Improvement of Engine Combustion and Emission Characteristics by Fuel Property Modulation
Abstract
:1. Introduction
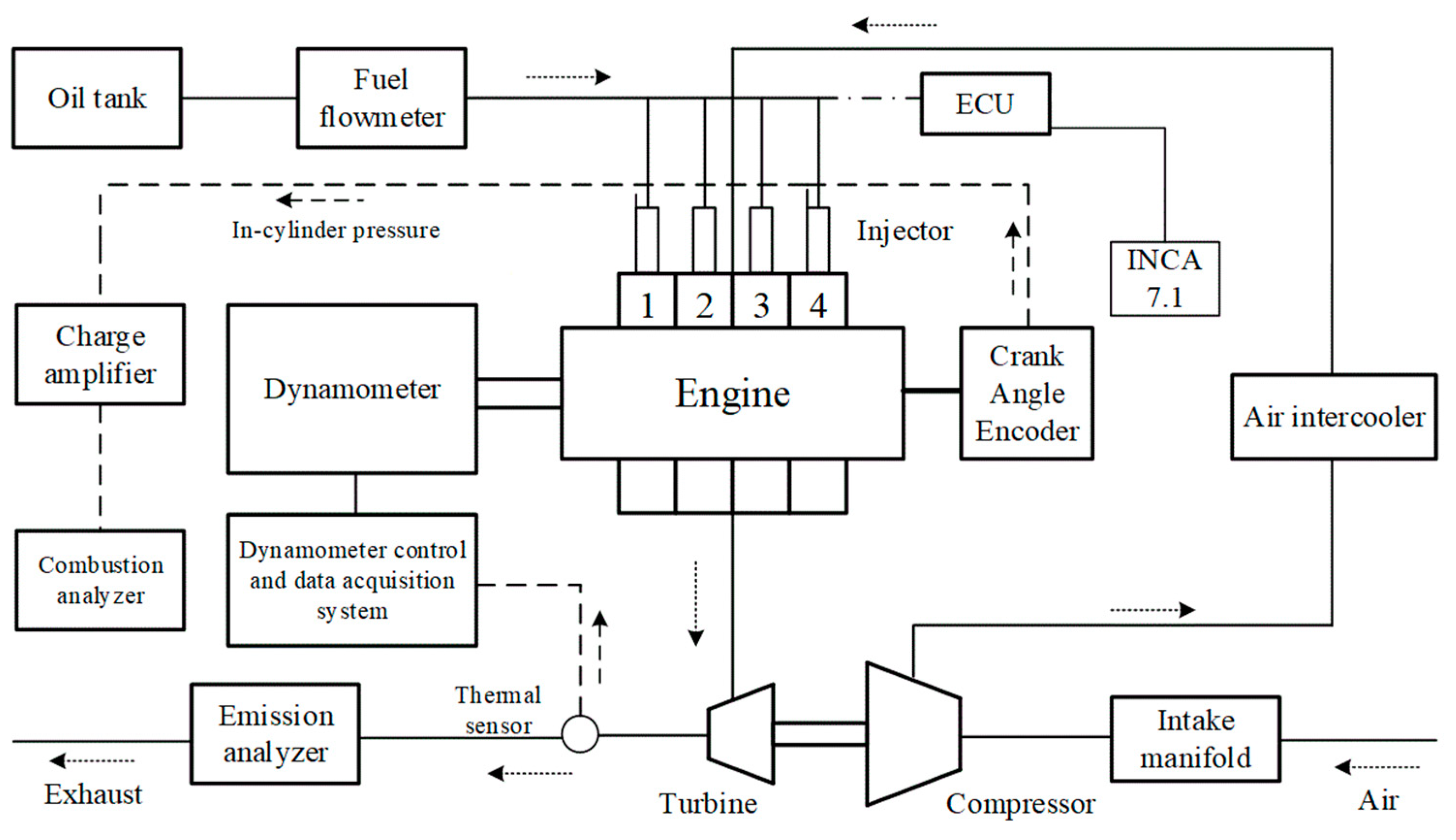
2. Experimental Parts
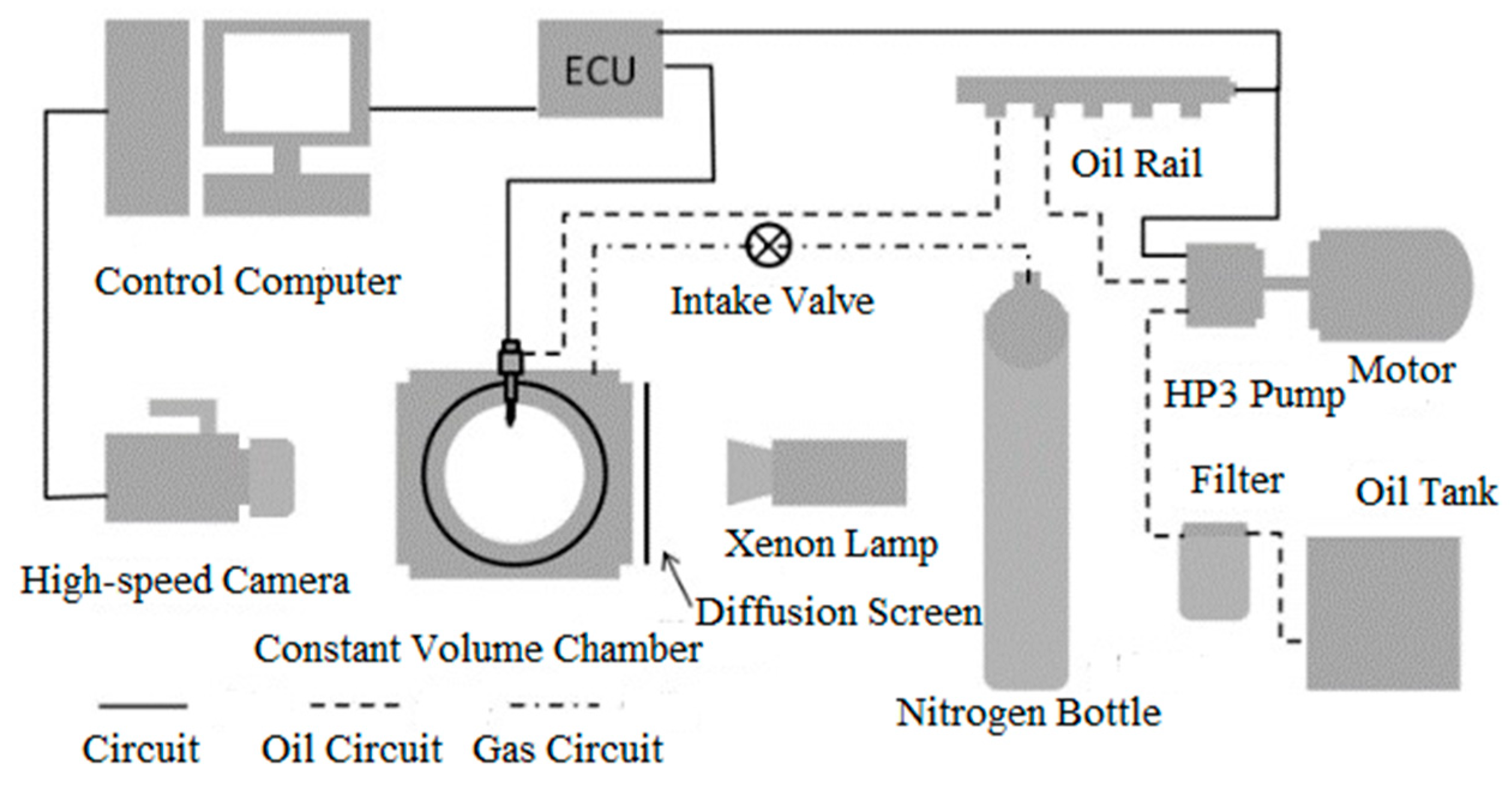
2.1. CVC Instrumentation
2.2. Test Fuel and Experiment Method
3. Results and Discussion
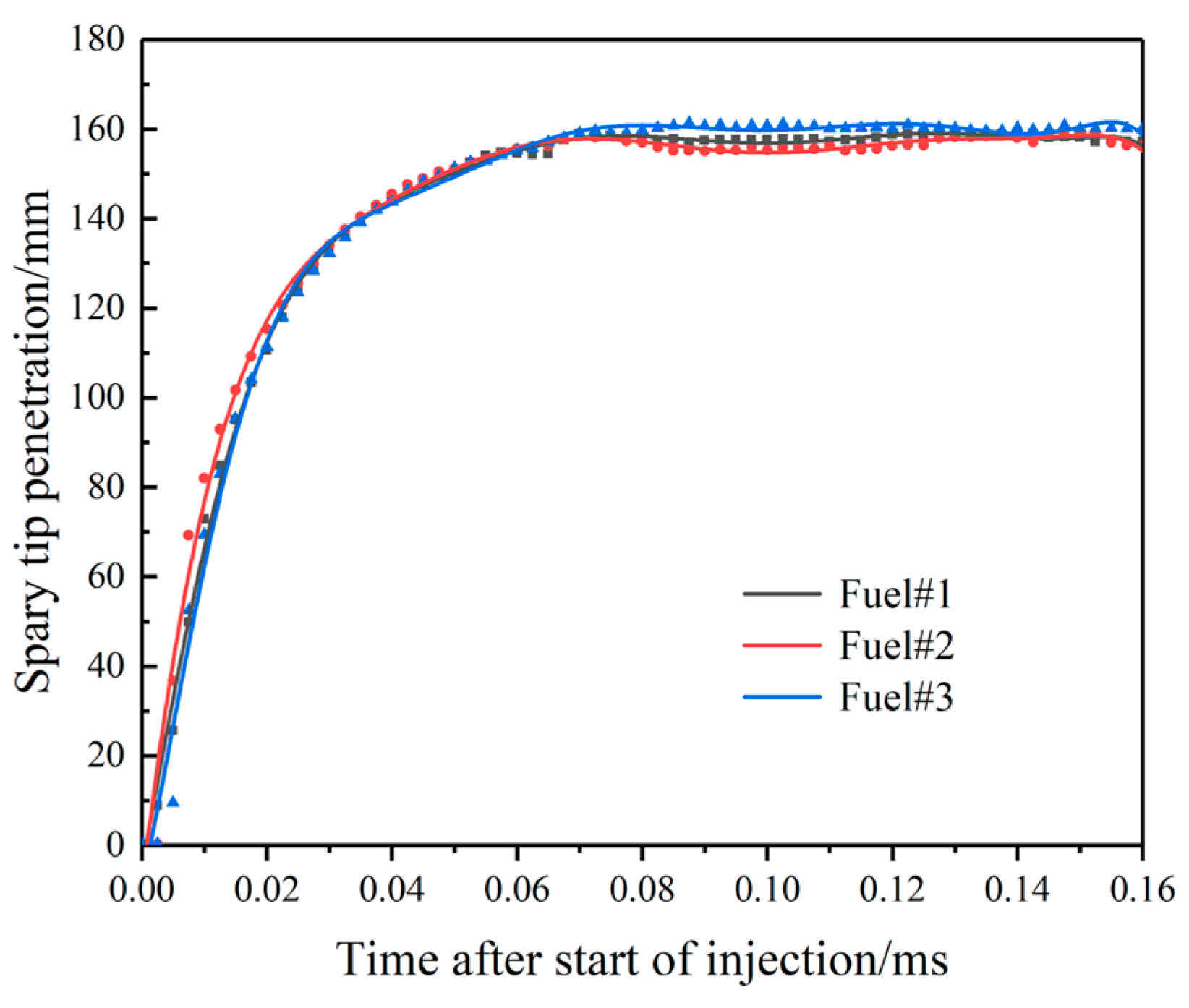
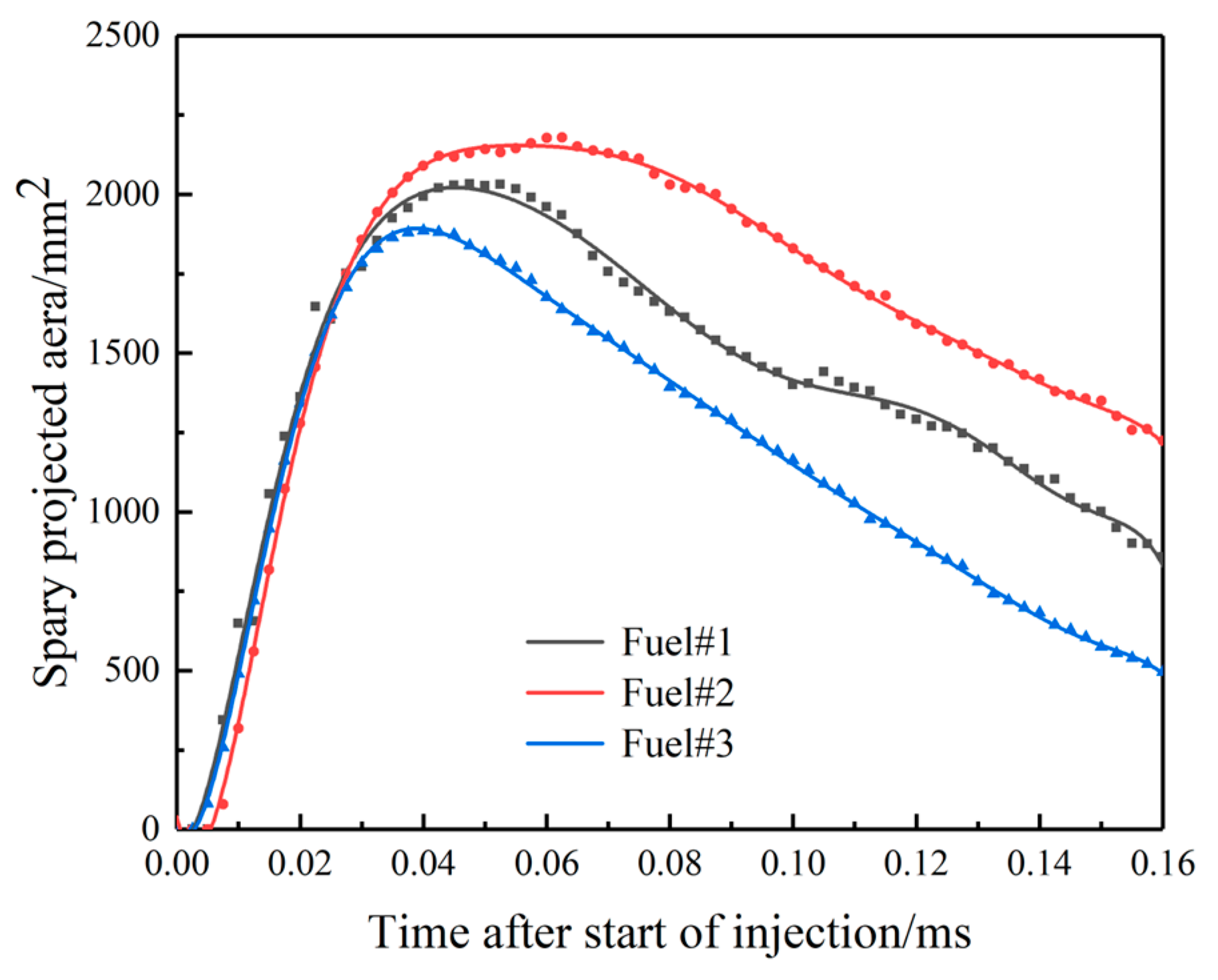
3.1. Effect of Fuel Volatility on Spray Characteristics
3.1.1. Effect of Injection Pressure on Fuel Spray Characteristics
3.1.2. Effect of Ambient Pressure on Spray Characteristics
3.2. Effect of Fuel Volatility on Combustion and Emissions
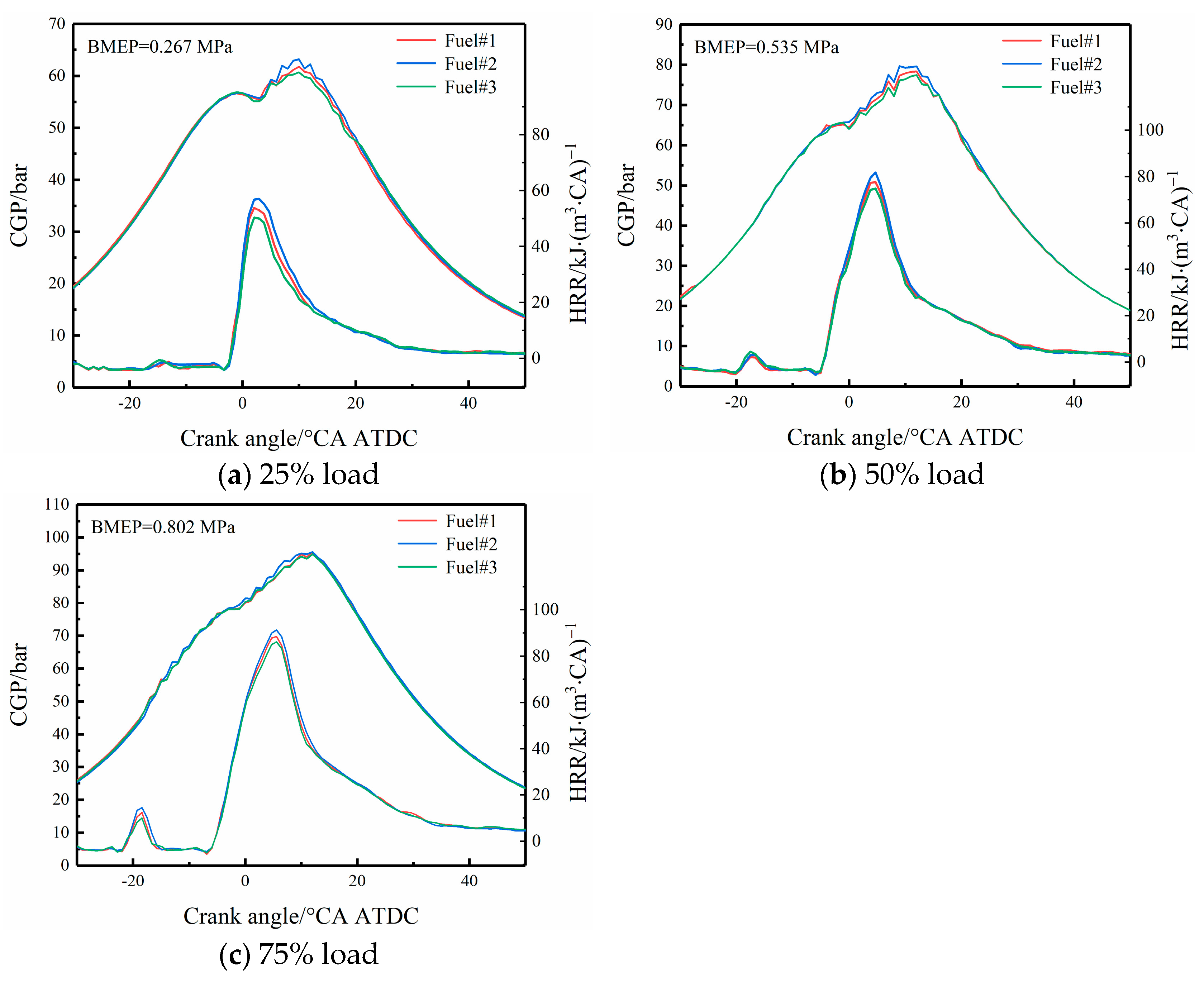
3.2.1. CGP and HRR
3.2.2. ID and CD
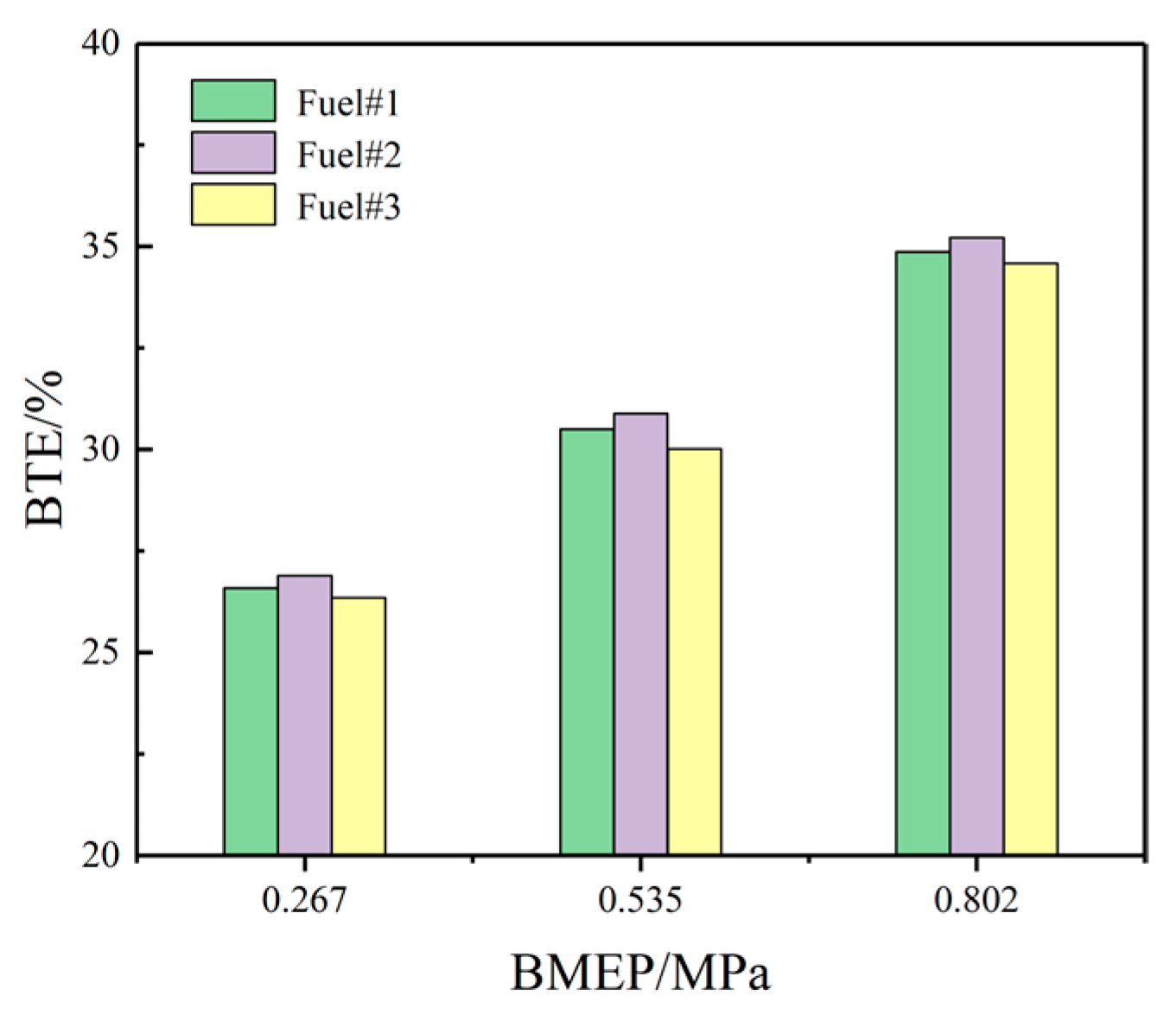
3.2.3. BTE
3.2.4. HC and CO Emission
3.2.5. NOx Emission
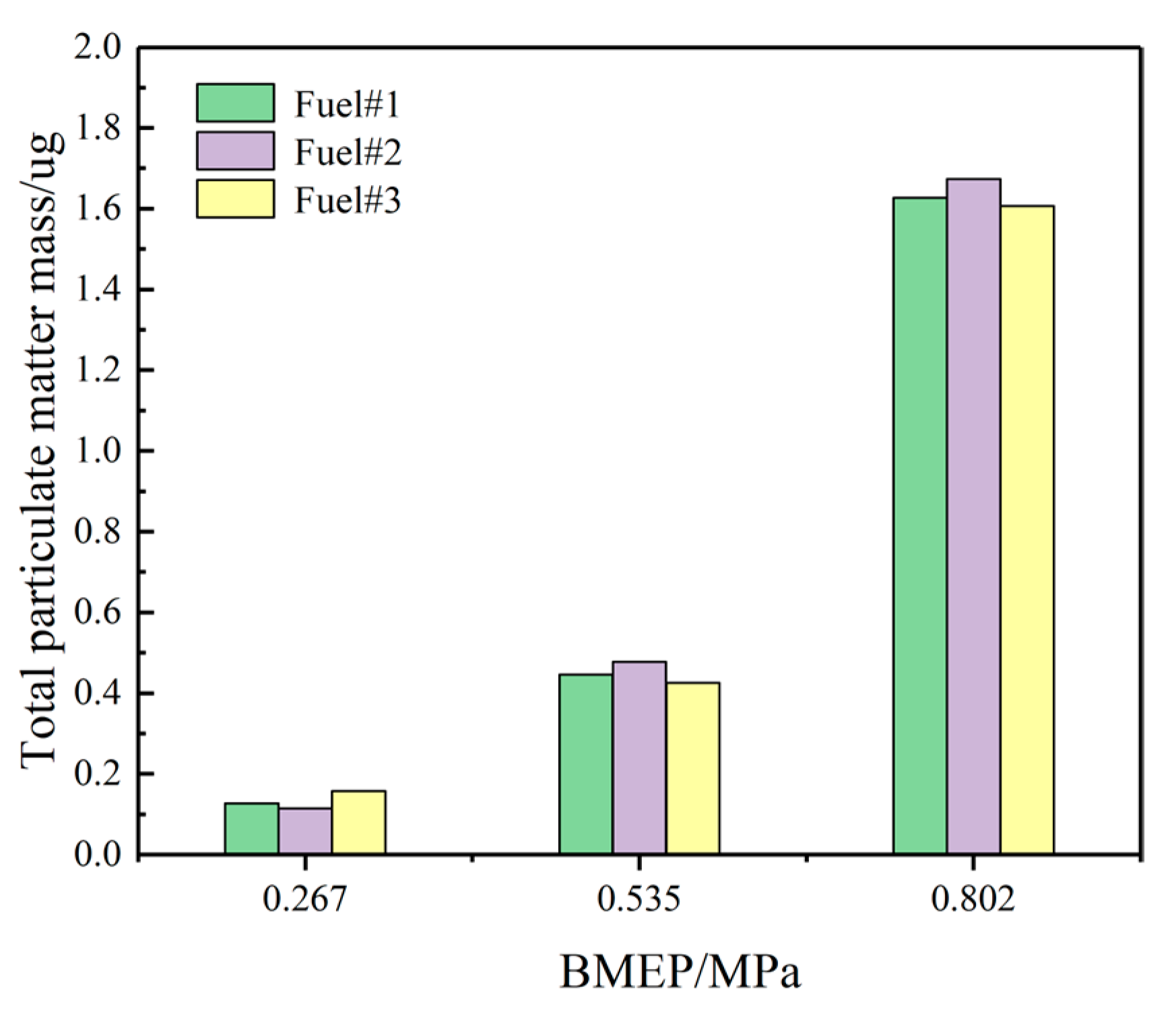
3.2.6. PMs Emissions
4. Conclusions
- (1)
- For fuel spraying, increasing rail pressure and decreasing ambient back pressure can increase the spray tip penetration and spray projected area. Specifically, as rail pressure increases from 80 MPa to 120 MPa and ambient pressure decreases from 10 MPa to 5 MPa, spray nozzle penetration spray increases by approximately 1–5%, and the projected area increases by 15–25%. At the same time, rail pressure has little effect on the spray cone angle, while increasing ambient back pressure can increase the spray cone angle. Although the volatile fuel reduces the spray tip penetration, the larger spray cone angle makes the total spray area larger, which is conducive to improving the degree of oil and gas mixing.
- (2)
- Improved fuel volatility effectively reduces CO emissions by about 8–10% and HC emissions by about 13–16%, but it increases NOx emissions by about 8–11%. Analyzing from the perspective of PM, it is necessary to combine the aromatic content of volatile fuels. Under low load conditions, it is recommended to use fuels with moderate volatility and aromatic content, and at medium and high loads, the volatility of the fuel has less weight on particulates and more weight on aromatics, so at medium and high loads, it is recommended to use fuels with less volatility and lower aromatic content.
- (3)
- With the increase in load, the peak CGP and HRR increased by 46.15% and 57.89%, respectively. The effect of volatility on cylinder pressure and combustion exothermic onset decreases. Redefine the ID and CD for different diesel volatile fuels with and without pre-injection heat release under different load conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Xu, H.; Jiang, C.; Wyszynski, M.L. Experimental study on microscopic and macroscopic characteristics of diesel spray with split injection. Fuel 2016, 174, 140–152. [Google Scholar] [CrossRef]
- Kook, S.; Pickett, L.M. Effect of fuel volatility and ignition quality on combustion and soot formation at fixed premixing conditions. SAE Int. J. Engines 2010, 2, 11–23. [Google Scholar] [CrossRef]
- Kitano, K.; Nishiumi, R.; Tsukasaki, Y.; Tanaka, T.; Morinaga, M. Effects of fuel properties on premixed charge compression ignition combustion in a direct injection diesel engine. SAE Tech. Pap. 2003. [Google Scholar] [CrossRef]
- Sluder, C.S.; Wagner, R.M.; Lewis, S.A.; Storey, J.M. Fuel property effects on emissions from high efficiency clean combustion in a diesel engine. SAE Tech. Pap. 2006. [Google Scholar] [CrossRef]
- Hutchison, B.R.M.; Wallace, J.S. Influence of fuel volatility on particulate matter emissions from a production DISI engine. Fuel 2021, 303, 121206. [Google Scholar] [CrossRef]
- Stanik, W. The effect of the cetane number improver on the ignition properties and combustion process of diesel fuel in a compression ignition engine. Naft.-Gaz-Sci. Technol. Oil Gas Ind. 2017, 73, 651–659. [Google Scholar]
- Ge, C.J.; Kim, Y.H.; Yoon, K.S.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, S.; Zhai, Y.; Liu, Q.; Wu, G.; Wu, H. Effect of Fuel Physicochemical Properties on Spray and Particulate Emissions. ACS Omega 2022, 7, 44251–44265. [Google Scholar] [CrossRef]
- Wu, H.; Xie, F.; Han, Y. Effect of cetane coupled with various engine conditions on diesel engine combustion and emission. Fuel 2022, 322, 124164. [Google Scholar] [CrossRef]
- Singh, A.P.; Agarwal, A.K. Experimental evaluation of sensitivity of low-temperature combustion to intake charge temperature and fuel properties. Int. J. Engine Res. 2018, 19, 732–757. [Google Scholar] [CrossRef]
- Xie, H.Z.; Song, L.B.; Xie, Y.Z.; Pi, D.; Shao, C.; Lin, Q. An Experimental Study on the Macroscopic Spray Characteristics of Biodiesel and Diesel in a Constant Volume Chamber. Energies 2015, 8, 5952–5972. [Google Scholar] [CrossRef]
- Chen, W.; Shuai, S.; Wang, J. Effect of the cetane number on the combustion and emissions of diesel engines by chemical kinetics modeling. Energy Fuels 2010, 24, 856–862. [Google Scholar] [CrossRef]
- Camille, H.; Chetankumar, P.; Lam, T.N.; Nilaphai, O.; Mounaïm-Rousselle, C. Effect of ABE and butanol blends with n-dodecane in different volume ratios on diesel combustion and soot characteristics in ECN spray a conditions. Fuel 2023, 345, 128099. [Google Scholar]
- Liu, S.; Zhu, Z.; Zhang, Z.; Gao, G.; Wei, Y. Effect of a cetane number (CN) improver on combustion and emission characteristics of a compression-ignition (CI) engine fueled with an ethanol–diesel blend. Energy Fuels 2010, 24, 2449–2454. [Google Scholar] [CrossRef]
- Han, Y.; Hu, S.; Sun, Y.; Sun, X.; Tan, M.; Xu, Y.; Tian, J.; Li, R.; Shao, Z. Compositional effect of gasoline on fuel economy and emissions. Energy Fuels 2018, 32, 5072–5080. [Google Scholar] [CrossRef]
- Wei, J.; Yin, Z.; Wang, C.; Lv, G.; Zhuang, Y.; Li, X.; Wu, H. Impact of aluminum oxide nanoparticles as an additive in diesel-methanol blends on a modern DI diesel engine. Appl. Therm. Eng. 2021, 185, 116372. [Google Scholar] [CrossRef]
- Wu, H.; Xie, F.; Han, Y.; Zhang, Q.; Li, Y. Effect of cetane coupled injection parameters on diesel engine combustion and emissions. Fuel 2022, 319, 123714. [Google Scholar] [CrossRef]
- Groendyk, M.; Rothamer, D. Effect of increased fuel volatility on CDC operation in a light-duty CIDI engine. Fuel 2017, 194, 195–210. [Google Scholar] [CrossRef]
- Burger, J.L.; Lovestead, T.M.; Gough, R.V.; Bruno, T.J. Characterization of the effects of cetane number improvers on diesel fuel volatility by use of the advanced distillation curve method. Energy Fuels 2014, 28, 2437–2445. [Google Scholar] [CrossRef]
- Bao, J.; Wang, H.; Wang, R.; Wang, Q.; Di, L.; Shi, C. Comparative experimental study on macroscopic spray characteristics of various oxygenated diesel fuels. Energy Sci. Eng. 2023, 11, 1579–1588. [Google Scholar] [CrossRef]
- Sekimoto, K.; Koss, A.R.; Gilman, J.B.; Selimovic, V.; Coggon, M.M.; Zarzana, K.J.; Yuan, B.; Lerner, B.M.; Brown, S.S.; Warneke, C.; et al. High-and low-temperature pyrolysis profiles describe volatile organic compound emissions from western US wildfire fuels. Atmos. Chem. Phys. 2018, 18, 9263–9281. [Google Scholar] [CrossRef]
- Chen, P.C.; Wang, W.C.; Roberts, W.L.; Fang, T. Spray and atomization of diesel fuel and its alternatives from a single-hole injector using a common rail fuel injection system. Fuel 2013, 103, 850–861. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Q.; Wang, Q.; Zhou, C.; Mo, C.; Wang, X. Experimental investigation of particle emissions under different EGR ratios on a diesel engine fueled by blends of diesel/gasoline/n-butanol. Energy Convers. Manag. 2016, 121, 212–223. [Google Scholar] [CrossRef]
- Teoh, Y.H.; Huspi, H.A.; How, H.G.; Sher, F.; Din, Z.U.; Le, T.D.; Nguyen, H.T. Effect of Intake Air Temperature and Premixed Ratio on Combustion and Exhaust Emissions in a Partial HCCI-DI Diesel Engine. Sustainability 2021, 13, 8593. [Google Scholar] [CrossRef]
- Loo, D.L.; Teoh, Y.H.; How, H.G.; Teh, J.S.; Andrei, L.C.; Starčević, S.; Sher, F. Applications Characteristics of Different Biodiesel Blends in Modern Vehicles Engines: A Review. Sustainability 2021, 13, 9677. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.S.; Huang, Z. Effect of n-pentanol addition on the combustion, performance and emission characteristics of a direct-injection diesel engine. Energy 2014, 70, 172–180. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, H.; Zhu, Z.; Lv, D.; Pan, Y.; Wei, H.; Zhuang, J. Effect of intake oxygen concentration on diesel–n-butanol blending combustion: An experimental and numerical study at low engine load. Energy Convers. Manag. 2018, 165, 53–65. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, J.E.; Sohn, Y.S. Demand Forecasting for Heavy-Duty Diesel Engines Considering Emission Regulations. Sustainability 2017, 9, 166. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Teoh, Y.H.; How, H.G.; Sher, F.; Le, T.D.; Nguyen, H.T.; Yaqoob, H. Fuel Injection Responses and Particulate Emissions of a CRDI Engine Fueled with Cocos nucifera Biodiesel. Sustainability 2021, 13, 4930. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, J.Y.; Yoo, B.O.; Song, J.H. Effects of Engine Load and Ternary Mixture on Combustion and Emissions from a Diesel Engine Using Later Injection Timing. Sustainability 2023, 15, 1391. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Li, Z.; Tan, H. Formation and emission characteristics of PAHs during pyrolysis and combustion of coal and biomass. Fuel 2024, 378, 132935. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Ma, Y.; Wu, G.; Yang, Z.; Fu, Q. Simulation Study on the Combustion and Emissions of a Diesel Engine with Different Oxygenated Blended Fuels. Sustainability 2024, 16, 631. [Google Scholar] [CrossRef]













| Properties | #1 | #2 | #3 |
|---|---|---|---|
| LowCalorific value (MJ/kg) | 42.94 | 42.99 | 42.95 |
| Density (25 °C) (kg/m3) | 818.8 | 818.8 | 820.6 |
| KinematicViscosity (25 °C) (mm2/s) | 3.4 | 3.4 | 3.4 |
| Surface Tension (10−3 N/m) | 24.9 | 24.5 | 24.4 |
| 50% distillation temperature (°C) | 244.4 | 234.6 | 259.8 |
| 90% distillation temperature (°C) | 330.8 | 338.6 | 360.3 |
| 95% distillation temperature (°C) | 342.6 | 359.1 | 361.8 |
| Sulfur content (mg/kg) | 3.7 | 3.8 | 4.1 |
| Cyclic aromatic hydrocarbon content (%) | 15.4 | 21.2 | 14.8 |
| Alkane content (%) | 50.1 | 47.3 | 37.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.; Liang, J.; Li, G.; Shao, Z.; Jiang, Z.; Feng, J. Improvement of Engine Combustion and Emission Characteristics by Fuel Property Modulation. Sustainability 2024, 16, 10764. https://doi.org/10.3390/su162310764
Liang K, Liang J, Li G, Shao Z, Jiang Z, Feng J. Improvement of Engine Combustion and Emission Characteristics by Fuel Property Modulation. Sustainability. 2024; 16(23):10764. https://doi.org/10.3390/su162310764
Chicago/Turabian StyleLiang, Kaijie, Jinguang Liang, Guowei Li, Zhengri Shao, Zhipeng Jiang, and Jincheng Feng. 2024. "Improvement of Engine Combustion and Emission Characteristics by Fuel Property Modulation" Sustainability 16, no. 23: 10764. https://doi.org/10.3390/su162310764
APA StyleLiang, K., Liang, J., Li, G., Shao, Z., Jiang, Z., & Feng, J. (2024). Improvement of Engine Combustion and Emission Characteristics by Fuel Property Modulation. Sustainability, 16(23), 10764. https://doi.org/10.3390/su162310764







