Catalytic Pyrolysis of Waste-Printed Circuit Boards Using a Cu/Fe Bimetal Synergistic Effect to Enhance Debromination
Abstract
1. Introduction
2. Experimental Section
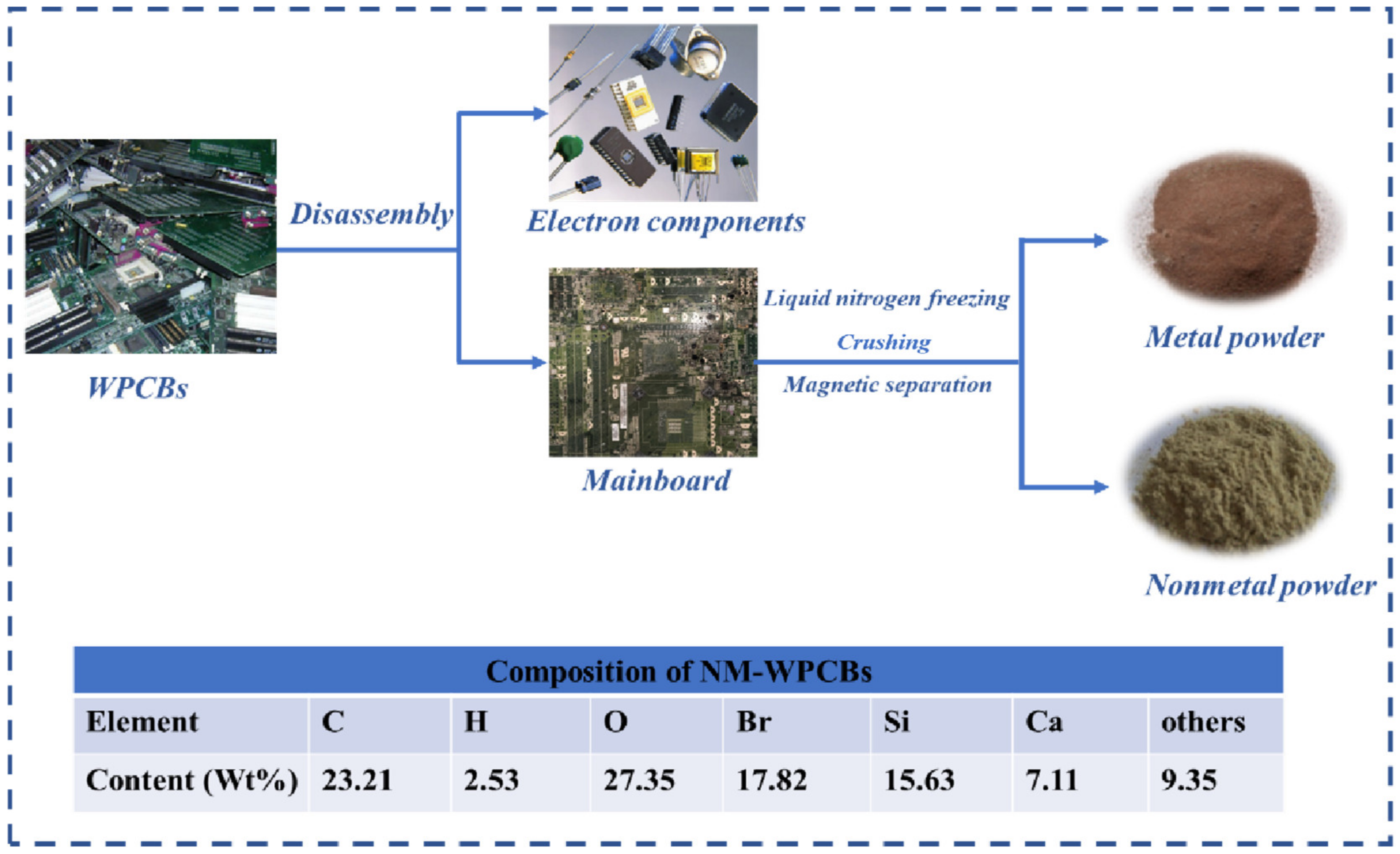
2.1. Materials
2.2. Preparation of Cu/Fe
2.3. In Situ Catalytic Pyrolysis Experiment
2.4. Characterization
3. Results and Discussion
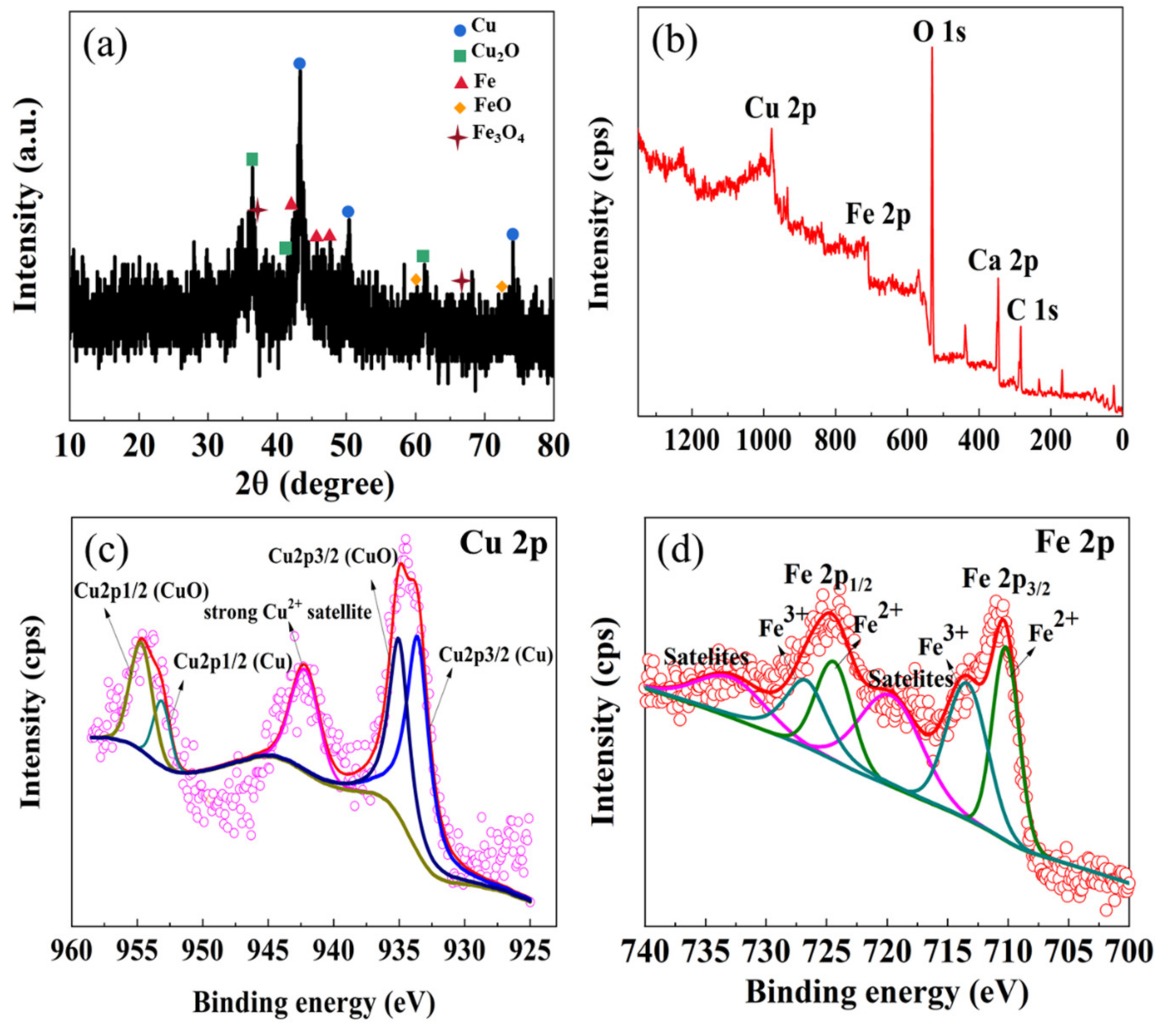
3.1. Characterization of Cu/Fe
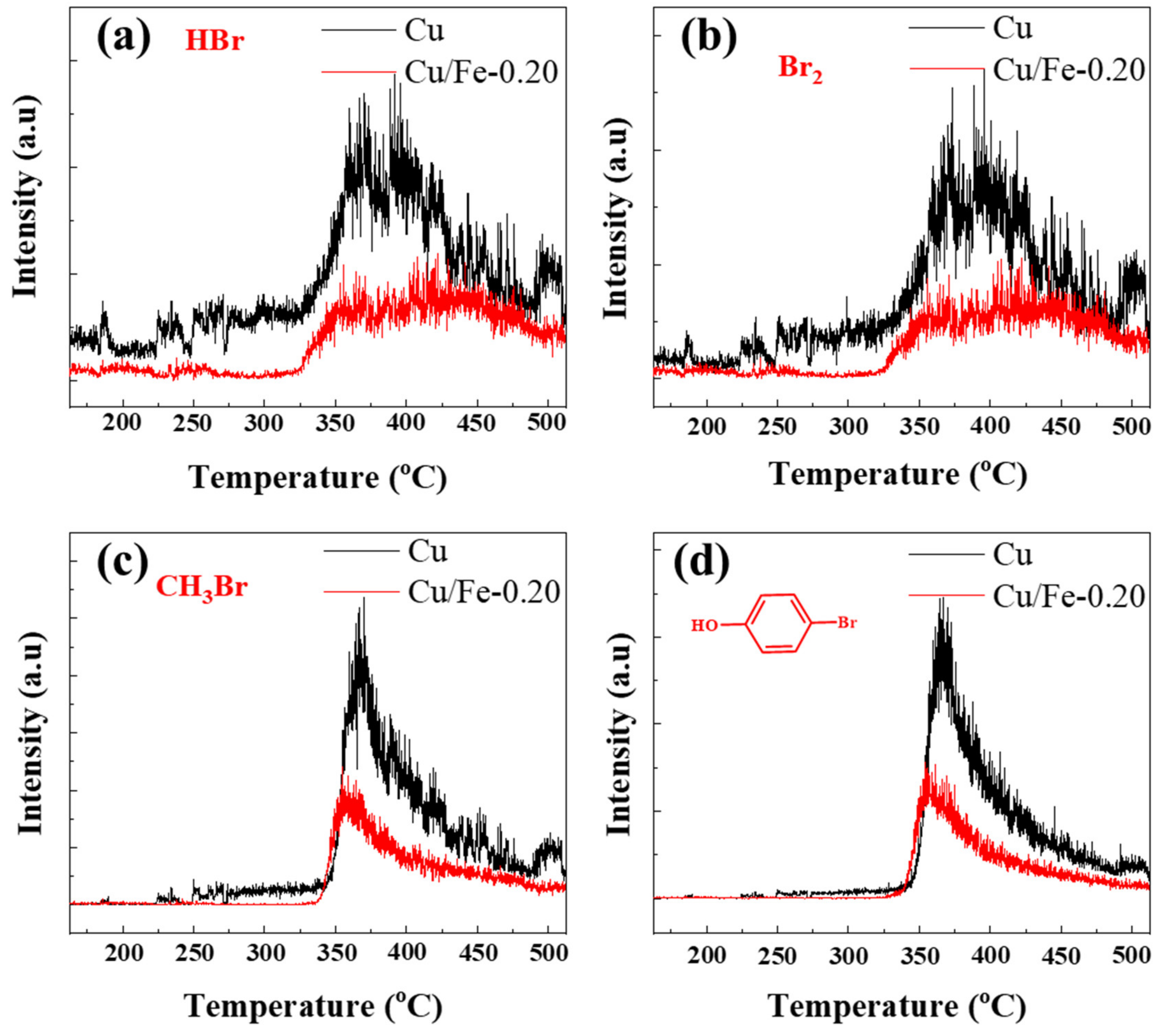
3.2. Factors on In Situ Catalytic Pyrolysis Debromination
3.3. In Situ Pyrolysis Catalytic Products
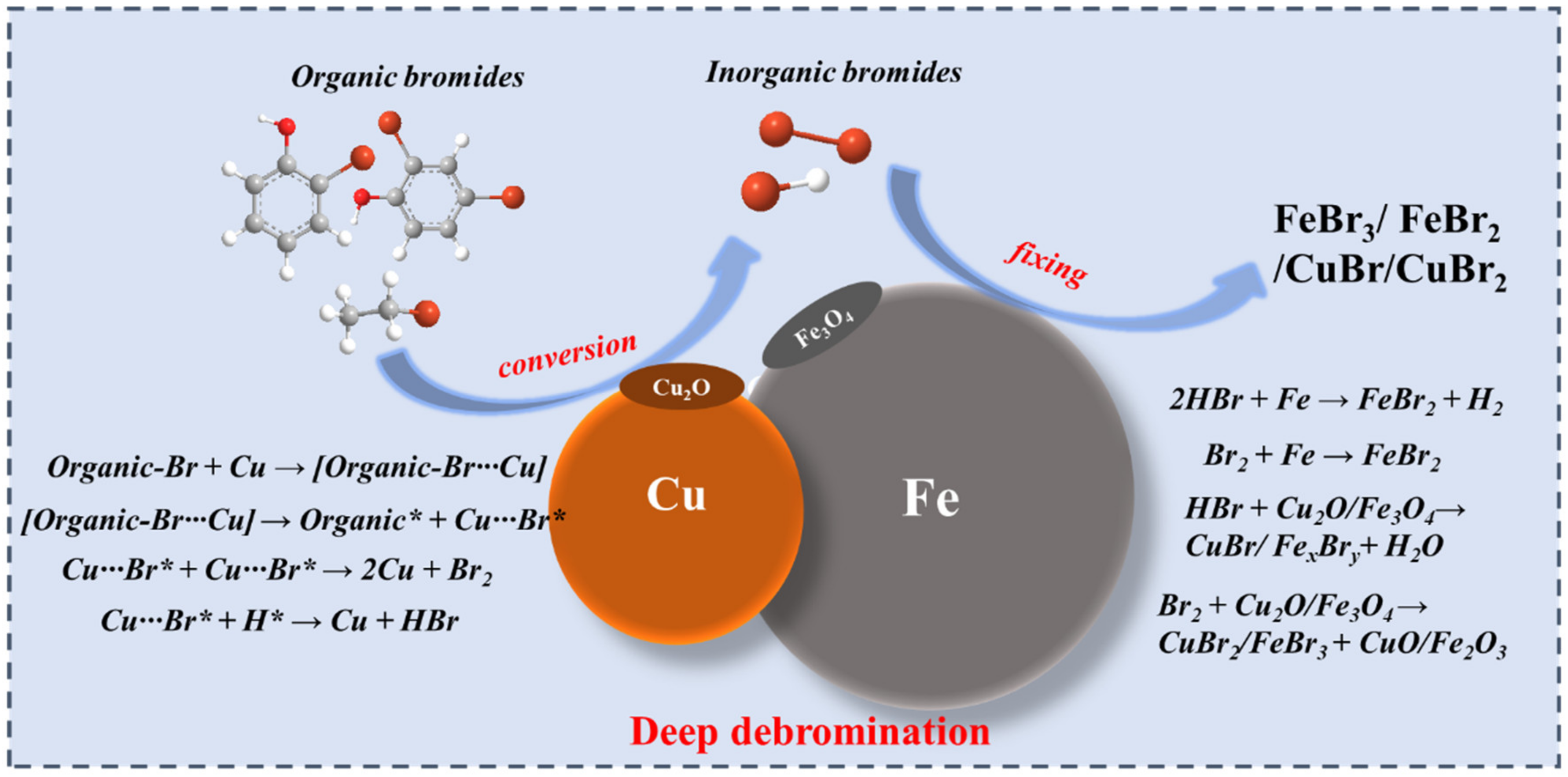
3.4. Debromination Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awasthi, A.; Zlamparet, G.; Zeng, X.; Li, J. Evaluating waste printed circuit boards recycling: Opportunities and challenges, a mini review. Waste Manag. Res. 2017, 35, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Lin, C.; Hui, D.; McKay, G. Waste Printed Circuit Board (PCB) Recycling Techniques. Topics Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, X.; Yao, Z.; Yin, Y.; Lin, K.; Liu, H.; Huang, J.; Ruan, J.; Qiu, R. Directional concentration of bromine from nonmetallic particles of crushed waste printed circuit boards by vacuum-gasification-condensation. J. Clean. Prod. 2019, 231, 462–467. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recy. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Hao, Y.; Fan, Z.Y.; Qian, G.R.; Zhou, M. Leaching behavior of copper powders from waste printed circuit board by electrogenerated chlorine and aeration. Mining Metall. Explora. 2013, 30, 79–84. [Google Scholar] [CrossRef]
- Hu, D.; Jia, Z.; Li, J.; Zhong, B.; Fu, W.; Luo, Y.; Jia, D. Characterization of Waste Printed Circuit Boards Nonmetals and its Reutilization as Reinforcing Filler in Unsaturated Polyester Resin. J. Polym. Environ. 2018, 26, 1311–1319. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, D.; Qiao, J.; Ju, Y.; Chen, Y.; Dionysiou, D.D. New insight into the cosolvent effect on the degradation of tetrabromobisphenol A (TBBPA) over millimeter-scale palladised sponge iron (Pd-s-Fe0) particles. Chem. Eng. J. 2019, 361, 1423–1436. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sust. Energ. Rev. 2016, 61, 433–450. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energ. Combust. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Liu, W.; Xu, J.; Han, J.; Jiao, F.; Qin, W.; Li, Z. Kinetic and Mechanism Studies on Pyrolysis of Printed Circuit Boards in the Absence and Presence of Copper. ACS Sustain. Chem. Eng. 2018, 7, 1879–1889. [Google Scholar] [CrossRef]
- Qin, B.; Lin, M.; Chen, X.; Xu, Z.; Fu, Y.; Hu, J.; Ruan, J. A novel approach for determining the accurate debromination time in the ball-milling process of nonmetallic particles from waste printed circuit boards by computation. J. Hazard. Mater. 2021, 410, 124611. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Lin, M.; Yao, Z.; Zhu, J.; Ruan, J.; Tang, Y.; Qiu, R. A novel approach of accurately rationing adsorbent for capturing pollutants via chemistry calculation: Rationing the mass of CaCO3 to capture Br-containing substances in the pyrolysis of nonmetallic particles of waste printed circuit boards. J. Hazard. Mater. 2020, 393, 122410. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yu, J.; Chen, T.; Yan, Q.; Song, Z.; Wang, B.; Sun, L. Influence of Fe based ZSM-5 catalysts on the vapor intermediates from the pyrolysis of brominated acrylonitrile-butadiene-styrene copolymer (Br-ABS). Fuel 2018, 230, 390–396. [Google Scholar] [CrossRef]
- Bhaskar, T.; Matsui, T.; Kaneko, J.; Uddin, M.A.; Muto, A.; Sakata, Y. Novel calcium based sorbent (Ca-C) for the dehalogenation (Br, Cl) process during halogenated mixed plastic (PP/PE/PS/PVC and HIPS-Br) pyrolysis. Green Chem. 2002, 4, 372–375. [Google Scholar] [CrossRef]
- Bhaskar, T.; Negoro, R.; Muto, A.; Sakata, Y. Prevention of chlorinated hydrocarbons formation during pyrolysis of PVC or PVDC mixed plastics. Green Chem. 2006, 8, 697. [Google Scholar] [CrossRef]
- Ma, C.; Kamo, T. Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/SiO2-Al2O3 catalyst. J. Anal. Appl. Pyrol. 2019, 138, 170–177. [Google Scholar] [CrossRef]
- Ma, C.; Kamo, T. Effect of steam-iron reaction on product characteristics and debromination during pyrolysis of epoxy-printed circuit boards. J. Hazard. Mater. 2019, 379, 120803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Xiao, Y.; Wei, C.; Han, D.; Huang, W. Catalytic debromination of tetrabromobisphenol A by Ni/nZVI bimetallic particles. Chem. Eng. J. 2016, 284, 1242–1250. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wang, F.; Bai, B.; Zhang, J.; Li, C.; Sun, Q.; Wang, Y.; Forson, K. Adsorption behavior and mechanism analysis of siloxane thickener for CO2 fracturing fluid on shallow shale soil. J. Mol. Liq. 2023, 376, 121394. [Google Scholar] [CrossRef]
- Li, O.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control strategy. Ocean Eng. 2024, 297, 117029. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Ma, S.; Shang, Z. Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem. Eng. J. 2016, 302, 663–669. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Han, D.; Huang, W.; Yang, C. New insights into the role of Ni loading on the surface structure and the reactivity of nZVI toward tetrabromo- and tetrachlorobisphenol A. Chem. Eng. J. 2017, 311, 173–182. [Google Scholar] [CrossRef]
- Lei, M.; Guo, S.; Wang, Z.; Zhu, L.; Tang, H. Ultrarapid and Deep Debromination of Tetrabromodiphenyl Ether over Noble-Metal-Free Cu/TiO2 Nanocomposites under Mild Conditions. Environ. Sci. Technol. 2018, 52, 11743–11751. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Zhang, W.; Wang, T.; Mei, M.; Chen, S.; Li, J. Mechanistic insights into catalysis of in-situ iron on pyrolysis of waste printed circuit boards: Comparative study of kinetics, products, and reaction mechanism. J. Hazard. Mater. 2022, 431, 128612. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhou, R.; Liu, Y.; Ma, D.; Wang, J.; Guan, Y.; Yao, Q.; Sun, M. Research on the pyrolysis characteristics and mechanisms of waste printed circuit boards at fast and slow heating rates. Waste Manag. 2022, 149, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Han, J.; Zhao, B.; Wang, Y.; Chen, W.; Xing, F. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses. J. Anal. Appl. Pyrol. 2018, 136, 132–145. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Xia, H.; Zhang, L.; Liu, D.; Shu, B. Catalytic pyrolysis of waste printed circuit boards to organic bromine: Reaction mechanism and comprehensive recovery. Environ. Sci. Pollut. R. 2023, 30, 108288–108300. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.H.; Ma, Q.A.; Yu, C.J.; Luo, Z.Y.; Cen, K.F. Influence of CaO additives on wheat-straw pyrolysis as determined by TG-FTIR analysis. J. Anal. Appl. Pyrol. 2010, 88, 199–206. [Google Scholar] [CrossRef]
- Hammoud, H.; Schmitt, M.; Bihel, F.; Antheaume, C.; Bourguignon, J.J. Direct Guanidinylation of Aryl and Heteroaryl Halides via Copper-Catalyzed Cross-Coupling Reaction. J. Org. Chem. 2012, 77, 417–423. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, L.; Huang, L.; He, W.; Li, H.; Wang, S.; Long, Z.; Sun, Z. Identification of Active Sites in Pt–Co Bimetallic Catalysts for CO Oxidation. ACS Appl. Energy Mater. 2021, 4, 11151–11161. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Song, H.; Kim, D.; Jeong, B.; Lee, J.; Shin, J.; Ryoo, R.; Park, J. Catalytic Synergy on PtNi Bimetal Catalysts Driven by Interfacial Intermediate Structures. ACS Catal. 2020, 10, 10459–10467. [Google Scholar] [CrossRef]
- Wu, C.; Liu, C.; Su, D.; Xin, H.; Fang, H.; Eren, B.; Zhang, S.; Murray, C.; Salmeron, M. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef]
- Altarawneh, M.; Ahmed, O.H.; Jiang, Z.T.; Dlugogorski, B.Z. Thermal Recycling of Brominated Flame Retardants with Fe2O3. J. Phys. Chem. A 2016, 120, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.H.; Altarawneh, M.; Al-Harahsheh, M.; Jiang, Z.T.; Dlugogorski, B.Z. Recycling of zincite (ZnO) via uptake of hydrogen halides. Phys. Chem. Chem. Phys. PCCP 2018, 20, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.; Isaacs, M.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M.; et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.; Song, H.; Jeong, B.; Lee, J.; Jung, Y.; Park, J. Metal Encapsulation-Driven Strong Metal–Support Interaction on Pt/Co3O4 during CO Oxidation. ACS Catal. 2023, 13, 5326–5335. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xi, Z.; Niu, B.; Gao, R.; Xu, Z. Catalytic Pyrolysis of Waste-Printed Circuit Boards Using a Cu/Fe Bimetal Synergistic Effect to Enhance Debromination. Sustainability 2024, 16, 3009. https://doi.org/10.3390/su16073009
Wang J, Xi Z, Niu B, Gao R, Xu Z. Catalytic Pyrolysis of Waste-Printed Circuit Boards Using a Cu/Fe Bimetal Synergistic Effect to Enhance Debromination. Sustainability. 2024; 16(7):3009. https://doi.org/10.3390/su16073009
Chicago/Turabian StyleWang, Jiahui, Zhen Xi, Bo Niu, Ruitong Gao, and Zhenming Xu. 2024. "Catalytic Pyrolysis of Waste-Printed Circuit Boards Using a Cu/Fe Bimetal Synergistic Effect to Enhance Debromination" Sustainability 16, no. 7: 3009. https://doi.org/10.3390/su16073009
APA StyleWang, J., Xi, Z., Niu, B., Gao, R., & Xu, Z. (2024). Catalytic Pyrolysis of Waste-Printed Circuit Boards Using a Cu/Fe Bimetal Synergistic Effect to Enhance Debromination. Sustainability, 16(7), 3009. https://doi.org/10.3390/su16073009




