Research Progress in the Joint Remediation of Plants–Microbes–Soil for Heavy Metal-Contaminated Soil in Mining Areas: A Review
Abstract
:1. Introduction
2. References Retrieval
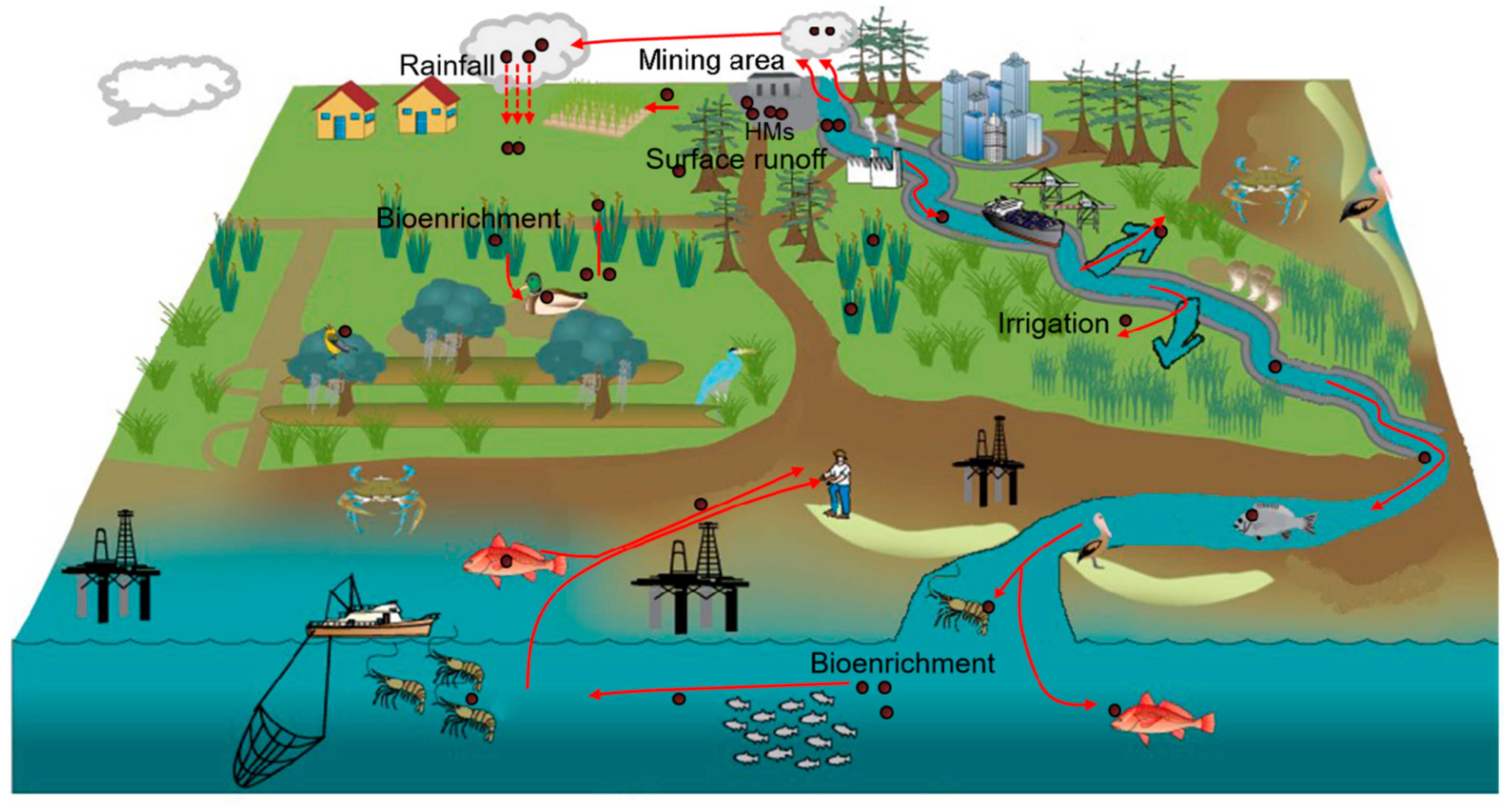
3. Characteristics of Heavy Metal Pollution in the Soil of Mining Areas
4. The Role of Plants in the Remediation of Heavy Metal Pollution in Mining Areas and Its Response Mechanism
4.1. The Role of Plants in the Remediation of HM-Polluted Sites
4.2. The Mechanism of Plant Response to HMs
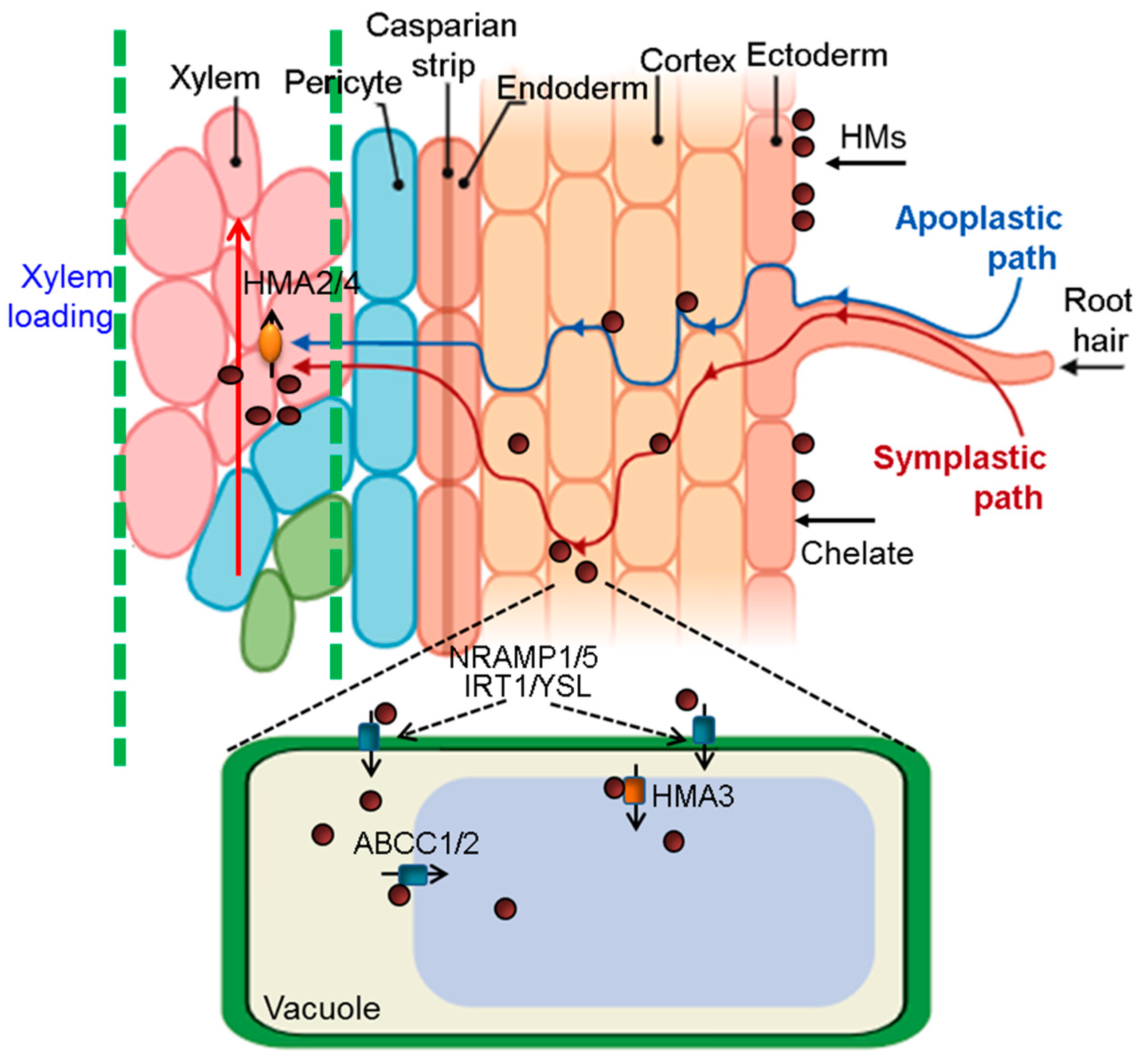
4.2.1. The Absorption and Transportation of HMs by Plants
4.2.2. The Allocation and Isolation of HMs
4.2.3. The Resistance of Plants to HMs
4.2.4. Response of Other Proteins to HMs
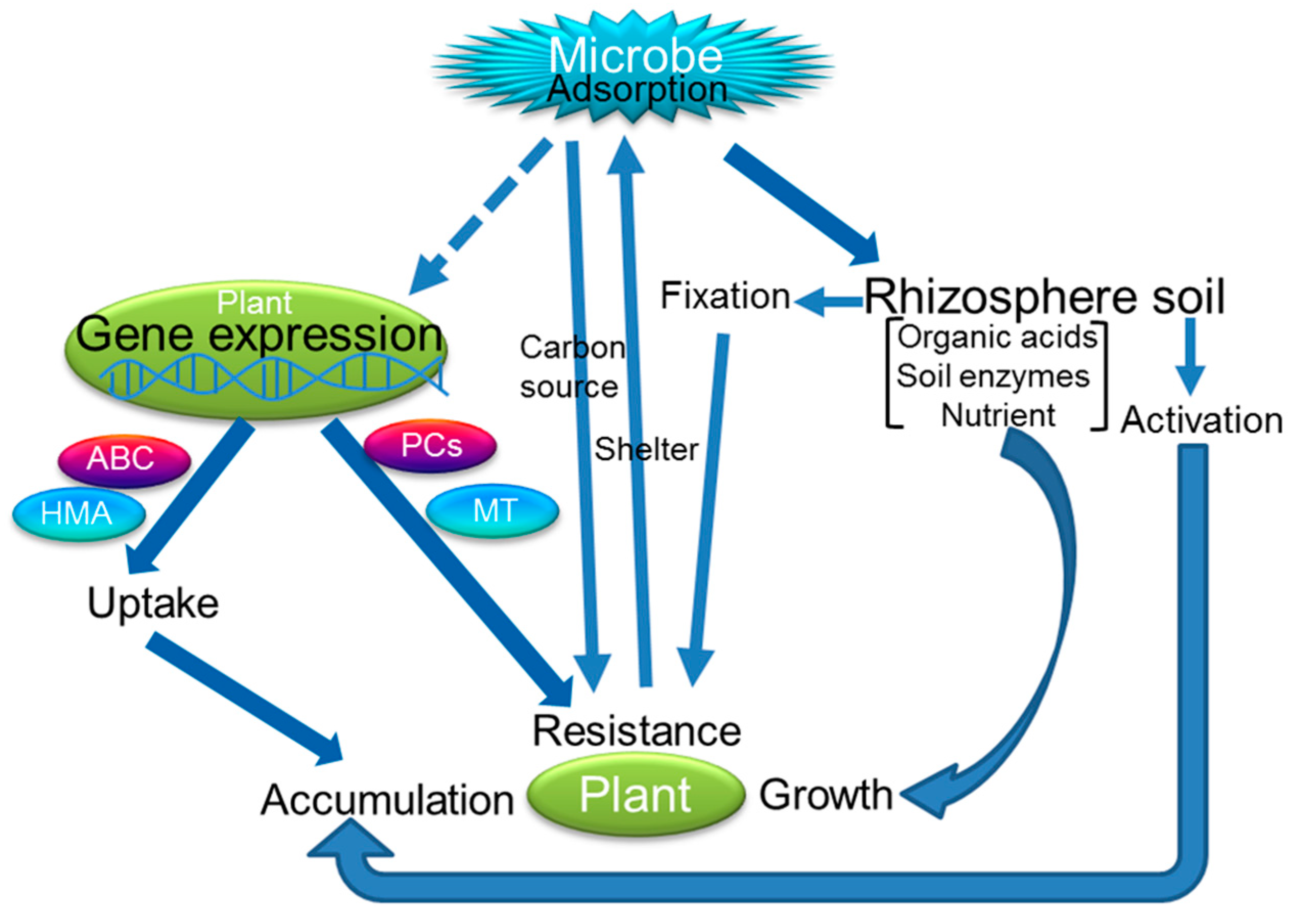
5. Joint Remediation of Plants–Microbes
5.1. Response of Microorganisms in Plants–Microbes Joint Remediation
5.2. The Mechanism of Plants–Microbes Joint Remediation
5.2.1. Promotion of Plant Growth
5.2.2. Enhanced Resistance to HMs
5.2.3. Increase in HM Accumulation
6. Advantages, Limitations, and Prospects of Plants–Microbes Joint Remediation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk assessment and source identification of heavy metal contamination by multivariate and hazard index analyses of a pipeline vandalised area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, S.; Wang, L. Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Sci. Rep. 2018, 8, 3256. [Google Scholar] [CrossRef] [PubMed]
- González-Méndez, B.; Webster, R.; Loredo-Portales, R.; Molina-Freaner, F.; Djellouli, R. Distribution of heavy metals polluting the soil near an abandoned mine in Northwestern Mexico. Environ. Earth Sci. 2022, 81, 176. [Google Scholar] [CrossRef]
- Huber, M.; Iakovleva, O. Zn-Pb Dumps, Environmental Pollution and Their Recultivation, Case of Ruda Śląska-Wirek, S Poland. Mining 2022, 2, 616–628. [Google Scholar] [CrossRef]
- Cai, L.M.; Xu, Z.C.; Qi, J.Y.; Feng, Z.Z.; Xiang, T.S. Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 2015, 127, 127–135. [Google Scholar] [CrossRef]
- Modabberi, S.; Tashakor, M.; Rajabian, N.; Khorasanipour, M.; Esmaeilzadeh, E.; Ambrosino, M.; Cicchella, D. Characterization and chemical fractionation of potentially toxic elements in soils of a pre-mining mineralized area; an evaluation of mobility and environmental risk. Environ. Geochem. Health 2023, 45, 4795–4815. [Google Scholar] [CrossRef]
- Skrobala, V.; Popovych, V.; Tyndyk, O.; Voloshchyshyn, A. Chemical pollution peculiarities of the Nadiya mine rock dumps in the Chervonohrad Mining District, Ukraine. Min. Miner. Depos. 2022, 16, 71–79. [Google Scholar] [CrossRef]
- Schneider, L.; Mariani, M.; Saunders, K.M.; Maher, W.A.; Harrison, J.J.; Fletcher, M.S.; Zawadzki, A.; Heijnis, H.; Haberle, S.G. How significant is atmospheric metal contamination from mining activity adjacent to the Tasmanian Wilderness World Heritage Area? A spatial analysis of metal concentrations using air trajectories models. Sci. Total Environ. 2019, 656, 250–260. [Google Scholar] [CrossRef]
- Stefanescu, L.; Alexandrescu, F. Environmental protection or subversion in mining? Planning challenges, perspectives and actors at the largest gold deposit in Europe. Land Use Policy 2019, 95, 103649. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, J.; Sun, S.; Shi, Q.; Feng, H.; Zhang, Y.; Cao, S. Health risk assessment of total exposure from cadmium in South China. Chemosphere 2021, 269, 128673. [Google Scholar] [CrossRef]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Wang, L.; Yin, X.; Gao, S.; Jiang, T.; Ma, C. In vitro oral bioaccessibility investigation and human health risk assessment of heavy metals in wheat grains grown near the mines in North China. Chemosphere 2020, 252, 126522. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, E.C.; Ferréol Bah, H.A.; Gomes Júnior, E.A.; dos Santos, N.R.; Costa, D.O.; Martinez, V.O.; Macêdo Pires, E.; Araújo Santana, J.V.; da Cerqueira, F.S.; Menezes-Filho, J.A. A Cross-Sectional Analysis Investigating Pregnant Women’s Renal Function and Its Association with Lead and Cadmium Exposures—The DSAN Birth Cohort Study in Recôncavo Baiano, Brazil. Toxics 2024, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Panqing, Y.; Abliz, A.; Xiaoli, S.; Aisaiduli, H. Human health-risk assessment of heavy metal–contaminated soil based on Monte Carlo simulation. Sci. Rep. 2023, 13, 7033. [Google Scholar] [CrossRef] [PubMed]
- Tepanosyan, G.; Sahakyan, L.; Maghakyan, N.; Saghatelyan, A. Combination of compositional data analysis and machine learning approaches to identify sources and geochemical associations of potentially toxic elements in soil and assess the associated human health risk in a mining city. Environ. Pollut. 2020, 261, 114210. [Google Scholar] [CrossRef]
- Dai, D.; Hu, H.; Wen, J.; Chen, H.; Chen, G.; Cui, X. Cunninghamia lanceolata (Lambert) Hooker: A Promising Candidate for Phytoremediation of Cd-Contaminated Soils. Forests 2024, 15, 115. [Google Scholar] [CrossRef]
- Zhakypbek, Y.; Kossalbayev, B.D.; Belkozhayev, A.M.; Murat, T.; Tursbekov, S.; Abdalimov, E.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. Reducing Heavy Metal Contamination in Soil and Water Using Phytoremediation. Plants 2024, 13, 1534. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Torres, D.; Donadio, F.; López, G.; Molina, R.; Obando, M.; Nievas, S.; Rosas, S.; Zeljković, S.Ć.; Díaz-Zorita, M.; De Diego, N.; et al. Previous Incubation of Bradyrhizobium japonicum E109 and Azospirillum argentinense Az39 (formerly A. brasilense Az39) Improves the Bradyrhizobium-Soybean Symbiosis. J. Soil Sci. Plant Nutr. 2022, 22, 4669–4682. [Google Scholar] [CrossRef]
- Li, Q.; Yao, S.; Wen, H.; Li, W.; Jin, L.; Huang, X. Improving Lead Phytoremediation Using Endophytic Bacteria Isolated from the Pioneer Plant Ageratina adenophora (Spreng.) from a Mining Area. Toxics 2024, 12, 291. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, P.; Zhang, S.; Zhang, S.; Sun, Y. Contribution of arbuscular mycorrhizal fungi and soil amendments to remediation of a heavy metal-contaminated soil using sweet sorghum. Pedosphere 2022, 32, 844–855. [Google Scholar] [CrossRef]
- Peroni, P.; Liu, Q.; Lizarazu, W.Z.; Xue, S.; Yi, Z.; Von Cossel, M.; Mastroberardino, R.; Papazoglou, E.G.; Monti, A.; Iqbal, Y. Biostimulant and Arbuscular Mycorrhizae Application on Four Major Biomass Crops as the Base of Phytomanagement Strategies in Metal-Contaminated Soils. Plants 2024, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Sow, S.; Ranjan, S.; Dharminder; Kumar, R.; Roy, D.K.; Kumar, S.; Kumar, A.; Srivastava, R.K.; Prasad, R.; et al. Unveiling the potential of plant growth promoting rhizobacteria (PGPR) in phytoremediation of heavy metal. Discov. Appl. Sci. 2024, 6, 324. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Qiu, Z.; Shahzad, S.M.; Nawaz, M.F.; Huang, J.; Naveed, S.; Li, L.; Wang, X.; Cheng, H. Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: A review. Environ. Pollut. 2023, 325, 121433. [Google Scholar] [CrossRef]
- Vesković, J.; Onjia, A. Environmental Implications of the Soil-to-Groundwater Migration of Heavy Metals in Mining Area Hotspots. Metals 2024, 14, 719. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, X.; Ge, J.; Liu, F.; Wu, C. Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J. Clean. Prod. 2020, 257, 120664. [Google Scholar] [CrossRef]
- Du, H.; Guo, P.; Wang, T.; Ma, M.; Wang, D. Significant bioaccumulation and biotransformation of methyl mercury by organisms in rice paddy ecosystems: A potential health risk to humans. Environ. Pollut. 2021, 273, 116431. [Google Scholar] [CrossRef]
- Radu, V.M.; Vîjdea, A.M.; Ivanov, A.A.; Alexe, V.E.; Dincă, G.; Cetean, V.M.; Filiuță, A.E. Research on the Closure and Remediation Processes of Mining Areas in Romania and Approaches to the Strategy for Heavy Metal Pollution Remediation. Sustainability 2023, 15, 15293. [Google Scholar] [CrossRef]
- Yang, S.; Ge, W.Y.; Chen, H.H.; Xu, W.L. Investigation of soil and groundwater environment in urban area during post-industrial era: A case study of brownfield in Zhenjiang, Jiangsu Province, China. China Geol. 2019, 2, 501–511. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, X.; Chen, Y.; Liu, Y. Source-specific probabilistic contamination risk and health risk assessment of soil heavy metals in a typical ancient mining area. Sci. Total Environ. 2024, 906, 167772. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; Yan, B.; Chen, T.; Lei, C.; Lin, H.Z.; Xiao, X.M. Contaminant characteristics and environmental risk assessment of heavy metals in the paddy soils from lead (Pb)-zinc (Zn) mining areas in Guangdong Province, South China. Environ. Sci. Pollut. Res. 2017, 24, 24387–24399. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tan, D.; Deng, S.; Lei, M. Pollution and ecological risk assessment of antimony and other heavy metals in soils from the world’s largest antimony mine area, China. Hum. Ecol. Risk Assess. Int. J. 2017, 24, 679–690. [Google Scholar] [CrossRef]
- Escarré, J.; Lefèbvre, C.; Raboyeau, S.; Dossantos, A.; Gruber, W.; Cleyet Marel, J.C.; Frérot, H.; Noret, N.; Mahieu, S.; Collin, C.; et al. Heavy Metal Concentration Survey in Soils and Plants of the Les Malines Mining District (Southern France): Implications for Soil Restoration. Water Air Soil Pollut. 2010, 216, 485–504. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelievre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Kramer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.N.; Trang, B.T.Q.; Tanaka, S.; Noi, N.V.; Son, L.T.; Noda, M.; Phuong, N.M.; Ueno, D.; Iwasaki, K. Heavy Metal Concentrations in Rice (Oryza sativa L.) Plants Grown in a Chromite Mining Area in Vietnam. Trop. Agric. Dev. 2011, 55, 135–141. [Google Scholar]
- Asensio, V.; Vega, F.A.; Singh, B.R.; Covelo, E.F. Effects of tree vegetation and waste amendments on the fractionation of Cr, Cu, Ni, Pb and Zn in polluted mine soils. Sci. Total Environ. 2013, 443, 446–453. [Google Scholar] [CrossRef]
- Boughattas, I.; Hattab, S.; Boussetta, H.; Sappin-Didier, V.; Viarengo, A.; Banni, M.; Sforzini, S. Biomarker responses of Eisenia andrei to a polymetallic gradient near a lead mining site in North Tunisia. Environ. Pollut. 2016, 218, 530–541. [Google Scholar] [CrossRef]
- Theologides; Christodoulos, P.; Christou; Anastasis; Costa; Costas; Kalavrouziotis; Ioannis, K.; Varnavas; Soterios, P. Assessment of toxic heavy metals concentrations in soils and wild and cultivated plant species in Limni abandoned copper mining site, Cyprus. J. Geochem. Explor. 2017, 178, 16–22. [Google Scholar] [CrossRef]
- Hao, Q.; Jiang, C. Heavy metal concentrations in soils and plants in Rongxi Manganese Mine of Chongqing, Southwest of China. Acta Ecol. Sin. 2015, 35, 46–51. [Google Scholar] [CrossRef]
- Nouri, M.; Haddioui, A.E.M. Assessment of metals contamination and ecological risk in ait Ammar abandoned iron mine soil, Morocco. Ekológia 2016, 35, 32–49. [Google Scholar] [CrossRef]
- Timofeev, I.; Kosheleva, N.; Kasimov, N. Contamination of soils by potentially toxic elements in the impact zone of tungstenmolybdenum ore mine in the Baikal region: A survey and risk assessment. Sci. Total Environ. 2018, 642, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Yildirim, D.; Sasmaz, A. Phytoremediation of As, Ag, and Pb in contaminated soils using terrestrial plants grown on Gumuskoy mining area (Kutahya Turkey). J. Geochem. Explor. 2017, 182, 228–234. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Olufemi, A.P.; Ogundele, L.T.; Okunnuwa, O.Q.; Toyeje, A.B.; Olowookere, C.J. Ecological and human health risk assessments of metals in soil and tailing from Ife-Ijesha gold mining area, Southwest Nigeria. Environ. Earth Sci. 2022, 81, 462. [Google Scholar] [CrossRef]
- Radi, N.; Hirche, A.; Boutaleb, A. Assessment of soil contamination by heavy metals and arsenic in Tamesguida abandoned copper mine area, Médéa, Algeria. Environ. Monit. Assess. 2022, 195, 247. [Google Scholar] [CrossRef]
- Zhao, M.; Zeng, S.; Liu, S.; Li, Z.; Jing, L. Metal accumulation by plants growing in China: Capacity, synergy, and moderator effects. Ecol. Eng. 2020, 148, 105790. [Google Scholar] [CrossRef]
- Guarino, C.; Zuzolo, D.; Marziano, M.; Baiamonte, G.; Morra, L.; Benotti, D.; Gresia, D.; Stacul, E.; Cicchella, D.; Sciarrillo, R. Identification of native-metal tolerant plant species in situ: Environmental implications and functional traits. Sci. Total Environ. 2019, 650, 3156–3167. [Google Scholar] [CrossRef]
- Khan, I.U.; Qi, S.S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.N.; Zhang, H.; Dai, Z.C.; Du, D.L. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffre, T.; Erskine, P.D.; Echevarria, G.; van Der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef]
- Salas-Luévano, M.A.; Mauricio-Castillo, J.A.; González-Rivera, M.L.; Vega-Carrillo, H.R.; Salas-Muñoz, S. Accumulation and phytostabilization of As, Pb and Cd in plants growing inside mine tailings reforested in Zacatecas, Mexico. Environ. Earth Sci. 2017, 76, 806. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Zaman, A.; Butt, T.A.; Mirza, C.R.; Iqbal, A.; Khan, A.H.A.; Yousaf, S.; Iqbal, M. Uptake and Distribution of Cadmium and Copper by Solanum lycopersicum L. and Changes in the Metabolite Production. Biol. Bull. 2023, 50, 390–399. [Google Scholar] [CrossRef]
- Velasco-Arroyo, B.; Curiel-Alegre, S.; Khan, A.H.A.; Rumbo, C.; Pérez-Alonso, D.; Rad, C.; De Wilde, H.; Pérez-de-Mora, A.; Barros, R. Phytostabilization of metal(loid)s by ten emergent macrophytes following a 90-day exposure to industrially contaminated groundwater. New Biotechnol. 2024, 79, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vila, M.; Arenas-Lago, D.; Rodriguez-Seijo, A.; Luisa Andrade, M.; Vega, F.A. Ability of Cytisus scoparius for phytoremediation of soils from a Pb/Zn mine: Assessment of metal bioavailability and bioaccumulation. J. Environ. Manag. 2019, 235, 152–160. [Google Scholar] [CrossRef]
- Li, S.; Liber, K. Influence of different revegetation choices on plant community and soil development nine years after initial planting on a reclaimed coal gob pile in the Shanxi mining area, China. Sci. Total Environ. 2017, 618, 1314–1323. [Google Scholar] [CrossRef]
- Samara, T.; Spanos, I.; Platis, P.; Papachristou, T.G. Heavy Metal Retention by Different Forest Species Used for Restoration of Post-Mining Landscapes, N. Greece. Sustainability 2020, 12, 4453. [Google Scholar] [CrossRef]
- Monfared, S.H.; Matinizadeh, M.; Shirvany, A.; Amiri, G.Z.; Fard, R.M.; Rostami, F. Accumulation of heavy metal in Platanus orientalis, Robinia pseudoacacia and Fraxinus rotundifolia. J. For. Res. 2012, 24, 391–395. [Google Scholar] [CrossRef]
- Sandell, F.E.; Carl, S.; Mulualem, T.; Stephen, S.; Christer, O.P. Biological traits of tropical trees suitable for restoration of copper-polluted lands. Ecol. Eng. 2019, 138, 118–125. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Li, J.; Liu, J.; Feng, S.; Guan, Y. Multi-criteria decision analysis of optimal planting for enhancing phytoremediation of trace heavy metals in mining sites under interval residual contaminant concentrations. Environ. Pollut. 2019, 255 Pt 2, 113255. [Google Scholar] [CrossRef] [PubMed]
- Ezura, H.; Gao, F.; Li, J.; Zhang, J.; Li, N.; Tang, C.; Bakpa, E.P.; Xie, J. Genome-wide identification of the ZIP gene family in lettuce (Lactuca sativa L.) and expression analysis under different element stress. PLoS ONE 2022, 17, e0274319. [Google Scholar]
- Li, N.; Meng, H.; Xing, H.; Liang, L.; Zhao, X.; Luo, K. Genome-wide analysis of MATE transporters and molecular characterization of aluminum resistance in Populus. J. Exp. Bot. 2017, 68, 5669–5683. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Shi, G. Genome-Wide Identification and Expression Profiling of Heavy Metal ATPase (HMA) Genes in Peanut: Potential Roles in Heavy Metal Transport. Int. J. Mol. Sci. 2024, 25, 613. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Y.; Liu, Y.; Zeng, G.; Hu, X.; Hu, X.; Zhou, L.; Guo, Y.; Li, J. Cadmium accumulation and apoplastic and symplastic transport in Boehmeria nivea (L.) Gaudich on cadmium-contaminated soil with the addition of EDTA or NTA. RSC Adv. 2015, 5, 47584–47591. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Luo, J.; Lux, A.; Kováč, J.; Wen, Y.; Zhou, Y.; Jan, J.; Liang, Y.; Li, T. The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J. Exp. Bot. 2017, 68, 739–751. [Google Scholar]
- He, B.Y.; Yu, D.P.; Chen, Y.; Shi, J.L.; Xia, Y.; Li, Q.S.; Wang, L.L.; Ling, L.; Zeng, E.Y. Use of low-calcium cultivars to reduce cadmium uptake and accumulation in edible amaranth (Amaranthus mangostanus L.). Chemosphere 2017, 171, 588–594. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Li, D.; Xu, X.; Li, C. Genome-Wide Analysis of the ZRT, IRT-Like Protein (ZIP) Family and Their Responses to Metal Stress in Populus trichocarpa. Plant Mol. Biol. Report. 2017, 35, 534–549. [Google Scholar] [CrossRef]
- Yamagata, A.; Murata, Y.; Namba, K.; Terada, T.; Fukai, S.; Shirouzu, M. Uptake mechanism of iron-phytosiderophore from the soil based on the structure of yellow stripe transporter. Nat. Commun. 2022, 13, 7180. [Google Scholar] [CrossRef]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C.; et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Ishikawa, S.; Fujimaki, S. Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza sativa L.) during Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification. Int. J. Mol. Sci. 2015, 16, 19111–19129. [Google Scholar] [CrossRef] [PubMed]
- Palusinska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maslinska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol. 2020, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Rono, J.K.; Liu, X.S.; Feng, S.J.; Yang, Z.M. Functional characterization of a new metallochaperone for reducing cadmium concentration in rice crop. J. Clean. Prod. 2020, 272, 123152. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Wang, R.; Zhang, J.; Owens, G. Cadmium adsorption by willow root: The role of cell walls and their subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef]
- Yu, H.; Wang, K.; Huang, H.; Zhang, X.; Li, T. The regulatory role of root in cadmium accumulation in a high cadmium-accumulating rice line (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 25432–25441. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Y.; Przybyłowicz, W.J.; Li, Z.; Barnabas, A.; Wu, L.; Luo, Y.; Mesjasz-Przybyłowicz, J. Elemental distribution by cryo-micro-PIXE in the zinc and cadmium hyperaccumulator Sedum plumbizincicola grown naturally. Plant Soil 2014, 388, 267–282. [Google Scholar] [CrossRef]
- Cui, J.L.; Zhao, Y.P.; Chan, T.S.; Zhang, L.L.; Tsang, D.C.W.; Li, X.D. Spatial distribution and molecular speciation of copper in indigenous plants from contaminated mine sites: Implication for phytostabilization. J. Hazard. Mater. 2020, 381, 121208. [Google Scholar] [CrossRef]
- Pittman, J.K.; Hirschi, K.D.; Weber, A. CAX-ing a wide net: Cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol. 2016, 18, 741–749. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef]
- Khan, N.M.; Ali, A.; Wan, Y.; Zhou, G. Genome-wide identification of heavy-metal ATPases genes in Areca catechu: Investigating their functionality under heavy metal exposure. BMC Plant Biol. 2024, 24, 484. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.T.; Maranon, T.; Murillo, J.M.; Redondo-Gomez, S. Response of Holm oak (Quercus ilex subsp. ballota) and mastic shrub (Pistacia lentiscus L.) seedlings to high concentrations of Cd and Tl in the rhizosphere. Chemosphere 2011, 83, 1166–1174. [Google Scholar]
- Gupta, D.K.; Pena, L.B.; Romero-Puertas, M.C.; Hernandez, A.; Inouhe, M.; Sandalio, L.M. NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant Cell Environ. 2017, 40, 509–526. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Laporte, D.; Moenne, A. Cadmium Accumulation Involves Synthesis of Glutathione and Phytochelatins, and Activation of CDPK, CaMK, CBLPK, and MAPK Signaling Pathways in Ulva compressa. Front. Plant Sci. 2021, 12, 669096. [Google Scholar] [CrossRef]
- Inouhe, M.; Sakuma, Y.; Chatterjee, S.; Datta, S.; Gupta, D.K. General Roles of Phytochelatins and Other Peptides in Plant Defense Mechanisms Against Oxidative Stress/Primary and Secondary Damages Induced by Heavy Metals. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 219–245. [Google Scholar]
- Ovecka, M.; Takac, T. Managing heavy metal toxicity stress in plants: Biological and biotechnological tools. Biotechnol. Adv. 2014, 32, 73–86. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2019, 39, 641–655. [Google Scholar] [CrossRef]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Jin, X.; Wu, Y. Root tolerance and biochemical response of Chinese lettuce (Lactuca sativa L.) genotypes to cadmium stress. PeerJ 2019, 7, e7530. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, R.; Ren, X.; Jia, H.; Hua, L.; Xu, H.; Lv, X.; Zhao, J.; Wei, T. Effects of salicylic acid, Epi-brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxicol. Environ. Saf. 2018, 157, 491–496. [Google Scholar] [CrossRef]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, X.; Guo, Y.; Zhang, J.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Enhanced phytoremdiation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 2018, 197, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous abscisic acid (ABA) promotes cadmium (Cd) accumulation in Sedum alfredii Hance by regulating the expression of Cd stress response genes. Environ. Sci. Pollut. Res. 2020, 27, 8719–8731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Tran, L.-S.P.; Xu, J. The R2R3-MYB transcription factor MYB49 regulates cadmium accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, X.; Huang, Y.; Han, Y.; Zhang, C.; Ren, Y.; Fan, T.; Xiao, F.; Liu, Y.; Cao, S. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2018, 42, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, B.; Wang, R.; He, J.; Xia, B.; Xue, Y.; Wang, R. Overexpression of a bacterial mercury transporter MerT in Arabidopsis enhances mercury tolerance. Biochem. Biophys. Res. Commun. 2017, 490, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Wang, D.L. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2020, 402, 123919. [Google Scholar] [CrossRef]
- Gao, H.; Xie, W.; Yang, C.; Xu, J.; Li, J.; Wang, H.; Chen, X.; Huang, C.F. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol. 2018, 217, 179–193. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Mondal, S. Heavy Metal Stress–Induced Activation of Mitogen-Activated Protein Kinase Signalling Cascade in Plants. Plant Mol. Biol. Rep. 2022, 41, 15–26. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Naamala, J.; Dakora, F.D. Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol. Fertil. Soils 2018, 54, 309–318. [Google Scholar] [CrossRef]
- Edulamudi, P.; Antony Masilamani, A.J.; Vanga, U.R.; Divi Venkata Ramana, S.G.; Konada, V.M. Biosorption and Symbiotic Potential of Horse Gram Rhizobia in Soils Contaminated with Cobalt. Curr. Microbiol. 2023, 80, 174. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. Comptes Rendus Biol. 2015, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pardo, B.; Zornoza, P. Mitigation of Cu stress by legume–Rhizobium symbiosis in white lupin and soybean plants. Ecotoxicol. Environ. Saf. 2014, 102, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. PeerJ 2019, 7, e6875. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Lu, Y.; Wei, J.; Qi, S.; Wu, B.; Cheng, S. Sustainable Remediation of Soil and Water Utilizing Arbuscular Mycorrhizal Fungi: A Review. Microorganisms 2024, 12, 1255. [Google Scholar] [CrossRef]
- Gil-Cardeza, M.L.; Müller, D.R.; Amaya-Martin, S.M.; Viassolo, R.; Gómez, E. Differential responses to high soil chromium of two arbuscular mycorrhizal fungi communities isolated from Cr-polluted and non-polluted rhizospheres of Ricinus communis. Sci. Total Environ. 2018, 625, 1113–1121. [Google Scholar] [CrossRef]
- Rubio-Santiago, J.; Hernández-Morales, A.; Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Carranza-Álvarez, C.; Rubio-Salazar, J.E.; Rosales-Loredo, S.; Pacheco-Aguilar, J.R.; Macías-Pérez, J.R.; et al. Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd. Plants 2023, 12, 498. [Google Scholar] [CrossRef]
- Cheng, X.; Cao, X.; Tan, C.; Liu, L.; Bai, J.; Liang, Y.; Cai, R. Effects of four endophytic bacteria on cadmium speciation and remediation efficiency of Sedum plumbizincicola in farmland soil. Environ. Sci. Pollut. Res. 2022, 29, 89557–89569. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Olmos Nicotra, M.F.; Escudero, L.; Breser, M.L.; Porporatto, C.; Agostini, E. Impact of double inoculation with Bradyrhizobium japonicum E109 and Azospirillum brasilense Az39 on soybean plants grown under arsenic stress. Plant Physiol. Biochem. 2019, 138, 26–35. [Google Scholar] [CrossRef]
- Vezza, M.E.; de Pramparo, R.P.; Wevar Oller, A.L.; Agostini, E.; Talano, M.A. Promising co-inoculation strategies to reduce arsenic toxicity in soybean. Environ. Sci. Pollut. Res. 2022, 29, 88066–88077. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Iqbal, A.; Nawaz, I.; Mirza, C.R.; Yousaf, S.; Farooq, G.; Ali, M.A.; Khan, A.H.A.; Iqbal, M. Enhanced uptake of Cd, Cr, and Cu in Catharanthus roseus (L.) G.Don by Bacillus cereus: Application of moss and compost to reduce metal availability. Environ. Sci. Pollut. Res. 2020, 27, 39807–39818. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Ruhil, T.; Gupta, V.K.; Verma, P. Microbial assisted multifaceted amelioration processes of heavy-metal remediation a clean perspective toward sustainable and greener future. Crit. Rev. Biotechnol. 2023, 44, 429–447. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2013, 11, 843–872. [Google Scholar] [CrossRef]
- Zhou, B.; Yao, W.; Wang, S.; Wang, X.; Jiang, T. The Metallothionein Gene, TaMT3, from Tamarix androssowii Confers Cd2+ Tolerance in Tobacco. Int. J. Mol. Sci. 2014, 15, 10398–10409. [Google Scholar] [CrossRef] [PubMed]
- Wekesa, C.; Muoma, J.O.; Reichelt, M.; Asudi, G.O.; Furch, A.C.U.; Oelmüller, R. The Cell Membrane of a Novel Rhizobium phaseoli Strain Is the Crucial Target for Aluminium Toxicity and Tolerance. Cells 2022, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Corticeiro, S.; Freitas, R.; Figueira, E. Different efficiencies of the same mechanisms result in distinct Cd tolerance within Rhizobium. Ecotoxicol. Environ. Saf. 2018, 150, 260–269. [Google Scholar] [CrossRef]
- Lakzian, A.; Murphy, P.; Giller, K.E. Transfer and loss of naturally-occurring plasmids among isolates of Rhizobium leguminosarum bv. viciae in heavy metal contaminated soils. Soil Biol. Biochem. 2007, 39, 1066–1077. [Google Scholar] [CrossRef]
- Deepika, K.V.; Raghuram, M.; Kariali, E.; Bramhachari, P.V. Biological responses of symbiotic Rhizobium radiobacter strain VBCK1062 to the arsenic contaminated rhizosphere soils of mung bean. Ecotoxicol. Environ. Saf. 2016, 134, 1–10. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Hui, C.M.; HungLi, M.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Oliveira, V.H.D.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in Populus trichocarpa. Environ. Exp. Bot. 2019, 171, 103925. [Google Scholar] [CrossRef]
- Cui, G.; Ai, S.; Chen, K.; Wang, X. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef] [PubMed]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2009, 20, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jing, R.; Wang, L.; Wu, N.; Guo, Z. Role of arbuscular mycorrhizal fungi in cadmium tolerance in rice (Oryza sativa L): A meta-analysis. Qual. Assur. Saf. Crop. Foods 2023, 15, 59–70. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Robert, F.; Schoefs, B.; Bertrand, M.; Henry, C.; Gianinazzi-Pearson, V.; Dumas-Gaudot, E.; Aschi-Smiti, S. Arbuscular mycorrhizal symbiosis elicits shoot proteome changes that are modified during cadmium stress alleviation in Medicago truncatula. BMC Plant Biol. 2011, 11, 75. [Google Scholar] [CrossRef]
- Bruno, L.B.; Anbuganesan, V.; Karthik, C.; Tripti; Rajkumar, M. Enhanced phytoextraction of multi-metal contaminated soils under increased atmospheric temperature by bioaugmentation with plant growth promoting Bacillus cereus. J. Environ. Manag. 2021, 289, 112553. [Google Scholar] [CrossRef]
- Shin, M.N.; Shim, J.; You, Y.; Myung, H.; Bang, K.S.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J. Hazard. Mater. 2012, 199–200, 314–320. [Google Scholar] [CrossRef]
- Vezza, M.E.; Olmos Nicotra, M.F.; Agostini, E.; Talano, M.A. Biochemical and molecular characterization of arsenic response from Azospirillum brasilense Cd, a bacterial strain used as plant inoculant. Environ. Sci. Pollut. Res. 2019, 27, 2287–2300. [Google Scholar] [CrossRef]
- Thomas, J.; Archana, G. Differential influence of heavy metals on plant growth promoting attributes of beneficial microbes and their ability to promote growth of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2023, 47, 102592. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas fluorescens promote photosynthesis, carbon fixation and cadmium phytoremediation of hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zeng, G.; Wu, X.; Wu, B.; Li, X.; Xu, H. Characteristics and in situ remediation effects of heavy metal immobilizing bacteria on cadmium and nickel co-contaminated soil. Ecotoxicol. Environ. 2020, 192, 110294. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Shamsy, R.; Liu, A.; Chen, S. Arbuscular mycorrhizal fungi-induced tolerance to chromium stress in plants. Environ. Pollut. 2023, 327, 121597. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2013, 375, 205–214. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, M.; Tan, S.; Yang, X.; Lan, Z.; Jiang, Q.; Ye, Z.; Jing, Y. Enhancement of arbuscular mycorrhizal fungus (Glomus versiforme) on the growth and Cd uptake by Cd-hyperaccumulator Solanum nigrum. Appl. Soil Ecol. 2015, 89, 44–49. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kazmi, S.Z.; Mirza, C.R.; Butt, T.A.; Gul, N.; Barros, R.; Yousaf, S.; Iqbal, M. Effect of Soil Amendments on the Enzymatic Profile of Soil when Nicotiana alata L. and Petunia hybrida L. were Irrigated with Synthetic Heavy Metal-contaminated Wastewater. Chiang Mai J. Sci. 2023, 50, e2023008. [Google Scholar] [CrossRef]
- Hao, X.; Xie, P.; Zhu, Y.G.; Taghavi, S.; Wei, G.; Rensing, C. Copper tolerance mechanisms of Mesorhizobium amorphae and its role in aiding phytostabilization by Robinia pseudoacacia in copper contaminated soil. Environ. Sci. Technol. 2015, 49, 2328–2340. [Google Scholar] [CrossRef]
- Du, B.; Pang, J.; Hu, B.; Allen, D.; Bell, T.; Pfautsch, S.; Netzer, F.; Dannenmann, M.; Zhang, S.; Rennenberg, H. N2-fixing black locust intercropping improves ecosystem nutrition at the vulnerable semi-arid Loess Plateau region, China. Sci. Total Environ. 2019, 688, 333–345. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Zhang, W.H.; Wang, Q.Y.; Qian, M.; Sheng, X.-F. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J. Hazard. Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef]
- Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- He, Y.M.; Yang, R.; Lei, G.; Li, B.; Li, Y. Arbuscular mycorrhizal fungi reduce cadmium leaching from polluted soils under simulated heavy rainfall. Environ. Pollut. 2020, 263, 114406. [Google Scholar] [CrossRef]
- Cornejo, P.; Pérez-Tienda, J.; Meier, S.; Valderas, A.; Borie, F.; Azcón-Aguilar, C.; Ferrol, N. Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil Biol. Biochem. 2013, 57, 925–928. [Google Scholar] [CrossRef]
- Gatasheh, M.K.; Shah, A.A.; Kaleem, M.; Usman, S.; Shaffique, S. Application of CuNPs and AMF alleviates arsenic stress by encompassing reduced arsenic uptake through metabolomics and ionomics alterations in Elymus sibiricus. BMC Plant Biol. 2024, 24, 667. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Srivastava, A.K.; El-sadek, M.S.A.; Kordrostami, M.; Tran, L.-S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2017, 29, 1065–1073. [Google Scholar] [CrossRef]
- Cicatelli, A.; Todeschini, V.; Lingua, G.; Biondi, S.; Torrigiani, P.; Castiglione, S. Epigenetic control of heavy metal stress response in mycorrhizal versus non-mycorrhizal poplar plants. Environ. Sci. Pollut. Res. Int. 2014, 21, 1723–1737. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Diao, F.; Ding, S.; Shi, Z.; Xu, J.; Hao, L.; Li, F.Y.; Guo, W. Differential effects of arbuscular mycorrhizal fungi on three salt-tolerant grasses under cadmium and salt stress. Land Degrad. Dev. 2022, 34, 506–520. [Google Scholar] [CrossRef]
- Ike, A.; Sriprang, R.; Ono, H.; Murooka, Y.; Yamashita, M. Bioremediation of cadmium contaminated soil using symbiosis between leguminous plant and recombinant rhizobia with the MTL4 and the PCS genes. Chemosphere 2007, 66, 1670–1676. [Google Scholar] [CrossRef]
- Shoaib, M.; Hussain, S.; Cheng, X.; Cui, Y.; Liu, H.; Chen, Q.; Ma, M.; Gu, Y.; Zhao, K.; Xiang, Q.; et al. Synergistic anti-oxidative effects of Pongamia pinnata against nickel mediated by Rhizobium pisi and Ochrobacterium pseudogrignonense. Ecotoxicol. Environ. Saf. 2021, 217, 112244. [Google Scholar] [CrossRef]
- Wu, K.; Luo, J.; Li, J.; An, Q.; Yang, X.; Liang, Y.; Li, T. Endophytic bacterium Buttiauxella sp. SaSR13 improves plant growth and cadmium accumulation of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2018, 25, 21844–21854. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]

| Mining Area | Country | Main Ores | HMs | Mean Concentration mg/kg | References |
|---|---|---|---|---|---|
| Tonglushan mine, Daye, Hubei | China | Cu | Cd | 1.46 | [31] |
| As | 43.25 | ||||
| Pb | 102.35 | ||||
| Cr | 90.51 | ||||
| Cu | 355.72 | ||||
| Ni | 32.31 | ||||
| Zn | 260.87 | ||||
| Lead (Pb)–zinc (Zn) mine, Guangdong | China | Pb, Zn | Cr | 30.91 | [32] |
| Ni | 20.25 | ||||
| Cd | 7.14 | ||||
| Cu | 57.8 | ||||
| Pb | 1093.03 | ||||
| Zn | 867.08 | ||||
| Mn | 358.77 | ||||
| Fe | 34,281.45 | ||||
| Xikuangshan antimony (Sb) mine, Hunan | China | Sb | Sb | 356.58 | [33] |
| Cu | 45.69 | ||||
| Zn | 486.42 | ||||
| As | 53.13 | ||||
| Cd | 9.98 | ||||
| Pb | 77.32 | ||||
| Les Malines mining district, Montpellier | France | Pb, Zn | Zn | 39,364 | [34] |
| Pb | 34,289 | ||||
| Cd | 225 | ||||
| As | 338 | ||||
| Ti | 3.5 | ||||
| Cartagena mining district, La Union | Spain | Ag, Pb, Zn, Cu, Fe | Cd | 49 | [35] |
| Cu | 274 | ||||
| Fe | 94,659 | ||||
| Mn | 8107 | ||||
| Pb | 4194 | ||||
| Zn | 23,361 | ||||
| Co Dinh mine, Thanh Hoa | Vietnam | Cr | 4353 | [36] | |
| Co | 341 | ||||
| Ni | 4440 | ||||
| Cu | 20.3 | ||||
| Zn | 106.6 | ||||
| Pb | 17.6 | ||||
| Touro mine, Galicia | Spain | Cu | Cr | 118 | [37] |
| Cu | 911 | ||||
| Ni | 15.3 | ||||
| Pb | 19.3 | ||||
| Zn | 78.2 | ||||
| Jebel Ressas mining site, Tunis | Tunisia | Pb and Zn | Cu | 14.25 | [38] |
| Mn | 306 | ||||
| Zn | 42,400 | ||||
| Pb | 14,500 | ||||
| Cd | 184 | ||||
| Limni mine, Cyprus | Cyprus | Cu | Zn | 4132 | [39] |
| Cu | 1534 | ||||
| Ni | 121.7 | ||||
| Cd | 6.4 | ||||
| Pb | 28.6 | ||||
| Rongxi Mn mine, Xiushan chongqing | China | Mn | Mn | 48,383 | [40] |
| Cd | 3.9 | ||||
| Cu | 80 | ||||
| Pb | 80.7 | ||||
| Zn | 131.2 | ||||
| Ait Ammar iron mine, Khouribga | Morocco | Fe | Cd | 2.12 | [41] |
| Cr | 134.6 | ||||
| Cu | 35 | ||||
| Zn | 90.8 | ||||
| Pb | 9.1 | ||||
| Fe | 156,461.5 | ||||
| Tungsten molybdenum ore mine, Zakamensk, Baikal region | The Buryat Republic | Tungsten (W) | Al | 70,500 | [42] |
| Mn | 2300 | ||||
| Fe | 55,000 | ||||
| As | 4.9 | ||||
| Cr | 47.8 | ||||
| Cu | 81 | ||||
| Ni | 31.7 | ||||
| Pb | 31.7 | ||||
| Zn | 133.5 | ||||
| Tongguan gold mine, Shaanxi | China | Gold (Au) | Hg | 2.91 | [43] |
| Cd | 2.45 | ||||
| Pb | 252 | ||||
| Cu | 46.4 | ||||
| Zn | 286 | ||||
| As | 16 | ||||
| Gumuskoy mining area, Kutahya | Turkey | Ag | As | 4771 | [44] |
| Ag | 37.78 | ||||
| Pb | 4320 | ||||
| The gold mining regions situated in the Ife–Ijesha axis, Osun State | Nigeria | Gold (Au) | Fe | 196.78 | [45] |
| Cd | 0.36 | ||||
| Cu | 3.78 | ||||
| Cr | 65.74 | ||||
| Pb | 6.12 | ||||
| Ni | 19.56 | ||||
| Zn | 10.78 | ||||
| Tamesguida abandoned copper mine area, Médéa | Algeria | Cu | Cu | 599.59 | [46] |
| Zn | 390.02 | ||||
| Cr | 93.05 | ||||
| As | 127.07 | ||||
| Pb | 70.04 | ||||
| Ni | 58.01 | ||||
| Fe | 74.3 |
| Microorganisms | HMs | Concentration | Resistance Mechanism | Transporters or Resistance Genes | References |
|---|---|---|---|---|---|
| Agrobacterium | Cd | 16.8 mg/L | Reactive oxygen species (ROS) | Metallothioneins | [115] |
| Rhizobia R. phaseoli strain B3 | Al | 0–5.4 mg/L | Repair and stabilize the membrane | ABC-transporters and novel proteins, extracellular exopolysaccharide | [116] |
| Rhizobia | Cd | 0–33.6 mg/L | Extracellular immobilization, periplasmic allocation, cytoplasmic sequestration, and biotransformation of toxic products | GSH | [117] |
| Rhizobia | Zn | 54–340 mg/kg | Plasmid transfer | [118] | |
| Rhizobia | As | 375–1500 mg/L | Changes extracellular polysaccharide composition | Carbohydrates, proteins, and uronic acids were significantly enhanced | [119] |
| Arbuscular mycorrhizal fungi | Cd | 1.12 mg/L | Changes the expression of Cd transporters and soil bacterial community | Expression of genes Nramp5 and HMA3 in root was up-regulated | [120] |
| Arbuscular mycorrhizal fungi | Cd | 81 mg/kg | Expression of PtMT2b was up-regulated | [121] | |
| Zn | 300 mg/kg | ||||
| Arbuscular mycorrhizal fungi | Cd | 0–20 mg/kg | Enhance P nutrition, promote growth | Up-regulated expression of AMF-inducible GmPTs and GmHMA19 | [122] |
| Arbuscular mycorrhizal fungi | Cd | 50.5 mg/L | Changes the redox | Up- regulated expression of an ATP-binding cassette (ABC) transporter (GintABC1) | [123] |
| Cu | 32 mg/L | ||||
| Arbuscular mycorrhizal fungi | Cd | Decreased the transfer factor | Improve the HMA3 gene expression in rice root | [124] | |
| Arbuscular mycorrhizal fungi | Cd | 2 mg/kg | A metabolic shift | The glycolysis-mediated mobilization of defense mechanisms | [125] |
| Bacillus cereus | Pb Zn Ni Cu Cd | 150 mg/L 400 mg/L 50 mg/L 200 mg/L 10 mg/L | Plant-beneficial metabolites, modulating the antioxidants | [126] | |
| Bacillus sp. MN3-4 | Pb | 50–1500 mg/L | Extracellular sequestration and intracellular accumulation | [127] | |
| Azospirillum brasilense | As | 1.88 mg/L | Indole Acetic acid | [110] | |
| Azospirillum brasilense | As | 1.88–37.5 mg/L | As resistance genes mediate the redox As transformation and extrusion outside the cell | ars operon | [128] |
| Pseudomonas and Enterobacter | Cu, Ni, Zn and Cd | 5–500 mg/L | Regulating the production of indole-3-acetic acid, phosphate solubilization, iron carrier, and hydrogen cyanide | [129] | |
| Pseudomonas fluorescens | Cd | 2.8 mg/L | Promote photosynthesis, carbon fixation | Photosynthetic genes and C4-pathway carbon fixation-related genes were significantly up-regulated | [130] |
| Paenibacillus sp. Bacillus sp. | Cd Ni | 18.98 mg/kg 108.12 mg/kg | Surface functional groups (-OH, -NH2, -COO, etc.) reduce the bioavailability of heavy metals | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, T.; Du, H.; Guo, P.; Wang, S.; Ma, M. Research Progress in the Joint Remediation of Plants–Microbes–Soil for Heavy Metal-Contaminated Soil in Mining Areas: A Review. Sustainability 2024, 16, 8464. https://doi.org/10.3390/su16198464
Li H, Wang T, Du H, Guo P, Wang S, Ma M. Research Progress in the Joint Remediation of Plants–Microbes–Soil for Heavy Metal-Contaminated Soil in Mining Areas: A Review. Sustainability. 2024; 16(19):8464. https://doi.org/10.3390/su16198464
Chicago/Turabian StyleLi, Hong, Tao Wang, Hongxia Du, Pan Guo, Shufeng Wang, and Ming Ma. 2024. "Research Progress in the Joint Remediation of Plants–Microbes–Soil for Heavy Metal-Contaminated Soil in Mining Areas: A Review" Sustainability 16, no. 19: 8464. https://doi.org/10.3390/su16198464






