Balancing Environmental Safety and Economic Feasibility: A Review of Soil Fluorine Management Strategies in South Korea
Abstract
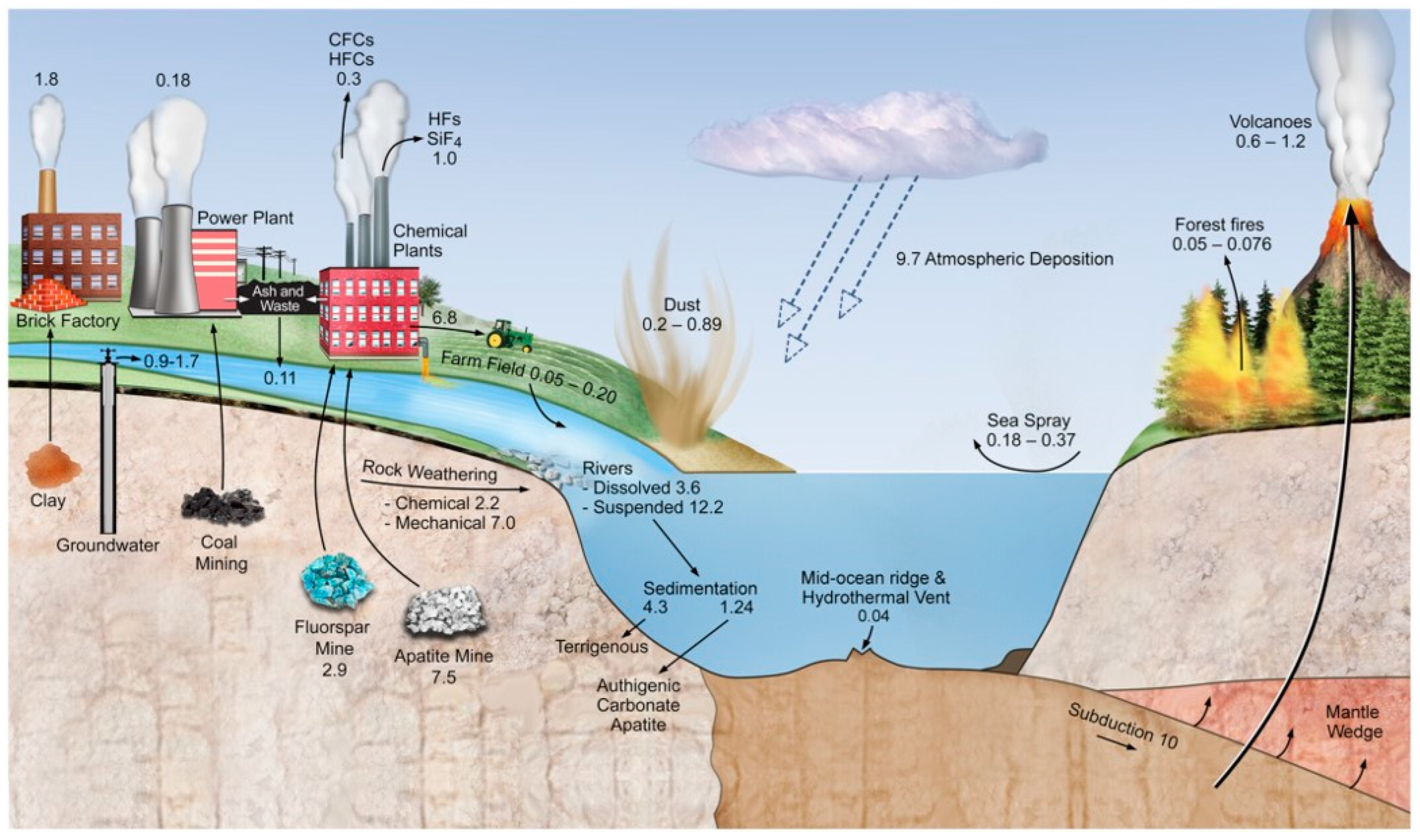
:1. Introduction
2. Origin and Sources of Fluorine
2.1. Natural Sources
2.1.1. Weathering
2.1.2. Volcanic Activity
2.1.3. Marine-Derived Components
2.1.4. Other Minor Sources
2.2. Anthropogenic Sources
2.2.1. Coal Combustion
2.2.2. Brick and Ceramic Manufacturing
2.2.3. Fluorine Emissions from Aluminum Smelting
2.2.4. Agricultural Sources
Fluoride Release during Phosphate Fertilizer Production
Fluoride Release during Phosphate Fertilizer Application
Other Agricultural Fluoride Sources
2.2.5. Fluoride Contamination by Various Industrial Sources
Mining and Waste Management
Fluoride in Steel Production
Glass and Other Industries
Fluorocarbons and Emerging Sources
2.2.6. Urban Fluoride Emissions and Concerns
2.2.7. Ubiquity and Persistence of Fluorinated Organic Compounds
Chlorofluorocarbons and Their Replacements
Trifluoroacetic Acid: A Persistent Byproduct
2.2.8. Fluoride in Petroleum
3. Soil Fluorine Management Strategies
3.1. Global Variations in Soil Fluorine Regulations
3.2. Balancing Soil Fluorine Standards in Korea
| No. | Country | Soil Quality Guidelines | Reference | |
|---|---|---|---|---|
| Land Use | Concentration (mg/kg) | |||
| 1 | Canada | Agriculture | 200 | CCME [142] |
| Agriculture/residential (Alberta) | 200 | |||
| Residential/parkland | 400 | |||
| Commercial/industrial | 2000 | |||
| 2 | Australia | Industrial waste (Victoria) | 450 | EPA, Victoria [146] |
| 3 | Switzerland | All regions | 400 | Slooff et al. [147] |
| 4 | The Netherlands | Regions with high clay content (>25%) | 500 | |
| Regions with very little or no clay content | 175 | |||
| 5 | Austria | Agricultural/residential (trigger value) | 200 | Carlon et al. [148] |
| Agricultural/residential (intervention value) | 1000 | |||
| 6 | Belgium | Special areas with high biological value | 45 | |
| Residential | 3950 | |||
| Industrial | 4690 | |||
| 7 | The Czech Republic | Agricultural | 500 | |
| 8 | Italy | Residential/public | 100 | |
| Agricultural | 2000 | |||
| 9 | Lithuania | Residential/recreational/agricultural | 200 | |
| 10 | Slovakia | Maximum allowable limits | 1000 | |
| Value for decontamination measures | 2000 | |||
| 11 | The United States (US) | Residential | 469 | USEPA [149] |
| 12 | Japan | All regions except agricultural | 4000 | Noh [13] |
3.3. Advantages, Disadvantages, and Challenges of Soil Fluorine Management Strategies: Navigating the Complexities
3.3.1. Proposed Soil Fluorine Management Strategies for Korea
3.3.2. Advantages—Benefits of Effective Soil Fluorine Management
3.3.3. Disadvantages—Limitations and Considerations
3.3.4. Challenges—Key Considerations for Implementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCME | Canadian Council of Ministers of the Environment |
| CFCs | Chlorofluorocarbons |
| EPA | Environmental Protection Agency |
| HF | Hydrogen fluoride |
| HCFCs | Hydrochlorofluorocarbons |
| HFCs | Hydrofluorocarbons |
| MC | Municipal solid waste compost |
| PBT | Persistent, bioaccumulative, and toxic |
| PFCs | Perfluorinated compounds |
| PFAS | Perfluoroalkyl and polyfluoroalkyl substances |
| PM | Particulate matter |
| SiF4 | Silicon tetrafluoride |
| SWL | Soil Worrisome Level |
| TFA | Trifluoroacetic acid |
| WHO | World Health Organization |
References
- Dehnen, S.; Schafer, L.L.; Lectka, T.; Togni, A. Fluorine: A Very Special Element and Its Very Special Impacts on Chemistry. Inorg. Chem. 2021, 60, 17419–17425. [Google Scholar] [CrossRef] [PubMed]
- Tavener, S.J.; Clark, J.H. Fluorine: Friend or foe? A green chemist’s perspective. Adv. Fluor. Sci. 2006, 2, 177–202. [Google Scholar] [CrossRef]
- Weinstein, L.H.; Davison, A. Fluorides in the Environment: Effects on Plants and Animals; Cabi Publishing: Wallingford, UK, 2004. [Google Scholar]
- Fuge, R. Fluorine in the environment, a review of its sources and geochemistry. Appl. Geochem. 2019, 100, 393–406. [Google Scholar] [CrossRef]
- Yoon, S.-U.; Oh, N.-R.; Kim, J.-S. Oral health conditions of college students in some regions based on fluorine awareness. J. Korea Contents Assoc. 2015, 15, 329–337. [Google Scholar] [CrossRef]
- Flemming, J.; Hannig, C.; Hannig, M. Caries management—The role of surface interactions in de-and remineralization-processes. J. Clin. Med. 2022, 11, 7044. [Google Scholar] [CrossRef]
- Tanouayi, G.; Gnandi, K.; Ouro-Sama, K.; Aduayi-Akue, A.A.; Ahoudi, H.; Nyametso, Y.; Solitoke, H.D. Distribution of fluoride in the phosphorite mining area of hahotoe–Kpogame (Togo). J. Health Pollut. 2016, 6, 84–94. [Google Scholar] [CrossRef]
- Mullane, D.; Baez, R.; Jones, S.; Lennon, M.; Petersen, P.; Rugg-Gunn, A.; Whelton, H. Fluoride and oral health. Community Dent. Health 2016, 33, 69–99. [Google Scholar] [CrossRef]
- Sharma, R.; Kaur, R. Insights into fluoride-induced oxidative stress and antioxidant defences in plants. Acta Physiol. Plant. 2018, 40, 181. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Nan, Z.; Zang, F.; Sun, H.; Zhang, Q.; Huang, W.; Bao, L. Accumulation, fractionation and health risk assessment of fluoride and heavy metals in soil-crop systems in northwest China. Sci. Total Environ. 2019, 663, 307–314. [Google Scholar] [CrossRef]
- Lim, G.-H.; Lee, H.-G.; Kim, H.-S.; Noh, H.-J.; Ko, H.-W.; Kim, J.-I.; Jo, H.-J.; Kim, H.-K. Evaluation of fluoride distribution, fate and transport characteristics in soils. J. Soil Groundw. Environ. 2018, 23, 90–103. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Noh, H.-J. Establishing science-based fluoride soil pollution standards: A critical approach. In Proceedings of the Unveiling Science-Based Standards for Fluoride Soil Remediation: A Conference on Rational Approaches, Dongja Art Hall, Seoul, Republic of Korea, 30 May 2024. [Google Scholar]
- Totsche, K.U.; Wilcke, W.; Körber, M.; Kobza, J.; Zech, W. Evaluation of Fluoride-Induced Metal Mobilization in Soil Columns. J. Environ. Qual. 2000, 29, 454–459. [Google Scholar] [CrossRef]
- Dehbandi, R.; Moore, F.; Keshavarzi, B. Provenance and geochemical behavior of fluorine in the soils of an endemic fluorosis belt, central Iran. J. Afr. Earth Sci. 2017, 129, 56–71. [Google Scholar] [CrossRef]
- Koga, K.T.; Rose-Koga, E.F. Fluorine. In Encyclopedia of Geochemistry; White, W.M., Ed.; Springer: Berlin, Germany, 2016; pp. 13–18. [Google Scholar]
- Robinson, W.; Edgington, G. Fluorine in soils. Soil Sci. 1946, 61, 341–354. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of fluorine. Glob. Biogeochem. Cycles 2020, 34, e2020GB006722. [Google Scholar] [CrossRef]
- Koritnig, S. Fluorine. In Handbook of Geochemistry; Springer: New York, NY, USA, 1978; p. 9B-90. [Google Scholar]
- Chipera, S.J.; Bish, D.L. Thermal evolution of fluorine from smectite and kaolinite. Clays Clay Miner. 2002, 50, 38–46. [Google Scholar] [CrossRef]
- Noble, D.C.; Smith, V.C.; Peck, L.C. Loss of halogens from crystallized and glassy silicic volcanic rocks. Geochim. Cosmochim. Acta 1967, 31, 215–223. [Google Scholar] [CrossRef]
- Sinha, S.; Jha, S.; Hazra, S. Influence of interflow carbonate-clay association for groundwater fluoride contamination in eastern Deccan, central India. Environ. Sci. Pollut. Res. 2023, 30, 56259–56272. [Google Scholar] [CrossRef]
- Fleischer, M.; Robinson, W. Some problems of the geochemistry of fluorine. In Studies in Analytical Geochemistry; Shaw, D.M., Ed.; University of Toronto Press: Toronto, ON, Canada, 1963; Volume 6, pp. 58–75. [Google Scholar]
- Usman, J.; Begum, S. Assessment of spatial and temporal variation in soil fluoride and organic matter relationship: Its implications for carbon sequestration potential. Fluoride 2022, 55, 329–342. [Google Scholar]
- Duraiswami, R.A.; Patankar, U. Occurrence of fluoride in the drinking water sources from Gad river basin, Maharashtra. J. Geol. Soc. India 2011, 77, 167–174. [Google Scholar] [CrossRef]
- Handa, B.K. Presentation and interpretation of fluoride ion concentrations in natural waters. In Proceedings of the Symposium on Fluorosis, Hyderabad, India, 3–5 July 1977; pp. 317–347. [Google Scholar]
- D’Alessandro, W. Human fluorosis related to volcanic activity: A review. WIT Trans. Biomed. Health 2006, 10, 10. [Google Scholar] [CrossRef]
- Bellomo, S.; Aiuppa, A.; D’Alessandro, W.; Parello, F. Environmental impact of magmatic fluorine emission in the Mt. Etna area. J. Volcanol. Geotherm. Res. 2007, 165, 87–101. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T. Contribution to the ongoing discussion on fluoride toxicity. Arch. Toxicol. 2021, 95, 2571–2587. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Ranjan, A. Fluoride Toxicity in Animals; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Flueck, W.T.; Smith-Flueck, J.A.M. Severe dental fluorosis in juvenile deer linked to a recent volcanic eruption in Patagonia. J. Wildl. Dis. 2013, 49, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Walser III, J.W.; Gowland, R.L.; Desnica, N.; Kristjánsdóttir, S. Hidden dangers? Investigating the impact of volcanic eruptions and skeletal fluorosis in medieval Iceland. Archaeol. Anthropol. Sci. 2020, 12, 77. [Google Scholar] [CrossRef]
- Williams-Jones, G.; Rymer, H. Hazards of volcanic gases. In The Encyclopedia of Volcanoes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 985–992. [Google Scholar]
- Balagizi, C.M.; Kasereka, M.M.; Cuoco, E.; Liotta, M. Rain-plume interactions at Nyiragongo and Nyamulagira volcanoes and associated rainwater hazards, East Africa. Appl. Geochem. 2017, 81, 76–89. [Google Scholar] [CrossRef]
- Friend, J.P. Natural Chlorine and Fluorine in the Atmosphere, Water and Precipitation; NASA: Washington, DC, USA; Scientific Assessment of Stratospheric Ozone: Boulder, CO, USA, 1989; Volume 2. [Google Scholar]
- Stewart, C.; Johnston, D.; Leonard, G.; Horwell, C.; Thordarson, T.; Cronin, S. Contamination of water supplies by volcanic ashfall: A literature review and simple impact modelling. J. Volcanol. Geotherm. Res. 2006, 158, 296–306. [Google Scholar] [CrossRef]
- Hong, S.-H.; Jang, M.-J.; Jung, S.-W.; Park, S.-W. A review on monitoring Mt. Baekdu Volcano using space-based remote sensing observations. Korean J. Remote Sens. 2018, 34, 1503–1517. [Google Scholar] [CrossRef]
- Sugawara, K. Migration of elements through phases of the hydrosphere and atmosphere. Chem. Earth’s Crust 1967, 2, 501–510. [Google Scholar]
- Carpenter, R. Factors controlling the marine geochemistry of fluorine. Geochim. Cosmochim. Acta 1969, 33, 1153–1167. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Chi, Q.; Wu, H.; Zhang, B.; Xu, S.; Han, Z.; Nie, L.; Liu, H.; Liu, D. Geochemical characteristics of fluorine (F) in mainland China’s pedosphere: On the basis of the China Geochemical Baselines project. J. Geochem. Explor. 2020, 219, 106635. [Google Scholar] [CrossRef]
- Neal, C.; Smith, C.J.; Walls, J.; Billingham, P.; Hill, S.; Neal, M. Comments on the hydrochemical regulation of the halogen elements in rainfall, stemflow, throughfall and stream waters at an acidic forested area in Mid-Wales. Sci. Total Environ. 1990, 91, 1–11. [Google Scholar] [CrossRef]
- Fu, T.; Fu, Y.; Li, C.; Dong, M.; Qi, C.; Wang, Z.; Chen, G.; Su, Q.; Xu, X.; Yu, H. Seawater intrusion-triggered high fluoride groundwater development on the eastern coast of China. Environ. Sci. Pollut. Res. 2024, 31, 11307–11320. [Google Scholar] [CrossRef] [PubMed]
- Wilkniss, P.; Bressan, D. Chemical processes at the air-sea interface: The behavior of fluorine. J. Geophys. Res. 1971, 76, 736–741. [Google Scholar] [CrossRef]
- Desboeufs, K.; Formenti, P.; Torres-Sánchez, R.; Schepanski, K.; Chaboureau, J.-P.; Andersen, H.; Cermak, J.; Feuerstein, S.; Laurent, B.; Klopper, D. Fractional solubility of iron in mineral dust aerosols over coastal Namibia: A link to marine biogenic emissions? Atmos. Chem. Phys. 2024, 24, 1525–1541. [Google Scholar] [CrossRef]
- Barnard, W.R.; Nordstrom, D.K. Flouride in precipitation—II. Implications for the geochemical cycling of fluorine. Atmos. Environ. (1967) 1982, 16, 105–111. [Google Scholar] [CrossRef]
- De Angelis, M.; Legrand, M. Origins and variations of fluoride in Greenland precipitation. J. Geophys. Res. Atmos. 1994, 99, 1157–1172. [Google Scholar] [CrossRef]
- Saether, O.; Andreassen, B.T.; Semb, A. Amounts and sources of fluoride in precipitation over southern Norway. Atmos. Environ. 1995, 29, 1785–1793. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, C.; Wei, J.; Dong, F.; Yang, W.; Hao, D.; Jia, Z.; Ji, Y. Geochemical process of groundwater fluoride evolution along global coastal plains: Evidence from the comparison in seawater intrusion area and soil salinization area. Chem. Geol. 2020, 552, 119779. [Google Scholar] [CrossRef]
- Lindner, B.L.; Frysinger, J.R. Bulk atmospheric deposition in the Charleston Harbor watershed. J. Coast. Res. 2007, 23, 1452–1461. [Google Scholar] [CrossRef]
- Lewandowska, A.; Falkowska, L.; Jóźwik, J. Factors determining the fluctuation of fluoride concentrations in PM10 aerosols in the urbanized coastal area of the Baltic Sea (Gdynia, Poland). Environ. Sci. Pollut. Res. 2013, 20, 6109–6118. [Google Scholar] [CrossRef]
- Mikkonen, H.G.; van de Graaff, R.; Mikkonen, A.T.; Clarke, B.O.; Dasika, R.; Wallis, C.J.; Reichman, S.M. Environmental and anthropogenic influences on ambient background concentrations of fluoride in soil. Environ. Pollut. 2018, 242, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Preunkert, S.; Legrand, M.; Wagenbach, D. Causes of enhanced fluoride levels in Alpine ice cores over the last 75 years: Implications for the atmospheric fluoride budget. J. Geophys. Res. Atmos. 2001, 106, 12619–12632. [Google Scholar] [CrossRef]
- Jayarathne, T.; Stockwell, C.E.; Yokelson, R.J.; Nakao, S.; Stone, E.A. Emissions of fine particle fluoride from biomass burning. Environ. Sci. Technol. 2014, 48, 12636–12644. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.J.; Manoharan, V.; Hedley, M.J.; Loganathan, P. Fluoride: A review of its fate, bioavailability, and risks of fluorosis in grazed-pasture systems in New Zealand. N. Z. J. Agric. Res. 2000, 43, 295–321. [Google Scholar] [CrossRef]
- Yang, N.; Tang, S.; Zhang, S.; Huang, W.; Chen, P.; Chen, Y.; Xi, Z.; Yuan, Y.; Wang, K. Fluorine in Chinese coal: A review of distribution, abundance, modes of occurrence, genetic factors and environmental effects. Minerals 2017, 7, 219. [Google Scholar] [CrossRef]
- Ketris, M.á.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Kang, Y.; Wu, B.; Sun, R.; Zhou, C.; Wu, D. Atmospheric emissions of F, As, Se, Hg, and Sb from coal-fired power and heat generation in China. Chemosphere 2013, 90, 1925–1932. [Google Scholar] [CrossRef]
- Yu, J.; Feng, F.; Wang, W.; Luo, K.; Chen, D.; Bai, G.; Li, Y.; Zheng, L.; Bai, A. Regularity of flourine release from fluorine-rich coal combustion in the fluorine poisoning area. Huan Jing Ke Xue = Huanjing Kexue 2004, 25, 43–46. [Google Scholar]
- Doley, D.; Hill, R.; Riese, R. Environmental fluoride in Australasia: Ecological effects, regulation and management. Clean Air Environ. Qual. 2004, 38, 35–55. [Google Scholar] [CrossRef]
- Shupe, J.L.; Bruner, R.H.; Seymour, J.L.; Alden, C.L. The pathology of chronic bovine fluorosis: A review. Toxicol. Pathol. 1992, 20, 274–288. [Google Scholar] [CrossRef]
- Fidanci, U.R.; Sel, T. The industrial fluorosis caused by a coal-burning power station and its effects on sheep. Turk. J. Vet. Anim. Sci. 2001, 25, 735–741. [Google Scholar]
- Zemek, F.; Heřman, M.; Kierdorf, H.; Kierdorf, U.; Sedláček, F. Spatial distribution of dental fluorosis in roe deer (Capreolus capreolus) from North Bohemia (Czech Republic) and its relationships with environmental factors. Sci. Total Environ. 2006, 370, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, U.; Bahelková, P.; Sedláček, F.; Kierdorf, H. Pronounced reduction of fluoride exposure in free-ranging deer in North Bohemia (Czech Republic) as indicated by the biomarkers skeletal fluoride content and dental fluorosis. Sci. Total Environ. 2012, 414, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Ando, M.; Tadano, M.; Asanuma, S.; Tamura, K.; Matsushima, S.; Watanabe, T.; Kondo, T.; Sakurai, S.; Ji, R.; Liang, C. Health effects of indoor fluoride pollution from coal burning in China. Environ. Health Perspect. 1998, 106, 239–244. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Belkin, H.E.; Zheng, B. Health impacts of domestic coal use in China. Proc. Natl. Acad. Sci. USA 1999, 96, 3427–3431. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, L.; Li, H. Environmental epidemic characteristics of coal-burning endemic fluorosis and the safety threshold of coal fluoride in China. Fluoride 2003, 36, 106–112. [Google Scholar]
- Gao, X.; Hu, Y.; Li, C.; Dai, C.; Li, L.; Ou, X.; Wang, Y. Evaluation of fluorine release from air deposited coal spoil piles: A case study at Yangquan city, northern China. Sci. Total Environ. 2016, 545, 1–10. [Google Scholar] [CrossRef]
- Ramya, S.; Deshmukh, V.; Khandekar, V.J.; Padmakar, C.; SuriNaidu, L.; Mahore, P.K.; Pujari, P.R.; Panaskar, D.; Labhasetwar, P.; Rao, V. Assessment of impact of ash ponds on groundwater quality: A case study from Koradi in Central India. Environ. Earth Sci. 2013, 69, 2437–2450. [Google Scholar] [CrossRef]
- Korea Electric Power Corporation. Statistics of Electric Power in Korea 2022; Korea Electric Power Corporation: Naju-si, Republic of Korea, 2023; p. 181. [Google Scholar]
- Xie, Z.; Wu, W.; Xu, J. Study on fluoride emission from soils at high temperature related to brick-making process. Chemosphere 2003, 50, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Troll, G.; Farzaneh, A. Fluorine loss in production of bricks and comparison with the loss in fluorine-bearing minerals. Interceram 1978, 4, 400–402. [Google Scholar]
- Fuge, R.; Hennah, T.J. Fluorine and heavy metals in the vicinity of brickworks. In Proceedings of the Trace Substances in Environmental Health, Annual Conference, Columbia; University of Missouri: Columbia, MO, USA, 1989; pp. 183–197. [Google Scholar]
- Bonvicini, G.; Fregni, A.; Palmonari, C. Fluorine compounds in gaseous emissions from industrial sources: The case of ceramic industries. Adv. Fluor. Sci. 2006, 1, 225–249. [Google Scholar] [CrossRef]
- Khalid, S.; Mansab, S. Effect of fluorides on air, water, soil and vegetation in peripheral areas of brick kiln of Rawalpindi. Pak. J. Bot. 2015, 47, 205–209. [Google Scholar]
- Schmidt, C.W. Modernizing Artisanal Brick Kilns: A Global Need. Environ. Health Perspect. 2013, 121, 242–249. [Google Scholar] [CrossRef]
- Ahmad, M.N.; van den Berg, L.J.; Shah, H.U.; Masood, T.; Büker, P.; Emberson, L.; Ashmore, M. Hydrogen fluoride damage to vegetation from peri-urban brick kilns in Asia: A growing but unrecognised problem? Environ. Pollut. 2012, 162, 319–324. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Ahmad, S.S.; Zia, A.; Iqbal, M.S.; Shah, H.; Mian, A.A.; Shah, R.U. Hydrogen fluoride effects on local mung bean and maize cereal crops from peri-urban brick kilns in South Asia. Fluoride 2014, 47, 315–319. [Google Scholar]
- Baum, E. Black carbon from brick kilns. In Proceedings of the Presentation for Clean Air Task Force, Boston, MA, USA, 7 April 2010. [Google Scholar]
- Tjahyono, N.; Gao, Y.; Wong, D.; Zhang, W.; Taylor, M.P. Fluoride emissions management guide (FEMG) for aluminium smelters. In Light Metals 2011; Lindsay, S.J., Ed.; Springer: Cham, Switzerland, 2011; pp. 301–306. [Google Scholar]
- Kvande, H. Production of primary aluminium. In Fundamentals of Aluminium Metallurgy; Elsevier: Amsterdam, The Netherlands, 2011; pp. 49–69. [Google Scholar]
- Alamdari, H. Aluminium production process: Challenges and opportunities. Metals 2017, 7, 133. [Google Scholar] [CrossRef]
- International Aluminium Institute. Fluoride Emissions. Available online: https://international-aluminium.org/statistics/fluoride-emissions/ (accessed on 4 September 2024).
- Robak, H. Aluminium plants and conifers in Norway. In Proceedings of the Air Pollution, Wageningen, The Netherlands, 22–27 April 1968; pp. 27–31. [Google Scholar]
- Krook, L.; Maylin, G.A. Industrial fluoride pollution. Chronic fluoride poisoning in Cornwall Island cattle. Cornell Vet. 1979, 69 (Suppl. 8), 1–70. [Google Scholar]
- Gilbert, O. Environmental effects of airborne fluorides from aluminium smelting at Invergordon, Scotland 1971–1983. Environ. Pollut. Ser. A Ecol. Biol. 1985, 39, 293–302. [Google Scholar] [CrossRef]
- Ouellet, M. Reduction of airborne fluoride emissions from Canadian aluminium smelters as revealed by snow chemistry. Sci. Total Environ. 1987, 66, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vike, E.; Håbjørg, A. Variation in fluoride content and leaf injury on plants associated with three aluminium smelters in Norway. Sci. Total Environ. 1995, 163, 25–34. [Google Scholar] [CrossRef]
- Rodriguez, J.H.; Wannaz, E.D.; Pignata, M.L.; Fangmeier, A.; Franzaring, J. Fluoride biomonitoring around a large aluminium smelter using foliage from different tree species. CLEAN–Soil Air Water 2012, 40, 1315–1319. [Google Scholar] [CrossRef]
- Talovskaya, A.V.; Osipova, N.A.; Filimonenko, E.A.; Polikanova, S.; Samokhina, N.; Yazikov, E.; Matveenko, I.A. Fluorine concentration in snow cover within the impact area of aluminium production plant (Krasnoyarsk city) and coal and gas-fired power plant (Tomsk city). IOP Conf. Ser. Earth Environ. Sci. 2015, 27, 012043. [Google Scholar] [CrossRef]
- Hufschmid, J.; Beveridge, I.; Coulson, G.; Gould, J. Bone fluoride concentrations of eastern grey kangaroos (Macropus giganteus) resident near an aluminium smelter in south-eastern Australia. Ecotoxicology 2011, 20, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, U.; Death, C.; Hufschmid, J.; Witzel, C.; Kierdorf, H. Developmental and post-eruptive defects in molar enamel of free-ranging eastern grey kangaroos (Macropus giganteus) exposed to high environmental levels of fluoride. PLoS ONE 2016, 11, e0147427. [Google Scholar] [CrossRef]
- Villalba, G.; Liu, Y.; Schroder, H.; Ayres, R.U. Global phosphorus flows in the industrial economy from a production perspective. J. Ind. Ecol. 2008, 12, 557–569. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- European Fertilizer Manufacturers’ Association. Booklet No. 4 of 8 Production of Phosphoric Acid. Best Available Techniques for Pollution Prevention and Control in the European Fertilizer Industry. Belgium. 2000. Available online: http://www.productstewardship.eu/fileadmin/user_upload/user_upload_prodstew/documents/Booklet_nr_4_Production_of_Phosphoric_Acid.pdf (accessed on 4 September 2024).
- Mirlean, N.; Roisenberg, A. Fluoride distribution in the environment along the gradient of a phosphate-fertilizer production emission (southern Brazil). Environ. Geochem. Health 2007, 29, 179–187. [Google Scholar] [CrossRef]
- Mezghani, I.; Elloumi, N.; Abdallah, F.B.; Chaieb, M.; Boukhris, M. Fluoride accumulation by vegetation in the vicinity of a phosphate fertilizer plant in Tunisia. Fluoride 2005, 38, 69–75. [Google Scholar]
- Dartan, G.; Taspinar, F.; Toroz, I. Analysis of fluoride pollution from fertilizer industry and phosphogypsum piles in agricultural area. J. Ind. Pollut. Control 2017, 33, 662. [Google Scholar]
- Ober, J.A. Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018; p. 204. [CrossRef]
- Loganathan, P.; Liu, Q.; Hedley, M.; Gray, C. Chemical fractionation of fluorine in soils with a long-term phosphate fertiliser history. Aust. J. Soil Res. 2007, 45, 390–396. [Google Scholar] [CrossRef]
- Hedley, M.; Loganathan, P.; Grace, N. Fertilizer-derived fluorine in grazed pasture systems. In Proceedings of the Australian Fertilizer Industry Conference, Hamilton Island, Queensland, Australia, 6–10 August 2007; pp. 1–24. [Google Scholar]
- Pickering, W. The mobility of soluble fluoride in soils. Environ. Pollut. Ser. B Chem. Phys. 1985, 9, 281–308. [Google Scholar] [CrossRef]
- Kundu, M.C.; Mandal, B. Assessment of potential hazards of fluoride contamination in drinking groundwater of an intensively cultivated district in West Bengal, India. Environ. Monit. Assess. 2009, 152, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Masuda, H.; Siddiqui, R.; Naseem, M. Sources of arsenic and fluoride in highly contaminated soils causing groundwater contamination in Punjab, Pakistan. Arch. Environ. Contam. Toxicol. 2009, 56, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Davis, R. Uptake of fluoride by ryegrass grown in soil treated with sewage sludge. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 277–284. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. Former. Pestic. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef]
- Key, B.D.; Howell, R.D.; Criddle, C.S. Fluorinated organics in the biosphere. Environ. Sci. Technol. 1997, 31, 2445–2454. [Google Scholar] [CrossRef]
- Scholz, L.M.; Kopittke, P.M.; Menzies, N.W.; Dalzell, S.A.; Macfarlane, D.C.; Wehr, J.B. Use of fluoride-containing water for the irrigation of soil–plant systems. J. Agric. Food Chem. 2015, 63, 4737–4745. [Google Scholar] [CrossRef]
- Botha, C.; Naude, T.; Minnaar, P.; Van Amstel, S.; Janse van Rensburg, S. Two outbreaks of fluorosis in cattle and sheep. J. South Afr. Vet. Assoc. 1993, 64, 165–168. [Google Scholar]
- Fuge, R.; Andrews, M.J. Fluorine in the UK environment. Environ. Geochem. Health 1988, 10, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Johnson, M.; Davidson, A.; Bradshaw, A. Fluoride in plants colonising fluorspar mine waste in the Peak District and Weardale. Environ. Pollut. (1970) 1976, 11, 9–23. [Google Scholar] [CrossRef]
- Andrews, S.; Cooke, J.; Johnson, M. Distribution of trace element pollutants in a contaminated ecosystem established on metalliferous fluorspar tailings. 3: Fluoride. Environ. Pollut. 1989, 60, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Semrau, K.T. Emission of fluorides from industrial processes—A review. J. Air Pollut. Control Assoc. 1957, 7, 92–108. [Google Scholar] [CrossRef]
- Yang, Q.X.; Xu, A.J.; Engström, F.; Han, F.L.; Xue, P.; He, D.F.; Björkman, B. Dissolution Behavior of Fluorine from AOD Slag after Treatments for Volume Stabilization. Appl. Mech. Mater. 2014, 587, 849–855. [Google Scholar] [CrossRef]
- Burns, K.N.; Allcroft, R. Fluorosis in Cattle. I. Occurrence and Effects in Industrial Areas of England and Wales 1954–57; Animal Disease Surveys; Report 2, Part 1; Ministry of Agriculture Fisheries and Food: London, UK, 1964.
- Villalba, G.; Ayres, R.U.; Schroder, H. Accounting for fluorine: Production, use, and loss. J. Ind. Ecol. 2007, 11, 85–101. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Air pollution control in municipal solid waste incinerators. In The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources; Khallaf, M., Ed.; 2011; Volume 1, Chapter 16; pp. 331–358. [Google Scholar]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2007, 29, 15. [Google Scholar]
- Sahariya, A.; Bharadwaj, C.; Emmanuel, I.; Alam, A. Fluoride toxicity in soil and plants: An overview. Asian J. Adv. Res. 2021, 4, 573–581. [Google Scholar]
- Sima, M.W.; Jaffé, P.R. A critical review of modeling Poly-and Perfluoroalkyl Substances (PFAS) in the soil-water environment. Sci. Total Environ. 2021, 757, 143793. [Google Scholar] [CrossRef]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Schaider, L.A.; Balan, S.A.; Blum, A.; Andrews, D.Q.; Strynar, M.J.; Dickinson, M.E.; Lunderberg, D.M.; Lang, J.R.; Peaslee, G.F. Fluorinated compounds in US fast food packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.M.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K.; Wang, Z. Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: Focus on the life cycle of C6-and C10-based products. Environ. Sci. Technol. Lett. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Boucher, J.M.; Scheringer, M.; Cousins, I.T.; Hungerbuhler, K. Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: Focus on the life cycle of C8-based products and ongoing industrial transition. Environ. Sci. Technol. 2017, 51, 4482–4493. [Google Scholar] [CrossRef]
- Paul, A.G.; Jones, K.C.; Sweetman, A.J. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009, 43, 386–392. [Google Scholar] [CrossRef]
- McCulloch, A. Fluorocarbons in the global environment: A review of the important interactions with atmospheric chemistry and physics. J. Fluor. Chem. 2003, 123, 21–29. [Google Scholar] [CrossRef]
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment; Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 147. [Google Scholar]
- Merrill, M.D.; Sleeter, B.M.; Freeman, P.A.; Liu, J.; Warwick, P.D.; Reed, B.C. Federal Lands Greenhouse Emissions and Sequestration in the United States—Estimates for 2005–14; US Geological Survey Scientific Investigation Report, 2018-5131; U.S. Geological Survey: Reston, VA, USA, 2018.
- Boutonnet, J.C.; Bingham, P.; Calamari, D.; Rooij, C.d.; Franklin, J.; Kawano, T.; Libre, J.-M.; McCul-Loch, A.; Malinverno, G.; Odom, J.M. Environmental risk assessment of trifluoroacetic acid. Hum. Ecol. Risk Assess. Int. J. 1999, 5, 59–124. [Google Scholar] [CrossRef]
- Solomon, K.R.; Velders, G.J.; Wilson, S.R.; Madronich, S.; Longstreth, J.; Aucamp, P.J.; Bornman, J.F. Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols. J. Toxicol. Environ. Health Part B 2016, 19, 289–304. [Google Scholar] [CrossRef]
- Wilson, J.; Marczewski, C. Comments on determination of fluorine in petroleum and petroleum process catalysts with a fluoride electrode. Anal. Chem. 1978, 50, 1584–1585. [Google Scholar] [CrossRef]
- González-Torres, M.; Pérez-Lombard, L.; Coronel, J.F.; Maestre, I.R.; Yan, D. A review on buildings energy information: Trends, end-uses, fuels and drivers. Energy Rep. 2022, 8, 626–637. [Google Scholar] [CrossRef]
- Blondes, M.S.; Shelton, J.L.; Engle, M.A.; Trembly, J.P.; Doolan, C.A.; Jubb, A.M.; Chenault, J.C.; Rowan, E.L.; Haefner, R.J.; Mailot, B.E. Utica shale play oil and gas brines: Geochemistry and factors influencing wastewater management. Environ. Sci. Technol. 2020, 54, 13917–13925. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.; Zou, C.; Cui, H.; Ni, Y.; Liu, J.; Wu, W.; Zhang, L.; Coyte, R.; Kondash, A. Recycling flowback water for hydraulic fracturing in Sichuan Basin, China: Implications for gas production, water footprint, and water quality of regenerated flowback water. Fuel 2020, 272, 117621. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Management Practices in the United States; ANL/EVS/R-09-1; TRN: US201106%%909; Argonne National Lab. (ANL): Argonne, IL, USA, 2009. [Google Scholar]
- Kim, J.; Hwang, Y.; Yoo, M.; Chen, S.; Lee, I.-M. Hydrogen fluoride (HF) substance flow analysis for safe and sustainable chemical industry. Environ. Sci. Pollut. Res. 2017, 24, 25137–25145. [Google Scholar] [CrossRef]
- CCME. Canadian Soil Quality Guidelines for The protection of Environmental and Human Health. Canadian Council of Ministers of the Environment. 2024. Available online: https://ccme.ca/en/results/97/ch/1,2,3,4,5,6 (accessed on 4 September 2024).
- Edition, F. Guidelines for drinking-water quality: Incorporating 1st and 2nd addenda. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the regulation of per-and polyfluoroalkyl substances (PFAS): A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Kim, M.Y. Improvement Measures for Soil Environment Conservation Act; 8983232013; Korea Legislation Research Institute: Sejong-si, Republic of Korea, 2001. [Google Scholar]
- EPA Victoria Australia. Industrial Waste Resource Guidelines. 1828.2: Waste Disposal Categories—Characteristics and Thresholds. 2009. Available online: https://www.epa.vic.gov.au/about-epa/publications/1828-2 (accessed on 24 September 2024).
- Slooff, W.; Eerens, H.; Janus, J. Integrated Criteria Document Fluorides; Report No.: 758474010; National Institute of Public Health and Environmental Protection: Bilthoven, The Netherlands, 1989; p. 191. [Google Scholar]
- Carlon, C. Derivation Methods of Soil Screening Values in Europe. A Review and Evaluation of National Procedures towards Harmonisation; European Commission, Joint Research Centre: Ispra, Italy, 2007. [Google Scholar]
- USEPA. Regional Screening Level Calculator; European Commission, Joint Research Centre: Ispra, Italy, 2024. [Google Scholar]
- Lee, C.-O.; Hong, S.-S.; Lee, B.-T.; Kim, G.-S.; Yun, H.-S. Spatial distribution of the dimension stone quarries in Korea. J. Petrol. Soc. Korea 2006, 15, 154–166. [Google Scholar]
- Lee, Y.-J.; Park, S.-Y. Regulations on Soil Fluoride Remediation to Be Revised: Stricter Than Toothpaste Standards. Korea Econ. Dly. 2024. Available online: https://www.hankyung.com/article/2023092570611 (accessed on 4 September 2024).
- Hallett, B.M.; Dharmagunawardhane, H.A.; Atal, S.; Valsami-Jones, E.; Ahmed, S.; Burgess, W.G. Mineralogical sources of groundwater fluoride in Archaen bedrock/regolith aquifers: Mass balances from southern India and north-central Sri Lanka. J. Hydrol. Reg. Stud. 2015, 4, 111–130. [Google Scholar] [CrossRef]
- Agarwal, R.; Srinivas, R. Severe neuropsychiatric manifestations and rhabdomyolysis in a patient with imidacloprid poisoning. Am. J. Emerg. Med. 2007, 25, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Del Piero, S.; Masiero, L.; Casellato, S. Influence of temperature on fluoride toxicity and bioaccumulation in the nonindigenous freshwater mollusk Dreissena polymorpha Pallas, 1769. Environ. Toxicol. Chem. 2012, 31, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, R.; Sharma, P.K.; Ivy, N.; Kumar, P.; Kant, N.; Jha, A.; Jha, P.K.; Gupta, P.K.; Sharma, P. Bioaccumulation of fluoride in plants and its microbially assisted remediation: A review of biological processes and technological performance. Processes 2021, 9, 2154. [Google Scholar] [CrossRef]
- Yuan, L.; Fei, W.; Jia, F.; Jun-Ping, L.; Qi, L.; Fang-Ru, N.; Xu-Dong, L.; Shu-Lian, X. Health risk in children to fluoride exposure in a typical endemic fluorosis area on Loess Plateau, north China, in the last decade. Chemosphere 2020, 243, 125451. [Google Scholar] [CrossRef]
- Rajak, P.; Roy, S.; Khatun, S.; Mandi, M.; Ganguly, A.; Das, K.; Dutta, A.; Nanda, S.; Ghanty, S.; Biswas, G. Fluoride contamination, toxicity and its potential therapeutic agents. Toxicol. Int. 2023, 29, 553–565. [Google Scholar] [CrossRef]
- Sunithaa, V.; Reddya, Y.S.; Suvarnaa, B.; Reddyb, B.M. Human health risk assessment (HHRA) of fluoride and nitrate using pollution index of groundwater (PIG) in and around hard rock terrain of Cuddapah, A.P. South India. Environ. Chem. Ecotoxicol. 2022, 4, 113–123. [Google Scholar] [CrossRef]
- Bombik, E.; Bombik, A.; Rymuza, K. The influence of environmental pollution with fluorine compounds on the level of fluoride in soil, feed and eggs of laying hens in Central Pomerania, Poland. Environ. Monit. Assess. 2020, 192, 178. [Google Scholar] [CrossRef]

| No. | Industry Sector | HF Consumption (Unit: tons) |
|---|---|---|
| 1 | Manufacture of chemicals and chemical products except pharmaceuticals and medicinal chemicals | 60,265 (37%) |
| 2 | Manufacture of electronic components, computers, and audiovisual communication equipment | 38,679 (24%) |
| 3 | Manufacture of other non-metallic mineral products | 31,547 (20%) |
| 4 | Manufacture of fabricated metal products except machinery and furniture | 8765 (5%) |
| 5 | Others | 21,867 (14%) |
| Total | - | 161,123 (100%) |
| Region | Fluorine Content | Land Uses |
|---|---|---|
| I | 400 | Croplands, rice paddies, orchards, residential areas, and schools |
| II | 400 | Forests, salt farms, playgrounds, and religious sites |
| III | 800 | Factories, gas stations, roads, and military sites |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.H.; Lee, S.H.; Bae, G.S.; Kim, H.W. Balancing Environmental Safety and Economic Feasibility: A Review of Soil Fluorine Management Strategies in South Korea. Sustainability 2024, 16, 8391. https://doi.org/10.3390/su16198391
Ji CH, Lee SH, Bae GS, Kim HW. Balancing Environmental Safety and Economic Feasibility: A Review of Soil Fluorine Management Strategies in South Korea. Sustainability. 2024; 16(19):8391. https://doi.org/10.3390/su16198391
Chicago/Turabian StyleJi, Chang Hwan, Soon Hong Lee, Gi Seong Bae, and Hyun Woo Kim. 2024. "Balancing Environmental Safety and Economic Feasibility: A Review of Soil Fluorine Management Strategies in South Korea" Sustainability 16, no. 19: 8391. https://doi.org/10.3390/su16198391






