Optimizing Biogas Production and Digestive Stability through Waste Co-Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Activated Sludge
2.2. Experimental Design
2.2.1. Experimental Conditions
Experimental Conditions for Experiment 1
Experimental Conditions for Experiment 2
2.3. Kinetic Model
2.4. Analytical Methods
2.5. Theoretical Methane Yield and Biodegradability
3. Results and Discussion
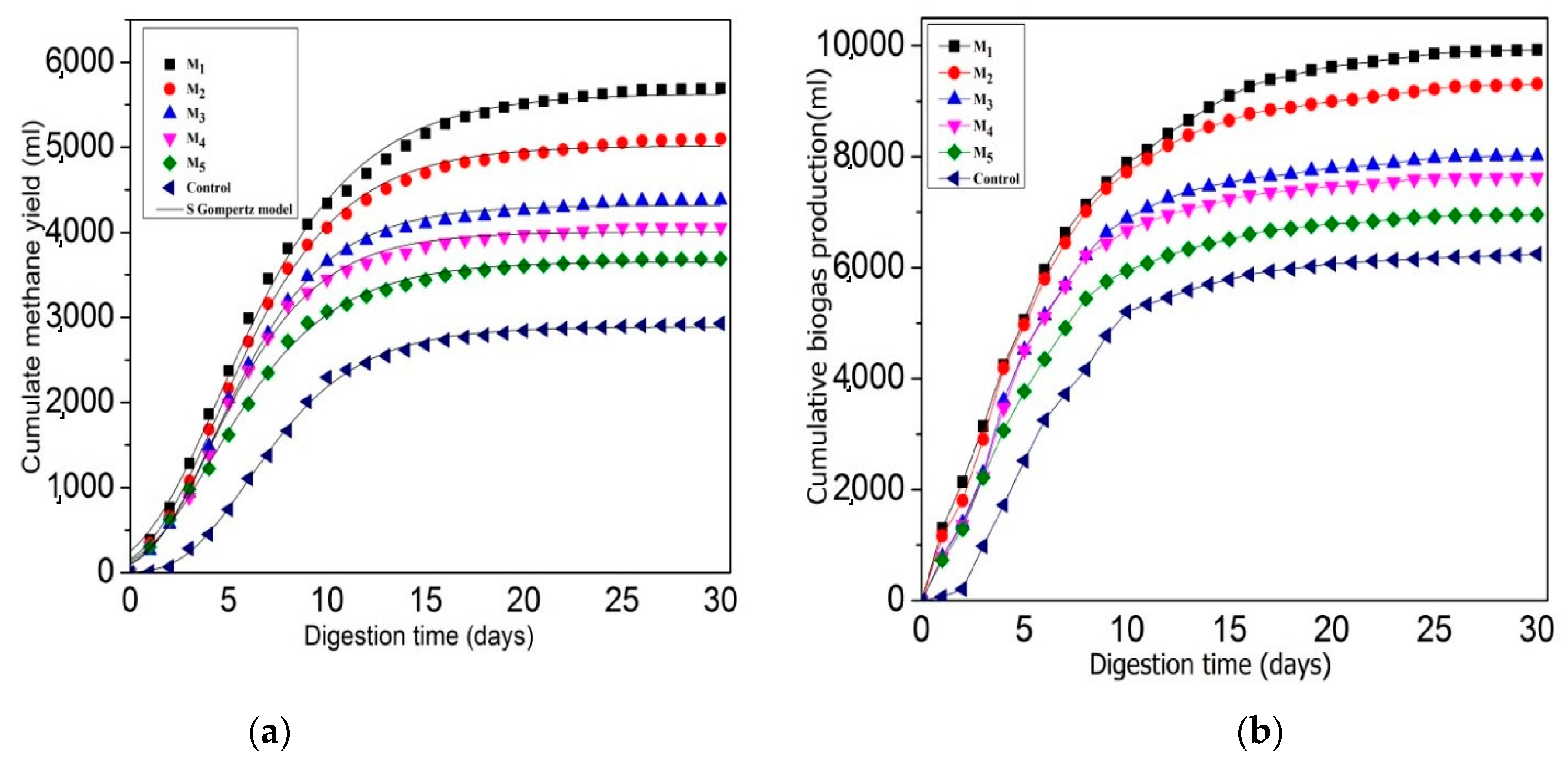
3.1. Effect of C/N Ratio on Anaerobic Digestion Performance and Stability
3.1.1. Effect of C/N Ratio for Biogas and Bio-Methane Yield
3.1.2. Effect of C/N Ratio for Digestate Stability
3.1.3. TS and VS Reduction
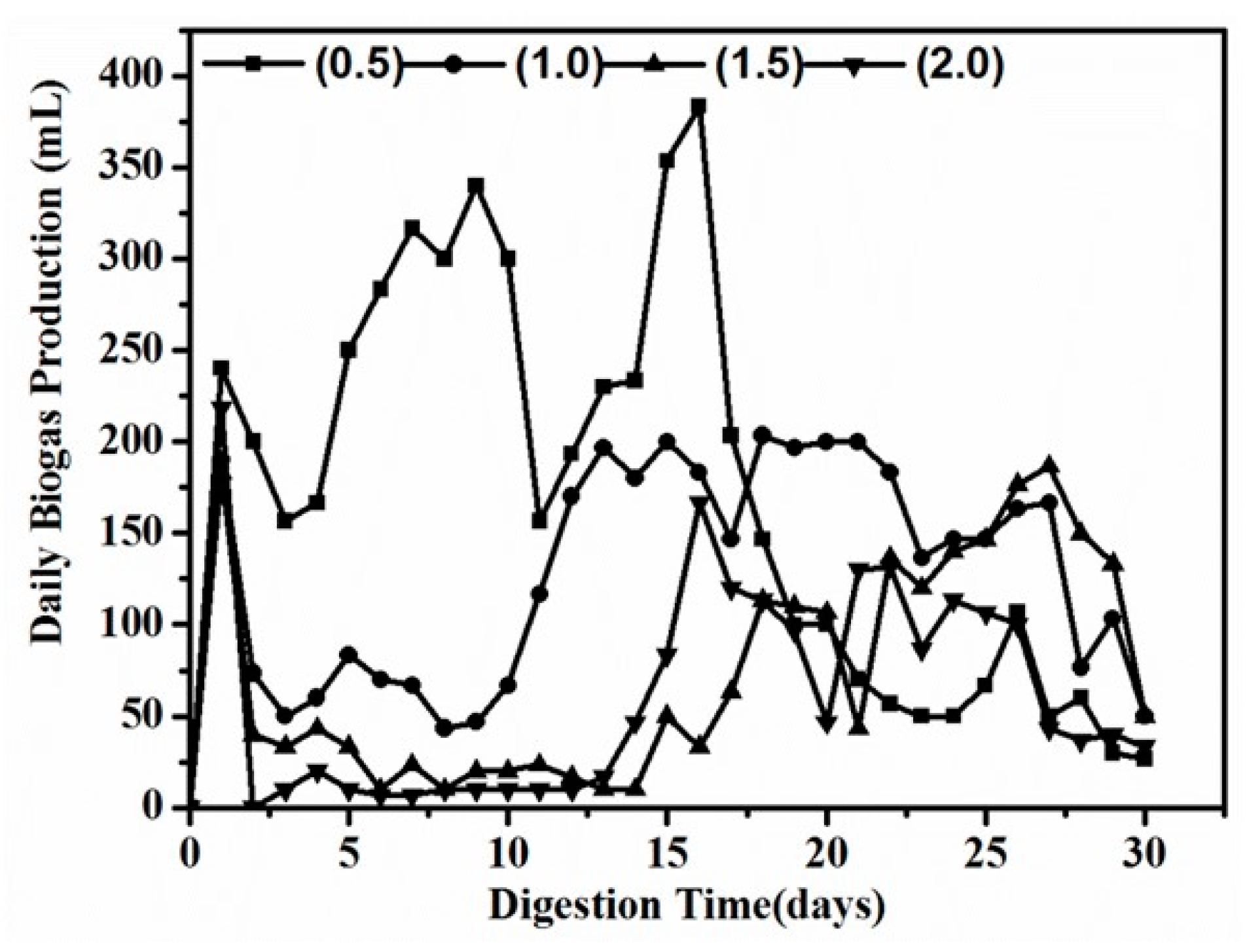
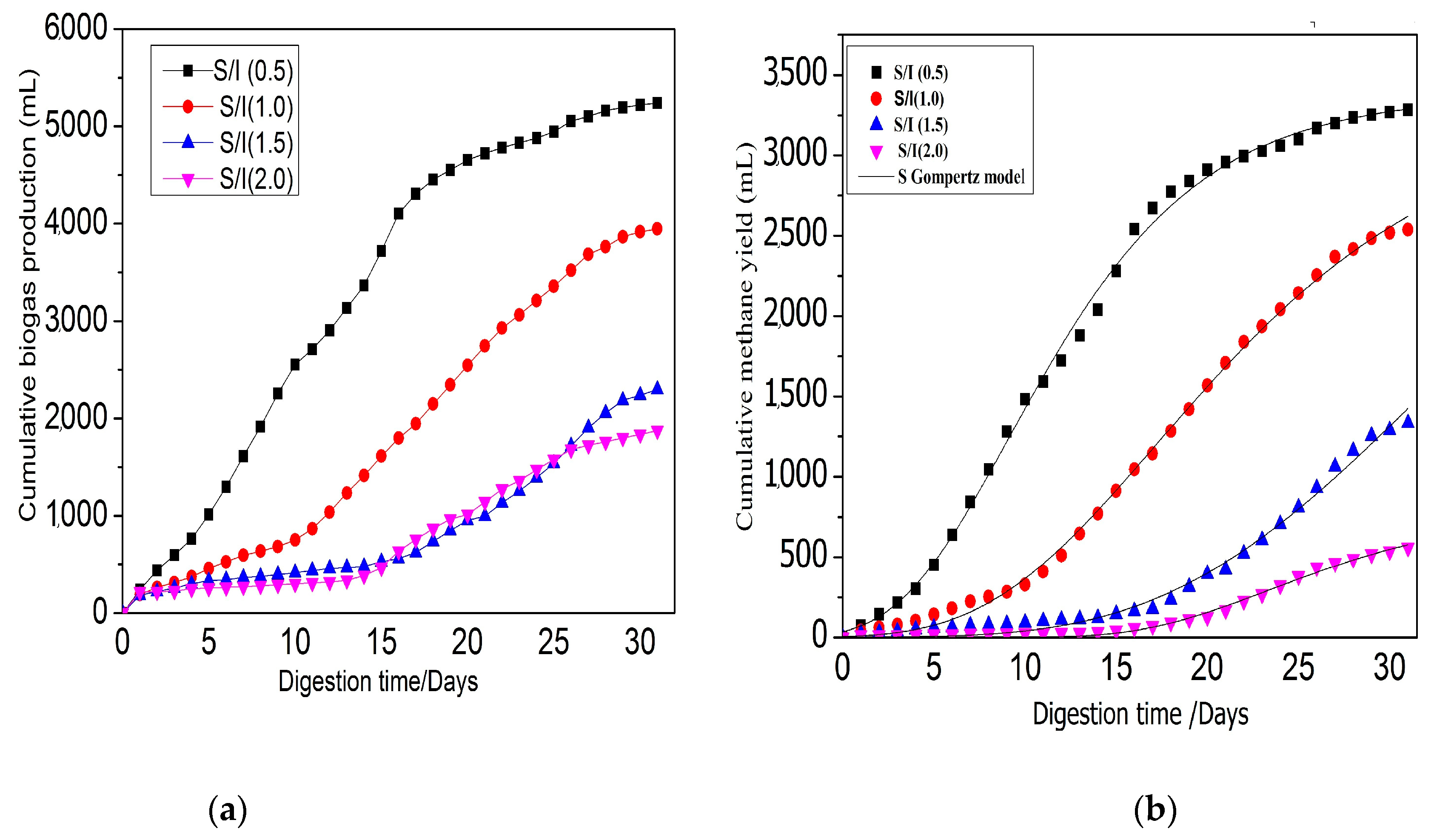
3.2. Effect of S/I Ratio on Anaerobic Digestion Performance and Stability
3.2.1. Effect of S/I Ratio for Biogas and Bio-Methane Yield
3.2.2. Effect of S/I Ratio on Digester Stability and PH
3.2.3. Graphically Examined Process Stability
3.2.4. Biodegradability and Methane Yield
3.3. Kinetic Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T.; Ahmad, N.; Sher, F.; Khan, S.U.D.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and emerging perspective of biomass based waste-to-energy technologies and their socio-environmental impact: A review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef]
- Lewis, A.W.; Yuen, S.T.S.; Smith, A.J.R. Detection of gas leakage from landfills us-ing infrared thermography—applicability and limitations. Waste Manag. Res. 2003, 21, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Jagaba, A.; Kutty, S.; Lawal, I.; Abubakar, S.; Hassan, I.; Zubairu, I.; Umaru, I.; Abdurrasheed, A.; Adam, A.; Ghaleb, A.; et al. Sequencing batch reactor technology for landfill leachate treatment: A state-of-the-art review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef]
- Krylova, N.I.; Khabiboulline, R.E.; Naumova, R.P.; Nagel, M.A. The influence of am-monium and methods for removal during the anaerobic treatment of poultry manure. J. Chem. Technol. Biotechnol. 1997, 70, 99–105. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, R.; Gikas, P.; Rapport, J.; Jenkins, B.; Li, X. Biogas production from municipal solid wastes using an integrated rotary drum and anaerobic-phased solids digester system. Bioresour. Technol. 2010, 101, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Kalavrouziotis, I. K (Ed.). Wastewater and Biosolids Management; Iwa Publishing: London, UK, 2017; p. 16. [Google Scholar] [CrossRef]
- The, L.; In, N. California Integrated Waste Management Board 2007 Annual Report 2007 Annual Report Overview: California, USA. 2007; pp. 1–15. Available online: https://dpw.lacounty.gov/epd/tf/Attachments/Minutes_Attachments/Attachments_031507.pdf (accessed on 28 March 2024).
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Davidson, O.; Hare, W.; Huq, S.; Karoly, D.; Kattsov, V.; et al. Climate Change 2007: Summary for Policymakers. Hemisphere 2007, 335, 12–17. [Google Scholar] [CrossRef]
- Lebiocka, M.; Piotrowicz, A. Co-digestion of sewage sludge and organic fraction of mu-nicipal solid waste. A comparison between laboratory and technical scales. Environ. Prot. Eng. 2012, 38, 157–162. [Google Scholar] [CrossRef]
- Kushwaha, A.; Mishra, V.; Gupta, V.; Goswami, S.; Gupta, P.K.; Singh, L.K.; Gupt, C.B.; Rakshit, K.; Goswami, L. Anaerobic digestion as a sustainable biorefinery concept for waste to energy conversion. In Waste-to-Energy Approaches Towards Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 129–163. [Google Scholar]
- World Bank. What a waste: A global review of solid waste management: Waste Composition. In Urban Development Series Knowledge Paper; World Bank: Washington, DC, USA, 2012; pp. 16–21. [Google Scholar]
- Chen, X.; Pang, Y.; Zou, D.; Yuan, H.; Liu, Y.; Zhu, B.; Li, X. Optimizing Acidogenic Process for Achieving High Biomethane Yield from Anaerobic Co-Digestion of Food Waste and Rice Straw Using Response Surface Methodology. J. Biobased Mater. Bioenergy 2014, 8, 512–518. [Google Scholar] [CrossRef]
- Chufo, A.; Yuan, H.; Zou, D.; Pang, Y.; Li, X. Biomethane production and physicochemical characterization of anaerobically digested teff (Eragrostis tef) straw pretreated by sodium hydroxide. Bioresour. Technol. 2015, 181, 214–219. [Google Scholar] [CrossRef]
- Callaghan, F.; Wase, D.; Thayanithy, K.; Forster, C. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass-Bioenergy 2002, 22, 71–77. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Yi, X.; Zhao, Y.; Jin, F.; Chen, L.; Hua, D. Study of two-phase anaerobic digestion of corn stover: Focusing on the conversion of volatile fatty acids and microbial characteris-tics in UASB reactor. Ind. Crops Prod. 2021, 160, 113097. [Google Scholar] [CrossRef]
- Kunatsa, T.; Xia, X. A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 2021, 344, 126311. [Google Scholar] [CrossRef] [PubMed]
- Stroot, P.G.; McMahon, K.D.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. digester performance. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Mshandete, A.; Kivaisi, A.; Rubindamayugi, M.; Mattiasson, B. Anaerobic batch co-digestion of sisal pulp and fish wastes. Bioresour. Technol. 2004, 95, 19–24. [Google Scholar] [CrossRef]
- Hajji, A.; Rhachi, M. The influence of particle size on the performance of anaerobic digestion of municipal solid waste. Energy Procedia 2013, 36, 515–520. [Google Scholar] [CrossRef]
- Neves, L.; Oliveira, R.; Alves, M. Fate of LCFA in the co-digestion of cow manure, food waste and discontinuous addition of oil. Water Res. 2009, 43, 5142–5150. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Lusk, P. Latest progress in anaerobic digestion. Biocycle 1999, 7, 40. [Google Scholar]
- Kuma, S.; Bharti, A.; Rich, R. Management of Organic Waste. Environ. Eng. 2012, 252, 83–90. [Google Scholar]
- Lopes, A.D.C.P.; Ebner, C.; Gerke, F.; Wehner, M.; Robra, S.; Hupfauf, S.; Bockreis, A. Residual municipal solid waste as co-substrate at wastewater treatment plants: An assessment of me-thane yield, dewatering potential and microbial diversity. Sci. Total Environ. 2022, 804, 149936. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.W.; Zhang, D.L.; Wang, M.X. Effects of the inoculum size, the volume of liq-uid medium and the size of flasks on the degradation of dibenzothiophene by Pseudomonas dela-fieldii. J. Environ. Sci. 2008, 20, 945–950. [Google Scholar]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J. Environ. Sci. Healthy Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L. Acetogenesis, Acetogenic Bacteria, and the Acetyl-CoA “Wood/Ljungdahl” Pathway: Past and Current Perspectives. In Acetogenesis. Chapman & Hall Microbiology Series; Drake, H.L., Ed.; Springer: Boston, MA, USA, 1994; pp. 3–60. [Google Scholar] [CrossRef]
- Al Afif, R.; Pfeifer, C. Enhancement of methane yield from cotton stalks by mechanical pre-treatment. Carbon Resour. Convers. 2021, 4, 164–168. [Google Scholar] [CrossRef]
- Oliva, A.; Papirio, S.; Esposito, G.; Lens, P.N. Pretreatment of lignocellulosic materials to enhance their methane potential. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Springer: Cham, Switzerland, 2022; pp. 85–120. [Google Scholar]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 2010, 101, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.R.; Zeshan; Yousaf, S.; Malik, R.N.; Visvanathan, C. Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour. Technol. 2015, 190, 451–457. [Google Scholar] [CrossRef]
- Owamah, H.; Ikpeseni, S.; Alfa, M.; Oyebisi, S.; Gopikumar, S.; Samuel, O.D.; Ilabor, S. Influence of inoculum/substrate ratio on biogas yield and kinetics from the anaerobic co-digestion of food waste and maize husk. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100558. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- López-Malo, A.; Alzamora, S.M.; Paris, M.J.; Lastra-Vargas, L.; Coronel, M.B.; Gómez, P.L.; Palou, E. Naturally occurring compounds–plant sources. In Antimicrobials in Food, 4th ed.; Davidson, P.M., Taylor, T.M., David, J.R.D., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 527–594. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- Gallert, C.; Henning, A.; Winter, J. Scale-up of anaerobic digestion of the biowaste fraction from domestic wastes. Water Res. 2003, 37, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Masih-Das, J.; Tao, W. Anaerobic co-digestion of food waste with liquid dairy manure or manure digestate: Co-substrate limitation and inhibition. J. Environ. Manag. 2018, 223, 917–924. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Allison, S.D. Cheaters, diffusion, and nutrients constrain decomposition by microbial en-zymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical methane potential (BMP) of food waste and primary sludge: Influence of inoculum pre-incubation and inoculum source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Sripitak, B. Biogas Production from Small Farm Cow Manure by High Solid Three-Stage Anaerobic Digester. Ph.D. Thesis, Prince of Songkla University, Songkhla, Thailand, 2022. [Google Scholar]
- Tanimu, M.I.; Ghazi TI, M.; Harun, R.M.; Idris, A. Effect of Carbon to Nitrogen Ratio of Food Waste on Biogas Methane Production in a Batch Mesophilic Anaerobic Digester. Int. J. Innov. Manag. Technol. 2014, 5, 116–119. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Q.; Zhao, X.; Cai, Y.; Ma, X.; Fu, J.; Wang, P.; Liu, W.; Ren, L. A review of the technologies used for preserving anaerobic digestion inoculum. Renew. Sustain. Energy Re-Views 2023, 188, 113804. [Google Scholar] [CrossRef]
- Widyarani Victor, Y.; Sriwuryandari, L.; Priantoro, E.A.; Sembiring, T.; Sintawardani, N. Influence of pH on biogas production in a batch anaerobic process of tofu wastewater. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 160, p. 012014. [Google Scholar]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.; Porrier, P.; Chamy, R. Effect of inoculum-substrate ratio on the start-up of solid waste anaerobic digesters. Water Sci. Technol. 2001, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of an-aerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioen-Gineered 2019, 10, 437–458. [Google Scholar]
- Li, H.L.; Guo, X.L.; Cao, F.F.; Wang, Y. Process evolution of dry anaerobic co-digestion of cattle manure with kitchen waste. Chem. Biochem. Eng. Q. 2014, 28, 161–166. [Google Scholar]
- Forster-Carneiro, T.; Pérez, M.; Romero, L.I. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour. Technol. 2008, 99, 6994–7002. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, F.; Yan, M.; Tang, S.; Duan, Z.; Sun, Y.; Li, Y. Effect of ammonia on an-aerobic digestion: Focusing on energy flow and electron transfer. Chem. Eng. J. 2023, 471, 144638. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, W.; Feng, L.; Li, J.; Xia, X.; Yu, H.; Liu, Y. Anaerobic Digestion Process and Biogas Production. In Biogas Plants: Waste Management, Energy Production and Carbon Footprint Reduction; Wiley: New York, NY, USA, 2024; pp. 1–35. [Google Scholar]
- Galeano, M.B.; Sulonen, M.; Ul, Z.; Baeza, M.; Baeza, J.A.; Guisasola, A. Bioelectro-chemical ammonium recovery from wastewater: A review. Chem. Eng. J. 2023, 15, 144855. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, J.; Wang, D.; Liang, J.; Zhou, L. Nitrogen removal performance of high ammonium and high salt wastewater by adding carbon source from food waste fer-mentation with different acidogenic metabolic pathways. Chemosphere 2022, 292, 13351. [Google Scholar] [CrossRef] [PubMed]
- Alexis, P.-O.B.; Torres-Lozada, P., 2nd; Marmolejo-Rebellón Luis, F., 3rd; Or-donez, L.J.A. Effect of Substrate-Inoculum Ratio on the Biochemical Methane Potential of Municipal Biowastes. Ing. Investig. Y Tecnol. 2015, 16, 515–526. [Google Scholar]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, J. Development of general Gompertz models and their simplified two-parameter forms based on specific microbial growth rate for microbial growth, bio-products and substrate consumption. Adv. Biotechnol. Microbiol. 2017, 4, 64–74. [Google Scholar]


| Parameter | Unit | FW | MSW | Inoculum |
|---|---|---|---|---|
| Total solid (TS) | % | 25.57 ± 0.8 | 96.31 ± 0.1 | 12.92 ± 1.2 |
| Volatile solid (VS) | % | 22.99 ± 0.5 | 88.97 ± 0.7 | 7.38 ± 1.0 |
| C (Total) | % | 53.56 ± 3.7 | 41.27 ± 0.1 | 27.21 ± 0.06 |
| N (Total) | % | 2.87 ± 0.3 | 0.1 ± 0.01 | 2.20 ± 0.07 |
| H (Total) | % | 7.45 ± 0.1 | 6.07 ± 0.3 | 4.10 ± 0.08 |
| S (Total) | % | 0.83 ± 0.5 | 0.01 ± 0.1 | 0.84 ± 0.02 |
| C/N | - | 18.7 ± 0.9 | 412.7 ± 7.0 | 12.4 ± 0.06 |
| Cellulose | % | 15.01 ± 0.5 | 63.20 ± 12.5 | 10.15 ± 0.02 |
| Hemi–Cellulose | % | 20.12 ± 1.6 | 15.50 ± 14.7 | 12.50 ± 1.8 |
| Lignin | % | 2.01 ± 1.7 | 4.17 ± 0.34 | 15.66 ± 0.7 |
| Mixtures | Food Waste (g) | MSW (g) | Mixtures Ratio | C/N Ratio |
|---|---|---|---|---|
| M1 | 40.3 | 1.8 | 22.39:1 | 20.9 |
| M2 | 30.9 | 3.9 | 7.92:1 | 25.1 |
| M3 | 24 | 6.01 | 4.0:1 | 31.1 |
| M4 | 20.5 | 6.9 | 2.97:1 | 35.2 |
| M5 | 19.2 | 9.3 | 2.06:1 | 42.1 |
| OLR (gVS/L) | S/I Ratio | Sludge Weight (g) | Food Weight (g) | MSW Weight (g) |
|---|---|---|---|---|
| 10 | 0.5 | 257 ± 0.19 | 22.55 ± 0.03 | 1.24 ± 0.03 |
| 10 | 1.0 | 128 ± 0.10 | 22.55 ± 0.03 | 1.24 ± 0.03 |
| 10 | 1.5 | 83 ± 0.08 | 22.55 ± 0.03 | 1.24 ± 0.03 |
| 10 | 2.0 | 65 ± 0.70 | 22.55 ± 0.03 | 1.24 ± 0.03 |
| Parameters | Units | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|---|
| CBP b | (mL) | 9930 | 9309 | 8020 | 7234 | 63 |
| SMY | (L/Kg vs.) | 454.44 | 432.81 | 380.17 | 343.02 | 303.07 |
| TS removed | (%) | 87 | 76 | 79 | 78 | 65 |
| VS removed | (%) | 89 | 86 | 84 | 80 | 70 |
| TMY a | (mL) | 630 | 616 | 597 | 587 | 576 |
| EMY b | (L/Kg vs.) | 456 | 422 | 368 | 354 | 312 |
| Alkalinity | (g/L) | 5.633 | 5.423 | 4.654 | 5.023 | 4.965 |
| Ammonia | (g/L) | 1.25 | 0.92 | 0.92 | 1.02 | 0.67 |
| COD | (g/L) | 23.92 | 27.05 | 25.31 | 35.37 | 30.84 |
| PH | 7.3 | 7.1 | 7.5 | 7.2 | 7.2 | |
| Hemi-Cellulose | (%) | 9.9 | 7.0 | 7.8 | 7.8 | 8.3 |
| Cellulose | (%) | 12.9 | 23.5 | 32.8 | 32.4 | 31.6 |
| Lignin | (%) | 1.6 | 5.4 | 7.5 | 12.4 | 17.4 |
| LCH | (%) | 26.6 | 37.1 | 45.4 | 53.8 | 57.7 |
| Parameters | Unit | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|---|
| SBY a | L/kg VS | 656 | 485 | 289 | 254 |
| CMY b | mL | 3543 | 2654 | 1234 | 587 |
| Biodegradability | % | 67 | 56 | 26 | 20 |
| TMY a | L/kg VS | 634 | 654 | 643 | 632 |
| EMY c | L/kg VS | 406 | 320 | 147 | 118 |
| Alkalinity | mg/L | 4765 | 4416 | 4533 | 5400 |
| Ammonia-N | mg/L | 990 ± 0.08 | 645 ± 0.04 | 664 ± 0.02 | 1075 ± 0.10 |
| COD | mg/L | 14,540 ± 0.97 | 6680 ± 3.21 | 5995 ± 2.88 | 6678 ± 3.65 |
| pH | 7.4 | 7.4 | 7.4 | 7.3 |
| Substrate | BGP (mL g VS−1) | Rm (mL g VS−1 d) | λ (d) | R2 |
|---|---|---|---|---|
| M1 | 5745 | 550 | 0.12 | 0.99 |
| M2 | 5231 | 545 | 0.23 | 0.99 |
| M3 | 4547 | 500 | 0.30 | 0.99 |
| M4 | 4231 | 490 | 0.30 | 0.99 |
| M5 | 3658 | 385 | 0.17 | 0.99 |
| S/I(0.5) | 3543 | 245 | 0.58 | 0.99 |
| S/I(1.0) | 3432 | 145 | 0.90 | 0.99 |
| S/I(1.5) | 4523 | 123 | 1.31 | 0.98 |
| S/I(2.0) | 905 | 55 | 2.48 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.M.; Javied, S.; Aslam, A.; Alamri, S.; Zaman, Q.u.; Hassan, A.; Noor, N. Optimizing Biogas Production and Digestive Stability through Waste Co-Digestion. Sustainability 2024, 16, 3045. https://doi.org/10.3390/su16073045
Ahmad RM, Javied S, Aslam A, Alamri S, Zaman Qu, Hassan A, Noor N. Optimizing Biogas Production and Digestive Stability through Waste Co-Digestion. Sustainability. 2024; 16(7):3045. https://doi.org/10.3390/su16073045
Chicago/Turabian StyleAhmad, Rao Muhammad, Sabiha Javied, Ambreen Aslam, Saud Alamri, Qamar uz Zaman, Ayesha Hassan, and Nabeela Noor. 2024. "Optimizing Biogas Production and Digestive Stability through Waste Co-Digestion" Sustainability 16, no. 7: 3045. https://doi.org/10.3390/su16073045
APA StyleAhmad, R. M., Javied, S., Aslam, A., Alamri, S., Zaman, Q. u., Hassan, A., & Noor, N. (2024). Optimizing Biogas Production and Digestive Stability through Waste Co-Digestion. Sustainability, 16(7), 3045. https://doi.org/10.3390/su16073045






