Induction of Time-Dependent Tolerance through Thermopriming in Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental and Priming Conditions
2.2. Growth Parameters
2.3. Leaf Compound Analysis
2.4. Data Analysis
3. Results
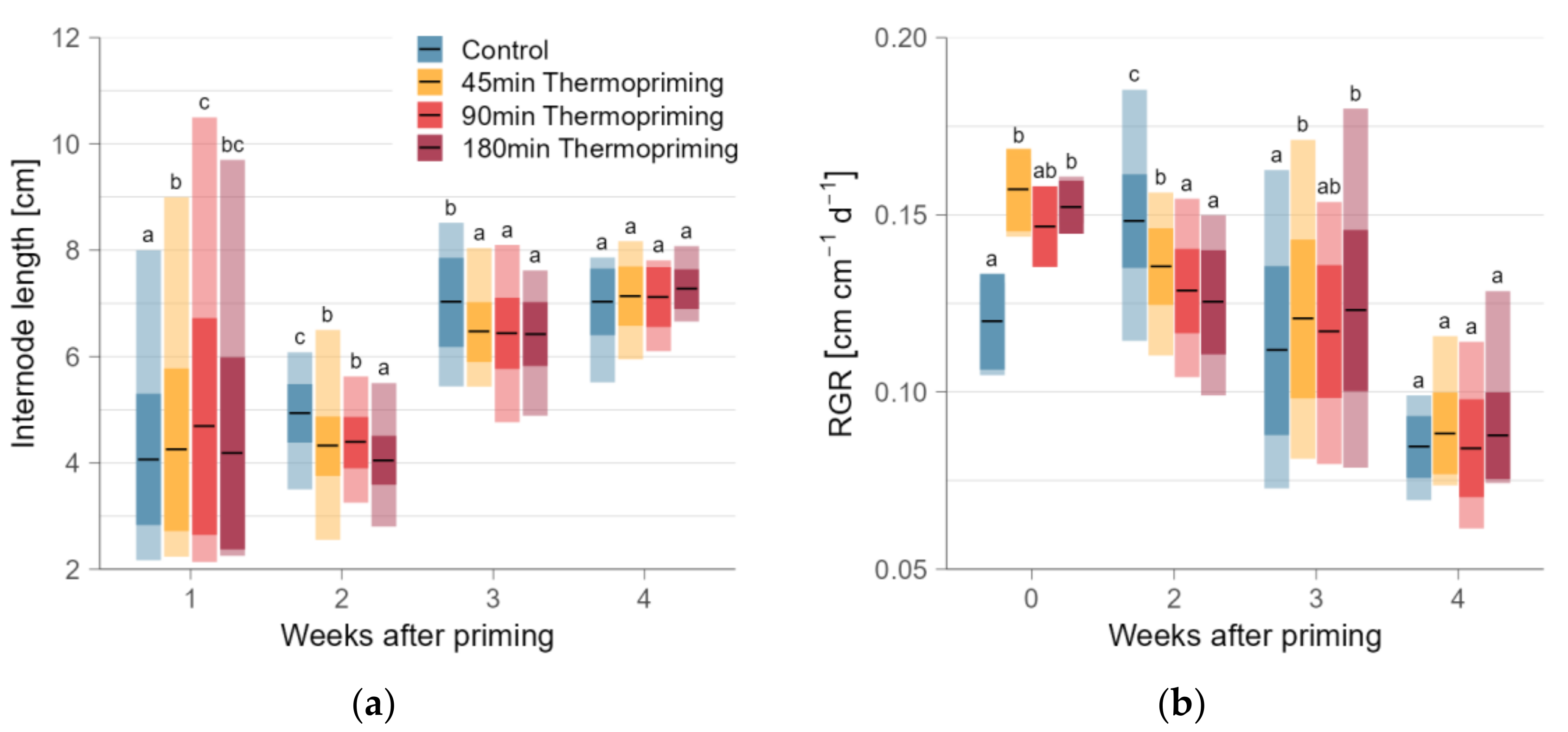
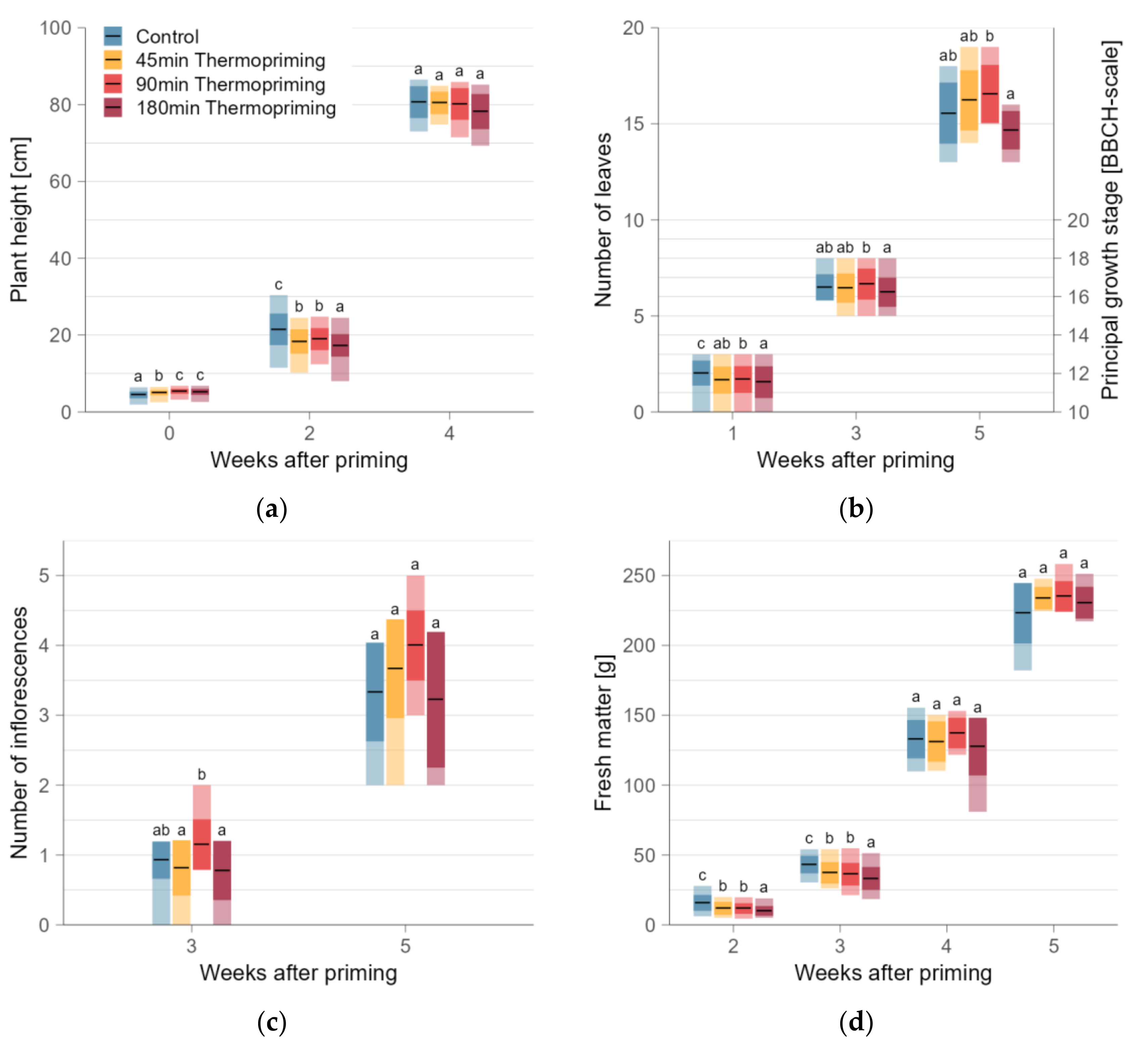
3.1. Plant Height after 90 min Thermopriming Most Similar to Control
3.2. Initially Delayed, but Later Accelerated Development by 90 min Thermopriming
3.3. Relative Growth Rates after 45 min Thermopriming Most Similar to Control
3.4. Decreased Biomass by Thermopriming—Most Pronounced after 180 min
3.5. Flavonols Increased after 90 min Thermopriming
3.6. Multiple Factor Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Priming Duration | WAS 1 | FM 2 | n | DM 3 | n | RGR 4 | n | Number of Inflorescences | n |
|---|---|---|---|---|---|---|---|---|---|
| [min] | [g] | [g] | [cm cm−1 d−1] | ||||||
| 0 | 5 | 33.2 ± 8.2 c | 39 | 2.1 ± 0.7 c | 30 | 0.12 ± 0.03 b | 183 | 0.8 ± 0.4 ab | 27 |
| 7 | 230.6 ± 11.4 a | 9 | 25.9 ± 1.9 a | 9 | 3.2 ± 1.0 a | 9 | |||
| 45 | 5 | 37.2 ± 7.7 b | 39 | 2.5 ± 0.8 b | 30 | 0.12 ± 0.03 b | 183 | 0.8 ± 0.4 a | 27 |
| 7 | 233.9 ± 8.0 a | 9 | 25.3 ± 2.0 a | 9 | 3.7 ± 0.7 a | 9 | |||
| 90 | 5 | 36.2 ± 8.1 b | 39 | 2.4 ± 0.7 b | 30 | 0.12 ± 0.03 a | 183 | 1.1 ± 0.4 b | 27 |
| 7 | 235.1 ± 10.9 a | 9 | 26.4 ± 2.8 a | 9 | 4.0 ± 0.5 a | 9 | |||
| 180 | 5 | 43.1 ± 6.3 a | 39 | 3.3 ± 1.1 a | 30 | 0.12 ± 0.03 a | 183 | 0.9 ± 0.3 a | 27 |
| 7 | 223.0 ± 21.6 a | 9 | 25.3 ± 2.8 a | 9 | 3.3 ± 0.7 a | 9 |
| Priming Duration | TCC 1 | TCarC 2 | TPC 3 | FCCatechin 4 | n | SLA 5 | n |
|---|---|---|---|---|---|---|---|
| [min] | [µg mg−1 DM−1] | [µg GAEs mg−1 DM−1] | [µg CEs mg−1 DM−1] | [cm g−1 DM−1] | |||
| 0 | 4.4 ± 0.6 b | 1.8 ± 0.4 b | 8.1 ± 1.6 c | 27.6 ± 9.6 b | 39 | 573.4 ± 170.6 a | 38 |
| 45 | 4.3 ± 0.5 ab | 1.8 ± 0.3 b | 7.4 ± 1.6 b | 28.5 ± 9.8 b | 39 | 574.0 ± 166.5 a | 38 |
| 90 | 4.4 ± 0.5 ab | 1.8 ± 0.4 b | 7.6 ± 1.4 bc | 27.6 ± 8.9 b | 39 | 574.9 ± 173.7 a | 39 |
| 180 | 4.3 ± 0.6 a | 1.6 ± 0.5 a | 6.8 ± 1.4 a | 23.8 ± 8.0 a | 39 | 644.5 ± 219.4 b | 39 |
References
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Park, B.-M.; Jeong, H.-B.; Yang, E.-Y.; Kim, M.-K.; Kim, J.-W.; Chae, W.; Lee, O.-J.; Kim, S.G.; Kim, S. Differential Responses of Cherry Tomatoes (Solanum lycopersicum) to Long-Term Heat Stress. Horticulturae 2023, 9, 343. [Google Scholar] [CrossRef]
- Zachariah, M.; Philip, S.; Pinto, I.; Vahlberg, M.; Singh, R.; Otto, F.; Barnes, C.; Kimutai, J. Extreme Heat in North America, Europe and China in July 2023 Made Much More Likely by Climate Change; Report; Grantham Institute for Climate Change, Imperial College London: London, UK, 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Hossain, M.A. Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier Science & Technology: San Diego, CA, USA, 2020; ISBN 978-0-12-817892-8. [Google Scholar]
- Lal, S.K.; Kumar, S.; Sheri, V.; Mehta, S.; Varakumar, P.; Ram, B.; Borphukan, B.; James, D.; Fartyal, D.; Reddy, M.K. Seed Priming: An Emerging Technology to Impart Abiotic Stress Tolerance in Crop Plants. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 41–50. ISBN 978-981-13-0031-8. [Google Scholar]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Turgut-Kara, N.; Arikan, B.; Celik, H. Epigenetic memory and priming in plants. Genetica 2020, 148, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Serano, N.L.G. Understanding the Molecular Basis of Thermopriming in Plants. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2019. [Google Scholar] [CrossRef]
- Röhlen-Schmittgen, S.; Körner, T.; Gierholz, R.; Hanten, S.; Roß, F.; Zinkernagel, J. Thermopriming in the early phase of tomato development leads to plant tolerance. Acta Hortic. 2023, 1372, 155–162. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat Priming During Early Reproductive Stages Enhances Thermo-Tolerance to Post-anthesis Heat Stress via Improving Photosynthesis and Plant Productivity in Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, L.; Gu, X.; Deng, X.; Qiu, Q.; Li, Q.; Zhang, Y.; Wang, M.; Deng, Y.; Wang, E.; et al. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019, 29, 379–390. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. Chromatin-Based Transcriptional Reprogramming in Plants under Abiotic Stresses. Plants 2022, 11, 1449. [Google Scholar] [CrossRef]
- Sanyal, R.P.; Misra, H.S.; Saini, A. Heat-stress priming and alternative splicing-linked memory. J. Exp. Bot. 2018, 69, 2431–2434. [Google Scholar] [CrossRef]
- Bäurle, I. Plant Heat Adaptation: Priming in response to heat stress. F1000Research 2016, 5, 2–3. [Google Scholar] [CrossRef]
- Charng, Y.-Y.; Mitra, S.; Yu, S.-J. Maintenance of abiotic stress memory in plants: Lessons learned from heat acclimation. Plant Cell 2023, 35, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, V.; Pandey, K.; Sopory, S.K.; Sanan-Mishra, N. Thermo-Priming Mediated Cellular Networks for Abiotic Stress Management in Plants. Front. Plant Sci. 2022, 13, 866409. [Google Scholar] [CrossRef] [PubMed]
- FAO. Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; FAO, Ed.; Food and Agricultural Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107649-1. [Google Scholar]
- United Nations. The Sustainable Development Goals Report 2023: Special Edition: Towards a Rescue Plan for People and Planet; United Nations Publications: New York, NY, USA, 2023; ISBN 978-92-1-101460-0. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; p. 129. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves; Edward Arnold: London, UK, 1982. [Google Scholar]
- Dörr, O.S.; Zimmermann, B.F.; Kögler, S.; Mibus, H. Influence of leaf temperature and blue light on the accumulation of rosmarinic acid and other phenolic compounds in Plectranthus scutellarioides (L.). Environ. Exp. Bot. 2019, 167, 103830. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- De Tayrac, M.; Lê, S.; Aubry, M.; Mosser, J.; Husson, F. Simultaneous analysis of distinct Omics data sets with integration of biological knowledge: Multiple Factor Analysis approach. BMC Genom. 2009, 10, 32. [Google Scholar] [CrossRef]
- Olas, J.J.; Apelt, F.; Annunziata, M.G.; John, S.; Richard, S.I.; Gupta, S.; Kragler, F.; Balazadeh, S.; Mueller-Roeber, B. Primary carbohydrate metabolism genes participate in heat-stress memory at the shoot apical meristem of Arabidopsis thaliana. Mol. Plant 2021, 14, 1508–1524. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A. Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 2013, 16, 661–666. [Google Scholar] [CrossRef]
- Javanmardi, J.; Rahemi, M.; Nasirzadeh, M. Responses of Tomato and Pepper Transplants to High-Temperature Conditioning. Int. J. Veg. Sci. 2014, 20, 374–391. [Google Scholar] [CrossRef]


| Experiments | |||
|---|---|---|---|
| Replicate: | 1 | 2 | 3 |
| Months: | June–August | August–September | September–October |
| Timing of thermopriming (week after sowing): | 2nd | 2nd | 2nd |
| Duration of experiment (weeks after thermopriming): | 5 | 3 | 3 |
| Duration of experiment (weeks after sowing): | 7 | 5 | 5 |
| Number of blocks: | 3 | 3 | 3 |
| Leaf parameters after thermopriming | |||
| Sample size per treatment, block, and week: | 3 | 5 | 5 |
| Total sample size per treatment and week: | 9 | 15 | 15 |
| Total sample size per treatment: | 45 | 45 | 45 |
| Growth parameters | |||
| Total sample size per treatment: | 45 | 45 | 45 |
| … decreasing after thermopriming each week by: | −9 | −15 | −15 |
| Priming Duration | Leaf Age | Developmental Stage | TCC 1 | TCarC 2 | TAC 3 | TPC 4 | FCCatechin 5 | n |
|---|---|---|---|---|---|---|---|---|
| [min] | [µg mg−1 DM−1] | [µg mg−1 DM−1] | [µg CyEs mg−1 DM−1] | [µg GAEs mg−1 DM−1] | [µg CEs mg−1 DM−1] | |||
| 0 | young | early 6 | 3.3 ± 1.1 a | 1.1 ± 0.4 b | 3.7 ± 1.1 a | 6.3 ± 2.3 a | 23.0 ± 6.3 a | 38–41 |
| late 7 | 4.2 ± 0.6 a | 1.8 ± 0.3 b | 4.3 ± 1.0 ab | 9.5 ± 2.8 b | 26.1 ± 8.0 a | 96 | ||
| mature | early | 3.6 ± 0.6 a | 1.0 ± 0.4 a | 4.2 ± 0.6 b | 4.6 ± 0.4 a | 22.0 ± 3.9 a | 30 | |
| late | 3.6 ± 0.7 a | 1.4 ± 0.5 c | 4.5 ± 0.8 b | 6.0 ± 1.4 b | 17.0 ± 4.9 a | 86 | ||
| 45 | young | early | 3.3 ± 0.8 a | 0.8 ± 0.8 a | 3.5 ± 0.9 a | 6.1 ± 1.8 a | 22.2 ± 6.5 a | 38–41 |
| late | 4.1 ± 0.6 a | 1.7 ± 0.3 ab | 4.3 ± 0.8 ab | 9.1 ± 2.5 ab | 26.4 ± 8.7 a | 96 | ||
| mature | early | 3.5 ± 0.5 a | 0.8 ± 0.6 a | 3.8 ± 0.6 ab | 4.7 ± 0.4 a | 22.2 ± 4.1 a | 29 | |
| late | 3.5 ± 0.5 a | 1.2 ± 0.6 b | 4.5 ± 0.7 ab | 5.7 ± 1.4 a | 17.0 ± 6.3 a | 87 | ||
| 90 | young | early | 3.4 ± 0.9 a | 0.8 ± 0.6 a | 3.4 ± 1.1 a | 6.1 ± 1.9 a | 22.4 ± 6.2 a | 37–40 |
| late | 4.1 ± 0.6 a | 1.7 ± 0.3 ab | 4.1 ± 0.8 a | 9.1 ± 2.6 ab | 25.6 ± 8.1 a | 96 | ||
| mature | early | 3.6 ± 0.4 a | 0.7 ± 0.8 a | 3.5 ± 0.7 a | 4.7 ± 0.5 a | 23.0 ± 2.8 a | 30 | |
| late | 3.6 ± 0.6 a | 1.2 ± 0.6 ab | 4.2 ± 0.6 a | 5.8 ± 1.6 ab | 16.4 ± 5.2 a | 87 | ||
| 180 | young | early | 3.3 ± 0.9 a | 0.6 ± 0.8 a | 3.6 ± 1.0 a | 6.2 ± 2.3 a | 22.3 ± 7.0 a | 37–40 |
| late | 4.1 ± 0.6 a | 1.6 ± 0.4 a | 4.3 ± 0.8 b | 8.7 ± 3.1 a | 25.2 ± 8.0 a | 96 | ||
| mature | early | 3.6 ± 0.5 a | 0.6 ± 0.9 a | 3.7 ± 0.7 a | 4.7 ± 0.7 a | 22.7 ± 5.1 a | 29 | |
| late | 3.6 ± 0.6 a | 1.0 ± 0.9 a | 4.3 ± 0.7 ab | 5.7 ± 1.4 ab | 17.5 ± 5.7 a | 86 |
| Priming Duration | Leaf Age | Developmental Stage | FCQuercetin 1 | n | Dualex Chlorophyll Index | Dualex Flavonol Index | n |
|---|---|---|---|---|---|---|---|
| [min] | [µg QEs mg−1 DM−1] | ||||||
| 0 | young | early 2 | 13.3 ± 2.5 ab | 38–41 | 28.06 ± 6.84 a | 0.50 ± 0.15 a | 315 |
| late 3 | 15.2 ± 2.5 a | 96 | 28.06 ± 6.84 b | 0.50 ± 0.15 b | 315 | ||
| mature | early | 13.9 ± 2.2 a | 30 | 25.78 ± 2.94 a | 0.40 ± 0.06 a | 234 | |
| late | 13.6 ± 2.4 a | 86 | 25.78 ± 2.94 a | 0.40 ± 0.06 b | 234 | ||
| 45 | young | early | 13.1 ± 1.9 ab | 38–41 | 28.26 ± 6.19 b | 0.50 ± 0.15 a | 315 |
| late | 15.1 ± 2.5 a | 96 | 28.26 ± 6.19 a | 0.50 ± 0.15 ab | 315 | ||
| mature | early | 13.5 ± 1.4 a | 29 | 27.47 ± 3.83 b | 0.40 ± 0.04 ab | 234 | |
| late | 13.5 ± 2.4 a | 87 | 27.47 ± 3.83 b | 0.40 ± 0.04 ab | 234 | ||
| 90 | young | early | 13.9 ± 2.8 b | 37–40 | 28.73 ± 6.11 c | 0.50 ± 0.15 a | 309 |
| late | 14.9 ± 2.5 a | 96 | 28.73 ± 6.11 a | 0.50 ± 0.15 a | 309 | ||
| mature | early | 14.0 ± 2.0 a | 30 | 27.73 ± 2.46 c | 0.40 ± 0.05 b | 229 | |
| late | 13.7 ± 2.4 a | 87 | 27.73 ± 2.46 b | 0.40 ± 0.05 ab | 229 | ||
| 180 | young | early | 12.8 ± 1.5 a | 37–40 | 28.13 ± 6.22 bc | 0.49 ± 0.15 a | 321 |
| late | 15.0 ± 2.5 a | 96 | 28.13 ± 6.22 a | 0.49 ± 0.15 a | 321 | ||
| mature | early | 13.3 ± 1.0 a | 29 | 27.89 ± 2.67 c | 0.39 ± 0.04 ab | 239 | |
| late | 13.6 ± 2.4 a | 86 | 27.89 ± 2.67 b | 0.39 ± 0.04 a | 239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körner, T.; Zinkernagel, J.; Röhlen-Schmittgen, S. Induction of Time-Dependent Tolerance through Thermopriming in Tomatoes. Sustainability 2024, 16, 1163. https://doi.org/10.3390/su16031163
Körner T, Zinkernagel J, Röhlen-Schmittgen S. Induction of Time-Dependent Tolerance through Thermopriming in Tomatoes. Sustainability. 2024; 16(3):1163. https://doi.org/10.3390/su16031163
Chicago/Turabian StyleKörner, Tobias, Jana Zinkernagel, and Simone Röhlen-Schmittgen. 2024. "Induction of Time-Dependent Tolerance through Thermopriming in Tomatoes" Sustainability 16, no. 3: 1163. https://doi.org/10.3390/su16031163





