Recent Trends in the Synthesis of Monomers for Furanoate Polyesters and Their Nanocomposites’ Fabrication as a Sustainable Packaging Material
Abstract
1. Introduction
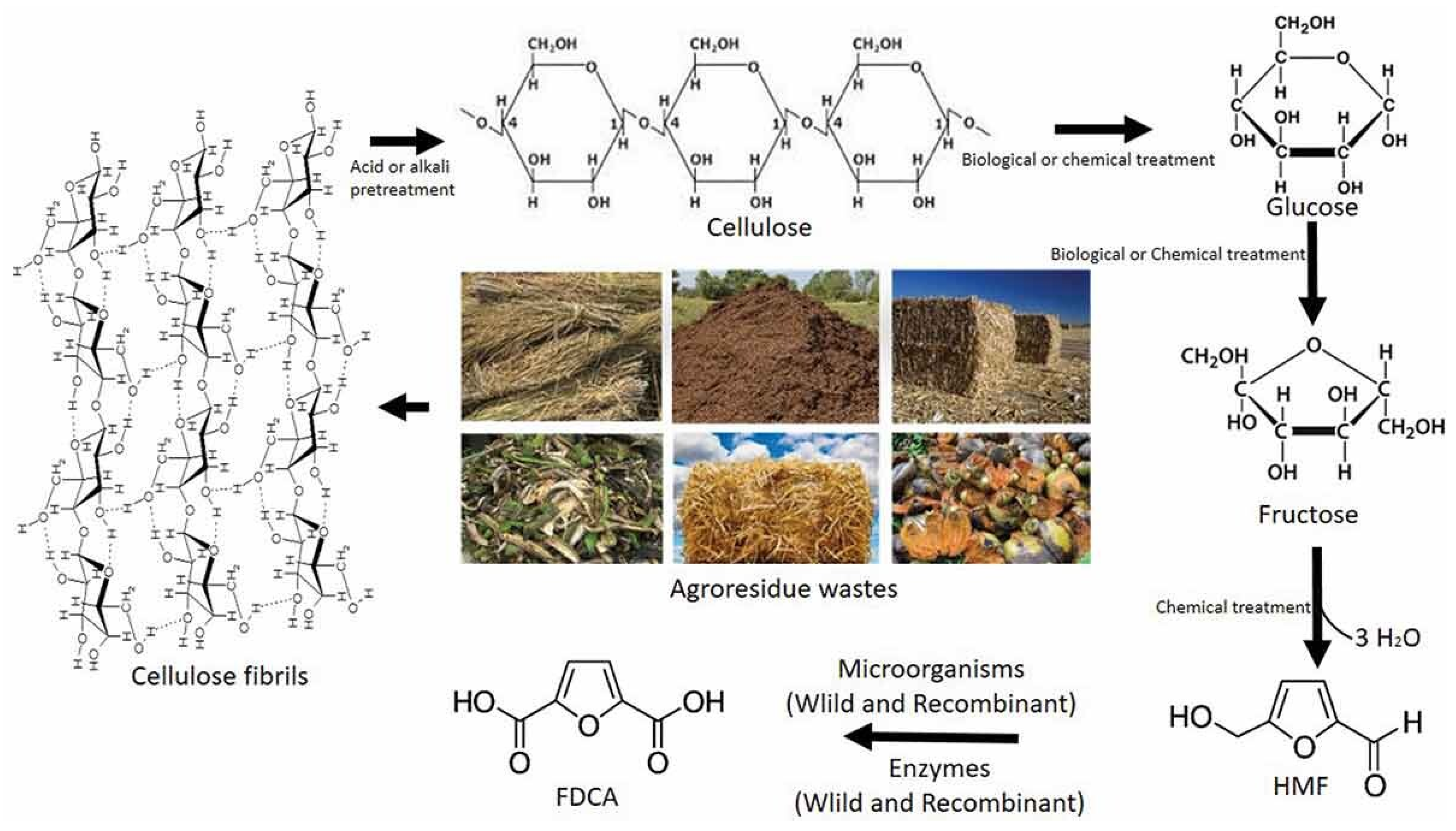
2. Recent Trends in the Synthesis of Monomers for Furanoate Polyesters
2.1. Synthesis Routes of FDCA
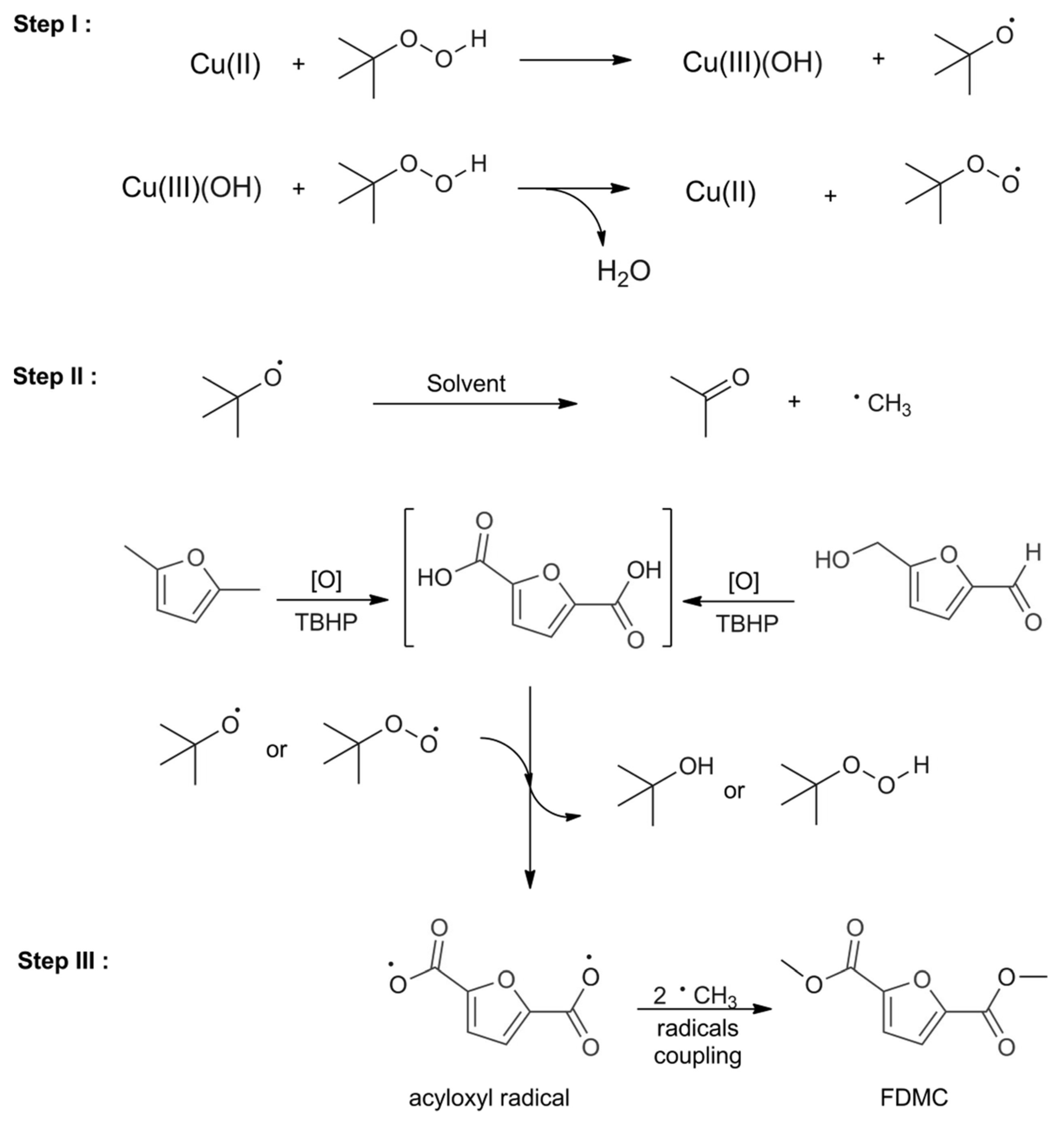
2.2. Synthesis Routes of DMFD
3. Furanoate-Based Nanocomposites
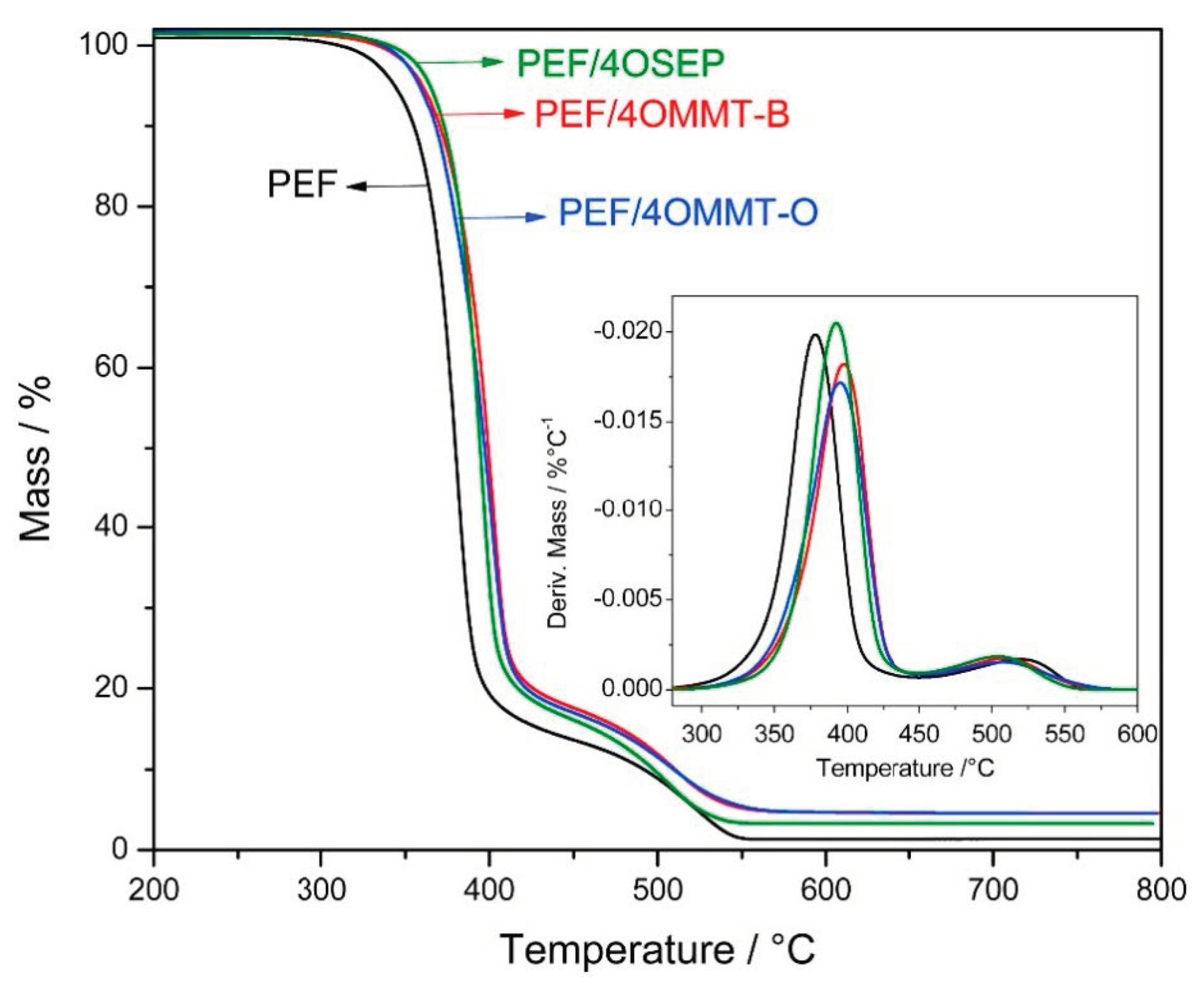
3.1. Poly(ethylene furanoate) (PEF)-Based Nanocomposites
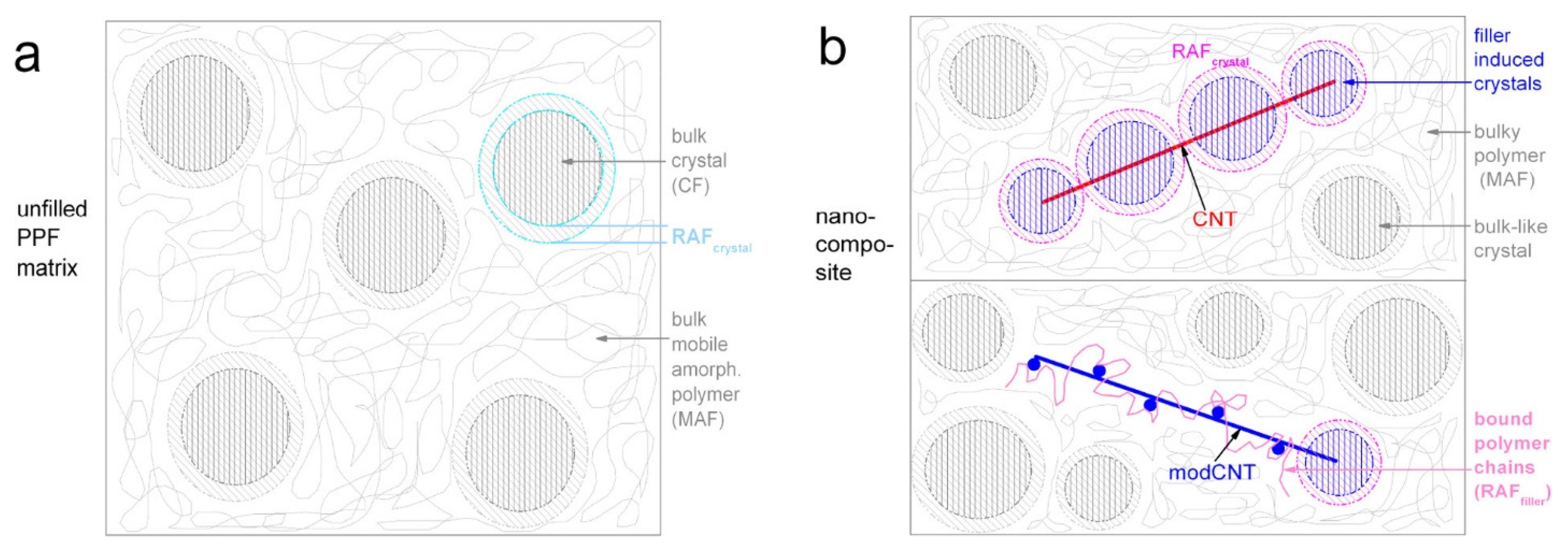
3.2. Poly(propylene furanoate) (PPF)-Based Nanocomposites
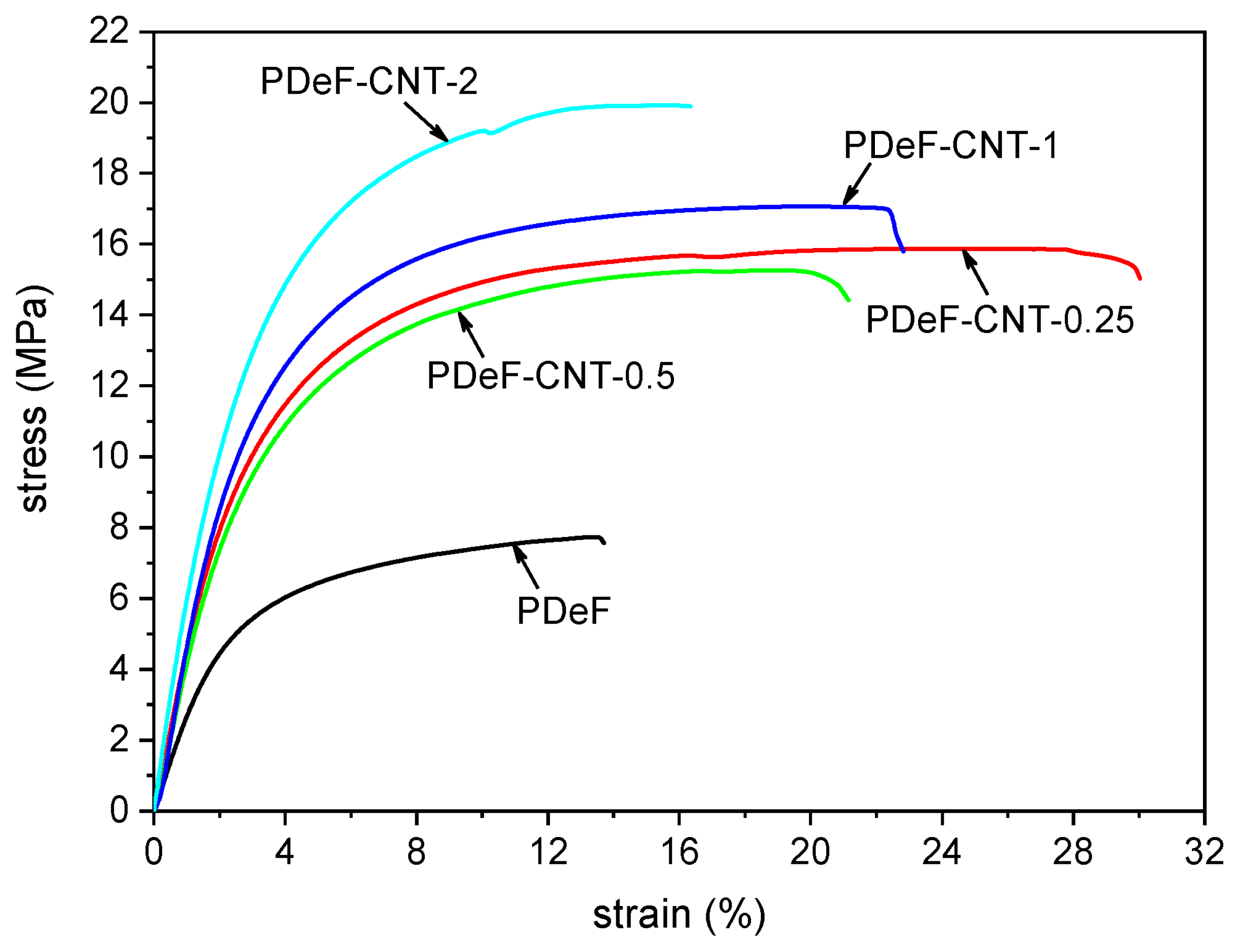
3.3. Poly(butylene furanoate) (PBF)-Based Nanocomposites
3.4. Other Furanoate-Based Nanocomposites
4. Challenges and Future Scope
- PEF, PPF, PBF, and other furan-based nanocomposites fabricated using different nanoparticles, like MWCNT’s, CNT’s, GO, and nano clays, improve the crystallinity of the overall material, resulting in improved thermal and mechanical properties.
- Metallic nanoparticles such as TiO2, ZnO, ZrO2, and Ag were incorporated into furan-based polyesters to improve the gas barrier and antimicrobial properties of nanocomposites to make them suitable for food-packaging applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvianti, F.; Maniar, D.; Boetje, L.; Loos, K. Green Pathways for the Enzymatic Synthesis of Furan-Based Polyesters and Polyamides; American Chemical Society: Washington, DC, USA, 2020; pp. 3–29. [Google Scholar]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased Materials for Food Packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Cakmak, H.; Sogut, E. Functional Biobased Composite Polymers for Food Packaging Applications. In Reactive and Functional Polymers Volume One; Springer International Publishing: Cham, Switzerland, 2020; pp. 95–136. [Google Scholar]
- Boldyreva, E.V.; Chus, U.A.; Klushin, V.A. Synthesis of 2,5-Furandicarboxylic Acid from Natural Raw Materials. Mater. Sci. Forum 2019, 945, 488–492. [Google Scholar] [CrossRef]
- Hwang, D.K.; Chung, S.; Kim, S.; Park, J.; Ryu, J.; Park, J.; Oh, D.X.; Jeon, H.; Koo, J.M. Exploring the Potential of 2,5-Furandicarboxylic Acid-Based Bioplastics: Properties, Synthesis, and Applications. Polym. Degrad. Stab. 2023, 218, 110539. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and Their Derived Products: A Review of Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA) Studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Meng, C.; Su, K.; Li, Z.; Zhang, M. Dimethyl 2,5-Furandicarboxylate Synthesis from 2,5-Furanodicarboxylic Acid and Dimethyl Carbonate in Presence of MgO-Al2O3 and Tetrabutylammonium Bromide. Mol. Catal. 2021, 510, 111707. [Google Scholar] [CrossRef]
- Jensen, M.H.; Riisager, A. Advances in the Synthesis and Application of 2,5-Furandicarboxylic Acid. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–170. [Google Scholar]
- Zhao, D.; Su, T.; Len, C.; Luque, R.; Yang, Z. Recent Advances in the Oxidative Esterification of 5-Hydroxymethylfurfural to Furan-2,5-Dimethylcarboxylate. Green Chem. 2022, 24, 6782–6789. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen Sorption and Transport in Amorphous Poly(Ethylene Furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(Ethylene Furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water Sorption in Poly(Ethylene Furanoate) Compared to Poly(Ethylene Terephthalate). Part 1: Equilibrium Sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Modification of Poly(Ethylene 2,5-Furandicarboxylate) with Biobased 1,5-Pentanediol: Significantly Toughened Copolyesters Retaining High Tensile Strength and O 2 Barrier Property. Biomacromolecules 2019, 20, 353–364. [Google Scholar] [CrossRef]
- Louw, J.; Farzad, S.; Görgens, J.F. Polyethylene Furanoate: Technoeconomic Analysis of Biobased Production. Biofuels Bioprod. Biorefining 2023, 17, 135–152. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Wang, J.; Ying, W.B.; Zhu, J. Fully Bio-Based Poly(Propylene Succinate-Co-Propylene Furandicarboxylate) Copolyesters with Proper Mechanical, Degradation and Barrier Properties for Green Packaging Applications. Eur. Polym. J. 2018, 102, 101–110. [Google Scholar] [CrossRef]
- Kainulainen, T.P.; Hukka, T.I.; Özeren, H.D.; Sirviö, J.A.; Hedenqvist, M.S.; Heiskanen, J.P. Utilizing Furfural-Based Bifuran Diester as Monomer and Comonomer for High-Performance Bioplastics: Properties of Poly(Butylene Furanoate), Poly(Butylene Bifuranoate), and Their Copolyesters. Biomacromolecules 2020, 21, 743–752. [Google Scholar] [CrossRef]
- Bianchi, E.; Soccio, M.; Siracusa, V.; Gazzano, M.; Thiyagarajan, S.; Lotti, N. Poly(Butylene 2,4-Furanoate), an Added Member to the Class of Smart Furan-Based Polyesters for Sustainable Packaging: Structural Isomerism as a Key to Tune the Final Properties. ACS Sustain. Chem. Eng. 2021, 9, 11937–11949. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Hamadneh, N.N.; Khan, W.A. Science and Applications of Tailored Nanostructures 50 4 Polymer Nanocomposites-Synthesis Techniques, Classification and Properties Outline; One Central Press: Manchester, UK, 2016. [Google Scholar]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Singhal, S.; Agarwal, S.; Mudoi, M.P.; Singhal, N.; Singh, R. Chemical Conversion of Furan Dicarboxylic Acid to Environmentally Benign Polyesters: An Overview. Biomass Convers. Biorefin. 2023, 13, 15619–15636. [Google Scholar] [CrossRef]
- Pandey, S.; Dumont, M.-J.; Orsat, V.; Rodrigue, D. Biobased 2,5-Furandicarboxylic Acid (FDCA) and Its Emerging Copolyesters’ Properties for Packaging Applications. Eur. Polym. J. 2021, 160, 110778. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent Trends in Nanotechnology Applications of Bio-Based Packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Itabaiana, I. Engineering the Future: Perspectives in the 2,5-Furandicarboxylic Acid Synthesis. Catal. Today 2020, 354, 211–217. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, S.; Lei, Y.; Chen, Z.; Yin, G. Synthesis of 2,5-Furandicarboxylic Acid by Catalytic Carbonylation of Renewable Furfural Derived 5-Bromofuroic Acid. Mol. Catal. 2018, 455, 204–209. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, J.; Chen, Z.; Yin, G.; Li, G. Catalytic Synthesis of 2,5-Furandicarboxylic Acid from Furoic Acid: Transformation from C5 Platform to C6 Derivatives in Biomass Utilizations. ACS Sustain. Chem. Eng. 2017, 5, 9360–9369. [Google Scholar] [CrossRef]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sustain. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Gawade, A.B.; Nakhate, A.V.; Yadav, G.D. Selective Synthesis of 2, 5-Furandicarboxylic Acid by Oxidation of 5-Hydroxymethylfurfural over MnFe2O4 Catalyst. Catal. Today 2018, 309, 119–125. [Google Scholar] [CrossRef]
- Ban, H.; Cheng, Y.; Wang, L.; Li, X. One-Pot Method for the Synthesis of 2,5-Furandicarboxylic Acid from Fructose: In Situ Oxidation of 5-Hydroxymethylfurfural and 5-Acetoxymethylfurfural over Co/Mn/Br Catalysts in Acetic Acid. Ind. Eng. Chem. Res. 2023, 62, 291–301. [Google Scholar] [CrossRef]
- Chen, G.; Wu, L.; Fan, H.; Li, B.G. Highly Efficient Two-Step Synthesis of 2,5-Furandicarboxylic Acid from Fructose without 5-Hydroxymethylfurfural (Hmf) Separation: In Situ Oxidation of Hmf in Alkaline Aqueous H2O/DMSO Mixed Solvent under Mild Conditions. Ind. Eng. Chem. Res. 2018, 57, 16172–16181. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Liu, G.; Wang, M.; Guo, C.; Shen, J. A Sustainable and Low-Cost Route to 2,5-Furandicarboxylic Acid by Carboxylation of Biomass-Based Furoic Acid and CO2. J. CO2 Util. 2023, 75, 102572. [Google Scholar] [CrossRef]
- Sahu, R.; Dhepe, P.L. Synthesis of 2,5-Furandicarboxylic Acid by the Aerobic Oxidation of 5-Hydroxymethyl Furfural over Supported Metal Catalysts. React. Kinet. Mech. Catal. 2014, 112, 173–187. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Yang, W.; Tang, X.; Li, W.; Luo, X.; Zhang, C.; Shen, C. Fast and Continuous Synthesis of 2,5-Furandicarboxylic Acid in a Micropacked-Bed Reactor. Chem. Eng. J. 2022, 442, 136110. [Google Scholar] [CrossRef]
- Yan, D.; Wang, G.; Gao, K.; Lu, X.; Xin, J.; Zhang, S. One-Pot Synthesis of 2,5-Furandicarboxylic Acid from Fructose in Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 1851–1858. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, W.; Su, R.; He, Z. Selective Synthesis of 2,5-Diformylfuran and 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Catalyzed by Magnetically Separable Catalysts. Energy Fuels 2017, 31, 533–541. [Google Scholar] [CrossRef]
- Liu, H.; Cao, X.; Wang, T.; Wei, J.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Liu, S.; Lin, L. Efficient Synthesis of Bio-Monomer 2,5-Furandicarboxylic Acid from Concentrated 5-Hydroxymethylfurfural or Fructose in DMSO/H2O Mixed Solvent. J. Ind. Eng. Chem. 2019, 77, 209–214. [Google Scholar] [CrossRef]
- Xie, W.; Liu, H.; Tang, X.; Zeng, X.; Sun, Y.; Ke, X.; Li, T.; Fang, H.; Lin, L. Efficient Synthesis of 2,5-Furandicarboxylic Acid from Biomass-Derived 5-Hydroxymethylfurfural in 1,4-Dioxane/H2O Mixture. Appl. Catal. A Gen. 2022, 630, 118463. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wen, M.; Zong, M.H.; Li, N. Synergistic Chemo/Biocatalytic Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural. Catal. Commun. 2020, 139, 105979. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.Y.; Zong, M.H.; Li, N. Sacrificial Substrate-Free Whole-Cell Biocatalysis for the Synthesis of 2,5-Furandicarboxylic Acid by Engineered Escherichia Coli. ACS Sustain. Chem. Eng. 2020, 8, 4341–4345. [Google Scholar] [CrossRef]
- Rajesh, R.O.; Godan, T.K.; Sindhu, R.; Pandey, A.; Binod, P. Bioengineering Advancements, Innovations and Challenges on Green Synthesis of 2, 5-Furan Dicarboxylic Acid. Bioengineered 2020, 11, 19–38. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, Z.; Tan, H.; Xu, Q.; Ouyang, J. Synthesis of 2,5-Furandicarboxylic Acid by a TEMPO/Laccase System Coupled WithPseudomonas PutidaKT2440. RSC Adv. 2020, 10, 21781–21788. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic Production of 2,5-Furandicarboxylic Acid: Recent Advances and Future Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 527–543. [Google Scholar] [CrossRef]
- Hsu, C.; Kuo, Y.; Liu, Y.; Tsai, S. Green Conversion of 5-hydroxymethylfurfural to Furan-2,5-dicarboxylic Acid by Heterogeneous Expression of 5-hydroxymethylfurfural Oxidase in Pseudomonas Putida S12. Microb. Biotechnol. 2020, 13, 1094–1102. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, X.; Zhou, X.; Xu, Y. Bioconversion of 5-Hydroxymethylfurfural (HMF) to 2,5-Furandicarboxylic Acid (FDCA) by a Native Obligate Aerobic Bacterium, Acinetobacter Calcoaceticus NL14. Appl. Biochem. Biotechnol. 2020, 192, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Godan, T.K.; Rajesh, R.O.; Loreni, P.C.; Kumar Rai, A.; Sahoo, D.; Pandey, A.; Binod, P. Biotransformation of 5-Hydroxymethylfurfural by Acinetobacter Oleivorans S27 for the Synthesis of Furan Derivatives. Bioresour. Technol. 2019, 282, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Li, Z. CoOx-MC (MC = Mesoporous Carbon) for Highly Efficient Oxidation of 5-Hydroxymethylfurfural (5-HMF) to 2,5-Furandicarboxylic Acid (FDCA). ACS Sustain. Chem. Eng. 2020, 8, 4801–4808. [Google Scholar] [CrossRef]
- Serrano, A.; Calviño, E.; Carro, J.; Sánchez-Ruiz, M.I.; Cañada, F.J.; Martínez, A.T. Complete Oxidation of Hydroxymethylfurfural to Furandicarboxylic Acid by Aryl-Alcohol Oxidase. Biotechnol. Biofuels 2019, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zong, M.; Zheng, G.; Li, N. One-Pot Enzyme Cascade for Controlled Synthesis of Furancarboxylic Acids from 5-Hydroxymethylfurfural by H 2 O 2 Internal Recycling. ChemSusChem 2019, 12, 4764–4768. [Google Scholar] [CrossRef]
- Rajesh, R.O.; Godan, T.K.; Rai, A.K.; Sahoo, D.; Pandey, A.; Binod, P. Biosynthesis of 2,5-Furan Dicarboxylic Acid by Aspergillus Flavus APLS-1: Process Optimization and Intermediate Product Analysis. Bioresour. Technol. 2019, 284, 155–160. [Google Scholar] [CrossRef]
- Karich, A.; Kleeberg, S.; Ullrich, R.; Hofrichter, M. Enzymatic Preparation of 2,5-Furandicarboxylic Acid (FDCA)—A Substitute of Terephthalic Acid—By the Joined Action of Three Fungal Enzymes. Microorganisms 2018, 6, 5. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, C.; Gao, L.; Liu, X.; You, S.; Qi, W.; Wang, K.; Guo, X.; Su, R.; Lu, H.; et al. Tandem Biocatalysis by CotA-TJ102@UIO-66-NH2 and Novozym 435 for Highly Selective Transformation of HMF into FDCA. Trans. Tianjin Univ. 2019, 25, 488–496. [Google Scholar] [CrossRef]
- Ge, T.; Liu, X.; Tang, J.; Liu, C.; Huang, J. Effect of Support on Oxidative Esterification of 2,5-Furandiformaldehyde to Dimethyl Furan-2,5-Dicarboxylate. Catalysts 2023, 13, 1430. [Google Scholar] [CrossRef]
- Sheet, N.; Osuga, R.; Arai, N.; Wiesfeld, J.J.; Suganuma, S.; Aoshima, T.; Fukuoka, A.; Hensen, E.J.M.; Nakajima, K. A Protection Strategy for High-Yield Synthesis of Dimethyl Furan-2,5-Dicarboxylate from 5-Hydroxymethylfurfural Using Methanol as an Acetalizing Agent. ChemCatChem 2024, 16, e202301259. [Google Scholar] [CrossRef]
- Cho, A.; Byun, S.; Cho, J.H.; Kim, B.M. AuPd-Fe3O4 Nanoparticle-Catalyzed Synthesis of Furan-2,5-Dimethylcarboxylate from 5-Hydroxymethylfurfural under Mild Conditions. ChemSusChem 2019, 12, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fang, H.; Qu, H.; Zhang, J.; Duan, X.; Yuan, Y. Fabrication of Supported Au-CuOx Nanohybrids by Reduction-Oxidation Strategy for Efficient Oxidative Esterification of 5-Hydroxymethyl-2-Furfural into Dimethyl Furan-2,5-Dicarboxylate. Appl. Catal. A Gen. 2018, 567, 80–89. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Vinu, A.; Kantam, M.L. Copper-Catalyzed Oxidative Methyl-Esterification of 5-Hydroxymethylfurfural Using TBHP as an Oxidizing and Methylating Reagent: A New Approach for the Synthesis of Furan-2,5-Dimethylcarboxylate. J. Catal. 2020, 389, 259–269. [Google Scholar] [CrossRef]
- Weerathunga, H.; Sarina, S.; Zhu, H.Y.; Waclawik, E.R. Oxidative Esterification of 5-Hydroxymethylfurfural into Dimethyl 2,5-Furandicarboxylate Using Gamma Alumina-Supported Gold Nanoparticles. ACS Omega 2021, 6, 4740–4748. [Google Scholar] [CrossRef]
- Li, F.; Li, X.L.; Li, C.; Shi, J.; Fu, Y. Aerobic Oxidative Esterification of 5-Hydroxymethylfurfural to Dimethyl Furan-2,5-Dicarboxylate by Using Homogeneous and Heterogeneous PdCoBi/C Catalysts under Atmospheric Oxygen. Green Chem. 2018, 20, 3050–3058. [Google Scholar] [CrossRef]
- van Strien, N.; Niskanen, J.; Berghuis, A.; Pöhler, H.; Rautiainen, S. Production of 2,5-Furandicarboxylic Acid Methyl Esters from Pectin-Based Aldaric Acid: From Laboratory to Bench Scale. ChemSusChem 2024, 17, e202300732. [Google Scholar] [CrossRef]
- Van Strien, N.; Rautiainen, S.; Asikainen, M.; Thomas, D.A.; Linnekoski, J.; Niemelä, K.; Harlin, A. A Unique Pathway to Platform Chemicals: Aldaric Acids as Stable Intermediates for the Synthesis of Furandicarboxylic Acid Esters. Green Chem. 2020, 22, 8271–8277. [Google Scholar] [CrossRef]
- Trapasso, G.; Chícharo, B.; Gherardi, T.; Redolfi-Bristol, D.; Aricò, F. Iron(III) Sulfate-Mediated Synthesis of 2,5-Furandicarboxylic Acid Dimethyl Ester from Galactaric Acid. Catalysts 2023, 13, 1114. [Google Scholar] [CrossRef]
- Trapasso, G.; Annatelli, M.; Dalla Torre, D.; Aricò, F. Synthesis of 2,5-Furandicarboxylic Acid Dimethyl Ester from Galactaric Acid via Dimethyl Carbonate Chemistry. Green Chemistry 2022, 24, 2766–2771. [Google Scholar] [CrossRef]
- Wiesfeld, J.J.; Osuga, R.; Suganuma, S.; Aoshima, T.; Hensen, E.J.M.; Nakajima, K. Oxidative Esterification of Acetal-Protected 5-Hydroxymethylfurfural to Dimethyl Furan-2,5-Dicarboxylate with High Recovery of Protecting Agent. ACS Sustain. Chem. Eng. 2024, 12, 450–458. [Google Scholar] [CrossRef]
- Stanley, J.; Terzopoulou, Z.; Klonos, P.A.; Zamboulis, A.; Xanthopoulou, E.; Koltsakidis, S.; Tzetzis, D.; Zemljič, L.F.; Lambropoulou, D.A.; Kyritsis, A.; et al. Effect of Monomer Type on the Synthesis and Properties of Poly(Ethylene Furanoate). Polymers 2023, 15, 2707. [Google Scholar] [CrossRef] [PubMed]
- Lotti, N.; Munari, A.; Gigli, M.; Gazzano, M.; Tsanaktsis, V.; Bikiaris, D.N.; Papageorgiou, G.Z. Thermal and Structural Response of in Situ Prepared Biobased Poly(Ethylene 2,5-Furan Dicarboxylate) Nanocomposites. Polymer 2016, 103, 288–298. [Google Scholar] [CrossRef]
- Kourtidou, D.; Karfaridis, D.; Kehagias, T.; Vourlias, G.; Bikiaris, D.N.; Chrissafis, K. Incorporating Graphene Nanoplatelets and Carbon Nanotubes in Biobased Poly(Ethylene 2,5-Furandicarboxylate): Fillers’ Effect on the Matrix’s Structure and Lifetime. Polymers 2023, 15, 401. [Google Scholar] [CrossRef]
- Xie, H.; Meng, H.; Wu, L.; Li, B.G.; Dubois, P. In-Situ Synthesis, Thermal and Mechanical Properties of Biobased Poly(Ethylene 2,5-Furandicarboxylate)/Montmorillonite (PEF/MMT) Nanocomposites. Eur. Polym. J. 2019, 121, 109266. [Google Scholar] [CrossRef]
- Martino, L.; Guigo, N.; van Berkel, J.G.; Sbirrazzuoli, N. Influence of Organically Modified Montmorillonite and Sepiolite Clays on the Physical Properties of Bio-Based Poly(Ethylene 2,5-Furandicarboxylate). Compos. B Eng. 2017, 110, 96–105. [Google Scholar] [CrossRef]
- Martino, L.; Niknam, V.; Guigo, N.; Gabriël Van Berkel, J.; Sbirrazzuoli, N. Morphology and Thermal Properties of Novel Clay-Based Poly(Ethylene 2,5-Furandicarboxylate) (PEF) Nanocomposites. RSC Adv. 2016, 6, 59800–59807. [Google Scholar] [CrossRef]
- Nasirudeen, M.B.; Hailes, H.C.; Evans, J.R.G. Preparation and Characterization of Biobased Poly(Ethylene-2,5-Furan Dicarboxylate)/Clay Nanocomposites. Niger. J. Basic Appl. Sci. 2018, 25, 114. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Dai, Y.; Zhang, Y.; Jiang, M.; Zhou, G. High Performance Biobased Poly(Ethylene 2,5-Furandicarboxylate) Nanocomposites for Food and Cosmetics Packaging Materials: PMDA Chain Extended and TiO2 NPs Functionalized. Arab. J. Chem. 2023, 16, 105228. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302, 1700012. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, J.; Zhang, Z.; Shi, F. Bio-Based Poly(Ethylene Furanoate)/ZnO Transparent Thin Films with Improved Water Vapor Barrier and Antibacterial Properties for Food Packaging Application. Mater. Res. Express 2022, 9, 115304. [Google Scholar] [CrossRef]
- Stanley, J.; Xanthopoulou, E.; Finšgar, M.; Zemljič, L.F.; Klonos, P.A.; Kyritsis, A.; Koltsakidis, S.; Tzetzis, D.; Lambropoulou, D.A.; Baciu, D.; et al. Synthesis of Poly(Ethylene Furanoate) Based Nanocomposites by In Situ Polymerization with Enhanced Antibacterial Properties for Food Packaging Applications. Polymers 2023, 15, 4502. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Xanthopoulou, E.; Zemljič, L.F.; Klonos, P.A.; Kyritsis, A.; Lambropoulou, D.A.; Bikiaris, D.N. Fabrication of Poly(Ethylene Furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties. Materials 2024, 17, 1606. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z. Synthesis and Characterization of In-Situ-Prepared Nanocomposites Based on Poly(Propylene 2,5-Furan Dicarboxylate) and Aluminosilicate Clays. Polymers 2018, 10, 937. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Papageorgiou, G.Z.; Kyritsis, A.; Pissis, P.; Bikiaris, D.N. Interfacial Interactions, Crystallization, and Molecular Dynamics of Renewable Poly(Propylene Furanoate) in Situ Filled with Initial and Surface Functionalized Carbon Nanotubes and Graphene Oxide. J. Phys. Chem. C 2020, 124, 10220–10234. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tarani, E.; Kasmi, N.; Papadopoulos, L.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal Decomposition Kinetics and Mechanism of In-Situ Prepared Bio-Based Poly(Propylene 2,5-Furan Dicarboxylate)/Graphene Nanocomposites. Molecules 2019, 24, 1717. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Janowska, I.; Pawlikowska, D.; Szymczyk, A.; Irska, I.; Lisiecki, S.; Stanik, R.; Gude, M.; Piesowicz, E. New Functional Nanocomposites Based on Poly(Trimethylene 2,5-Furanoate) and Few Layer Graphene Prepared by in Situ Polymerization. Express Polym. Lett. 2018, 12, 530–542. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, K.; Jiang, Z.; Qiu, Z. Biobased Poly(1,3-Propylene 2,5-Furandicarboxylate)-Carbon Nanotubes Nanocomposites with Enhanced Thermal, Mechanical Properties and Crystallization Behavior. J. Polym. Environ. 2022, 30, 555–561. [Google Scholar] [CrossRef]
- Pan, S.; Jiang, Z.; Qiu, Z. Influence of Low Contents of Cellulose Nanocrystals on the Crystallization Behavior of Biobased Poly(Propylene 2,5-Furandicarboxylate). Giant 2024, 17, 100212. [Google Scholar] [CrossRef]
- Mahmud, S.; Wang, J.; Shao, N.; Xiong, Z.; Zhang, R.; Zhu, J. Nucleation and Crystallization of Poly(Propylene 2,5-Furan Dicarboxylate) by Direct Blending of Microcrystalline Cellulose: Improved Tensile and Barrier Properties. Cellulose 2020, 27, 9423–9436. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Jiang, M.; Wang, G.; Wang, R.; Wu, G.; Zhou, G. Renewable Poly(Butene 2, 5-Furan Dicarboxylate) Nanocomposites Constructed by TiO2 Nanocubes: Synthesis, Crystallization, and Properties. Polym. Degrad. Stab. 2021, 189, 109591. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Silva, N.H.C.S.; Freire, C.S.R.; Andrade, M.; Mendes, A.; Silvestre, A.J.D. Furanoate-Based Nanocomposites: A Case Study Using Poly(Butylene 2,5-Furanoate) and Poly(Butylene 2,5-Furanoate)-Co-(Butylene Diglycolate) and Bacterial Cellulose. Polymers 2018, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, L.; Terzopoulou, Z.; Vlachopoulos, A.; Klonos, P.A.; Kyritsis, A.; Tzetzis, D.; Papageorgiou, G.Z.; Bikiaris, D. Synthesis and Characterization of Novel Polymer/Clay Nanocomposites Based on Poly (Butylene 2,5-Furan Dicarboxylate). Appl. Clay Sci. 2020, 190, 105588. [Google Scholar] [CrossRef]
- Lu, H.; Tang, S.-Y.; Yun, G.; Li, H.; Zhang, Y.; Qiao, R.; Li, W. Modular and Integrated Systems for Nanoparticle and Microparticle Synthesis—A Review. Biosensors 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.V.; Liyaskina, E.V.; Parchaykina, M.V.; Kuzmenko, T.P.; Kurgaeva, I.V.; Revin, V.D.; Ullah, M.W. Bacterial Cellulose-Based Polymer Nanocomposites: A Review. Polymers 2022, 14, 4670. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Qiu, Z. In Situ Synthesis, Crystallization Behavior, and Mechanical Property of Biobased Poly(Hexamethylene 2,5-Furandicarboxylate)/Multiwalled Carbon Nanotube Nanocomposites. Ind. Eng. Chem. Res. 2022, 61, 9745–9754. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Bortolotti, M.; Pegoretti, A.; Bikiaris, D.N. Mechanical and Functional Properties of Novel Biobased Poly(Decylene-2,5-Furanoate)/Carbon Nanotubes Nanocomposite Films. Polymers 2020, 12, 2459. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Terzopoulou, Z.; Kyritsis, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Molecular dynamics in nanocomposites based on renewable poly (butylene 2, 5-furan-dicarboxylate) in situ reinforced by montmorillonite nanoclays: Effects of clay modification, crystallization, and hydration. J. Phys. Chem. 2020, 124, 7306–7317. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Papadopoulos, L.; Klonos, P.A.; Terzopoulou, Z.; Aït Hocine, N.; Benelfellah, A.; Papageorgiou, G.Z.; Kyritsis, A.; Bikiaris, D.N. Calorimetric and Dielectric Study of Renewable Poly(Hexylene 2,5-Furan-Dicarboxylate)-Based Nanocomposites in Situ Filled with Small Amounts of Graphene Platelets and Silica Nanoparticles. Polymers 2020, 12, 1239. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A.; Ihuoma, D.E. Recently Emerging Trends in Polymer Nanocomposites Packaging Materials. Polym.-Plast. Technol. Mater. 2019, 58, 1054–1109. [Google Scholar] [CrossRef]




| A/A | Product | Catalyst | Medium | Monomer Yield | Ref. |
|---|---|---|---|---|---|
| 1 | FDCA | Comamonas testosteroni SC1588 cells | Laccase-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) | 87% | [1] |
| 2 | FDCA | Pseudomonas putida S12 strain | 5-hydroxymethylfurfural oxidase (HMFO) | 70% | [44] |
| 3 | FDCA | Acinetobacter calcoaceticus NL14 | Sodium carbonate (Na2CO3) | 100% | [45] |
| 4 | FDCA | Acinetobacter oleivorans S27 | - | 100% | [46] |
| 5 | FDCA | CoOx-MC (MC = mesoporous carbon) | Water using O2 as the oxidant | 95.3% | [47] |
| 6 | FDCA | Aryl-alcohol oxidase | Phosphate | 100% | [48] |
| 7 | FDCA | Galactose oxidase (GO) and alcohol dehydrogenases (ADHs) | Water | 95% | [49] |
| 8 | FDCA | Aspergillus flavus APLS-1 | Phosphate | 67% | [50] |
| 9 | FDCA | Aryl alcohol oxidase (AAO)/Agrocybe aegerita (AaeUPO)/galactose oxidase (GAO) | Phosphate | 80% | [51] |
| 10 | FDCA | Laccase (CotA-TJ102@UIO-66-NH2) and Novozym 435 | Dimethylformamide (DMF) | 95.5% | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanley, J.; Fras Zemljič, L.; Lambropoulou, D.A.; Bikiaris, D.N. Recent Trends in the Synthesis of Monomers for Furanoate Polyesters and Their Nanocomposites’ Fabrication as a Sustainable Packaging Material. Sustainability 2024, 16, 8632. https://doi.org/10.3390/su16198632
Stanley J, Fras Zemljič L, Lambropoulou DA, Bikiaris DN. Recent Trends in the Synthesis of Monomers for Furanoate Polyesters and Their Nanocomposites’ Fabrication as a Sustainable Packaging Material. Sustainability. 2024; 16(19):8632. https://doi.org/10.3390/su16198632
Chicago/Turabian StyleStanley, Johan, Lidija Fras Zemljič, Dimitra A. Lambropoulou, and Dimitrios N. Bikiaris. 2024. "Recent Trends in the Synthesis of Monomers for Furanoate Polyesters and Their Nanocomposites’ Fabrication as a Sustainable Packaging Material" Sustainability 16, no. 19: 8632. https://doi.org/10.3390/su16198632
APA StyleStanley, J., Fras Zemljič, L., Lambropoulou, D. A., & Bikiaris, D. N. (2024). Recent Trends in the Synthesis of Monomers for Furanoate Polyesters and Their Nanocomposites’ Fabrication as a Sustainable Packaging Material. Sustainability, 16(19), 8632. https://doi.org/10.3390/su16198632









