Environmental Impact Assessment of a Plant Cell-Based Bio-Manufacturing Process for Producing Plant Natural Product Ingredients
Abstract
:1. Introduction
2. Methodology
2.1. Life Cycle Assessment
2.1.1. Aim and Scope
2.1.2. Standards, Software and Database
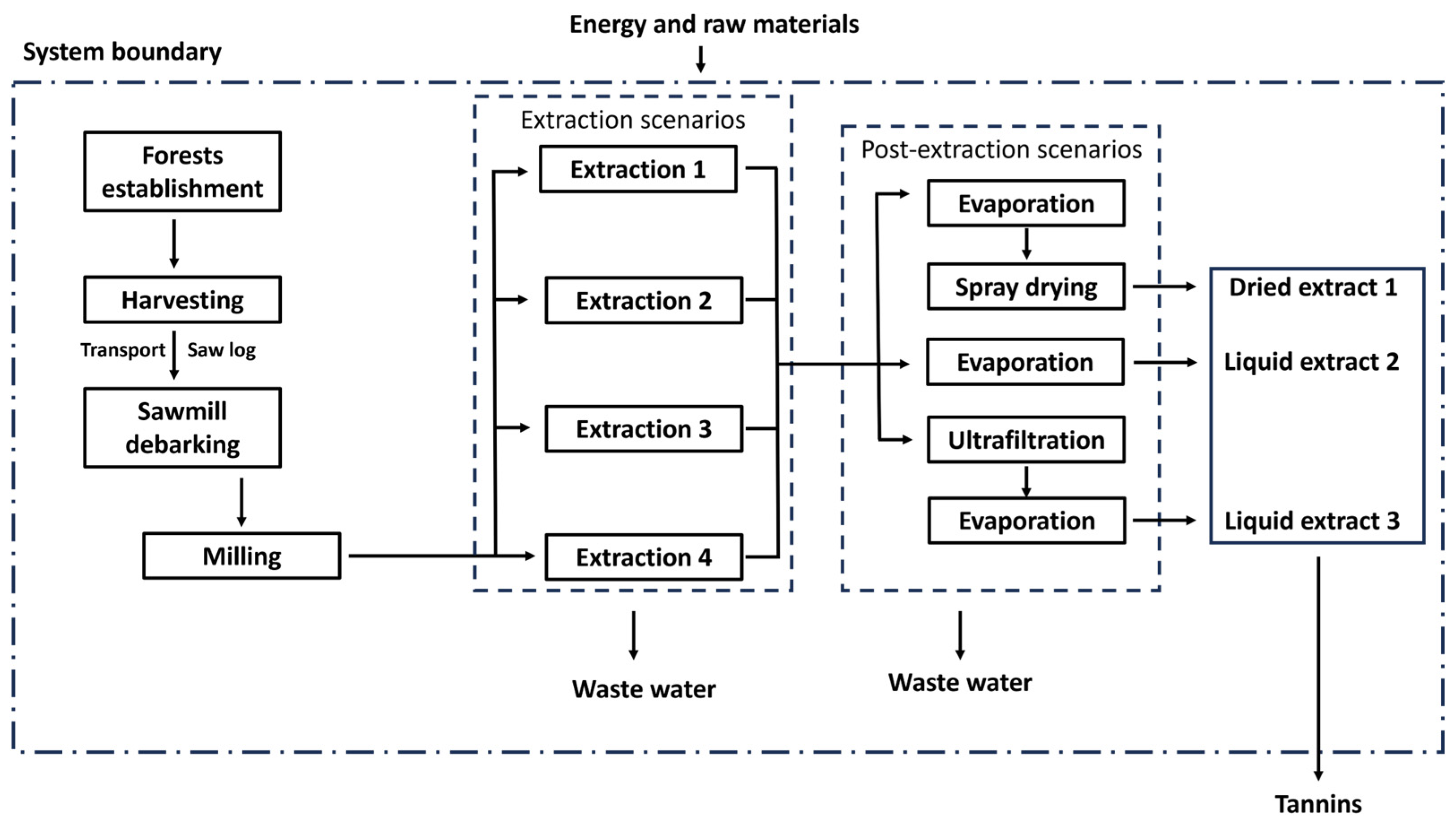
2.1.3. System Boundaries
2.1.4. Life Cycle Inventory (LCI)
2.1.5. Life Cycle Impact Assessment (LCIA) Method
2.2. Material Circularity Index
3. Results and Discussion
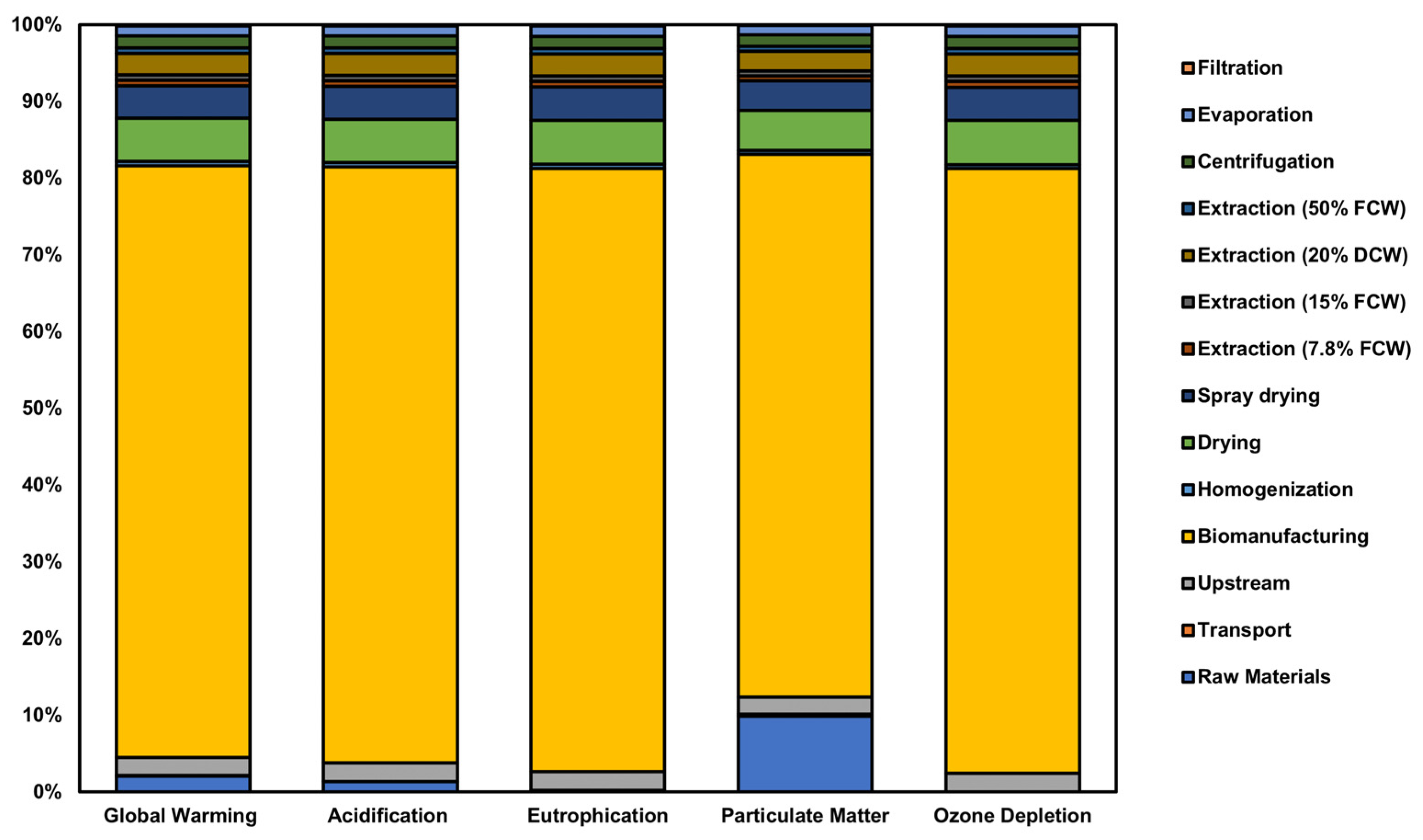
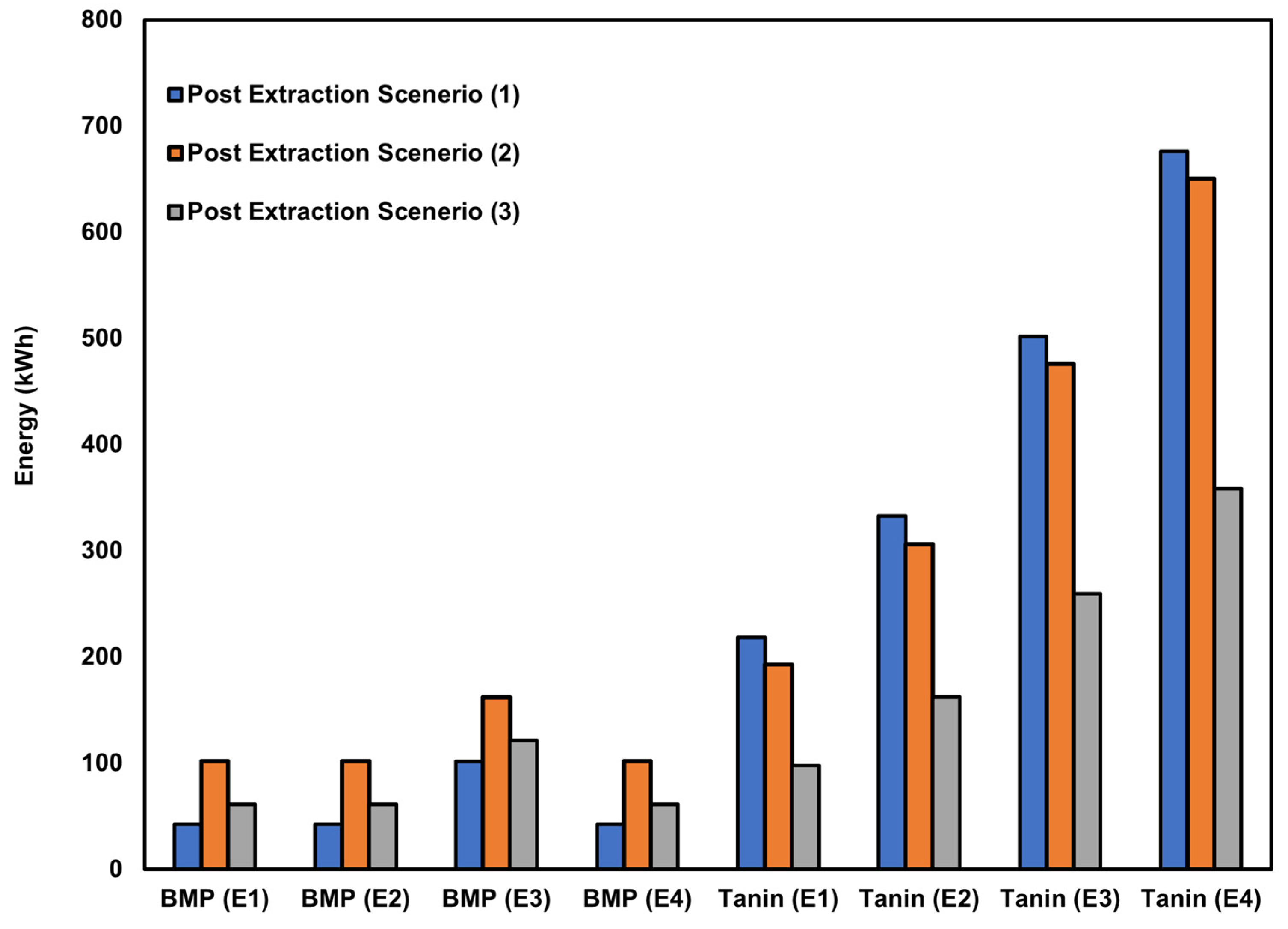
3.1. Environmental Impact Assessment
3.2. Material Circularity Index (MCI) of the Biomanufacturing Process
3.3. Biomanufacturing Process and Wild Harvest Approach
4. Conclusions
4.1. Environmental Impact Insights
4.2. Energy Source Influence
4.3. Material Circularity Index
4.4. Comparative Analysis with the Wild Harvest Approach
4.5. Recommendations for Improvement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelsen, A.; Jagger, P.; Babigumira, R.; Belcher, B.; Hogarth, N.J.; Bauch, S.; Börner, J.; Smith-Hall, C.; Wunder, S. Environmental income and rural livelihoods: A global-comparative analysis. World Dev. 2014, 64, S12–S28. [Google Scholar] [CrossRef] [PubMed]
- Magrach, A.; Sanz, M.J. Environmental and social consequences of the increase in the demand for ‘superfoods’ world-wide. People Nat. 2020, 2, 267–278. [Google Scholar] [CrossRef]
- Hudson, A.; Milliken, W.; Timberlake, J.; Giovannini, P.; Fijamo, V.; Massunde, J.; Chipanga, H.; Nivunga, M.; Ulian, T. Natural Plant Resources for Sustainable Development: Insights from Community Use in the Chimanimani Trans-Frontier Conservation Area, Mozambique. Hum. Ecol. 2020, 48, 55–67. [Google Scholar] [CrossRef]
- Leão, T.C.C.; Lobo, D.; Scotson, L. Economic and Biological Conditions Influence the Sustainability of Harvest of Wild Animals and Plants in Developing Countries. Ecol. Econ. 2017, 140, 14–21. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, S.; Fan, L.; Zeng, Y.; Yang, Y.; Zhao, Y.; Lee, T.M. Does higher demand for medicinal plants lead to more harvest? Evidence from the dual trade of Nardostachy jatamansi and Fritillaria cirrhosa and Tibetan people’s harvesting behavior. Front. Ecol. Evol. 2023, 11, 1145928. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, L.; Hu, S.; Poha; Jiayangji; Wan, A.K.Y.; Zeng, Y.; Yang, Y.; Duan, B.; Lee, T.M. Understanding indigenous people’s traditional Chinese medicinal plants harvesting preferences to guide sustainable management. People Nat. 2023, 5, 1174–1186. [Google Scholar] [CrossRef]
- Opuogulaya, R.; Gbosidom, V.L.; Ekiyor, T.H. Assessing the Sustainability of Thryonomys swinderianus Hunting in Ogoni Land. Asian J. Biol. 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Jaiswal, P.; Singh, A.V.; Yadav, V.K.; Kumar, A.; Kumari, N. Chapter 18—Phytochemical studies in the field of plant tissue culture. In Advances in Plant Tissue Culture; Chandra Rai, A., Kumar, A., Modi, A., Singh, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 395–405. [Google Scholar] [CrossRef]
- Jaiswal, N.; Verma, Y.; Misra, P. Elicitation enhanced the production of bioactive compound and biomass accumulation in callus cultures of Glycyrrhiza glabra L. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 427–436. [Google Scholar] [CrossRef]
- Nielsen, E.; Temporiti, M.E.E.; Cella, R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep. 2019, 38, 1199–1215. [Google Scholar] [CrossRef]
- Patel, H.; Krishnamurthy, R. Elicitors in Plant Tissue Culture. J. Pharmacogn. Phytochem. 2013, 2, 60–65. [Google Scholar]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K.-M. Cellular agriculture—Industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Nygren, H.; Nohynek, L.; Puupponen-Pimiä, R.; Heiniö, R.L.; Maiorova, N.; Rischer, H.; Ritala, A. Plant cell cultures as food-aspects of sustainability and safety. Plant Cell Rep. 2020, 39, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.M.; Heiniö, R.L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—A concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kärkkäinen, E.; Häkkinen, S.T.; Nohynek, L.; Ritala, A.; Rischer, H.; Tuomisto, H.L. Life cycle assessment of plant cell cultures. Sci. Total Environ. 2022, 808, 151990. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A. Life Cycle Assessment: A review of the methodology and its application to sustainability. Curr. Opin. Chem. Eng. 2013, 2, 273–277. [Google Scholar] [CrossRef]
- Ding, T.; Bianchi, S.; Ganne-Chedeville, C.; Kilpelainen, P.; Haapala, A.; Raty, T. Life cycle assessment of tannin extraction from spruce bark. Iforest-Biogeosci. For. 2017, 10, 807–814. [Google Scholar] [CrossRef]
- ISO 14040: 2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO. Environmental Management-Life Cycle Assessment-Requirements and Guidelines; ISO: Geneva, Switzerland, 2006; Volume 14044. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- GreenDelta, G. OpenLCA Software; GreenDelta GmbH: Berlin, Germany, 2019. [Google Scholar]
- Thévenot, A.; Rivera, J.L.; Wilfart, A.; Maillard, F.; Hassouna, M.; Senga-Kiesse, T.; Le Féon, S.; Aubin, J. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018, 170, 1260–1267. [Google Scholar] [CrossRef]
- Rocchi, L.; Paolotti, L.; Cortina, C.; Fagioli, F.F.; Boggia, A. Measuring circularity: An application of modified Material Circularity Indicator to agricultural systems. Agric. Food Econ. 2021, 9, 9. [Google Scholar] [CrossRef]
- Rigamonti, L.; Mancini, E. Life cycle assessment and circularity indicators. Int. J. Life Cycle Assess. 2021, 26, 1937–1942. [Google Scholar] [CrossRef]





| Biomanufacturing Processes | Description | Energy (kWh) | |
|---|---|---|---|
| Production Process | Upstream Processing | Preparation of VSC inoculum and media | 68.0 |
| Biomanufacturing | Growth of plant cells in the bioreactor | 2184.0 | |
| Homogenisation | Cell disruption in solvent | 15.0 | |
| Drying | Removal of water from the system | 160.0 | |
| Adsorption | Use of resins to recover biomolecules from conditioned media | 0.0 | |
| Extraction | Extraction 1 (7.8% FCW) | Extraction | 20.0 |
| Extraction 2 (15% FCW) | Extraction | 20.0 | |
| Extraction 3 (20% DCW) | Extraction | 80.0 | |
| Extraction 4 (50% FCW) | Extraction | 20.0 | |
| Post- Extraction | Post-Extraction Scenario 1 | Centrifugation | 22.1 |
| Spray Drying | 60.0 | ||
| Post- Extraction Scenario 2 | Centrifugation | 22.1 | |
| Post-Extraction Scenario 3 | Evaporation | 37.5 | |
| Filtration | 3.6 |
| Energy Source | Embodied Carbon Factor (kgCO2e/kWh) | Embodied Acidification Factor (kgSO2e/kWh) | Embodied Eutrophication Factor (kgPe/kWh) | Embodied Particulate Matter Factor (kgPM2.5e/kWh) | Embodied Ozone Depletion Factor (kgCFC11e/kWh) |
|---|---|---|---|---|---|
| Energy Mix | 4.66 × 10−2 | 1.90 × 10−4 | 1.71 × 10−5 | 8.14 × 10−6 | 4.20 × 10−7 |
| Geothermal | 7.54 × 10−2 | 2.60 × 10−4 | 3.87 × 10−5 | 1.60 × 10−4 | 2.50 × 10−8 |
| Hydropower | 4.31 × 10−3 | 1.33 × 10−5 | 1.50 × 10−6 | 9.90 × 10−6 | 4.99 × 10−7 |
| Wind | 1.34 × 10−2 | 5.88 × 10−5 | 1.05 × 10−5 | 3.23 × 10−5 | 5.83 × 10−9 |
| Solar | 7.84 × 10−2 | 3.50 × 10−4 | 5.17 × 10−5 | 1.80 × 10−4 | 3.35 × 10−7 |
| Materials | Mass Per Volume (kg/L) | Supplier Location (A) | GBL Location (B) | Mode of Transport | Distance from A to B (km) |
|---|---|---|---|---|---|
| MS Salts | 0.0043 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| B5 Vitamin Stock 1000X | 0.0010 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| Sucrose | 0.0300 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| Casein hydrolysate | 0.0005 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| PVP-10 | 0.0015 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| 6-BA | 0.000001 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| Kinetin | 0.000001 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| NAA | 0.000001 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| 2,4-D | 0.000003 | Glasgow, UK PA4 9RF | Edinburgh UK EH26 0PL | Lorry | 96.1 |
| Water | 1.000000 | Edinburgh UK EH26 0PL | Edinburgh UK EH26 0PL | N/A | 0.0 |
| Material | Embodied Carbon Factor (kgCO2e/kWh) | Embodied Acidification Factor (kgSO2e/kWh) | Embodied Eutrophication Factor (kgPe/kWh) | Embodied Particulate Matter Factor (kgPM2.5e/kWh) | Embodied Ozone Depletion Factor (kgCFC11e/kWh) |
|---|---|---|---|---|---|
| MS salt | 3.90 × 10−2 | 1.40 × 10−5 | 5.91 × 10−8 | 0.00 × 100 | 0.00 × 100 |
| B5 Vitamin stock, 1000X | 1.95 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| Sucrose | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 |
| Casein hydrolysate | 2.05 × 100 | 1.07 × 10−2 | 8.00 × 10−4 | 4.20 × 10−3 | 1.56 × 10−5 |
| PVP-10 | 1.95 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| 6-BA | 1.95 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| Kinetin | 1.95 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| NAA | 1.42 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| 2,4-D | 1.95 × 100 | 5.87 × 10−3 | 4.40 × 10−4 | 2.58 × 10−3 | 4.01 × 10−7 |
| Water | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 |
| Tannin Extraction | Description | Energy (kWh) | |
|---|---|---|---|
| Extraction | Extraction | Extraction | 22.80 |
| Post-Extraction | Post-Extraction Scenario 1 | Evaporation | 211.1 |
| Spray Drying | 7.600 | ||
| Post-Extraction Scenario 2 | Evaporation | 192.8 | |
| Post-Extraction Scenario 3 | Ultrafiltration | 0.410 | |
| Evaporation | 97.50 |
| Ingredients | Raw Materials | Waste | ||
|---|---|---|---|---|
| Recycled Content (%) | Reused Content (%) | Recycled Fraction (%) | Reused Fraction (%) | |
| MS Salts | 0 | 0 | 0.19 | 0 |
| B5 Vitamin Stock 1000X | 0 | 0 | 0.19 | 0 |
| Sucrose | 0 | 0 | 0.19 | 0 |
| Casein hydrolysate | 0 | 0 | 0.19 | 0 |
| PVP-10 | 0 | 0 | 0.19 | 0 |
| 6-BA | 0 | 0 | 0.19 | 0 |
| Kinetin | 0 | 0 | 0.19 | 0 |
| NAA | 0 | 0 | 0.19 | 0 |
| 2,4-D | 0 | 0 | 0.19 | 0 |
| Products (Output) | Life Span (Months) | Industry Average Life Span (Years) |
|---|---|---|
| Liquid extract [7.8, 25, 50% FCW] | 24 | 2 |
| Dried extract (Powder) | 25 | 3 |
| Liquid Extract 2 (20% DCW) | 26 | 4 |
| Evaporated extract (100X) | 27 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluyemi, G.F.; Afolabi, R.O.; Zamora, S.C.; Li, Y.; McElroy, D. Environmental Impact Assessment of a Plant Cell-Based Bio-Manufacturing Process for Producing Plant Natural Product Ingredients. Sustainability 2024, 16, 8515. https://doi.org/10.3390/su16198515
Oluyemi GF, Afolabi RO, Zamora SC, Li Y, McElroy D. Environmental Impact Assessment of a Plant Cell-Based Bio-Manufacturing Process for Producing Plant Natural Product Ingredients. Sustainability. 2024; 16(19):8515. https://doi.org/10.3390/su16198515
Chicago/Turabian StyleOluyemi, Gbenga F., Richard O. Afolabi, Samuel Casasola Zamora, Yuan Li, and David McElroy. 2024. "Environmental Impact Assessment of a Plant Cell-Based Bio-Manufacturing Process for Producing Plant Natural Product Ingredients" Sustainability 16, no. 19: 8515. https://doi.org/10.3390/su16198515






