Abstract
A byproduct from orange juice processing known as pastazzo represents a significant organic waste stream. Rich in essential oils and known for its inhibitory effect on plant germination, pastazzo could serve as a valuable input for agricultural purposes. This study assesses the effects of a 40% w/v orange pastazzo water extract (OPWE) produced by hydrodynamic cavitation on the germination of two species, one of economic interest (Lactuca sativa L.) and one common weed (Chenopodium album L.). Three dilutions of OPWE in water (25%; 50%; 75%) were compared to a control treatment in four experiments, using (i) seeds in Petri dishes; (ii) seeds in commercial substrate; (iii) C. album seeds and transplanted L. sativa in commercial substrate; and (iv) other weeds in an open-field plantation of L. sativa. Highly rich in limonene, OPWE applied at higher concentrations in Petri dishes caused the effective inhibition of germination in C. album and a germination delay in L. sativa. Similar results were observed in the germination of the two species in commercial substrate, with none of the dilutions affecting L. sativa biomass. In the field experiment, despite a relatively low number of weeds in the control treatment, higher OPWE concentrations reduced the number of grasses and forbs, largely confirming the inhibitory effects. We conclude that OPWE produced with hydrodynamic cavitation, an efficient and affordable method of extraction, represents an effective crop treatment due to the species-specific effects of its constituent limonene on plant germination. Further tests are essential to understand the extent to which OPWE interacts with other species and types of substrate.
1. Introduction
The increasing need for food, which is linked to both population growth and the environmental threats engendered by current intensive agricultural practices, poses great challenges in terms of the safety, sustainability, and affordability of its production. In 2009, the European Commission adopted the EU 2009/128/EC Directive on the Sustainable Use of Pesticides (SUP), which, despite widespread concerns [1,2], has been boosted by the Commission’s Farm-to-Fork [3] strategy proposing a 50% reduction in the use of chemical pesticides by 2030, in line with other European initiatives [4,5,6,7,8]. Along with their usefulness, pesticides and herbicides pose dangerous threats to the environment [9,10] and people [11,12]. For instance, the most common glyphosate has been classified as a “probable cancerogenic” [13], while other widely used post-emergence selective herbicides (e.g., quizalofop-p-ethyl and cycloxydim), have been found to affect commercial plant species and in turn human health [10,14,15,16]. Therefore, it is important to incentivize the reduction in the use of such substances in the environment (as indicated by the National Action Plans in the EU) [17] and to identify suitable “green” alternatives that address both the immediate needs of farmers and the increased resistance to pesticides that plants and other organisms gain over time [18].
In their various organs, plants produce secondary metabolites [19,20,21] such as phenolics, steroids, saponins, terpenoids, alkaloids, and flavonoids—whose functions include the regulation of plant growth and defense against abiotic and biotic stresses [22,23]. Within a plant, the production of these substances varies depending on the plant tissues, growth stages, and environmental conditions, such as soil characteristics, water availability, and the intensity, quality, and duration of light [24,25,26]. Some of these molecules work as allelochemicals, as they can interact with other organisms. In some cases, they may reduce germination and stunt the development of other plants [27,28,29], interfering with their enzymatic and photosynthetic activity and the growth of their root system, thus affecting their capacity to absorb nutrients [30,31], the division and elongation of their cells and the permeability of their membranes [32]. Essential oils are complex mixtures of secondary metabolites such as volatile organic compounds (e.g., monoterpenes, sesquiterpenes, aliphatic alcohols, ketones, aldehydes, acids, etc.) that are responsible for the volatile aroma.
In the Citrus genus, essential oils are mainly contained in the rind of the fruit, and as they are particularly rich in the volatile monoterpene d-limonene [33], they are used in the food industry and more recently have been found to be of interest for the agricultural sector [34]. Among Citrus, oranges represent the major production in the Mediterranean. The southern regions of Spain and Italy produce 3.4 and 1.5 megatons per year, respectively, which correspond to 80% of all European orange production; in Italy, Sicily is the region with the greatest production (~1 MT/year) [35].
The orange production and transformation industry generates a considerable amount of residue as damaged fruits (~15%) and orange byproducts from processing fruits, namely pastazzo, which is a highly fluid (75–85% water) slurry composed of residues such as peels, seeds, fibers, and pulp, representing 55–65% of the mass in the orange juice industrial chain and whose cost of disposal is very high [36].
Although pastazzo is defined as an agricultural product by the EC Treaty of Amsterdam [37], its use is still limited. When used as livestock feed according to the EC Directive 98/67 [38], it needs to be mixed with other foods due to the d-limonene inhibitory effect on the animal’s anaerobic digestion [39,40]; whereas when used in biogas and energy production, the transportation to the anaerobic digestion plants [36] and the energy demand for dehydration make the process too expensive [41,42,43,44].
Despite these limitations, pastazzo is rich in essential oils—which makes it a potentially valuable product. However, the most common methods of producing these oils are (a) water vapor extraction, which lowers the quality of the essential oil due to the high temperature, and (b) methods based on solvents, which require high energy consumption and have environmental repercussions [45]. More recently, an alternative extraction technique, known as hydrodynamic cavitation, has been proven effective, scalable, and efficient in terms of its energy demand, complying with the principles of green extraction of natural products [46].
Hydrodynamic cavitation occurs when a liquid passes through a device that induces a flow restriction, increasing its velocity and kinetic energy. This, in turn, causes the flow pressure to drop below the vapor pressure of the particular liquid, resulting in the formation of cavities. These cavities start collapsing, generating a substantial amount of energy in the surrounding liquid and resulting in significant pressures (500 MPa) and extreme heat over a short time [47,48], which cause cell enlargement or destruction, releasing the cell metabolites (plant bioactives, enzymes, proteins, etc.) in the liquid [47]. In addition, hydrodynamic cavitation generates considerable amounts of extract, in the form of a nanoemulsion, of the order of tens of liters in a short time; therefore, it is considered a green alternative to more common extraction methods. The technique has been successfully applied to citrus pastazzo for the extraction of bioactive compounds (e.g., pectin, flavonoids, essential oils, pigments, and dyes) [46] that could be used in different economic sectors.
Pastazzo water extracts have never been tested for agricultural use, so although it is known that citrus essential oils may have an inhibitory effect on seed germination, it is still completely unknown to what extent the pastazzo water extract produced via hydrodynamic cavitation would be effective if used as a biological herbicide. Therefore, the aim of this study is to assess (a) the extent to which orange pastazzo water extract (OPWE) produced via hydrodynamic cavitation may be effective in inhibiting seed germination of a weed (Chenopodium album L.) and a species of economic interest (Lactuca sativa L.), and (b) any effects on the growth parameters of Lactuca sativa L.
The responses of these two species were compared in a series of experiments, ranging from the laboratory scale to the open field.
2. Materials and Methods
2.1. Production and Characterization of Orange Pastazzo Water Extract (OPWE)
In March 2022, organic orange pastazzo produced by a juice factory was delivered to the laboratory and kept refrigerated at 4 °C for several days until manufacturing the water extract via hydrodynamic cavitation [49,50]. A total of 48.5 kg of pastazzo was finely ground with a blender, mixed in water (121 L), and processed in hydrodynamic cavitation for 35 min from 10.5 °C to 47 °C according to [50]. Once produced, the orange pastazzo water extract (OPWE) was analyzed to determine its terpene composition using gas chromatography and mass spectrometry conditions as described by [51], except that a DB-WAX UI (Agilent, Santa Clara, CA, USA) capillary column (60 m, 0.25 mm i.d., 0.5 m df) was used. Data were acquired and analyzed using Agilent MassHunter software 1.0 to obtain deconvoluted peak spectra, which were matched against the NIST 11 spectral library for tentative identification. Kovats’ retention indices were calculated for further compound confirmation and compared with those reported in the literature for the used chromatographic column. When available, authentic standards were also injected to obtain a positive identification.
The OPWE reaction (pH) was measured using a probe (XS Instruments, Carpi, MO, Italy). Then, OPWE was refrigerated at 4 °C for the whole duration of the study.
2.2. OPWE Effects on the Germination of Chenopodium album L. and Lactuca sativa L. in Petri Dish
Commercial lettuce seeds (Lactuca sativa L.) and lamb’s quarters seeds (Chenopodium album L.) collected from wild plants in a field (43°56′ N, 10°55′ E) were used for this experiment. For each species, 40 Petri dishes (Ø 9 cm) were prepared, considering four replicates per treatment and four treatments: 100%, 75%, 50%, and 25% dilutions with distilled water, plus control (CTRL), i.e., distilled water. A disc of filter paper was placed on the bottom of each dish, and 10 lettuce seeds or 25 lamb’s quarters seeds were placed at regular distances in each dish. At the beginning of the experiment, 7 mL of the relevant solution was applied to each Petri dish, and further liquid was added when the filter paper became dry (on average, the application occurred once a week in all dishes). Petri dishes were incubated at 22 °C in the dark, and the number of new germinations (seeds showing evident radicles of about 1 mm) was noted daily until no new germinations were observed. Eventually, lettuce seeds were monitored for 12 days and lamb’s quarters seeds for 35 days.
2.3. OPWE Effects on the Germination of Chenopodium album L. and Lactuca sativa L. Sown on Commercial Substrate
This experiment was set up to assess the germination with OPWE applied on a commercial substrate, thus with the probability of buffer effect and volatilization. In mid-March 2022, 12 small pots (0.75 L, 18 cm × 13 cm × 10 cm) for each species were prepared with a commercial horticultural substrate (COMPO BIO, Münster, Germany), three replicates per treatment (75%, 50%, 25%) and CTRL (i.e., water) and 30 seeds were sown per pot at a regular distance and ~2 mm of depth, for a total of 360 seeds for both species. Dilutions were applied every five days (the interval was decided on the basis of the observation of new sprouting and the assumption of the volatilization of inhibitory compounds) by pouring uniformly 100 mL of treatment on the surface of the relative pots. The pots were placed in controlled laboratory conditions at 22 ± 1 °C and a photoperiod of 8 h of light per day. Daily, the pots were monitored, and the number of new germinations was noted. The monitoring period was 20 days for lettuce and 58 days for lamb’s quarters.
2.4. OPWE Effects on the Germination of Chenopodium album L. on Commercial Substrate with Transplanted Lactuca sativa L.
This experiment was set up to assess the effect of OPWE on the germination of lamb’s quarters sown in pots with commercial substrate, in combination with transplanted lettuce, in order to assess the effects on lettuce growth. Lettuce was previously grown in a sowing tray filled with commercial substrate (as in the previous experiment, described in Section 2.3) until the third true leaf was fully expanded. Then, on 18 May 2022, 20 pots (1.5 L) were prepared with a mixture of the same commercial substrate and volcanic lapilli (3:1 in volume). In each pot, one lettuce plant with 5 young leaves was transplanted, and 25 seeds of lamb’s quarters were sown at about 2 mm depth. The pots were placed outdoors, under a shading mesh to shield the plants from direct solar radiation and irrigated daily for one minute (300 mL) to keep the substrate moist. A 200 mL volume of the OPWE treatment (75%, 50%, and 25%) and water for control (CTRL) were poured on the surfaces of the pots (five per treatment) every six days, three times in total. Lamb’s quarters emergence was monitored approximately every other day by noting the number of new germinations (epicotyl sprouting from the substrate) for 30 days. Then, on 15 June, the lettuce plants were cut, and the final biomass was measured: diameter of the head, fresh weight, and dry weight after drying at 70 °C until reaching constant weight.
2.5. OPWE Effects on Weed Germination in an Open Field Plantation of Lactuca sativa L.
This experiment was conducted to assess the effect of OPWE on weed germination in a field experiment with lettuce. Lettuce was previously sown in a seed tray filled with commercial substrate. When lettuce plants reached the height of 8 cm in May 2022, they were transplanted in a tilled open field (Borgo Virgilio, Mantova, Italy, 45°05′40′’ N–10°47′30′’ E), placing 40 plants in four rows at 0.5 m distance between rows and 20 cm between plants. The soil was loamy (50% sand, 40% silt, and 10% clay) with a pH measured in water of 8.5. The total organic carbon (C) was 10.7 g kg−1, and the total nitrogen (N) was 1.15 g kg−1. Assimilable phosphorous (P) and exchangeable potassium (K) were 76.4 and 810 mg kg−1, respectively. An organic fertilizer (organic N 3%, P2O5 3%, organic C 25%) was applied before the transplant. Drip irrigation supplied water twice a day for one minute (daily average of 4.1 mm). The application of the treatments (75%, 50%, and 25) and water (CTRL) occurred by pouring 250 mL of the relative OPWE dilution uniformly around 10 plants of lettuce along the row for each treatment, covering a radius of 15 cm. The applications were conducted every other week on three dates: 29 May, 12 June, and 26 June. After 70 days (on the 15 July), assuming that weed species had the same probability of germinating inside the experimental plot, the composition of the naturally spread weeds was observed around three sides of the lettuce heads to avoid double counting along the row. The number of weeds by genus or species was noted during in-field observations, and the identification was refined ex-post by photo interpretation. Then, lettuce biomass was assessed: the average diameter of the lettuce head was calculated as the average of three diameters; then, each plant was cut, the fresh weight and the dry weight after drying at 70 °C until reaching constant weight.
2.6. Statistical Analysis
The statistical analysis of the germinations in Petri dishes and in the commercial substrate (Section 2.3 and Section 2.4) was carried out in the R.4.1.3 Stat environment [52]. The proportional cumulative germination (propCum) expressed by the number of germinating seeds throughout the experiment with respect to the total number of seeds was used with the “drcSeedGerm” R packages and the specific function “drm” to fit the response curve by using a proper non-linear function [51,53]. The latter function also gives the results of the Wald test on fitted parameters. Three levels of rejections of the null hypothesis (Ho) of parameters equal to zero are taken into account and indicated by asterisks: (i) * p-value < 0.05 (ii) ** p-value <0.01 (iii) *** p-value < 0.001. A four-parameter logistic model was used in the work, whose mathematical representation is
where d is the higher asymptotic limit (the upper limit), c is the lower asymptotic limit (the lower limit), T50 is the time value in days at the inflection point, and b is the slope at the inflection point. The germination process is described by “time-to-event data”, and the end time is the day for each inspection interval. The median germination time (time required for 50% of viable seeds to germinate, T50), final germination out of the total number of seeds, and their relative standard errors were calculated. The comparison between germination metrics was also carried out by using a specific function of the R “drc” package based on t-statistics and p-values for the null hypothesis that the ratio of parameters equals 1 or that the difference equals 0. A public repository is available for code and data https://github.com/alfcrisci/repo_ugolini_pastazzo (accessed on 5 March 2023). Data is under CC by the 4.0 Creative Commons license. In the experiment described in Section 2.6, the comparison between lettuce biomass in different treatments was carried out by ANOVA when the assumption of normality (Kolmogorov–Smirnov test) and the homogeneity (Levene median test) of variance were satisfied, followed by Tukey’s test for the post hoc comparison of means, or using non-parametric Kruskal–Wallis test when the conditions were not satisfied. The comparison between different percentages of weeds was conducted by the Chi-square goodness of fit test between coupled treatments with at least five observations in each treatment, assuming as the null hypothesis that the germination of weeds does not change across the plot.
3. Results
3.1. Terpene Characterization of the Orange Pastazzo Water Extract (OPWE)
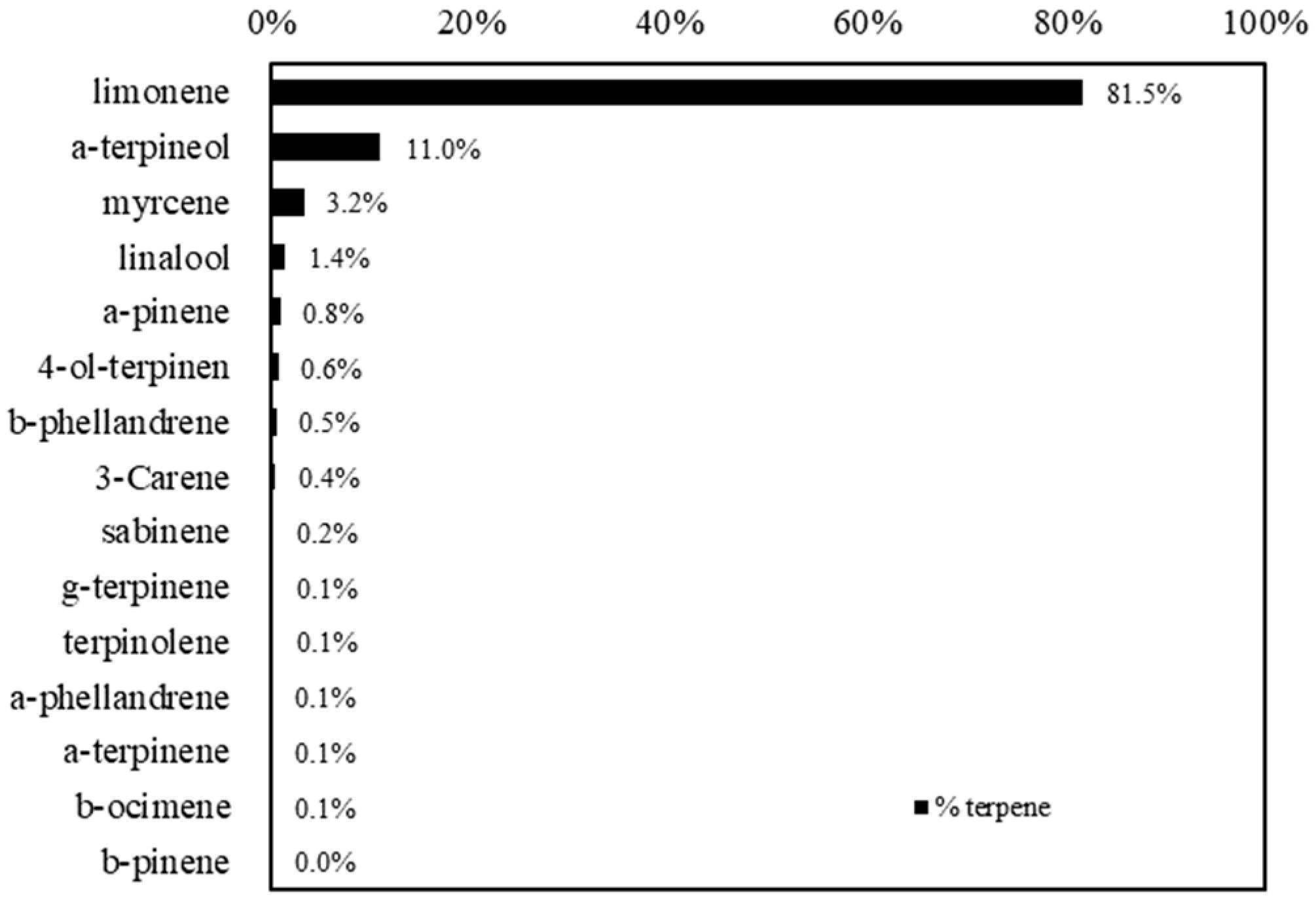
OPWE produced by hydrodynamic cavitation is a slightly acidic liquid (pH around 6.4) with a fine solid residue corresponding to the amount of pastazzo used in the process. The terpene composition of the liquid part, filtered from solid residues, showed the prevalence of monoterpenes, mainly limonene (1543 mg/L, 81.5%), followed by a-terpineol (227 mg/L, 11%), myrcene (49 mg/L, 3.2%), and linalool (30 mg/L, 1.4%), while all the other monoterpenes were present in concentrations less than 6 mg/L (Figure 1).
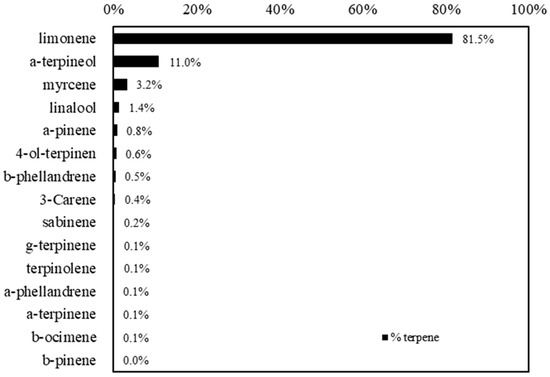
Figure 1.
Terpene composition of the orange pastazzo water extract (OPWE) used in the experiments.
3.2. OPWE Effects on the Germination of Lactuca sativa L. and Chenopodium album L. in Petri Dishes
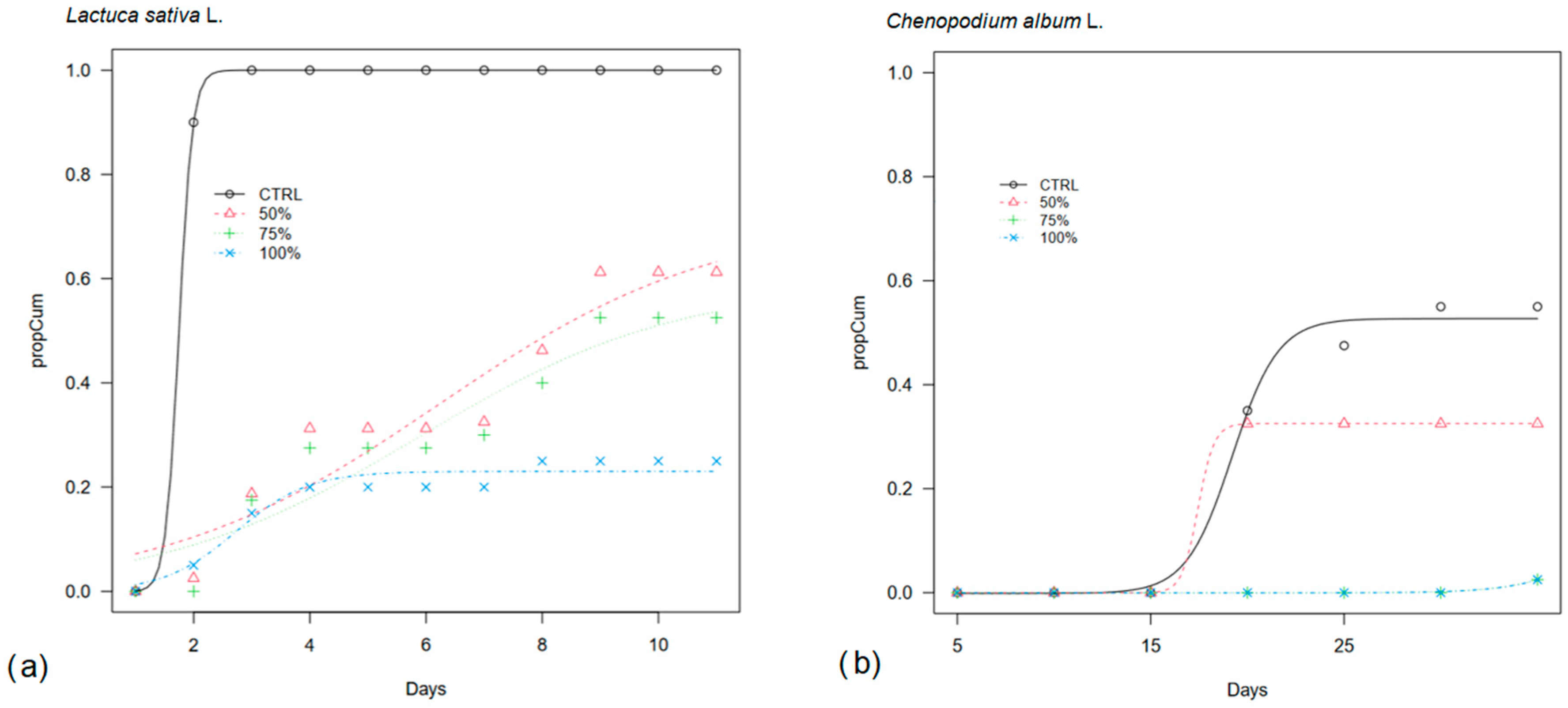
In this experiment, lettuce germination was mostly inhibited when pure OPWE was applied (Figure 2a), resulting in a share of 23%, and it was partially inhibited at 75% and 50%, with final germination of 59% and 72%, respectively. In addition, in these OPWE treatments, germination was slower than in CTRL with T50 occurring around day 6 vs. day 2 in CTRL (Table 1).
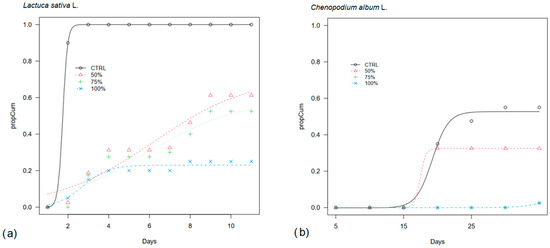
Figure 2.
Cumulative germination curves (propCum) of (a) lettuce (Lactuca sativa L.) and (b) lamb’s quarter (Chenopodium album L.) seeds in a Petri dish treated with pure orange pastazzo water extract (100%) and at 50% and 75% dilution of OPWE in distilled water (50%, 75%) and distilled water as control (CTRL).

Table 1.
Final germination (%), Median germination time (time to reach 50% of the final germinated seeds, T50, day) in Lactuca sativa L. and Chenopodium album L. in Petri dish treated with dilutions of orange pastazzo water extract at 25%, 50% and 75% in distilled water and compared to distilled water as control (CTRL). Small letters indicate the significant differences between treatments at p-value < 0.001.
Lamb’s quarters were also substantially inhibited (only 2% germinations) in the most concentrated OPWE treatments (75% and 100%) (Figure 2b). Although generally low, it recorded the highest final germination in 50% and in CTRL (33% and 53%, respectively). CTRL and 50% were the only treatments in which 50% viable seed germinated within a shorter period of 19 and 17 days, respectively (Table 1).
3.3. OPWE Effects on the Germination of Lactuca sativa L. and Chenopodium album L. Sown in Commercial Substrate
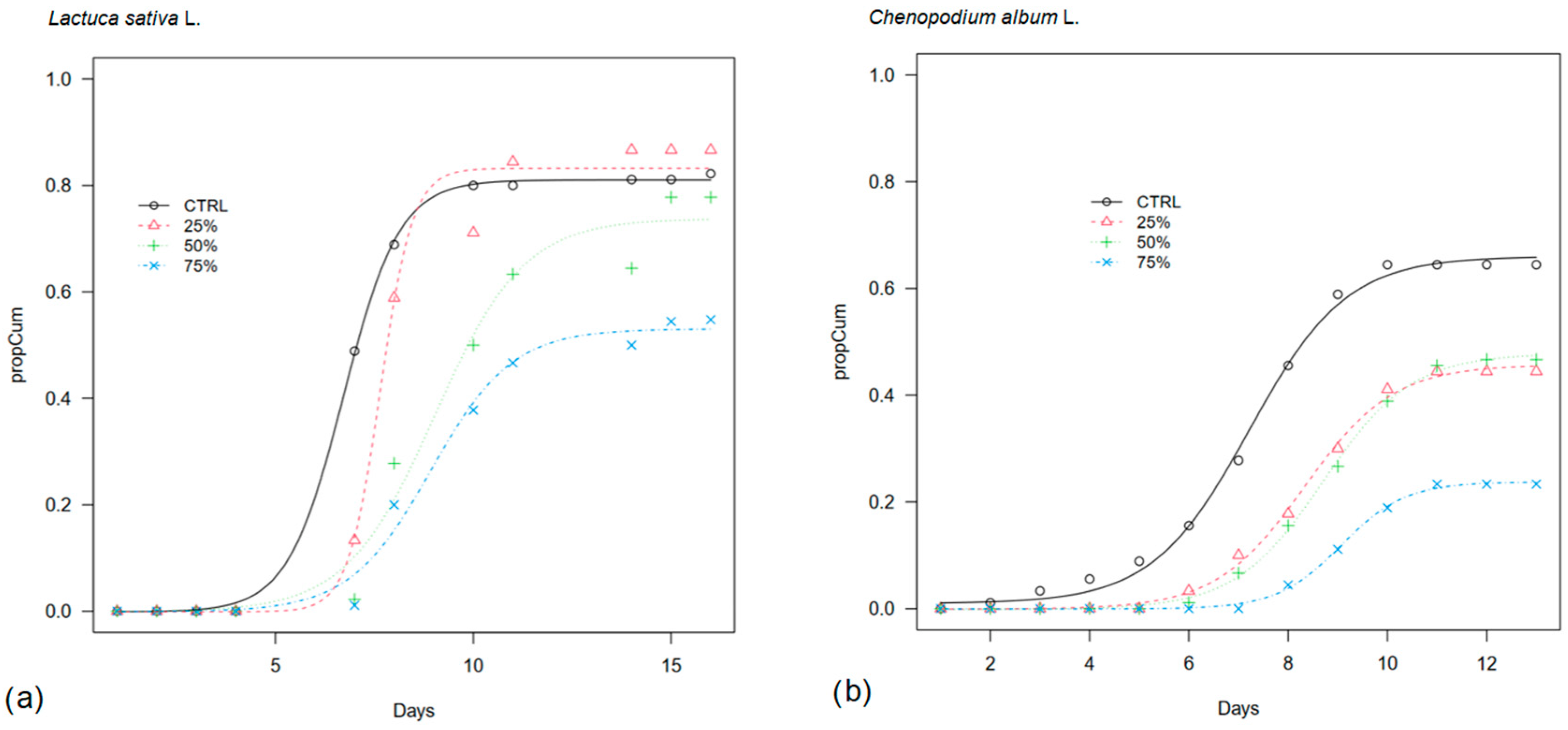
When sown in commercial substrate, lettuce again showed a delayed germination in OPWE treatments with respect to control, with 50% of seeds germinating by day 7 in CTRL, day 8 in 25%, and day 9 in 50% and 75% OPWE (Figure 3). However, the final germination in the diluted treatments was high and comparable to control (81% vs. 83% and 74% in CTRL, 25%, and 50% OPWE, respectively), while the lowest share was found in 75% (53%).
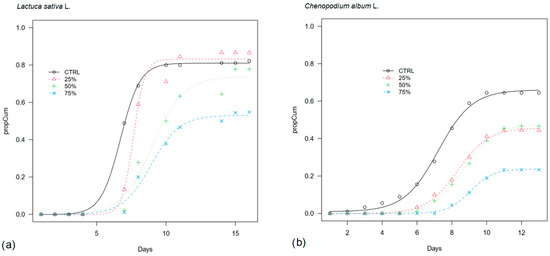
Figure 3.
Cumulative germination curve (propCum) of (a) lettuce (Lactuca sativa L.) and (b) lamb’s quarter (Chenopodium album L.) seeds (N seeds = 30) on commercial substrate treated with orange pastazzo water extracts (OPWE) at 25%, 50% and 75% concentration in water and water as control (CTRL). Day 0 in lamb’s quarter germination corresponds to day 42 from sowing.
Lamb’s quarters started germinating in the control in which the median germination time was reached around day 49, and the final germination was 66%. In 25%, 50%, and 75%, T50 occurred 1–2 days after the control and final germination shares were lower (46%, 48%, and 24%, respectively) (Table 2).

Table 2.
Final germination (%), Median germination time (Time to reach 50% of final germinated seeds, T50, day) in lettuce (Lactuca sativa L.) and lamb’s quarter (Chenopodium album L.) (N seeds = 30) grown on commercial substrate treated with orange pastazzo water extracts (OPWE) at 25%, 50% and 75% concentration in water and water as control (CTRL). T50 in lamb’s quarters indicates the number of days after day 42 (days from sowing). Small letters indicate the statistical differences between treatments at p-value < 0.001.
Lettuce growth in OPWE treatments at 25% and 50% was enhanced, resulting in greater length (plant height and root) and leaf area, although no differences were found in the dry biomass (Table 3).

Table 3.
Growth parameters of lettuce (Lactuca sativa L.) grown on a commercial substrate treated with orange pastazzo water extracts (OPWE) at 25%, 50%, and 75% concentration in water and water as control (CTRL). Means ± standard deviations are shown. Small letters indicate the statistical differences between treatments are shown at p < 0.05.
3.4. OPWE Effect on the Germination of Chenopodium album L. on Commercial Substrate with Transplanted Lactuca sativa L.
In the outdoor experiment with pots filled in with commercial substrate and transplanted lettuce, lamb’s quarters’ germination was low in all treatments: the highest final germination was recorded in the control (26 ± 11%), while lower and decreasing percentages were observed with increasing OPWE concentration: 14 ± 8% in 25%, 4 ± 4% in 50% and 0 ± 0%, in 75%. In addition, during the experiment, we observed the presence of ants, which may have been attracted by the OPWE, interfering in the experiment by taking away the seeds.
Regarding the lettuce growth, at the end of the experiment, the lettuce head diameter, height, and number of leaves were comparable between treatments (Table 4).

Table 4.
Germination of lamb’s quarters (Chenopodium album L.) (N seeds per pot = 25) and final growth parameters of transplanted lettuce (Lactuca sativa L.) on a commercial substrate treated with orange pastazzo water extracts (OE) at 25%, 50%, and 75% concentration and water as control (CTRL). Means ±standard deviations are shown. Small letters indicate the statistical differences between treatments are shown at p < 0.05.
3.5. OPWE Effects on Weeds’ Germination with Transplanted Lactuca sativa L. in the Field
The experiment in an open field on a lettuce plantation with OPWE treatments did not evidence significant differences regarding the total number of weeds among the treatments, but a general trend in which the number of grasses seemed fewer in the treatments with higher OPWE concentrations. The most common weeds were forbs (representing, on average, 86% of weeds), with matted sandmat (Euphorbia serpens Kunth) as the most abundant in all treatments. A few other weeds were observed (Table 5), among which, despite the small numbers, Canada thistle (Cirsium arvense (L.) Scop., 1771) and lamb’s quarters (Chenopodium alba L.) showed a trend with greater presence in control and in 75%, while among the grasses, foxtail millet (Setaria italica (L.) P. Beauv.) was the most abundant, with greater presence in OPWE treatments than in control, while barnyard grass (Echinochloa crus-galli (L.) P. Beauv.) was more numerous in the control.

Table 5.
Number (N) and percentage (%) of weed species growing around the transplanted lettuce (Lactuca sativa L.) in a field plantation treated with orange pastazzo water extracts (OPWE) at 25%, 50%, and 75% concentration in water and water as control (CTRL). The Chi-square test has been used to compare each species percentage between coupled treatments (with at least 5 observations in each treatment). Small letters indicate the statistical differences between treatments are shown at α < 0.05.
Regarding lettuce biomass, in OPWE treatments, some parameters were slightly lower than in control, such as lettuce head diameter and fresh and dry biomass in 50% treatment and biomass in 75% treatment (Table 6).

Table 6.
Final growth parameters of lettuce (Lactuca sativa L.) (N per treatment: 10) in a field plantation treated with orange pastazzo water extracts (OPWE) at 25%, 50%, and 75% concentration in water and with water as control (CTRL). Means ± standard deviations are shown. Small letters indicate the statistical differences between treatments are shown at p < 0.05.
4. Discussion
This study shows the results of the application of an orange pastazzo water extract produced using hydrodynamic cavitation on seed germination at different stages. OPWE was produced as an emulsion of stable terpenic composition, highly rich in limonene and a-terpineol monoterpenes relative to essential oils of different orange cultivars—in line with other studies [54,55,56,57,58,59,60]. Orange essential oils or pure limonene have demonstrated some inhibitory effect on seed germination [61,62], but no tests have been carried out so far with OPWE. We decided to use one species known as a weed and one species of economic interest not only to assess the OPWE effect on germination but also to assess any effect on the growth of the species of economic interest.
The experiments In Petri dishes with OPWE applied directly on the seeds confirmed its inhibitory capacity, as greater inhibition was clearly observed at higher concentrations (100% and 75%), especially in the weeds. Indeed, germination of lamb’s quarters was completely inhibited at 100% and 75%, while lettuce germination was reduced by about 25% and 40% with respect to control at 75% and 100% concentrations, respectively, and it also was delayed by about 4 days. In a previous study [63], it was claimed that the behavior of different species to allelochemicals can be explained by the size of the seeds, as the volume and contact area of teguments may play a role in the imbibition capacity as well as by the following biological activity. This would explain the sensitivity of lamb’s quarters to OPWE at the highest concentrations despite the longer time needed for the germination to start.
Again, in the indoor experiment with seeds sown on commercial substrate, most inhibition was observed at the highest concentration (75%), with a reduction in germination by about 26% in lettuce and 38% in lamb’s quarters as compared to the control, which might be due to the buffering effect of the substrate—likely due to biological processes or volatilization. At intermediate concentrations (25% and 50%), the inhibition still was more evident in lamb’s quarters than in lettuce. Again, lettuce showed slower germination as compared to the control, as 50% of viable seeds in such treatments germinated 3–4 days after 50% of seeds in control. This confirms a species-specific effect whose reasons should be further investigated. Among the two species, lamb’s quarter looked more sensitive even at lower concentrations (25% and 50%), even though it needed more time to germinate.
From the literature, there is evidence that some citrus species, such as Citrus junos [64,65] and Citrus reticulata [66], are rich in abscisic acid-β-D-glucopyranosyl ester, which may be the cause of inhibition, while other studies claim that some monoterpenes have phytotoxic properties, which cause anatomical and physiological changes in plant seedlings (e.g., reduction in phytohormones, carbohydrates, fatty acids, mitochondria activity due to inhibition of DNA synthesis or disruption of membranes surrounding mitochondria and nuclei [67,68]. Therefore, we may assume that OPWE easily interferes with the seed development when it is in contact with seeds, like during the imbibition, and depending on the seed characteristics regardless of the species’ typical time for germination. This effect is likely associated with the presence of monoterpenes, specifically limonene. Monoterpenes have been found to have a phytotoxic effect on seeds, especially altering the mitochondrial functions, which mostly rule seed development, leading to the production of reactive oxygen species and cell membrane disruption [67]. Among monoterpenes, other studies confirm the negative effect of limonene on seed development and seedling growth as found in Amaranthus viridis L. [69], Allium cepa L., Chenopodium album L. and other species [68,70,71].
The effect may decrease if volatilization of monoterpenes occurs, i.e., in commercial substrate and in an open field, allowing some germination even at higher OPWE concentrations. We also mention that the application of OPWE on the surface of the commercial substrate produced a solid biofilm, which might have also provided a mechanical resistance to the sprouting of epicotyls, decreasing the probability of germinated seeds underneath the layer developing and growing up.
In the in-pot experiment with commercial substrate outdoors, lamb’s quarters germination was very low in all treatments as well as in the control, but again, it showed a decreasing trend at increasing OPWE concentrations and total inhibition at 75%, apparently associated with the allelopathic effects of OPWE. The general low germination rate in all treatments was due to the appearance of ants, likely attracted by OPWE and then spreading across the pots of the experiment. In addition, in this experiment, we also observed a sporadic presence of fungi (Coprinus plicatilis Fr.) in the pots with OPWE, which could have benefited from the presence of degradable cellulose of OPWE solid residue.
Regarding the effects on the plant biomass, lettuce seedlings treated with OPWE at lower concentrations (25% and 50%) developed more than in the other treatments, resulting in enhanced leaf area and plant and root length, confirming the effectiveness of such products on plant growth at low concentrations. When OPWE was applied to transplanted lettuce in pots, final biometric parameters and biomass were similar in all treatments, despite a slightly positive trend at higher concentrations (75%), in contrast to the previous experiment and similarly to other studies—although these used different types of citrus byproducts, such as pure orange pastazzo after slight dehydration [72] and citrus pulp [73]. In contrast, in the open-field experiment, OPWE affected lettuce head diameter and weight, especially at 50% and 75% concentrations. Literature reports inconsistent results on the effects of limonene on plant growth, sometimes evidencing how effects may be cultivar-specific [71]. Regarding the effects on other weeds, which are critical in agricultural production, in the in-field experiment, we assumed a similar probability of anemophilous weed seeds spreading across the small experiment trial. We found the prevalence of Euphorbia serpens Kunth regardless of the OPWE treatments, a species originating in South America but almost naturalized in Europe [74]. The low number of other weed species did not allow consistent comments; however, interesting patterns were observed for Setaria italica (L.) P. Beauv. and Abutilon theophrasti Medik, which seemed to be slightly advantaged by OPWE, and for barnyard grass (Echinochloa crus-galli (L.) P. Beauv.) and johnsongrass (Sorghum halepense L.), which were affected in all OPWE treatments. The authors of [75] observed a similar knock-down effect on barnyard grass and johnsongrass treated with pelargonic acid. Also, Canada thistle (Cirsium arvense (L.) Scop., 1771) and, to a lesser extent, lamb’s quarters (Chenopodium album L.) showed reduced presence in the OPWE treatments up to 50% concentration, but surprisingly as high as in control at 75%. This might be explained by concurrent factors, such as different seed distribution or lower competition with lettuce for light or other resources in the treatment where lettuce was smaller (75%) or volatilization of limonene as the application of OPWE was carried out less frequently than in the other experiments.
This study offers insights into the use of OPWE as a bio-herbicide, although many aspects should be further investigated and limitations overcome. Indeed, OPWE is not a standard product since pastazzo characteristics may change depending on the type of processed orange cultivars or the processes and harvesting practices. However, the limonene concentration of OPWE is in line with the concentration in essential oils, confirming the effectiveness of the extraction method. Another aspect that should be taken into consideration is the volatilization of the volatile component and its interaction with the substrate’s characteristics. Indeed, the application of OPWE dilutions was carried out arbitrarily based on the observation of new emergences (except in the experiment in the open field, for logistic reasons). During the experiments, we observed the formation of an OPWE biofilm, resulting in a mechanical resistance to seedling emergence. Unfortunately, we could not perform the analysis of the terpene composition of the substrate/soil during the experiments or tests to understand the relationship between OPWE and the substrate/soil microorganisms besides weather and management practices, which affect the decomposition and the mineralization of the OPWE.
Therefore, further investigations should focus on OPWE applications on different soil types and plant species to assess to what extent the allelopathic effect is soil- and species-specific and assess the replicability of the results.
Despite such knowledge gaps, this study is a first attempt to demonstrate how novel and effective hydrodynamic cavitation is to produce a low-cost orange pastazzo water extract with potential interest as bio-herbicide against lamb’s quarters and likely other weeds. The extraction of cell compounds (including limonene) with this technique is known to be effective and efficient (producing high yields within a few minutes of process time), making it a novel route characterized by effectiveness, reliability, efficiency, and affordability for the use and valorization of orange waste [49].
5. Conclusions
This study reports remarkable results of experiments regarding the use of a water extract of orange waste produced by a juice processing factory via hydrodynamic cavitation. The study confirmed the effectiveness of the technique to produce a water extract rich in limonene, a monoterpene with known inhibitory effects on many plant species. The orange pastazzo water extract was tested at different concentrations on lettuce and lamb’s quarters seeds in different settings, from laboratory to in-field trials. Experiments showed an inhibitory effect on lamb’s quarters in Petri dishes and when sown in commercial substrate, likely due to the toxic effect of the limonene on the seed functional activity, although this effect was less remarkable in lettuce, evidencing a species-specific effect. OPWE affected lettuce biomass with some inconsistencies: seedlings growing on commercial substrates and transplanted lettuce in the soil of the open field trial were affected negatively at higher concentrations, while transplanted lettuce in the commercial substrate was not affected, leaving question marks on the effects of limonene on plants at different stages, and on the relationship between OPWE and the substrate. Allelopathic effects were slightly noticed on some weeds in the open field trial, although further research is needed to validate this result. In conclusion, hydrodynamic cavitation is a novel technique with high potential to produce water extracts from agricultural waste with efficacy as bio-herbicides. However, future research is needed to validate the results and assess OPWE effects on other species, substrates, and application methodologies.
Author Contributions
Conceptualization, F.U.; methodology, F.U., F.M. and M.M.; formal analysis, A.C. and F.U.; investigation, F.U., S.B., L.A., A.D.P. and G.C.; data curation, A.C., G.C. and A.D.P.; writing—original draft preparation, F.U., A.C., A.D.P. and M.M.; writing—review and editing, F.U., F.M. and F.Z.; supervision, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available at https://github.com/alfcrisci/repo_ugolini_pastazzo (accessed on 5 March 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission. Directive on the Sustainable Use of Pesticides. Available online: https://food.ec.europa.eu/system/files/2022-06/pesticides_sud_eval_2022_reg_2022-305_en.pdf (accessed on 28 February 2024).
- Eurostat. Agri-Environmental Indicator—Consumption of Pesticides. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides (accessed on 28 February 2024).
- European Commission. European Green Deal, the Commission’s Strategy from Farm-to-Fork. Available online: https://www.euronews.com/green/green-series/farm-to-fork (accessed on 28 February 2024).
- European Commission. EU Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives. Available online: https://commission.europa.eu/document/020f7141-d73d-4191-853e-c5918a52f9f3_en (accessed on 28 February 2024).
- European Commission. Pathway to a Healthy Planet for All—EU Action Plan: Towards Zero Pollution for Air, Water and Soil. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52021DC0400&from=EN (accessed on 28 February 2024).
- European Commission. EU Soil Strategy for 2030—Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate. Available online: https://environment.ec.europa.eu/publications/eu-soil-strategy-2030_en (accessed on 28 February 2024).
- European Commission. Pollinators Initiative. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52018DC0395&from=EN (accessed on 28 February 2024).
- European Commission. Chemicals Strategy for Sustainability—Towards a Toxic-Free Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2020%3A667%3AFIN (accessed on 28 February 2024).
- Bakadir, K.; Kassale, A.; Barouni, K.; Lakhmiri, R.; Albourine, A.J. Retention of a compound of herbicides, 2,4-dichlorophenoxy acetic acid, to a soil in the absence and in the presence of Cu(II) and Zn(II) cations. J. Mater. Environ. Sci. 2016, 7, 1056–1063. [Google Scholar]
- Deshmukh, U.S.; Dhabe, S.P. Sub-chronic toxicity studies of Quizalofop-p-ethyl in female Wistar albino rats. Eur. J. Environ. Ecol. 2015, 2, 186–189. [Google Scholar]
- EFSA. The 2013 European Union Report on Pesticide Residues in Food. Available online: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2015.4038 (accessed on 28 February 2024). [CrossRef]
- Cherry, N.; Beach, J.; Senthilselvan, A.; Burstyn, I. Pesticide Use and Asthma in Alberta Grain Farmers. Int. J. Environ. Res. Public Health 2018, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- IARC. Evaluation of Five Organophosphate Insecticides and Herbicides. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/MonographVolume112-1.pdf (accessed on 28 February 2024).
- Deveci, A.; Aksoy, O.; Al, G. Investigation of the effects of quizalofop-P-ethyl on pollen germination of Hyacinthus orientalis L. Caryologia 2017, 70, 77–81. [Google Scholar] [CrossRef]
- Aksoy, O.; Deveci, A.; Kızılırmak, S.; Billur Akdeniz, G.A. Phytotoxic Effect of Quizalofop-P-Ethyl on Soybean (Glycine max L.). J. Biol. Environ. Sci. 2013, 7, 49–55. [Google Scholar]
- Rosculete, C.A.; Bonciu, E.; Rosculete, E.; Olaru, L.A. Determination of the Environmental Pollution Potential of Some Herbicides by the Assessment of Cytotoxic and Genotoxic Effects on Allium cepa. Int. J. Environ. Res. Public Health 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- European Union. Conference on the Future of Europe, Report on the Final Outcome. Available online: https://prod-cofe-platform.s3.eu-central-1.amazonaws.com/qtde64rjnkdaf5u2j54ocssxyn9w?response-content-disposition=inline%3B%20filename%3D%22Book_CoFE_Final_Report_EN_full.pdf%22%3B%20filename%2A%3DUTF-8%27%27Book_CoFE_Final_Report_EN_full.pdf&response-content-type=application%2Fpdf&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA3LJJXGZPDFYVOW5V%2F20221214%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20221214T072925Z&X-Amz-Expires=300&X-Amz-SignedHeaders=host&X-Amz-Signature=cbca5f502518d735fbc9b9de743e905497dc273fd71c00fec967c0857c381941 (accessed on 12 December 2023).
- Gut, L.; Schilder, A.; Isaacs, R.; McManus, P.; Landis, J.; Sanchez, J. Managing the community of pests and beneficials. In Fruit Crop Ecology and Management Extension Bulletin, 1st ed.; Landis, J.N., Sanchez, J.E., Bird, G.W., Edson, C.E., Isaacs, R., Lehnert, R.H., Schilder, A.M.C., Swinton, S.M., Eds.; Michigan State University: East Lansing, MI, USA, 2002; pp. 34–75. [Google Scholar]
- Ahmed, S.A.; El-Rokiek, K.G.; El-Masry, R.R.; Messiha, N.K. The efficiency of allelochemicals in the seed powder of Eruca sativa in controlling weeds in Pisum sativum. Middle East J. Agric. Res. 2014, 3, 757–762. [Google Scholar]
- Putnam, A.R.; DeFrank, J. Use of phytotoxic plant residues for selective weed control. Crop Prot. 1983, 2, 173–181. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Chapter Two—Allelopathy and the Role of Allelochemicals in Plant Defence. In Advances in Botanical Research–How Plants Communicate with Their Biotic Environment, 1st ed.; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. ISBN 978-0-12-801431-8. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Zimdahl, R.L. Fundamentals of Weed Science, 5th ed.; Academic Press: Cambridge, MA, USA, 2018; p. 715. ISBN 978-0-12-811143-7. [Google Scholar]
- Podda, A.; Pollastri, S.; Bartolini, P.; Pisuttu, C.; Pellegrini, E.; Nali, C.; Cencetti, G.; Michelozzi, M.; Frassinetti, S.; Giorgetti, L.; et al. Drought stress modulates secondary metabolites in Brassica oleracea L. convar. acephala (DC) Alef, var. sabellica L. J. Sci. Food Agric. 2019, 99, 5533–5540. [Google Scholar] [CrossRef]
- Guarino, S.; Abbate, L.; Mercati, F.; Fatta Del Bosco, S.; Motisi, A.; Arif, M.A.; Cencetti, G.; Palagano, E.; Michelozzi, M. Citrus varieties with different tolerance grades to Tristeza virus show dissimilar volatile terpene profiles. Agronomy 2021, 11, 1120. [Google Scholar] [CrossRef]
- Srikrishnah, S.; Begam, U.J. Review on Use of Plant Extracts in Weed Control. Curr. Trends Biomed. Eng. Biosci. 2019, 18, 555993. [Google Scholar] [CrossRef]
- Khanh, T.D.; Trung, K.H.; Xuan, T. Allelopathy of Barnyardgrass (Echinochloa crusgalli) Weed: An Allelopathic Interaction with Rice (Oryza sativa L.). Vietnam J. Agric. Sci. 2018, 1, 97–116. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Barnyard grass-induced rice allelopathy and momilactone B. J. Plant Physiol. 2011, 168, 1016–1020. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Eurostat. EU Production and Trade in Oranges. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20190107-1 (accessed on 10 March 2023).
- Comparetti, A.; Febo, P.; Greco, C.; Mammano, M.M.; Orlando, S. Sicilian potential biogas production from Citrus industry by-product. In Proceedings of the 11th International AIIA Conference “Biosystems Engineering Addressing the Human Challenges of the 21st Century”, Bari, Italy, 5–8 July 2016; pp. 169–173. Available online: https://iris.unipa.it/retrieve/handle/10447/244144/459895/2017_AIIA_SicilianPotentialBiogasProductionCitrusIndustryBy-product.pdf (accessed on 10 March 2023).
- European Commission. Consolidated Version of the Treaty Establishing the European Community. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:11997E/TXT&rid=1 (accessed on 12 December 2022).
- European Commission. Commission Directive 98/67/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0067 (accessed on 12 December 2022).
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Forgács, G.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J.; Hováth, I.S. Methane production from citrus wastes: Process development and cost estimation. J. Chem. Technol. Biotechnol. 2012, 87, 250–255. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochem. Eng. J. 2016, 109, 9–18. [Google Scholar] [CrossRef]
- Martinez, J.; Jorge, C.; Rosas, M.J.G. Influence of biochar addition in the anaerobic digestion of complex substrates: Sewage sludge and orange peels. In Proceedings of the SUM2016, Third Symposium on Urban Mining and Circular Economy, Bergamo, Italy, 23–25 May 2016. [Google Scholar]
- Siles, J.A.; Vargas, F.; Gutiérrez, M.C.; Chica, A.F.; Martín, M.A. Integral valorisation of waste orange peel using combustion, biomethanisation and co-composting technologies. Bioresour. Technol. 2016, 211, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Panno, D.; Volpe, R.; Messineo, A. Upgrade of citrus waste as a biofuel via slow pyrolysis. J. Anal. Appl. Pyrolysis 2015, 115, 66–76. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Techno-economic and environmental assessment of essential oil extraction from Oregano (Origanum vulgare) and Rosemary (Rosmarinus officinalis) in Colombia. J. Clean. Prod. 2016, 112, 172–181. [Google Scholar] [CrossRef]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies Alba Mill.) Needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-thermal, energy efficient hydrodynamic cavitation for food processing, process intensification and extraction of natural bioactives: A review. Ultrason. Sonochem. 2023, 98, 106504. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Sawant, O.; Sonawane, S.K.; Show, P.L.; Waghamare, A.; Hilares, R.; Dos Santos, J.C. Novel, Nonthermal, Energy efficient, industrially scalable hydrodynamic cavitation–Applications in food processing Food. Rev. Int. 2020, 36, 668–691. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; de Carlo, A.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; et al. Volatile Compounds of Lemon and Grapefruit IntegroPectin. Molecules 2021, 26, 51. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.; Crisci, A.; Albanese, L.; Cencetti, G.; Maienza, A.; Michelozzi, M.; Zabini, F.; Meneguzzo, F. Effects of silver fir (Abies alba Mill.) needle extract produced via hydrodynamic cavitation on seed germination. Plants 2021, 10, 1399. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 5 June 2022).
- Onofri, A.; Benincasa, P.; Mesgaran, M.B.; Ritz, C. Hydrothermal-time-to-event models for seed germination. Eur. J. Agron. 2018, 101, 129–139. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lei, Z.; Zhang, G.; Pilon, A.C.; Huhman, D.V.; Xie, R.; Xi, W.; Zhou, Z.; Sumner, L.W. Metabolite profiles of essential oils in citrus peels and their taxonomic implications. Metabolomics 2015, 11, 952–963. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.N.; Lima, M.I.S. Allelopathic effects of orange (Citrus sinensis L.) peel essential oil. Acta Bot. Bras. 2012, 26, 256–259. [Google Scholar] [CrossRef]
- Pino, J.A.; Cuevas-Glory, L.; Sauri-Duch, E. Volatile Constituents of Peel and Leaf Oils of Cajel Orange (Citrus sinensis [L.] Osbeck). J. Essent. Oil-Bear. Plants 2010, 13, 742–746. [Google Scholar] [CrossRef]
- Jabalpurwala, F.A.; Smoot, J.M.; Rouseff, R.L. A comparison of citrus blossom volatiles. Phytochemistry 2009, 70, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Boussaada, O.; Chemli, R. Chemical Composition of Essential Oils from Flowers, Leaves and Peel of Citrus aurantium L. var. amara from Tunisia. J. Essent. Oil-Bear. Plants 2006, 23, 870–889. [Google Scholar] [CrossRef]
- Türker, M.; Battal, A.G.; Gulluce, M.; Sahin, F.; Erez, M.; Yildirim, N. Allelopathic effects of plants extracts on physiological and cytological processes during maize seed germination. Allelopath. J. 2008, 21, 273–286. [Google Scholar]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Pellissier, F. Improved germination bioassays for allelopathy research. Acta Physiol. Plant. 2013, 35, 23–30. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Tanaka, Y. Allelopathic potential of Citrus junos fruit waste from food processing industry. Bioresour. Technol. 2004, 94, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Assessment of allelopathic potential of shoot powder of lemon balm. Sci. Hortic. 2003, 97, 419–423. [Google Scholar] [CrossRef]
- Suwanagul, D.; Pitaksaringkarn, W.; Suwanagul, A. Allelopathic potential of Citrus reticulata peel extract for weed control. Rev. Int. Am. Stud. 2016, 6, 17–21. [Google Scholar] [CrossRef]
- Ishii-Iwamoto, E.L.; Coelho, E.M.P.; Reis, B.; Moscheta, I.S.; Bonato, C.M. Effects of Monoterpenes on Physiological Processes During Seed Germination and Seedling Growth. Curr. Bioact. Compd. 2012, 8, 50–64. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Vaid, S.U.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Phytotoxicity of limonene against Amaranthus viridis L. Bioscan 2011, 6, 163–165. [Google Scholar]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z. Naturforschung C 2007, 62, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Oksanen, E.J.; Holopainen, J.K. Effects of limonene on the growth and physiology of cabbage (Brassica oleracea L.) and carrot (Daucus carota L.) plants. J. Sci. Food Agric. 2004, 84, 1319–1326. [Google Scholar] [CrossRef]
- Consoli, S.; Caggia, C.; Russo, N.; Randazzo, C.L.; Continella, A.; Modica, G.; Cacciola, S.O.; Faino, L.; Reverberi, M.; Baglieri, A.; et al. Sustainable Use of Citrus Waste as Organic Amendment in Orange Orchards. Sustainability 2023, 15, 2482. [Google Scholar] [CrossRef]
- Abbate, C.; Tuttobene, R.; Avola, G.; Gennari, M. The Effect of Citrus Pulp Amendment on Sunflower Production and the Dissipation of the Herbicide Aclonifen. Ital. J. Agron. 2007, 3, 341–347. [Google Scholar] [CrossRef]
- Sîrbu, C.; Șuşnia, I. New records in the alien flora of Romania: Euphorbia serpens and E. glyptosperma. J. Plant Dev. 2018, 25, 135–144. [Google Scholar] [CrossRef]
- Kanatas, P.; Zavra, S.-M.; Tataridas, A.; Gazoulis, I.; Antonopoulos, N.; Synowiec, A.; Travlos, I. Pelargonic Acid and Caraway Essential Oil Efficacy on Barnyardgrass (Echinochloa crus-galli (L.) P.Beauv.) and Johnsongrass (Sorghum halepense (L.) Pers.). Agronomy 2022, 12, 1755. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).