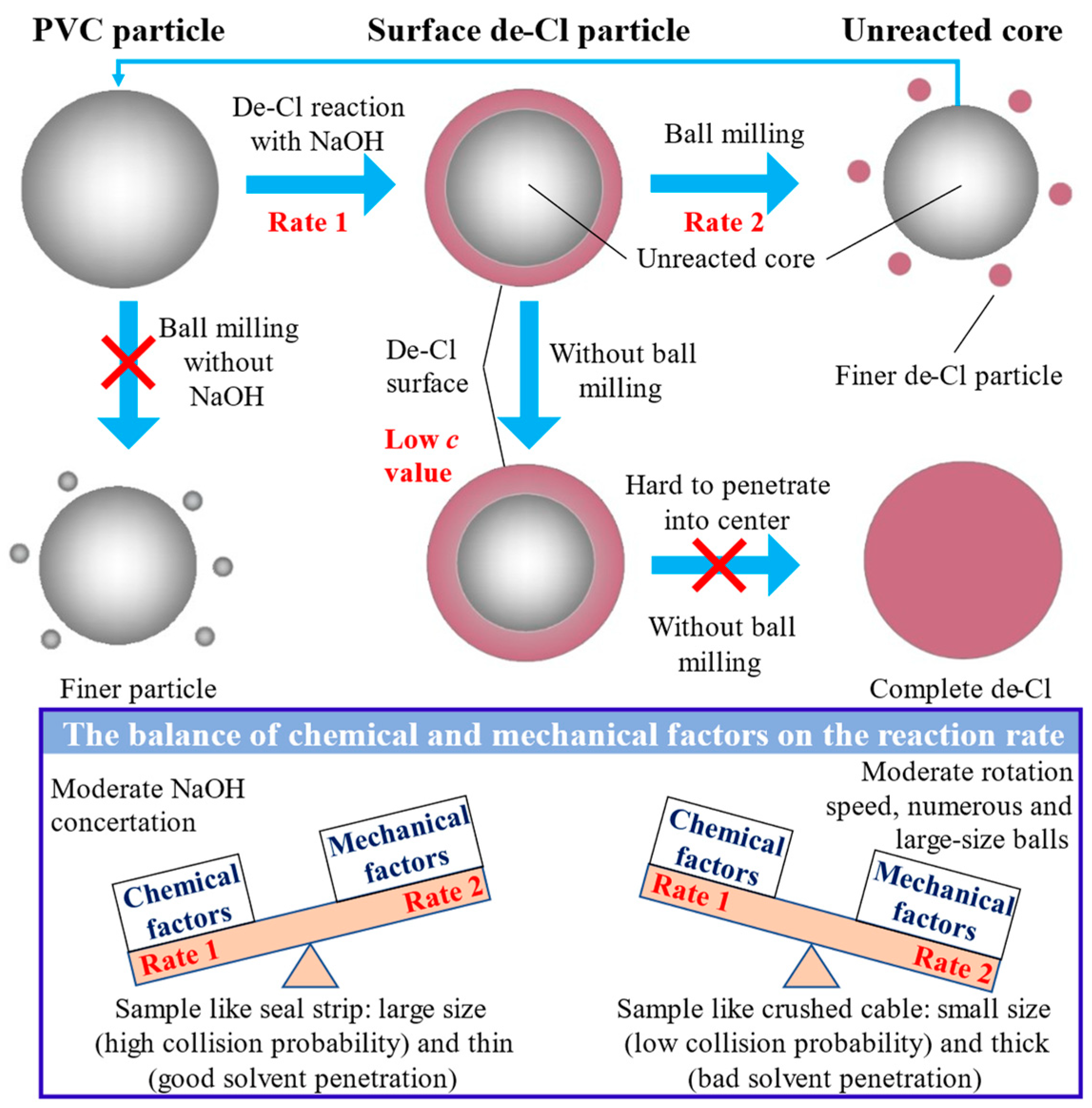
4.1. Pretreatment
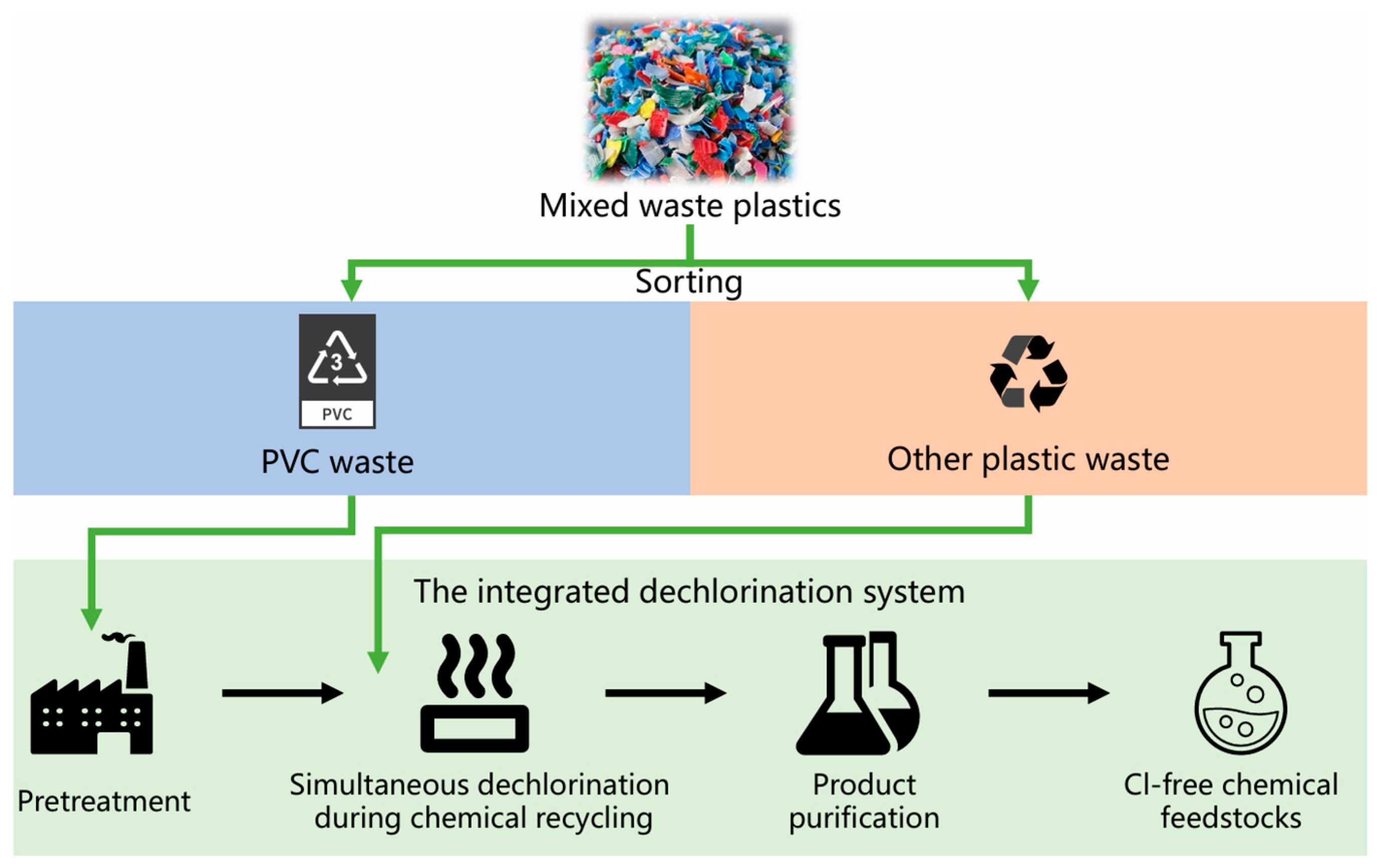
4.2. Simultaneous Dechlorination during Chemical Recycling
4.3. Product Purification
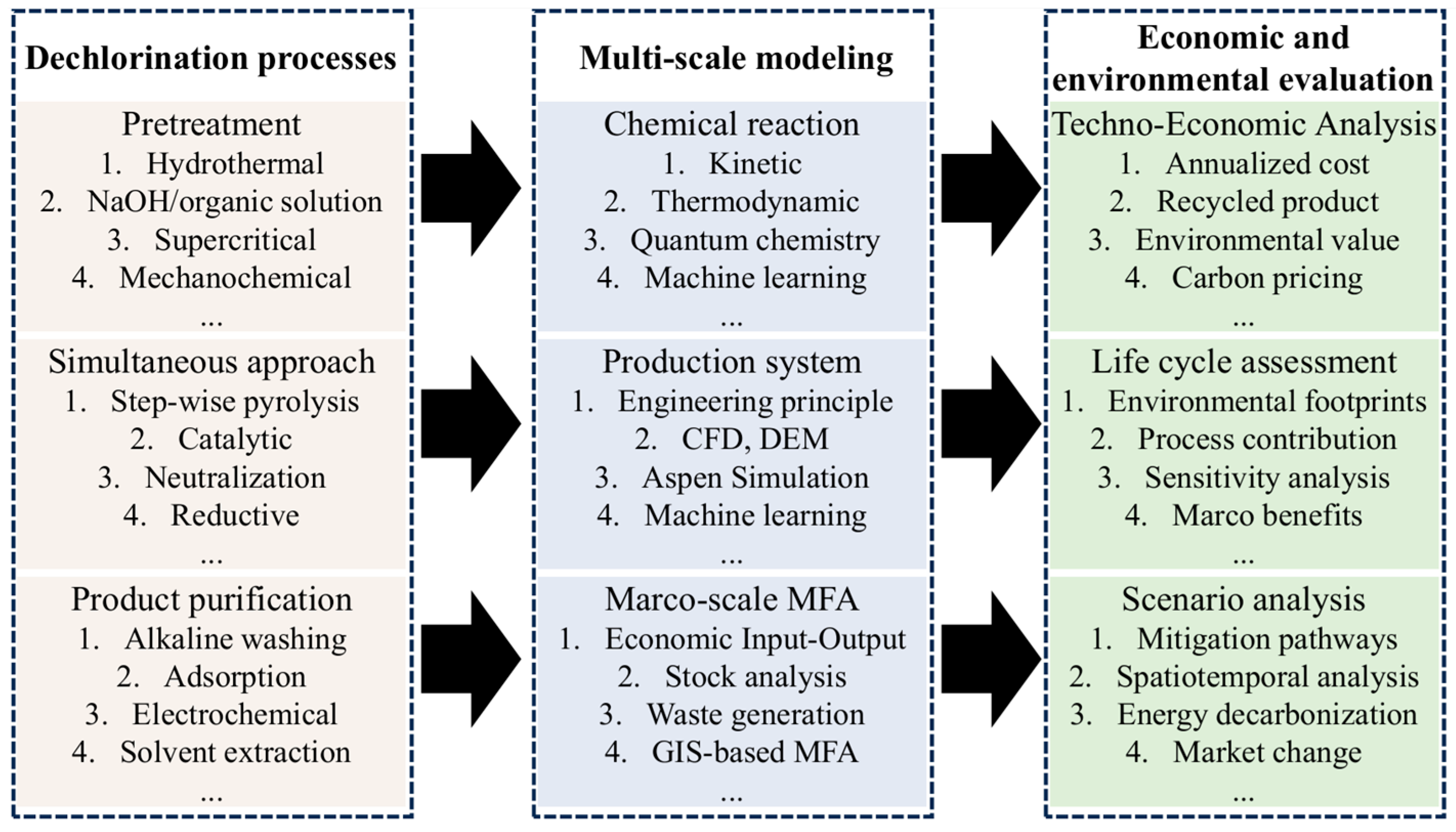
5. Multi-Scale Model Construction
5.1. Microscale Model Construction
5.1.1. Pretreatment Dechlorination Reaction Model
5.1.2. Simultaneous Dechlorination during Chemical Recycling Reaction Model
5.1.3. Dechlorination and Purification Model for Products
5.2. Modeling of the Production System of PVC Dechlorination
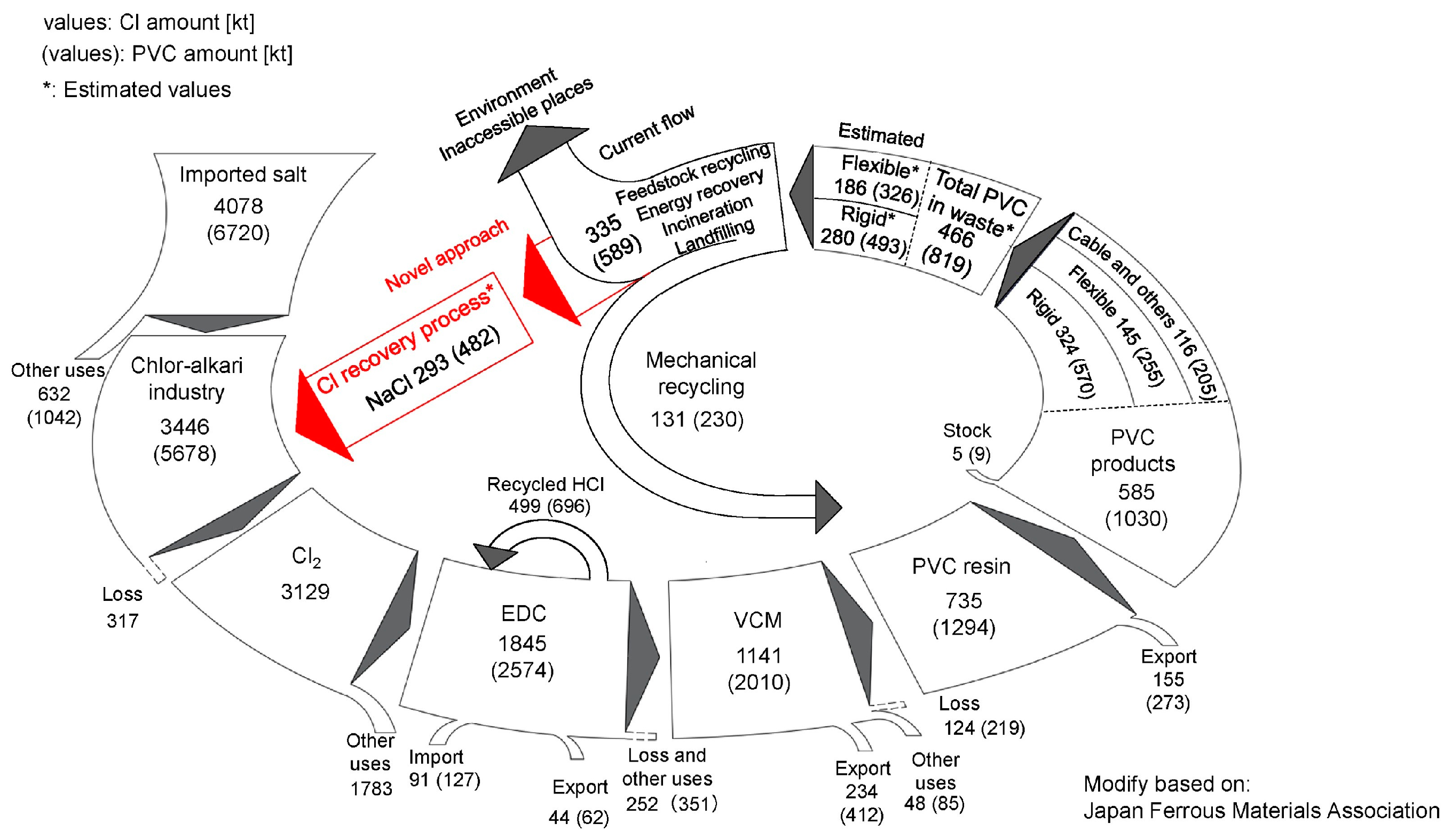
5.3. Macro-Scale MFA
6. TEA and LCA of Dechlorination Technology for PVC Waste
6.1. TEA
6.2. LCA
6.3. Scenario Analysis
7. Conclusions
- (1)
- Most current studies focused on experimental research involving single-step dechlorination processes for PVC waste. To achieve a high degree of dechlorination in the recovered hydrocarbons, we suggested an integrated dechlorination system. This method could achieve dechlorination under mild conditions—avoiding extreme conditions that increase energy consumption and reagent use—while still meeting the required chlorine content in the final product.
- (2)
- To identify design improvements and application potential, we summarized the mechanisms, material and energy flows of the dechlorination process from both micro and macro model levels. Currently, constructing multi-scale models faces challenges, such as parameter uncertainty and inconsistent data quality. To address these issues, future research should focus on validating and optimizing models through experiments, simulations and up-scaling applications to improve predictive capability and stability. Additionally, establishing standardized data protocols and shared platforms is recommended to enhance data quality and accessibility.
- (3)
- We also analyzed the TEA and LCA of the dechlorination process for PVC waste. Currently, TEA and LCA for PVC waste face challenges such as market demand, regulatory policies, product quality positioning and the complexity of environmental impact assessments. By summarizing various environmental and economic indicators, we proposed sustainable criteria for promoting these emerging technologies from laboratory to industrial applications. Stakeholders should consider both the economic feasibility and the potential for PVC waste dechlorination and plastic chemical recycling.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Drewniok, M.P.; Gao, Y.; Cullen, J.M.; Cabrera Serrenho, A. What to Do about Plastics? Lessons from a Study of United Kingdom Plastics Flows. Environ. Sci. Technol. 2023, 57, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Rabiu, M.K.; Jaeger-Erben, M. Reducing single-use plastic in everyday social practices: Insights from a living lab experiment. Resour. Conserv. Recycl. 2024, 200, 107303. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Singh, R.K.; Das, H.T.; Dubey, S.; Kumar, V. Impact of aquatic microplastics and nanoplastics pollution on ecological systems and sustainable remediation strategies of biodegradation and photodegradation. Sci. Total Environ. 2022, 806, 151358. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jia, S.; Ramakrishna, S. Accelerating Plastic Circularity: A Critical Assessment of the Pathways and Processes to Circular Plastics. Processes 2023, 11, 1457. [Google Scholar] [CrossRef]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.-S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef]
- Palm, E.; Nilsson, L.J.; Ahman, M. Electricity-based plastics and their potential demand for electricity and carbon dioxide. J. Clean. Prod. 2016, 129, 548–555. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Kobeticova, K.; Cerny, R. Ecotoxicity assessment of short- and medium-chain chlorinated paraffins used in polyvinyl-chloride products for construction industry. Sci. Total Environ. 2018, 640, 523–528. [Google Scholar] [CrossRef]
- Pelegrini, K.; Pereira, T.C.B.; Maraschin, T.G.; Teodoro, L.D.S.; Basso, N.R.D.S.; Galland, G.L.B.D.; Ligabue, R.A.; Bogo, M.R. Micro- and nanoplastic toxicity: A review on size, type, source, and test-organism implications. Sci. Total Environ. 2023, 878, 162954. [Google Scholar] [CrossRef]
- MacLeo, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Soares, J.; Miguel, I.; Venancio, C.; Lopes, I.; Oliveira, M. Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021, 412, 125227. [Google Scholar] [CrossRef]
- Feng, B.; Jing, Y.; Liu, X.; Guo, Y.; Wang, Y. Waste PVC upcycling: Transferring unmanageable Cl species into value-added Cl-containing chemicals. Appl. Catal. B-Environ. 2023, 331, 122671. [Google Scholar] [CrossRef]
- Tomatis, M.; Greer, A.J.; Oster, K.; Tedstone, A.; Cuellar-Franca, R.M.; Garforth, A.; Hardacre, C.; Azapagic, A. Environmental assessment of a novel ionic-liquid based method for recycling of PVC in composite materials. Sci. Total Environ. 2023, 887, 163999. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Chemical recycling of plastic wastes with alkaline earth metal oxides: A review. Sci. Total Environ. 2023, 905, 167251. [Google Scholar] [CrossRef]
- Kumagai, S.; Lu, J.; Fukushima, Y.; Ohno, H.; Kameda, T.; Yoshioka, T. Diagnosing chlorine industrial metabolism by evaluating the potential of chlorine recovery from polyvinyl chloride wastes-A case study in Japan. Resour. Conserv. Recycl. 2018, 133, 354–361. [Google Scholar] [CrossRef]
- Kots, P.A.; Vance, B.C.; Quinn, C.M.; Wang, C.; Vlachos, D.G. A two-stage strategy for upcycling chlorine-contaminated plastic waste. Nat. Sustain. 2023, 6, 1258–1267. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Yu, X.; Qi, Y. A high-efficiency and low-temperature subcritical water dechlorination strategy of polyvinyl chloride using coal fly ash (CFA) and coal gangue (CG) as enhancers. J. Clean. Prod. 2020, 260, 121085. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Song, J.; Sima, J.; Zhu, C.; Huang, Q. Dechlorination and fuel gas generation in chemical looping conversion of waste PVC over inherently Na/Ca/K-containing bauxite residue-based oxygen carriers. Waste Manag. 2023, 168, 211–220. [Google Scholar] [CrossRef]
- Mahmud, R.; Moni, S.M.; High, K.; Carbajales-Dale, M. Integration of techno-economic analysis and life cycle assessment for sustainable process design—A review. J. Clean. Prod. 2021, 317, 128247. [Google Scholar] [CrossRef]
- Sun, N.; Wang, P.; Jian, X.; Hao, M.; Yan, X.; Chen, W.-Q. Material Flow analysis of plastics from provincial household appliances in China: 1978-2016. Waste Manag. 2022, 153, 156–166. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.-z.; Deng, R.; Luo, Y.-h. Oxygen-Induced Enhancement in Low-Temperature Dechlorination of PVC: An Experimental and DFT Study on the Oxidative Pyrolysis Process. ACS Sustain. Chem. Eng. 2021, 9, 2835–2843. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Guo, X. Dechlorination of PVC at low temperature by solvothermal treatment with alkaline additives. Process Saf. Environ. Prot. 2024, 183, 945–951. [Google Scholar] [CrossRef]
- Hapipi, A.M.; Suda, H.; Uddin, M.A.; Kato, Y. Dechlorination of Polyvinyl Chloride under Superheated Steam with Catalysts and Adsorbents. Energy Fuels 2018, 32, 7792–7799. [Google Scholar] [CrossRef]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Practical dechlorination of polyvinyl chloride wastes in NaOH/ethylene glycol using an up-scale ball mill reactor and validation by discrete element method simulations. Waste Manag. 2019, 99, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yuan, G.; Yin, L.; Chen, D.; He, P.; Wang, H. Morphological characteristics of polyvinyl chloride (PVC) dechlorination during pyrolysis process: Influence of PVC content and heating rate. Waste Manag. 2016, 58, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kumagai, S.; Saito, Y.; Yoshioka, T.; Huang, X.; Shao, Y.; Ran, J.; Sun, L. Recent Advancements in Pyrolysis of Halogen-Containing Plastics for Resource Recovery and Halogen Upcycling: A State-of-the-Art Review. Environ. Sci. Technol. 2024, 58, 1423–1440. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Sima, J.; Zhu, Y.; Du, X.; Williams, P.T.; Huang, Q. Dechlorination of waste polyvinyl chloride (PVC) through non-thermal plasma. Chemosphere 2023, 338, 139535. [Google Scholar] [CrossRef]
- Romero, A.; Moreno, I.; Escudero, L.; Yuste, R.; Pizarro, P.; Moreno, J.M.; Serrano, D.P. Dechlorination of a real plastic waste pyrolysis oil by adsorption with zeolites. J. Environ. Chem. Eng. 2024, 12, 112638. [Google Scholar] [CrossRef]
- Zou, D.; Wang, X.; Wu, C.; Li, T.; Wang, M.; Liu, S.; Wang, Q.; Shimaoka, T. Dechlorination of Municipal Solid Waste Incineration Fly Ash by Leaching with Fermentation Liquid of Food Waste. Sustainability 2020, 12, 4389. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tsai, Y.-C. Dry dechlorination of solid-derived fuels obtained from food waste and polyvinyl chloride. Sci. Total Environ. 2022, 841, 156745. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Ieshige, M.; Okuwaki, A. Dechlorination behaviour of flexible poly(vinyl chloride) in NaOH/EG solution. Polym. Degrad. Stab. 2008, 93, 1822–1825. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, P.; Cui, X.; Geng, F.; Guo, Q. Kinetics study on hydrothermal dechlorination of poly(vinyl chloride) by in-situ sampling. Environ. Technol. Innov. 2021, 23, 101703. [Google Scholar] [CrossRef]
- Tito, E.; dos Passos, J.S.; Rombola, A.G.; Torri, C.; Bensaid, S.; Pirone, R.; Biller, P. Sequential hydrothermal dechlorination and liquefaction of PVC. Energy Convers. Manag. 2024, 304, 118228. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef]
- Moellnitz, S.; Khodier, K.; Pomberger, R.; Sarc, R. Grain size dependent distribution of different plastic types in coarse shredded mixed commercial and municipal waste. Waste Manag. 2020, 103, 388–398. [Google Scholar] [CrossRef]
- Balaz, M.; Bujnakova, Z.; Achimovicova, M.; Tesinsky, M.; Balaz, P. Simultaneous valorization of polyvinyl chloride and eggshell wastes by a semi-industrial mechanochemical approach. Environ. Res. 2019, 170, 332–336. [Google Scholar] [CrossRef]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Machine learning-based discrete element reaction model for predicting the dechlorination of poly (vinyl chloride) in NaOH/ethylene glycol solvent with ball milling. Chem. Eng. J. Adv. 2020, 3, 100025. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, B.; Zhu, M. An Overview on Recycling of Waste Poly(Vinyl Chloride). Green Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Cruz, P.P.R.; da Silva, L.C.; Fiuza, R.A., Jr.; Polli, H. Thermal dehydrochlorination of pure PVC polymer: Part I-thermal degradation kinetics by thermogravimetric analysis. J. Appl. Polym. Sci. 2021, 138, e50598. [Google Scholar] [CrossRef]
- Wu, J.; Papanikolaou, K.G.; Cheng, F.; Addison, B.; Cuthbertson, A.A.; Mavrikakis, M.; Huber, G.W. Kinetic Study of Polyvinyl Chloride Pyrolysis with Characterization of Dehydrochlorinated PVC. ACS Sustain. Chem. Eng. 2024, 12, 7402–7413. [Google Scholar] [CrossRef]
- Cupples, A.M.; Spormann, A.M.; McCarty, P.L. Vinyl chloride and cis-dichloroethene dechlorination kinetics and microorganism growth under substrate limiting conditions. Environ. Sci. Technol. 2004, 38, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Amos, B.K.; Christ, J.A.; Abriola, L.M.; Pennell, K.D.; Löffler, F.E. Experimental evaluation and mathematical modeling of microbially enhanced tetrachloroethene (PCE) dissolution. Environ. Sci. Technol. 2007, 41, 963–970. [Google Scholar] [CrossRef]
- Zullo, F.M.; Liu, M.; Zou, S.; Yestrebsky, C.L. Mechanistic and computational studies of PCB 151 dechlorination by zero valent magnesium for field remediation optimization. J. Hazard. Mater. 2017, 337, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Soares, D.; Vilarinho, C.; Castro, F. Kinetics of thermal de-chlorination of PVC under pyrolytic conditions. Waste Manag. 2012, 32, 847–851. [Google Scholar] [CrossRef]
- Peng, Y.; Dai, L.; Dai, A.; Wu, Q.; Zou, R.; Liu, Y.; Ruan, R.; Wang, Y. Catalytic process toward green recycling of polyvinyl chloride: A study on thermodynamic, kinetic and pyrolysis characteristics. J. Anal. Appl. Pyrolysis 2022, 168, 105719. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, H.; Qing, T.; Zhang, J.; Tian, K. Transformation and kinetics of chlorine-containing products during pyrolysis of plastic wastes. Chemosphere 2021, 284, 131348. [Google Scholar] [CrossRef]
- Al-Yaari, M.; Dubdub, I. Pyrolytic Behavior of Polyvinyl Chloride: Kinetics, Mechanisms, Thermodynamics, and Artificial Neural Network Application. Polymers 2021, 13, 4359. [Google Scholar] [CrossRef]
- Chibante, V.G.; Fonseca, A.M.; Salcedo, R.R. Modeling dry-scrubbing of gaseous HCl with hydrated lime in cyclones with and without recirculation. J. Hazard. Mater. 2010, 178, 469–482. [Google Scholar] [CrossRef]
- Scala, F.; D’Ascenzo, M.; Lancia, A. Modeling flue gas desulfurization by spray-dry absorption. Sep. Purif. Technol. 2004, 34, 143–153. [Google Scholar] [CrossRef]
- Guo, X.; Yin, L.; Hu, L.; Cao, J.; Shen, H.; Xu, J.; Hu, Y.; Chen, D. Numerical simulation of wet deacidification process of sludge incineration flue gas. Fuel 2020, 280, 118480. [Google Scholar] [CrossRef]
- Pan, C.; Wang, W.; Zhang, Y.; Nam, J.C.; Wu, F.; You, Z.; Xu, J.; Li, J. Porous graphitized carbon-supported FeOCl as a bifunctional adsorbent-catalyst for the wet peroxide oxidation of chlorinated volatile organic compounds: Effect of mesopores and mechanistic study. Appl. Catal. B-Environ. 2023, 330, 122659. [Google Scholar] [CrossRef]
- Pré, P.; Delage, F.; Le Cloirec, P. A model to predict the adsorber thermal behavior during treatment of volatile organic compounds onto wet activated carbon. Environ. Sci. Technol. 2002, 36, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Arjang, S.; Motahari, K.; Saidi, M. Experimental and Modeling Study of Organic Chloride Compounds Removal from Naphtha Fraction of Contaminated Crude Oil Using Sintered γ-Al2O3 Nanoparticles: Equilibrium, Kinetic, and Thermodynamic Analysis. Energy Fuels 2018, 32, 4025–4039. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Lai, C.; Sun, X. Beyond the standard two-film theory: Computational fluid dynamics simulations for carbon dioxide capture in a wetted wall column. Chem. Eng. Sci. 2018, 184, 103–110. [Google Scholar] [CrossRef]
- Janda, V.; Vasek, P.; Bizova, J.; Belohlav, Z. Kinetic models for volatile chlorinated hydrocarbons removal by zero-valent iron. Chemosphere 2004, 54, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Bokare, V.; Jung, J.-l.; Chang, Y.-Y.; Chang, Y.-S. Reductive dechlorination of octachlorodibenzo-p-dioxin by nanosized zero-valent zinc: Modeling of rate kinetics and congener profile. J. Hazard. Mater. 2013, 250, 397–402. [Google Scholar] [CrossRef]
- Chambon, J.C.; Bjerg, P.L.; Scheutz, C.; Baelum, J.; Jakobsen, R.; Binning, P.J. Review of reactive kinetic models describing reductive dechlorination of chlorinated ethenes in soil and groundwater. Biotechnol. Bioeng. 2013, 110, 1–23. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Prytula, M.T. Kinetics of the Sequential Microbial Reductive Dechlorination of Hexachlorobenzene. Environ. Sci. Technol. 2000, 34, 4001–4009. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.-q.; Zhang, X.-d.; Liu, J.-w.; Zhou, Y.-y. Dechlorination of organochloride waste mixture by microwave irradiation before forming solid recovered fuel. Waste Manag. 2017, 62, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Murena, F.; Gioia, F. Solvent extraction of chlorinated compounds from soils and hydrodechlorination of the extract phase. J. Hazard. Mater. 2009, 162, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Lu, J.; Gu, D.; Li, G.; Liu, Y.; Rao, P.; Fang, S.; Zhang, N. Modeling and optimization of electrodeionization process for the energy-saving of ultrapure water production. J. Clean. Prod. 2022, 372, 133754. [Google Scholar] [CrossRef]
- Istrate, I.-R.; Galvez-Martos, J.-L.; Vazquez, D.; Guillen-Gosalbez, G.; Dufour, J. Prospective analysis of the optimal capacity, economics and carbon footprint of energy recovery from municipal solid waste incineration. Resour. Conserv. Recycl. 2023, 193, 106943. [Google Scholar] [CrossRef]
- Lotfi, K.; Bonakdari, H.; Ebtehaj, I.; Mjalli, F.S.; Zeynoddin, M.; Delatolla, R.; Gharabaghi, B. Predicting wastewater treatment plant quality parameters using a novel hybrid linear-nonlinear methodology. J. Environ. Manag. 2019, 240, 463–474. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Y.; Ding, X.; Liu, Q.; Ma, C.-A. Electrocatalytic dechlorination of chloropicolinic acid mixtures by using palladium-modified metal cathodes in aqueous solutions. Electrochim. Acta 2016, 210, 762–772. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, T.; Wang, S.; Chen, Y.; Cong, L.; Qu, Y. Dechlorination of 4-chlorophenol to phenol in bioelectrochemical systems. J. Hazard. Mater. 2013, 244–245, 743–749. [Google Scholar] [CrossRef]
- Mao, X.; Ciblak, A.; Baek, K.; Amiri, M.; Loch-Caruso, R.; Alshawabkeh, A.N. Optimization of electrochemical dechlorination of trichloroethylene in reducing electrolytes. Water Res. 2012, 46, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Borjigin, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Fukushima, Y.; Yoshioka, T. Practical dehalogenation of automobile shredder residue in NaOH/ethylene glycol with an up-scale ball mill reactor. J. Mater. Cycles Waste Manag. 2020, 22, 1620–1629. [Google Scholar] [CrossRef]
- Tongamp, W.; Kano, J.; Suzuta, Y.; Saito, F.; Themelis, N.J. Relation between mechanochemical dechlorination rate of polyvinyl chloride and mill power consumption. J. Mater. Cycles Waste Manag. 2009, 11, 32–37. [Google Scholar] [CrossRef]
- Wang, F.; Wang, S.; Li, Z.; You, H.; Aviso, K.B.; Tan, R.R.; Jia, X. Water footprint sustainability assessment for the chemical sector at the regional level. Resour. Conserv. Recycl. 2019, 142, 69–77. [Google Scholar] [CrossRef]
- Parvatker, A.G.; Tunceroglu, H.; Sherman, J.D.; Coish, P.; Anastas, P.; Zimmerman, J.B.; Eckelman, M.J. Cradle-to-Gate Greenhouse Gas Emissions for Twenty Anesthetic Active Pharmaceutical Ingredients Based on Process Scale-Up and Process Design Calculations. ACS Sustain. Chem. Eng. 2019, 7, 6580–6591. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Ihara, I.; Elgarahy, A.M.; Ayyad, A.; Mehta, N.; Ng, K.H.; El-Monaem, E.M.A.M.; Eltaweil, A.S.; Hosny, M.; et al. Materials, fuels, upgrading, economy, and life cycle assessment of the pyrolysis of algal and lignocellulosic biomass: A review. Environ. Chem. Lett. 2023, 21, 1419–1476. [Google Scholar] [CrossRef]
- Adrianto, L.R.; Pfister, S. Prospective environmental assessment of reprocessing and valorization alternatives for sulfidic copper tailings. Resour. Conserv. Recycl. 2022, 186, 106567. [Google Scholar] [CrossRef]
- Lu, J.; Kumagai, S.; Ohno, H.; Kameda, T.; Saito, Y.; Yoshioka, T.; Fukushima, Y. Deducing targets of emerging technologies based on ex ante life cycle thinking: Case study on a chlorine recovery process for polyvinyl chloride wastes. Resour. Conserv. Recycl. 2019, 151, 104500. [Google Scholar] [CrossRef]
- Pachon, E.R.; Vaskan, P.; Raman, J.K.; Gnansounou, E. Transition of a South African sugar mill towards a biorefinery. A feasibility assessment. Appl. Energy 2018, 229, 1–17. [Google Scholar] [CrossRef]
- Kalyva, A.E.; Vagia, E.C.; Konstandopoulos, A.G.; Srinivasa, A.R.; T-Raissi, A.; Muradov, N.; Kakosimos, K.E. Hybrid photo-thermal sulfur-ammonia water splitting cycle: Thermodynamic analysis of the thermochemical steps. Int. J. Hydrogen Energy 2017, 42, 9533–9544. [Google Scholar] [CrossRef]
- Chou, J.-S.; Yeh, K.-C. Life cycle carbon dioxide emissions simulation and environmental cost analysis for building construction. J. Clean. Prod. 2015, 101, 137–147. [Google Scholar] [CrossRef]
- Lu, J.; Xu, J.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Separation mechanism of polyvinyl chloride and copper components from swollen electric cables by mechanical agitation. Waste Manag. 2019, 93, 54–62. [Google Scholar] [CrossRef]
- Aki, H.; Wakui, T.; Yokoyama, R. Development of an energy management system for optimal operation of fuel cell based residential energy systems. Int. J. Hydrogen Energy 2016, 41, 20314–20325. [Google Scholar] [CrossRef]
- Ghoroghi, A.; Rezgui, Y.; Petri, I.; Beach, T. Advances in application of machine learning to life cycle assessment: A literature review. Int. J. Life Cycle Assess. 2022, 27, 433–456. [Google Scholar] [CrossRef]
- Villares, M.; Isildar, A.; van der Giesen, C.; Guinee, J. Does ex ante application enhance the usefulness of LCA? A case study on an emerging technology for metal recovery from e-waste. Int. J. Life Cycle Assess. 2017, 22, 1618–1633. [Google Scholar] [CrossRef]
- Wang, J.; Chan, F.K.S.; Johnson, M.F.; Chan, H.K.; Cui, Y.; Chen, J.; Zhu, Y.-G.; Chen, W.-Q. Material flow analysis of chemical additives in plastics: A critical review. Crit. Rev. Environ. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Lase, I.S.; Tonini, D.; Caro, D.; Albizzati, P.F.; Cristóbal, J.; Roosen, M.; Kusenberg, M.; Ragaert, K.; Van Geem, K.M.; Dewulf, J.; et al. How much can chemical recycling contribute to plastic waste recycling in Europe? An assessment using material flow analysis modeling. Resour. Conserv. Recycl. 2023, 192, 106916. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakajima, K.; Yoshizawa, Y.; Matsubae-Yokoyama, K.; Nagasaka, T. Analyzing Polyvinyl Chloride in Japan with the Waste Input−Output Material Flow Analysis Model. J. Ind. Ecol. 2009, 13, 706–717. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, N.; Hu, S. Industrial metabolism of PVC in China: A dynamic material flow analysis. Resour. Conserv. Recycl. 2013, 73, 33–40. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, C.; Li, F.; Liu, H.; Yang, J. Stocks and flows of polyvinyl chloride (PVC) in China: 1980–2050. Resour. Conserv. Recycl. 2020, 154, 104584. [Google Scholar] [CrossRef]
- Chen, L.; Liu, W.; Yang, T.; Nowack, B. Probabilistic material flow analysis of eight commodity plastics in China: Comparison between 2017 and 2020. Resour. Conserv. Recycl. 2023, 191, 106880. [Google Scholar] [CrossRef]
- Amadei, A.M.; Rigamonti, L.; Sala, S. Exploring the EU plastic value chain: A material flow analysis. Resour. Conserv. Recycl. 2023, 197, 107105. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Piwowarska, D.; Festinger, N.; Chrusciel, J.J. Risks Associated with the Presence of Polyvinyl Chloride in the Environment and Methods for Its Disposal and Utilization. Materials 2024, 17, 173. [Google Scholar] [CrossRef]
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2021, 407, 124399. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Su, F.; Chen, Y.; Wang, T.; Ali, W.; Jin, H.; Xiong, L.; Ma, Y.; Liu, Z.; Zou, H. Co-exposure to PVC microplastics and cadmium induces oxidative stress and fibrosis in duck pancreas. Sci. Total Environ. 2024, 927, 172395. [Google Scholar] [CrossRef] [PubMed]
- Ayres, R.U. The Life Cycle of Chlorine, Part III. J. Ind. Ecol. 1998, 2, 93–115. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S.; Shi, L. Industrial metabolism of chlorine in a chemical industrial park: The Chinese case. J. Clean. Prod. 2016, 112, 4367–4376. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wei, S.; Dong, J.; Zhao, J.; Qian, G. Assessing the chlorine metabolism and its resource efficiency in chlor-alkali industrial symbiosis—A case of Shanghai Chemical Industry Park. J. Clean. Prod. 2022, 380, 134934. [Google Scholar] [CrossRef]
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The fate of chlorine during MSW incineration: Vaporization, transformation, deposition, corrosion and remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
- Zaki, M.T.; Rowles, L.S. A Critical Review of Data Science Applications in Resource Recovery and Carbon Capture from Organic Waste. ACS ES T Eng. 2023, 3, 1424–1467. [Google Scholar] [CrossRef]
- Kaushik, L.K.; Muthukumar, P. Life cycle Assessment (LCA) and Techno-economic Assessment (TEA) of medium scale (5–10 kW) LPG cooking stove with two-layer porous radiant burner. Appl. Therm. Eng. 2018, 133, 316–326. [Google Scholar] [CrossRef]
- Samiee, L.; Goodarzvand-Chegini, F.; Khalili-Garakani, A.; Kargari, N.; Rahmanian, N. Feasibility study of organochlorine waste processing and recycling in the vinyl chloride production unit: Techno-economic assessment and emission estimation. J. Mater. Cycles Waste Manag. 2023, 25, 3530–3544. [Google Scholar] [CrossRef]
- Herbes, C.; Roth, U.; Wulf, S.; Dahlin, J. Economic assessment of different biogas digestate processing technologies: A scenario-based analysis. J. Clean. Prod. 2020, 255, 120282. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V.; Shanmugam, K.; Souihi, N.; Tysklind, M. Advancing game changing academic research concepts to commercialization: A Life Cycle Assessment (LCA) based sustainability framework for making informed decisions in Technology Valley of Death (TVD). Resour. Conserv. Recycl. 2018, 133, 404–416. [Google Scholar] [CrossRef]
- Ogmundarson, O.; Sukumara, S.; Herrgard, M.J.; Fantke, P. Combining Environmental and Economic Performance for Bioprocess Optimization. Trends Biotechnol. 2020, 38, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Wang, L.; Wang, Z.; Cooper, N.J.; Elimelech, M. Directing the research agenda on water and energy technologies with process and economic analysis. Energy Environ. Sci. 2023, 16, 714–722. [Google Scholar] [CrossRef]
- Sinke, P.; Swartz, E.; Sanctorum, H.; van der Giesen, C.; Odegard, I. Ex-ante life cycle assessment of commercial-scale cultivated meat production in 2030. Int. J. Life Cycle Assess. 2023, 28, 234–254. [Google Scholar] [CrossRef]
- Ferdous, J.; Bensebaa, F.; Pelletier, N. Integration of LCA, TEA, Process Simulation and Optimization: A systematic review of current practices and scope to propose a framework for pulse processing pathways. J. Clean. Prod. 2023, 402, 136804. [Google Scholar] [CrossRef]
- Nordahl, S.L.; Scown, C.D. Recommendations for life-cycle assessment of recyclable plastics in a circular economy. Chem. Sci. 2024, 15, 9397–9407. [Google Scholar] [CrossRef]
- Liang, Q.; Yu, L. Assessment of carbon emission potential of polyvinyl chloride plastics. E3S Web Conf. 2023, 393, 01031. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Mudersbach, M.; Juergens, M.; Pohler, M.; Spierling, S.; Venkatachalam, V.; Endres, H.-J.; Barner, L. Life Cycle Assessment in a Nutshell-Best Practices and Status Quo for the Plastic Sector. Macromol. Rapid Commun. 2023, 2300466. [Google Scholar] [CrossRef]
- Yadav, G.; Singh, A.; Dutta, A.; Uekert, T.; DesVeaux, J.S.; Nicholson, S.R.; Tan, E.C.D.; Mukarakate, C.; Schaidle, J.A.; Wrasman, C.J.; et al. Techno-economic analysis and life cycle assessment for catalytic fast pyrolysis of mixed plastic waste. Energy Environ. Sci. 2023, 16, 3638–3653. [Google Scholar] [CrossRef]
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the life cycle assessment of chemical recycling of plastic waste e A review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Costa, L.P.; Vaz de Miranda, D.M.; Pinto, J.C. Critical Evaluation of Life Cycle Assessment Analyses of Plastic Waste Pyrolysis. ACS Sustain. Chem. Eng. 2022, 10, 3799–3807. [Google Scholar] [CrossRef]
- Lu, J.; Kumagai, S.; Fukushima, Y.; Ohno, H.; Kameda, T.; Saito, Y.; Yoshioka, T. Combined Experiment, Simulation, and Ex-ante LCA Approach for Sustainable Cl Recovery from NaCl/Ethylene Glycol by Electrodialysis. Ind. Eng. Chem. Res. 2020, 59, 20112–20122. [Google Scholar] [CrossRef]
- Lu, J.; Kumagai, S.; Fukushima, Y.; Ohno, H.; Borjigin, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Sustainable Advance of Cl Recovery from Polyvinyl Chloride Waste Based on Experiment, Simulation, and Ex Ante Life-Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 14112–14123. [Google Scholar] [CrossRef]
- Igos, E.; Benetto, E.; Meyer, R.; Baustert, P.; Othoniel, B. How to treat uncertainties in life cycle assessment studies? Int. J. Life Cycle Assess. 2019, 24, 794–807. [Google Scholar] [CrossRef]
- Korkmaz, P.; Gardumi, F.; Avgerinopoulos, G.; Blesl, M.; Fahl, U. A comparison of three transformation pathways towards a sustainable European society—An integrated analysis from an energy system perspective. Energy Strategy Rev. 2020, 28, 100461. [Google Scholar] [CrossRef]
- Mueller, C.; Hoffrichter, A.; Wyrwoll, L.; Schmitt, C.; Trageser, M.; Kulms, T.; Beulertz, D.; Metzger, M.; Duckheim, M.; Huber, M.; et al. Modeling framework for planning and operation of multi-modal energy systems in the case of Germany. Appl. Energy 2019, 250, 1132–1146. [Google Scholar] [CrossRef]
- Papineau, M.; Yassin, K.; Newsham, G.; Brice, S. Conditional demand analysis as a tool to evaluate energy policy options on the path to grid decarbonization. Renew. Sustain. Energy Rev. 2021, 149, 111300. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Lei, J.; Zi, Y. A cross-scale modelling and decarbonisation quantification approach for navigating Carbon Neutrality Pathways in China. Energy Convers. Manag. 2023, 297, 117733. [Google Scholar] [CrossRef]
- Han, M.; Wu, Y.; Sun, J.; Geng, X.; Gao, X.; Zhou, T.; Lu, J. Carbon feasibility of terminating plastic waste leakage by landfill mining: A case study based on practical projects in China. Sci. Total Environ. 2024, 906, 167461. [Google Scholar] [CrossRef]
- Lu, J.; Tang, J.; Shan, R.; Li, G.; Rao, P.; Zhang, N. Spatiotemporal analysis of the future carbon footprint of solar electricity in the United States by a dynamic life cycle assessment. Iscience 2023, 26, 106188. [Google Scholar] [CrossRef]
- Tang, J.; Xiao, X.; Han, M.; Shan, R.; Gu, D.; Hu, T.; Li, G.; Rao, P.; Zhang, N.; Lu, J. China’s Sustainable Energy Transition Path to Low-Carbon Renewable Infrastructure Manufacturing under Green Trade Barriers. Sustainability 2024, 16, 3387. [Google Scholar] [CrossRef]
- Xue, N.; Lu, J.; Gu, D.; Lou, Y.; Yuan, Y.; Li, G.; Kumagai, S.; Saito, Y.; Yoshioka, T.; Zhang, N. Carbon footprint analysis and carbon neutrality potential of desalination by electrodialysis for different applications. Water Res. 2023, 232, 119716. [Google Scholar] [CrossRef]
- Abrha, H.; Cabrera, J.; Dai, Y.; Irfan, M.; Toma, A.; Jiao, S.; Liu, X. Bio-Based Plastics Production, Impact and End of Life: A Literature Review and Content Analysis. Sustainability 2022, 14, 4855. [Google Scholar] [CrossRef]
- Bocque, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-Based and Bio-Based Plasticizers: Chemical Structures to Plasticizing Properties. J. Polym. Sci. Part A-Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Martellini, T.; Russo, A.; Cincinelli, A.; Santini, S.; Lofrumento, C.; Baini, M.; Ciattini, S.; Conti, L.; Mostardini, F.; Mercatelli, L.; et al. Bioplastics on marine sandy shores: Effects on the key species Talitrus saltator (Montagu, 1808). Sci. Total Environ. 2023, 876, 162811. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.J.; Townsend, T.G. Life-Cycle Assumptions of Landfilled Polylactic Acid Underpredict Methane Generation. Environ. Sci. Technol. Lett. 2016, 3, 166–169. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Zuk, T.; Pietraszek, J.; Rytlewski, P.; Moraczewski, K.; Stepczynska, M. Electrostatic separation of binary mixtures of some biodegradable polymers and poly(vinyl chloride) or poly(ethylene terephthalate). Polimery 2016, 61, 835–843. [Google Scholar] [CrossRef]
- Adachi, W.; Kumagai, S.; Shao, Z.; Saito, Y.; Yoshioka, T. Selective recovery of pyrolyzates of biodegradable (PLA, PHBH) and common plastics (HDPE, PP, PS) during co-pyrolysis under slow heating. Sci. Rep. 2024, 14, 16476. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, J.; Zhao, Y.; Li, G.; Chi, Y.; Weiss-Hortala, E.; Nzihou, A.; Luo, G.; Ye, C. Hydrogen-Rich and Clean Fuel Gas Production from Co-pyrolysis of Biomass and Plastic Blends with CaO Additive. ACS Omega 2022, 7, 36050–36904. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, H.; Nakajima, D.; Goto, S.; Sugita, K.; Wu, W.; Kawamoto, K. HCl emission during co-pyrolysis of demolition wood with a small amount of PVC film and the effect of wood constituents on HCl emission reduction. Fuel 2008, 87, 3155–3157. [Google Scholar] [CrossRef]

| Dechlorination Method | The Characteristic of the Method | Experimental Factors | Reference |
|---|---|---|---|
| Pyrolysis under low temperature (200–300 °C) | Through the breaking of chemical bonds between atoms during low-temperature pyrolysis to achieve dechlorination | Temperature, different gas atmosphere (inert gas, air, oxygen) | [21] |
| Solvothermal dechlorination method | Taking advantage of elimination and substitution reactions to achieve dechlorination | Temperature, reaction time, different alkaline additives(NaOH, KOH, K2CO3) | [22] |
| Metal oxide catalytic technology | Incorporating catalysts during dechlorination process can enhance dechlorination efficiency, lowering the pyrolysis reaction temperature. | Different metal oxide catalysts (TiO2, MgO, CoO) | [23] |
| Mechanochemical approach | Combine chemical methods (NaOH) to make the surface rigid and physical method (ball milling) for crushing in order to achieve better dechlorination | Chemical factors (e.g., NaOH concentration) and mechanical factors (e.g., ball size, milling speed) | [24] |
| Pyrolysis under high temperature | Pyrolysis under high temperature decomposes PVC plastic into small molecular compounds | The PVC content in mixed plastic (0–12%), heating rate (10–60 °C/min) | [26] |
| Catalytic dechlorination | Utilizing waste materials for catalyst offers a sustainable and cost-effective solution for PVC waste. | The interaction between metal oxides and alkalinity additives | [27] |
| Electrolysis method | Decomposing PVC plastic into Cl gas and chemicals through electrochemical reactions. | Treatment time, discharge power on dechlorination efficiency | [28] |
| Adsorption technology for liquid products (e.g., pyrolysis oil) | Zeolite adsorbents have the characteristics of recyclability, which can be regenerated at high temperature. | Temperature, different sodium zeolites as adsorbents | [30] |
| Adsorption technology for gas products (e.g., HCl) | Using organic acids and kitchen waste sludge for gas products dechlorination | Concentrations and pH of organic acids | [31] |
| Adsorption and steam pressurization technology for solid products | Achieving efficient dechlorination through the combined use of alkaline adsorption and pressurization techniques | The type and number of alkaline adsorbents on dechlorination efficiency | [32] |
| TRL | Scale | TEA | LCA |
|---|---|---|---|
| 1 | Concepts | Extended (Ex ante) TEA | Extended (Ex ante) LCA |
| 2 | Lab-scale | ||
| 3 | Bench scale | ||
| 4 | Engineering scale | Traditional TEA | Extended LCA |
| 5 | Full scale | Traditional LCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Han, M.; Gu, D.; Bi, Z.; Gu, N.; Hu, T.; Li, G.; Zhang, N.; Lu, J. PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment. Sustainability 2024, 16, 8331. https://doi.org/10.3390/su16198331
Tian Y, Han M, Gu D, Bi Z, Gu N, Hu T, Li G, Zhang N, Lu J. PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment. Sustainability. 2024; 16(19):8331. https://doi.org/10.3390/su16198331
Chicago/Turabian StyleTian, Yuan, Mengqi Han, Dungang Gu, Zhujie Bi, Nannan Gu, Tingting Hu, Guanghui Li, Nan Zhang, and Jiaqi Lu. 2024. "PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment" Sustainability 16, no. 19: 8331. https://doi.org/10.3390/su16198331
APA StyleTian, Y., Han, M., Gu, D., Bi, Z., Gu, N., Hu, T., Li, G., Zhang, N., & Lu, J. (2024). PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment. Sustainability, 16(19), 8331. https://doi.org/10.3390/su16198331







