Optimisation of Process Parameters to Maximise the Oil Yield from Pyrolysis of Mixed Waste Plastics
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Preparing the Feedstock for Pyrolysis
2.2. Experimental Set up and Pyrolysis of MWP
- (1)
- Gas
- (2)
- Char
- (3)
- Wax Analysis
3. Design of Experiments (DoE) Using RSM
4. Statistical Analysis of the Developed Model
5. Results and Discussion
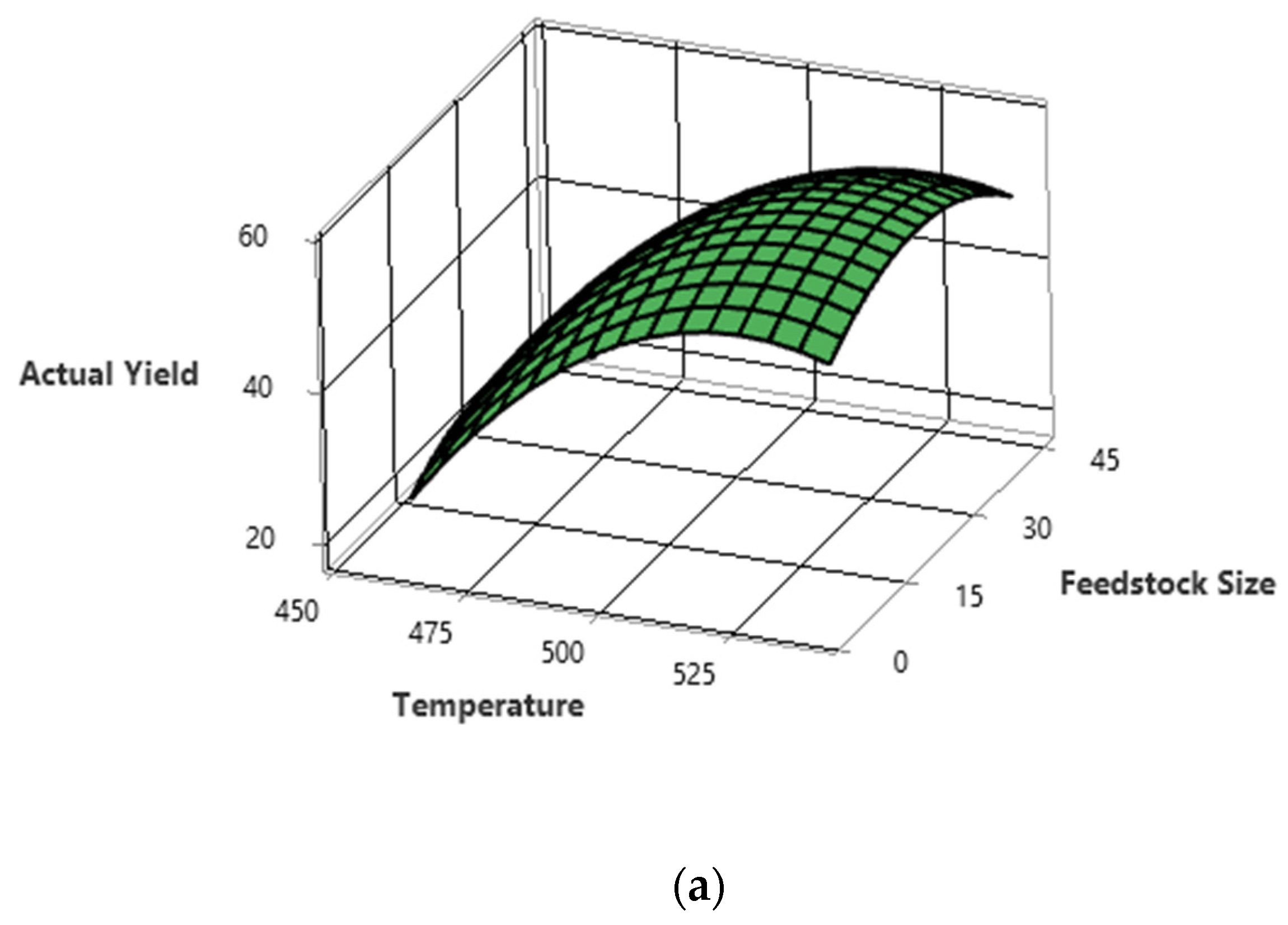
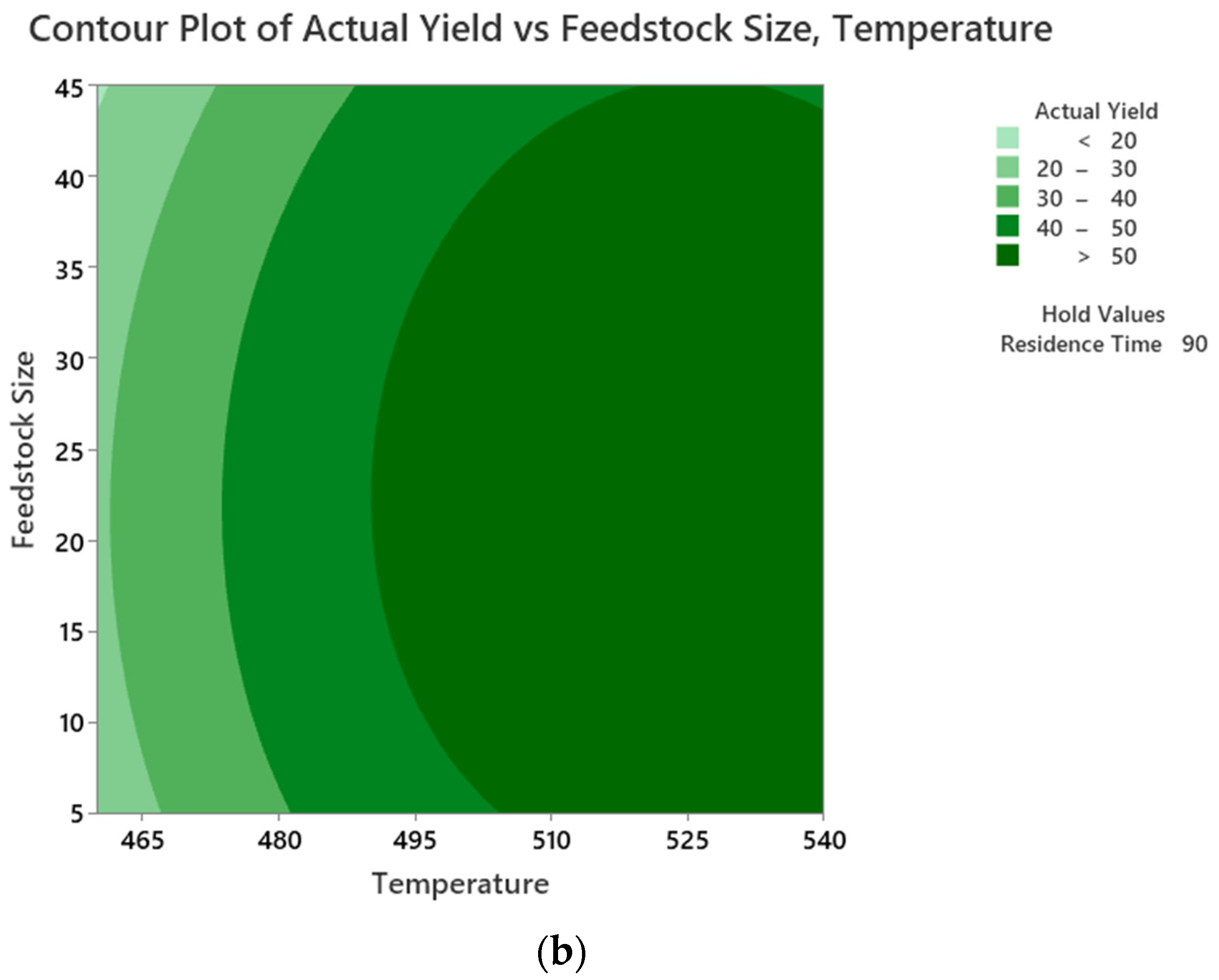
5.1. Optimisation of Liquid Oil Yield
−0.007139 A × A − 0.001775 B × B − 0.01759 D × D + 0.002608 A × B + 0.00111 A × D + 0.00011 B × D
5.2. Analysis of Variance (ANOVA)
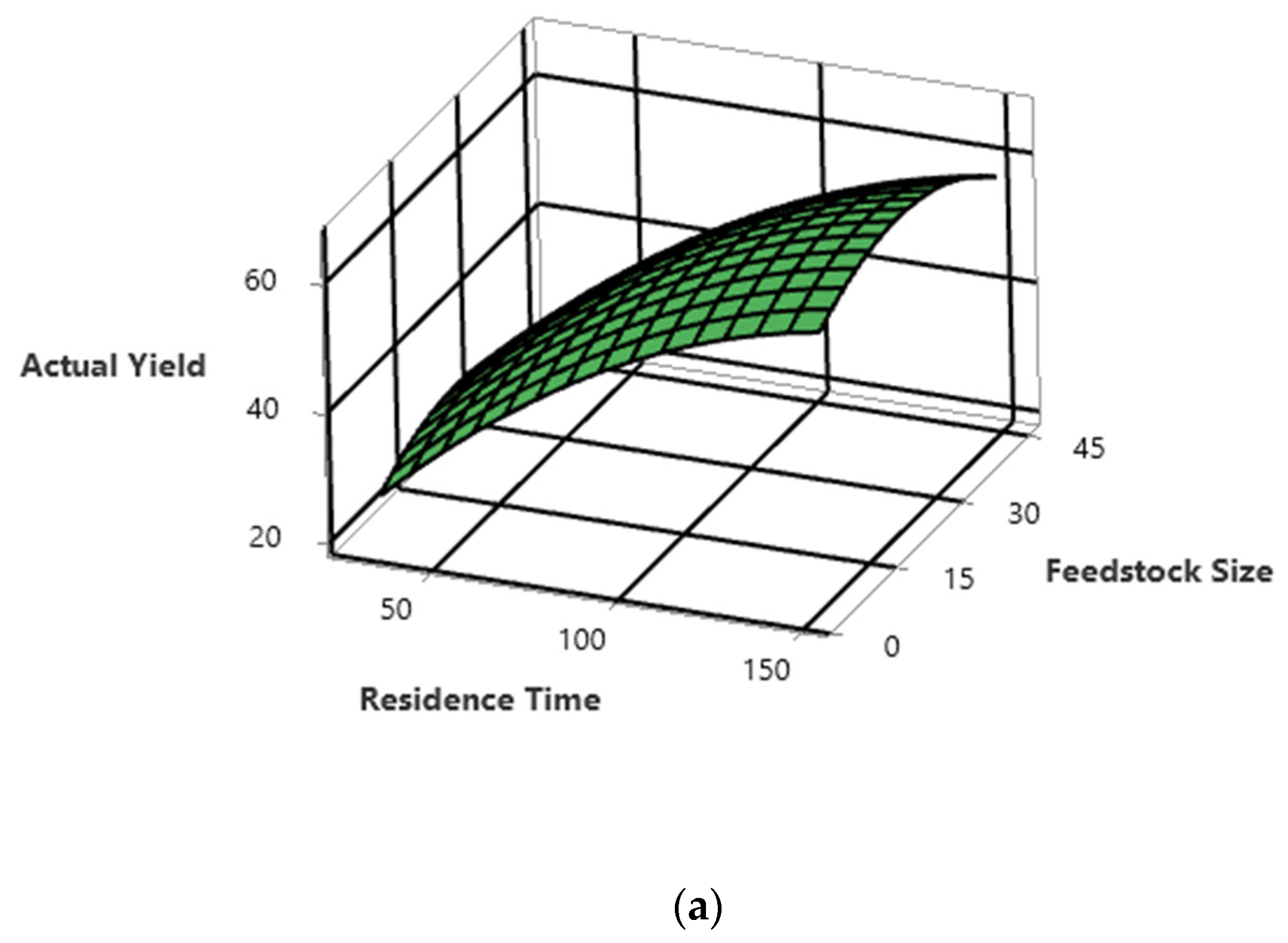
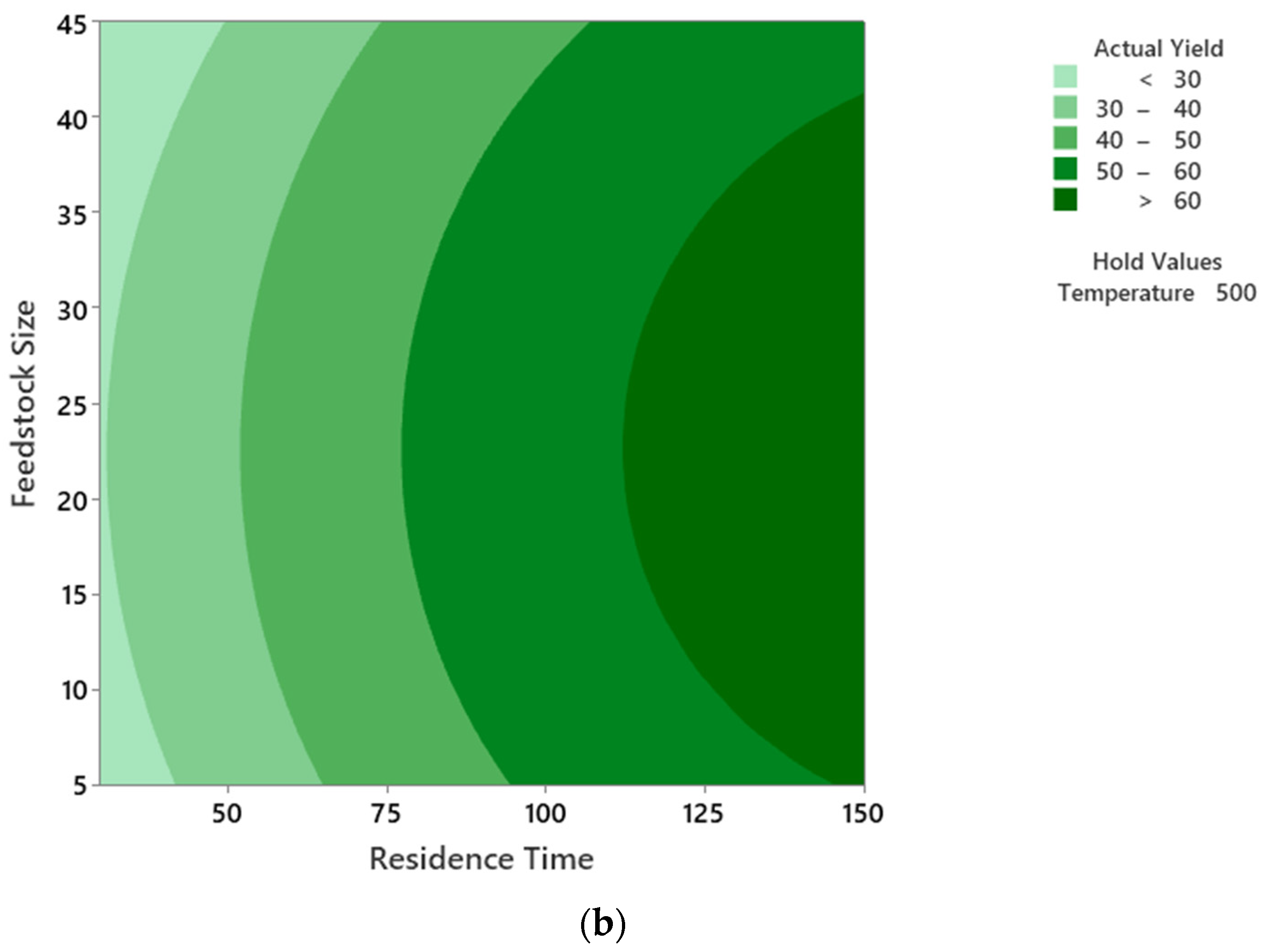
5.3. Interactions of the Process Parameters on PPO Yield
5.4. Characterisation of PPO under Optimum Conditions
5.5. FTIR Analysis of PPO
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nanda, S.; Berruti, F. Thermochemical conversion of plastic waste to fuels: A review. Environ. Chem. Lett. 2021, 19, 123–148. [Google Scholar] [CrossRef]
- Statistica. Global Plastic Production 1950–2020. 2021. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 25 January 2024).
- Zhang, K.; Shi, H.; Peng, J.; Wang, Y.; Xiong, X.; Wu, C.; Lam, P.K. Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018, 630, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Gourmelon, G. Global plastic production rises, recycling lags. Vital Signs 2015, 22, 91–95. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Statistica. Plastic Material Production Worldwide by Region 2019. 2019. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0111913 (accessed on 25 January 2024).
- Kumar, R.; Mishra, M.; Singh, S.; Kumar, A. Experimental evaluation of waste plastic oil and its blends on a single cylinder diesel engine. J. Mech. Sci. Technol. 2016, 30, 4781–4789. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M. GHG emission and carbon sequestration potential from MSW of Indian metro cities. Urban Clim. 2014, 8, 30–41. [Google Scholar] [CrossRef]
- Lee, K.-H.; Shin, D.-H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Selvaganapathy, T.; Muthuvelayudham, R.; Jayakumar, M. Process parameter optimization study on thermolytic polystyrene liquid fuel using response surface methodology (RSM). Mater. Today Proc. 2020, 26, 2729–2739. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Optimization of process parameters by response surface methodology (RSM) for catalytic pyrolysis of waste high-density polyethylene to liquid fuel. J. Environ. Chem. Eng. 2014, 2, 115–122. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Durodola, O.; Ogunjuyigbe, A.S.; Munda, J.L. Effects of operating factors on the bio-oil produced from pyrolysis of plastic wastes using response surface methodology. In Proceedings of the 2019 IEEE PES/IAS PowerAfrica, Abuja, Nigeria, 20–23 August 2019; pp. 527–532. [Google Scholar]
- Pinto, F.; Paradela, F.; Gulyurtlu, I.; Ramos, A.M. Prediction of liquid yields from the pyrolysis of waste mixtures using response surface methodology. Fuel Process. Technol. 2013, 116, 271–283. [Google Scholar] [CrossRef]
- Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I.; Nogueira, C.; Matos, A. Response surface methodology optimization applied to rubber tyre and plastic wastes thermal conversion. Fuel 2010, 89, 2217–2229. [Google Scholar] [CrossRef]
- Prabha, B.; Ramesh, D.; Sriramajayam, S.; Uma, D. Optimization of Pyrolysis Process Parameters for Fuel Oil Production from the Thermal Recycling of Waste Polypropylene Grocery Bags Using the Box–Behnken Design. Recycling 2024, 9, 15. [Google Scholar] [CrossRef]
- Dutta, N.; Mondal, P.; Gupta, A. Optimization of process parameters using response surface methodology for maximum liquid yield during thermal pyrolysis of blend of virgin and waste high-density polyethylene. J. Mater. Cycles Waste Manag. 2022, 24, 1182–1193. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Koh, W.; Brown, R.J.; Senadeera, W.; O’Hara, I.; Moghaddam, L. Biodiesel production from non-edible beauty leaf (Calophyllum inophyllum) oil: Process optimization using response surface methodology (RSM). Energies 2014, 7, 5317–5331. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Wu, S.; Kong, X.; Fang, Y.; Liu, J. Optimizing the conditions for the microwave-assisted pyrolysis of cotton stalk for bio-oil production using response surface methodology. Waste Biomass Valorization 2017, 8, 1361–1369. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, P.; Mondal, P. Biofuels production from pine needles via pyrolysis: Process parameters modeling and optimization through combined RSM and ANN based approach. Fuel 2022, 310, 122230. [Google Scholar] [CrossRef]
- Vasile, C.; Brebu, M.; Sakata, Y.; Pakdel, H.; Roy, C.; Miranda, R. Solid waste treatment by pyrolysis methods. J. Environ. Prot. Ecol. 2002, 3, 230e235. [Google Scholar]
- Thorat, P.; Warulkara, S.; Sathonea, H. Pyrolysis of waste plastic to produce Liquid Hydroocarbons. Adv. Polym. Sci. Technol. Int. J. 2013, 3, 14–18. [Google Scholar]
- Sembiring, F.; Purnomo, C.W.; Purwono, S. Catalytic Pyrolysis of Waste Plastic Mixture. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 012020. [Google Scholar]
- Lopez-Urionabarrenechea, A.; De Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- Singh, R.; Ruj, B.; Sadhukhan, A.; Gupta, P.; Tigga, V. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- Almohamadi, H.; Alamoudi, M.; Ahmed, U.; Shamsuddin, R.; Smith, K. Producing hydrocarbon fuel from the plastic waste: Techno-economic analysis. Korean J. Chem. Eng. 2021, 38, 2208–2216. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.; Nizami, A. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.; Chowdhury, A.A.; Schaller, D.; Jahirul, M. Uncovering the differences: A comparison of properties of crude plastic pyrolytic oil and distilled and hydrotreated plastic diesel produced from waste and virgin plastics as automobile fuels. Fuel 2023, 350, 128743. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, H.-y.; Xing, W.-l.; Song, L.-h.; Yang, L.; Yang, D.; Shu, X. Effects of various additives on the pyrolysis characteristics of municipal solid waste. Waste Manag. 2018, 78, 621–629. [Google Scholar] [CrossRef]
- Hasan, M.; Rasul, M.; Jahirul, M.; Khan, M. Fast pyrolysis of macadamia nutshell in an auger reactor: Process optimization using response surface methodology (RSM) and oil characterization. Fuel 2023, 333, 126490. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuels 1999, 13, 188–196. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef]
- López, A.; De Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A.; Torres, A. Pyrolysis of municipal plastic wastes II: Influence of raw material composition under catalytic conditions. Waste Manag. 2011, 31, 1973–1983. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Prabir, B. Biomass Gasification and Pyrolysis: Practical Design and Theory; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Fernández, Y.; Arenillas, A.; Menéndez, J.Á. Microwave Heating Applied to Pyrolysis; INTECH Open Access Publisher: London, UK, 2011. [Google Scholar]
- Joo, H.; Guin, J.A. Continuous upgrading of a plastics pyrolysis liquid to an environmentally favorable gasoline range product. Fuel Process. Technol. 1998, 57, 25–40. [Google Scholar] [CrossRef]
- Mani, M.; Subash, C.; Nagarajan, G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl. Therm. Eng. 2009, 29, 2738–2744. [Google Scholar] [CrossRef]
- Mani, M.; Nagarajan, G.; Sampath, S. Characterisation and effect of using waste plastic oil and diesel fuel blends in compression ignition engine. Energy 2011, 36, 212–219. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Convers. Manag. 2015, 105, 951–956. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Lee, K.-H. Effects of the types of zeolites on catalytic upgrading of pyrolysis wax oil. J. Anal. Appl. Pyrolysis 2012, 94, 209–214. [Google Scholar] [CrossRef]
- Miskolczi, N.; Ateş, F.; Borsodi, N. Comparison of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part II: Contaminants, char and pyrolysis oil properties. Bioresour. Technol. 2013, 144, 370–379. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Artetxe, M.; Amutio, M.; Elordi, G.; Bilbao, J. Steam activation of pyrolytic tyre char at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Williams, P.T. Yield and composition of gases and oils/waxes from the feedstock recycling of waste plastic. In Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; Wiley: Hoboken, NJ, USA, 2006; pp. 285–313. [Google Scholar]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar] [CrossRef]
- Heras, F.; Jimenez-Cordero, D.; Gilarranz, M.A.; Alonso-Morales, N.; Rodriguez, J. Activation of waste tire char by cyclic liquid-phase oxidation. Fuel Process. Technol. 2014, 127, 157–162. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawa, K. Fuel oil production from municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Procedia 2014, 47, 180–188. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Martín-Lara, M.; Calero, M.; Blázquez, G. Environmental impact of different scenarios for the pyrolysis of contaminated mixed plastic waste. Green Chem. 2024. advance article. [Google Scholar] [CrossRef]
- Hermanns, R.; Kraft, A.; Hartmann, P.; Meys, R. Comparative life cycle assessment of pyrolysis–recycling Germany’s sorted mixed plas-tic waste. Chem. Ing. Tech. 2023, 95, 1259–1267. [Google Scholar] [CrossRef]
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J. How to Apply Response Surface Methodology; ASQC: Milwaukee, WI, USA, 1990; Volume 8, p. 3. [Google Scholar]
- Wong, S.L.; Ngadi, N.; Amin, N.A.S.; Abdullah, T.A.T.; Inuwa, I.M. Pyrolysis of low density polyethylene waste in subcritical water optimized by response surface methodology. Environ. Technol. 2016, 37, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Azargohar, R.; Dalai, A. Production of activated carbon from Luscar char: Experimental and modeling studies. Microporous Mesoporous Mater. 2005, 85, 219–225. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.; Ferreira, H.S.; Matos, G.D.; David, J.; Brandão, G.; da Silva, E.P.; Portugal, L.; Dos Reis, P.; Souza, A. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.O. Designs of Experiments: Statistical Principles of Research Design and Analysis; Duxbury Press: New York, NY, USA, 2000. [Google Scholar]
- Cho, M.-H.; Jung, S.-H.; Kim, J.-S. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels 2010, 24, 1389–1395. [Google Scholar] [CrossRef]
- Moreira, R.; dos Reis Orsini, R.; Vaz, J.M.; Penteado, J.C.; Spinacé, E.V. Production of biochar, bio-oil and synthesis gas from cashew nut shell by slow pyrolysis. Waste Biomass Valorization 2017, 8, 217–224. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.-C.; Karonis, D. Alternative diesel from waste plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef]
- Khatha, W.; Ekarong, S.; Somkiat, M.; Jiraphon, S. Fuel Properties, Performance and Emission of Alternative Fuel from Pyrolysis of Waste Plastics. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 012001. [Google Scholar]
- Janyalertadun, A.; Santaweesuk, C.; Sanongraj, S. Fuel production, performance, and emission of a CI engine using waste plastics oil. World J. Eng. 2017, 14, 114–120. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A.; Dimitriadis, A. Catalyst evaluation for waste cooking oil hydroprocessing. Fuel 2012, 93, 638–641. [Google Scholar] [CrossRef]
- Belbessai, S.; Azara, A.; Abatzoglou, N. Recent Advances in the Decontamination and Upgrading of Waste Plastic Pyrolysis Products: An Overview. Processes 2022, 10, 733. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Makarem, M.; Rahimpour, M. Light olefins/bio-gasoline production from biomass. In Bioenergy Systems for the Future; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–148. [Google Scholar]
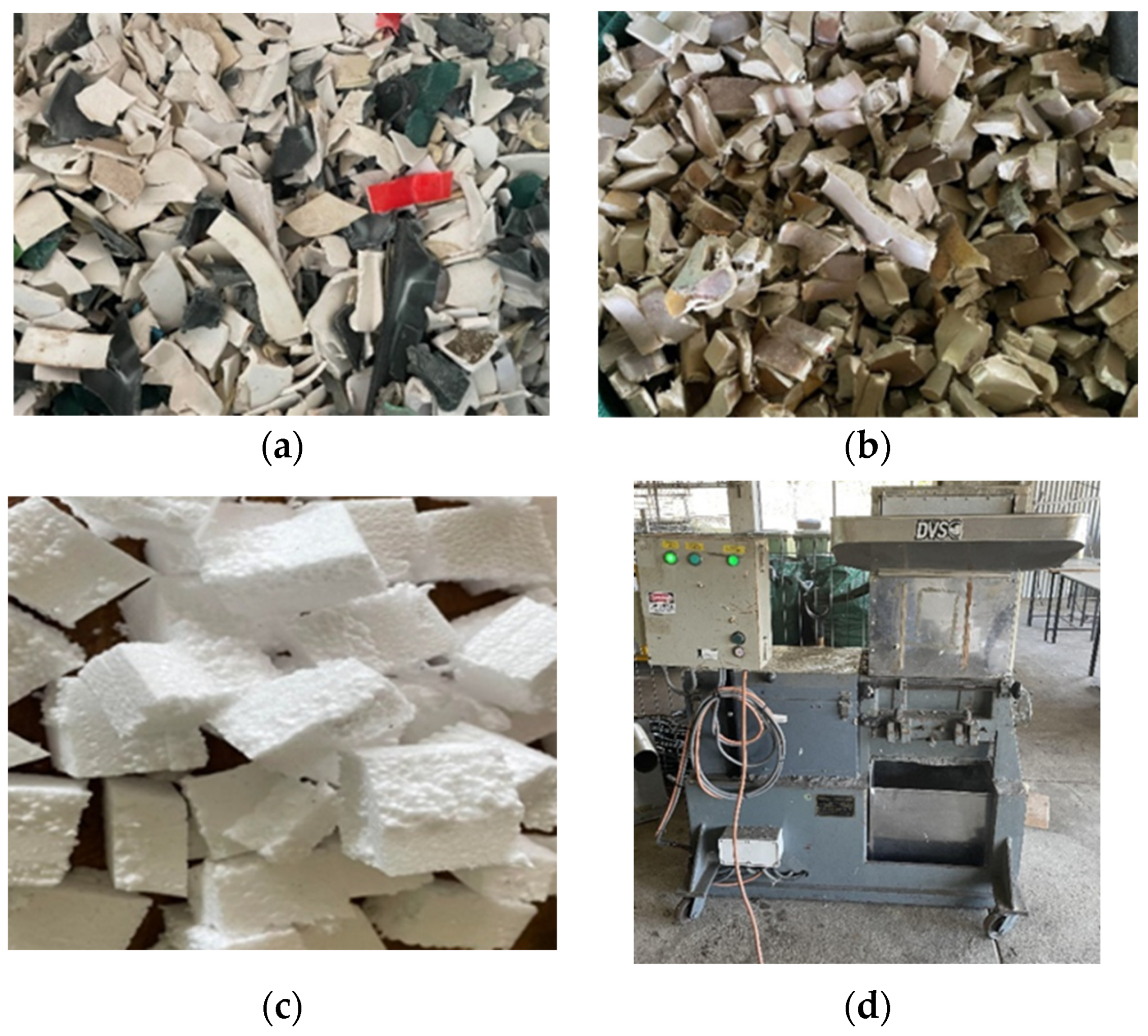





| Variables | Unit | Symbol Coded | Range and Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Temperature | °C | A | 460 | 500 | 540 |
| Residence time | min | B | 30 | 90 | 150 |
| Feedstock size | mm | D | 5 | 25 | 45 |
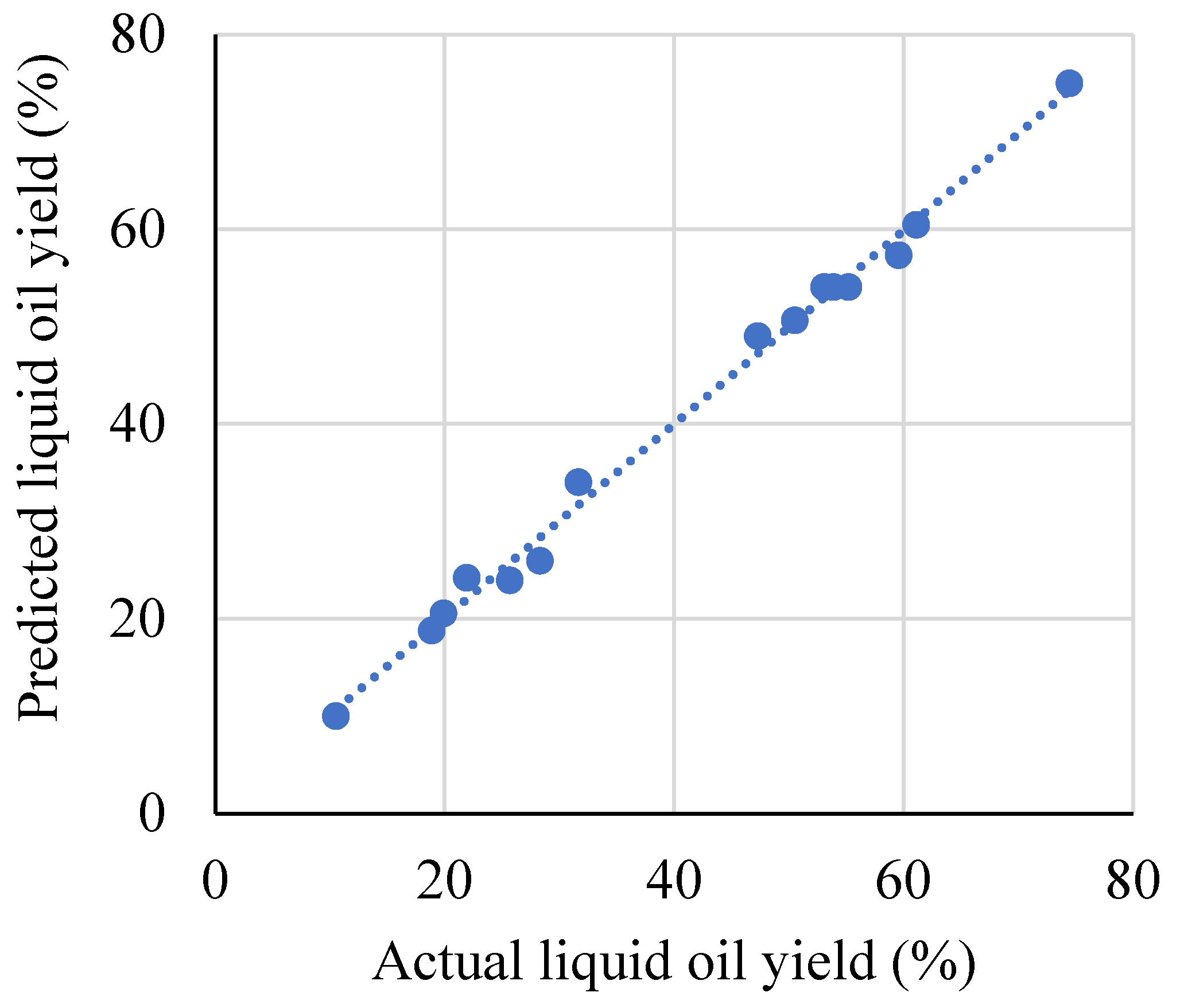
| Run | Temp. (°C) | Residence Time (min) | Feedstock Size (mm) | Liquid Oil Yield (%) | |
|---|---|---|---|---|---|
| Actual | Predicted | ||||
| 1 | 500 (0) | 150 (1) | 5 (−1) | 61.09 | 60.4563 |
| 2 | 500 (0) | 90 (0) | 25 (0) | 55.19 | 54.0533 |
| 3 | 460 (−1) | 150 (1) | 25 (0) | 31.65 | 34.0075 |
| 4 | 460 (−1) | 90 (0) | 5 (−1) | 25.67 | 23.9462 |
| 5 | 500 (0) | 30 (−1) | 45 (1) | 19.91 | 20.5438 |
| 6 | 540 (1) | 90 (0) | 5 (−1) | 50.53 | 50.6387 |
| 7 | 500 (0) | 90 (0) | 25 (0) | 53.90 | 54.0533 |
| 8 | 540 (1) | 90 (0) | 45 (1) | 47.30 | 49.0238 |
| 9 | 540 (1) | 150 (1) | 25 (0) | 74.47 | 74.9950 |
| 10 | 500 (0) | 30 (−1) | 5 (−1) | 21.94 | 24.1888 |
| 11 | 500 (0) | 150 (1) | 45 (1) | 59.57 | 57.3212 |
| 12 | 540 (1) | 30 (−1) | 25 (0) | 28.31 | 25.9525 |
| 13 | 460 (−1) | 30 (−1) | 25 (0) | 10.53 | 10.0050 |
| 14 | 460 (−1) | 90 (0) | 45 (1) | 18.89 | 18.7812 |
| 15 | 500 (0) | 90 (0) | 25 (0) | 53.07 | 54.0533 |
| Analysis | Property | Amount (wt%) |
|---|---|---|
| Ultimate | C | 79.9 |
| H | 12.6 | |
| S | 0.00 | |
| N | 0.00 | |
| O | 5.1 | |
| Chlorine HHV (MJ/kg) | 1.13 44.4 | |
| Proximate | Moisture content | 1.37 |
| Volatile matter | 92.90 | |
| Fixed carbon | 1.14 |
| Source | DF | Adj SS | Adj MS | F Value | p Value | Remark |
|---|---|---|---|---|---|---|
| Model | 9 | 5190.04 | 576.67 | 93.52 | 0.0000 | Significant |
| A | 1 | 1620.80 | 1620.80 | 262.84 | 0.0000 | Significant |
| B | 1 | 2667.79 | 2667.79 | 432.62 | 0.0000 | Significant |
| D | 1 | 22.98 | 22.98 | 3.73 | 0.1114 | Not significant |
| A2 | 1 | 481.68 | 481.68 | 78.11 | 0.0003 | Significant |
| B2 | 1 | 150.84 | 150.84 | 24.46 | 0.0043 | Significant |
| D2 | 1 | 182.69 | 182.69 | 29.63 | 0.0028 | Significant |
| A × B | 1 | 156.75 | 156.75 | 25.42 | 0.0040 | Significant |
| A × D | 1 | 3.15 | 3.15 | 0.51 | 0.5067 | Not significant |
| B × D | 1 | 0.07 | 0.07 | 0.01 | 0.9222 | Not significant |
| Lack-of-Fit | 3 | 28.55 | 9.52 | 8.34 | 0.1090 | Not significant |
| Pure Error | 2 | 2.28 | 1.14 | |||
| Total | 14 | 5220.88 |
| Parameters | Objective | Minimum Threshold | Maximum Boundary |
|---|---|---|---|
| Temperature (°C) | Within range | 460 | 550 |
| Residence time (min) | Within range | 30 | 150 |
| Feedstock particle size (mm) | Within range | 5 | 45 |
| Yield of PPO (%) | Maximise | 10.53 | 74.47 |
| Run | Temp. (°C) | Residence Time (min) | Particle Size (mm) | Exp. PPO Yield (%) | Predicted PPO Yield (%) | Error (%) |
|---|---|---|---|---|---|---|
| 1 | 536 | 150 | 24 | 74.77 | 75.14 | 0.49 |
| 2 | 536 | 150 | 24 | 75.92 | 75.14 | 1.03 |
| 3 | 536 | 150 | 24 | 75.98 | 75.14 | 1.11 |
| Avg. | 536 | 150 | 24 | 75.55 | 75.14 | 0.88 |
| Fuel Properties | Unit | Testing Methods | Equipment Used |
|---|---|---|---|
| Flash point | °C | D93B | Pensky Martins closed-cup apparatus (Koehler instrument company, INC., Bohemia, NY, USA) |
| Calorific value | kJ/kg | D4809 | Parr 6200 calorimeter (Parr Instrument Company, Chicago, IL, USA) |
| Density | kg/L | D4052 | DM40 Mettler Toledo density meter (METTLER TOLEDO, Columbus, OH, USA) |
| Cetane index (CI) | - | D4737 A | Ignition quality tester (CFR Engines, Ottawa, ON, Canada) |
| Lubricity | µm | D6079 | Ducom lubricity meter (Ducom instruments, New York, NY, USA) |
| Kinematic viscosity (KV) | cSt | D7042 | Stabinger viscometer SVM 3000 (Anton Paar, Gurugram, India) |
| Sulfur content | (mg/kg) | D5453 | XPLORER sulfur analyser (TE Instrument, Delft, The Netherlands) |
| Water content | wt% | D2709 | Centrifuge sigma (Thermo Fisher Scientific, Waltham, MA, USA) |
| Properties | ASTM Std. | EN Std. | Aus. Std. Diesel | Crude PPO | CPPO [63] | CPPO [64] | CPPO [65] |
|---|---|---|---|---|---|---|---|
| HHV (MJ/kg) | 42–46 | - | 44–46 | 44.15 | 43.58 | 37.72 | 45.24 |
| KV (mm2/s) | 1.9–4.1 | 2–4.5 | 2–4.5 | 1.318 | 2.3 | 1.66 | 3.11 |
| CI | 40 min | 46 min | 46 min | 32 | 66.7 | - | 46.7 |
| Density@15 °C (g/cm2) | - | 0.82–0.84 | 0.82–0.85 | 0.832 | 0.8027 | 0.899 | 0.823 |
| Flash point (°C) | 52 min | 55 min | 61.5 min. | <20 | 48 | 35 | 54 |
| Water content (wt. %) | 0.05 | 0.02 max | 0.02 max. | 0.125 | 0.051 | - | - |
| Sulfur content (mg/kg) | 15 max | 10 max | 10 max. | 5.12 | 42.9 | - | - |
| Lubricity (WSD, µm) | 520 max | 460 max | 460 max. | 220 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, F.; Rasul, M.G.; Chowdhury, A.A.; Jahirul, M.I. Optimisation of Process Parameters to Maximise the Oil Yield from Pyrolysis of Mixed Waste Plastics. Sustainability 2024, 16, 2619. https://doi.org/10.3390/su16072619
Faisal F, Rasul MG, Chowdhury AA, Jahirul MI. Optimisation of Process Parameters to Maximise the Oil Yield from Pyrolysis of Mixed Waste Plastics. Sustainability. 2024; 16(7):2619. https://doi.org/10.3390/su16072619
Chicago/Turabian StyleFaisal, Farjana, Mohammad Golam Rasul, Ashfaque Ahmed Chowdhury, and Md Islam Jahirul. 2024. "Optimisation of Process Parameters to Maximise the Oil Yield from Pyrolysis of Mixed Waste Plastics" Sustainability 16, no. 7: 2619. https://doi.org/10.3390/su16072619





