Study of a New Photocatalytic Film Process Combined with a Constructed Wetland and an Analysis of Reoxygenation Pathways in a Water Body
Abstract
1. Introduction
2. Materials and Methods
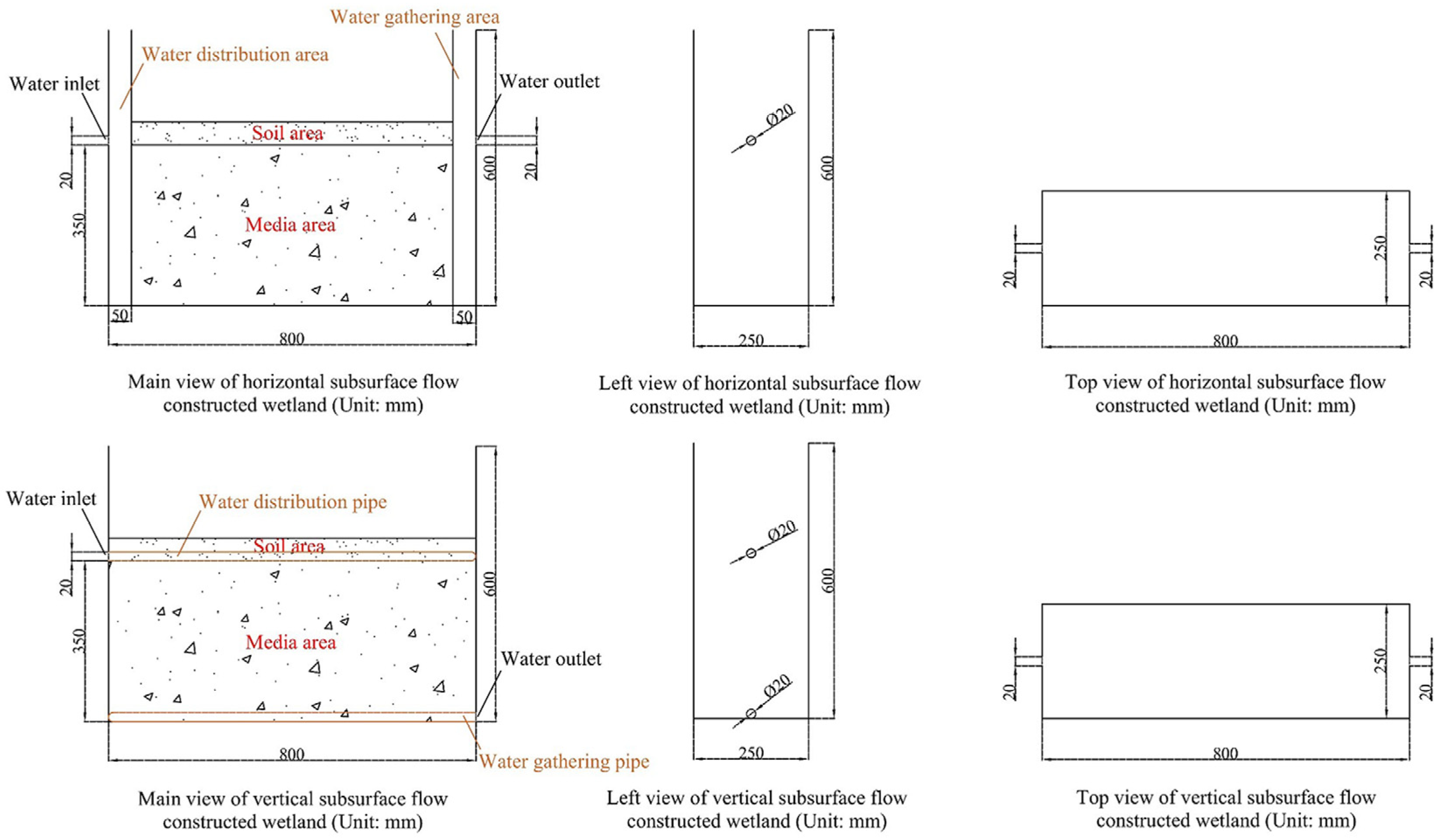
2.1. Construction and Operation of the Experimental Setup
2.2. Water Sampling and Analysis
2.3. PCR Amplification and Sequencing Library Construction
2.4. Statistical and Analysis
3. Results
3.1. Overall Treatment Performance
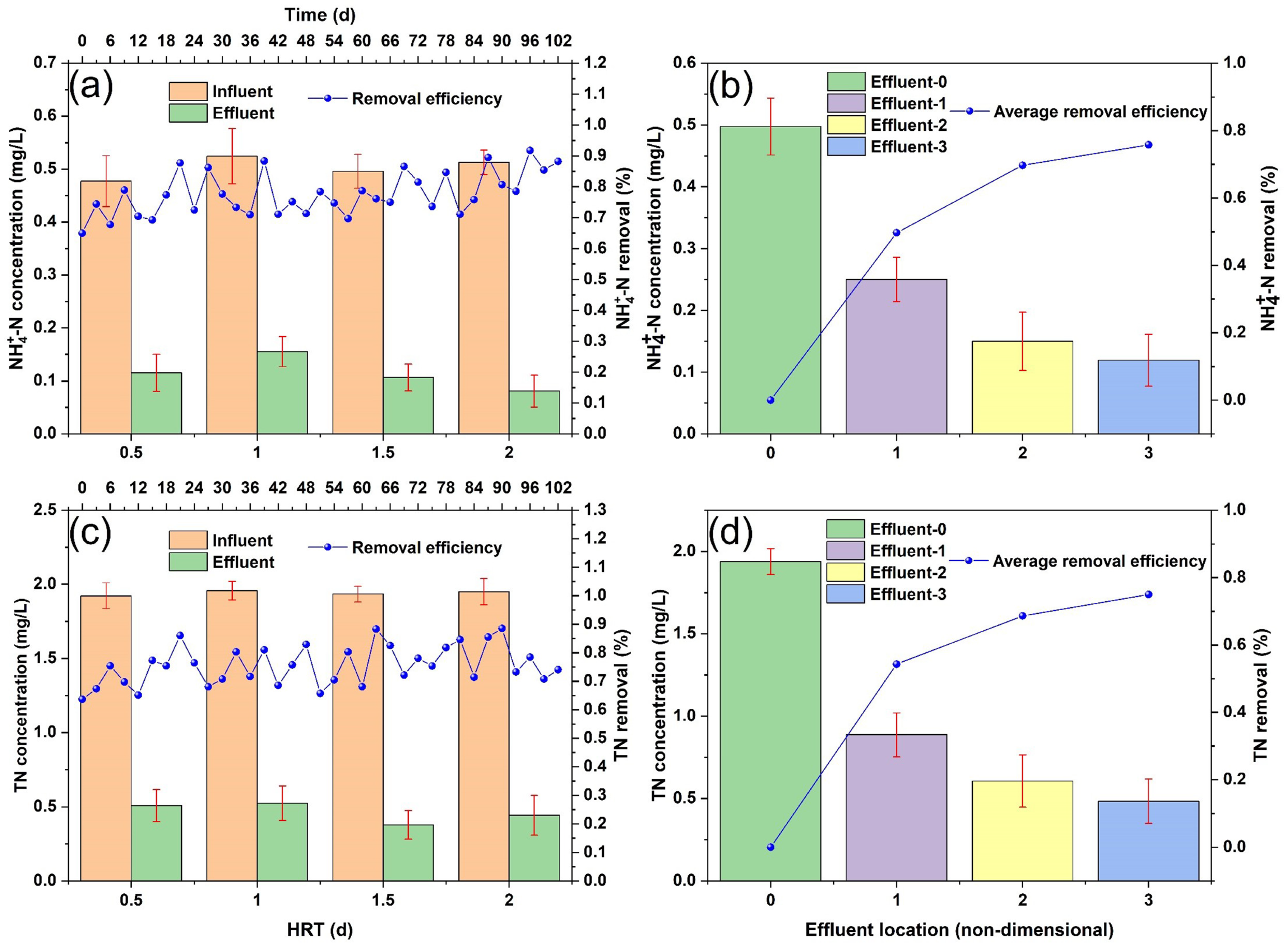
3.1.1. Nitrogen Removal
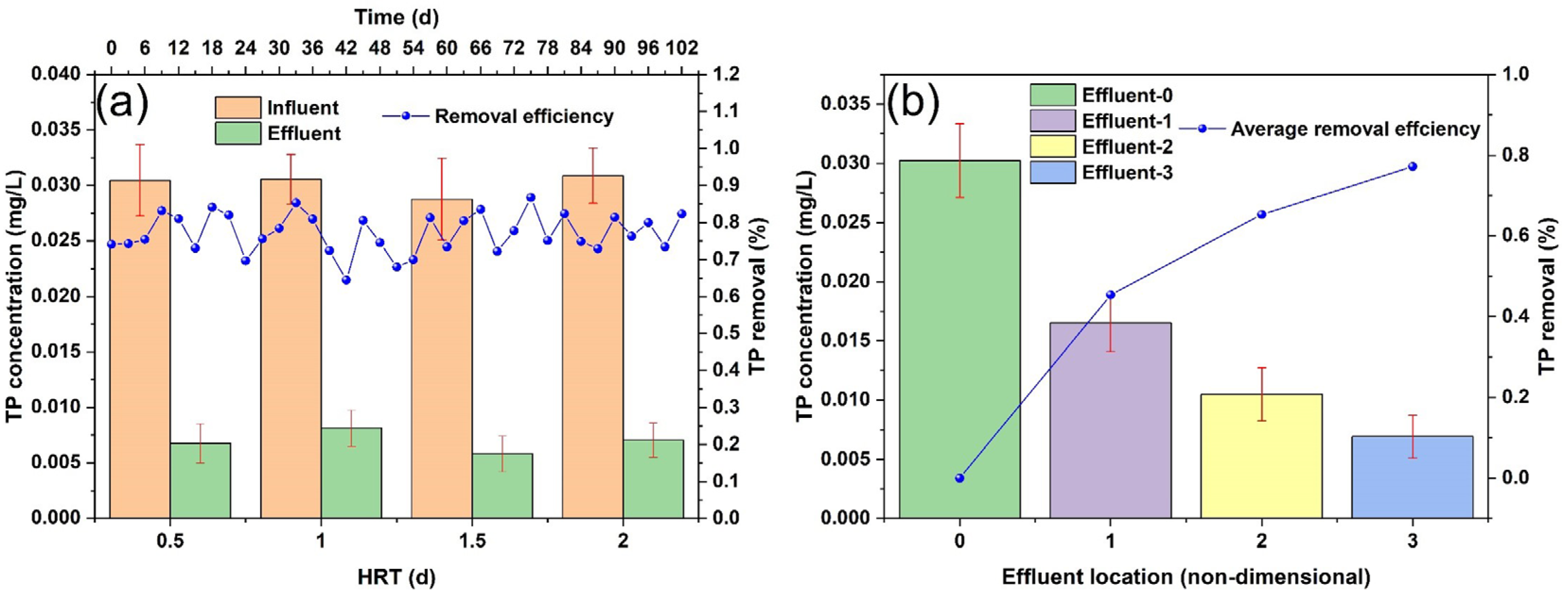
3.1.2. Phosphorus Removal
3.1.3. COD Removal
3.1.4. Chlorophyll a Removal
3.2. Changes in the Physicochemical Environment
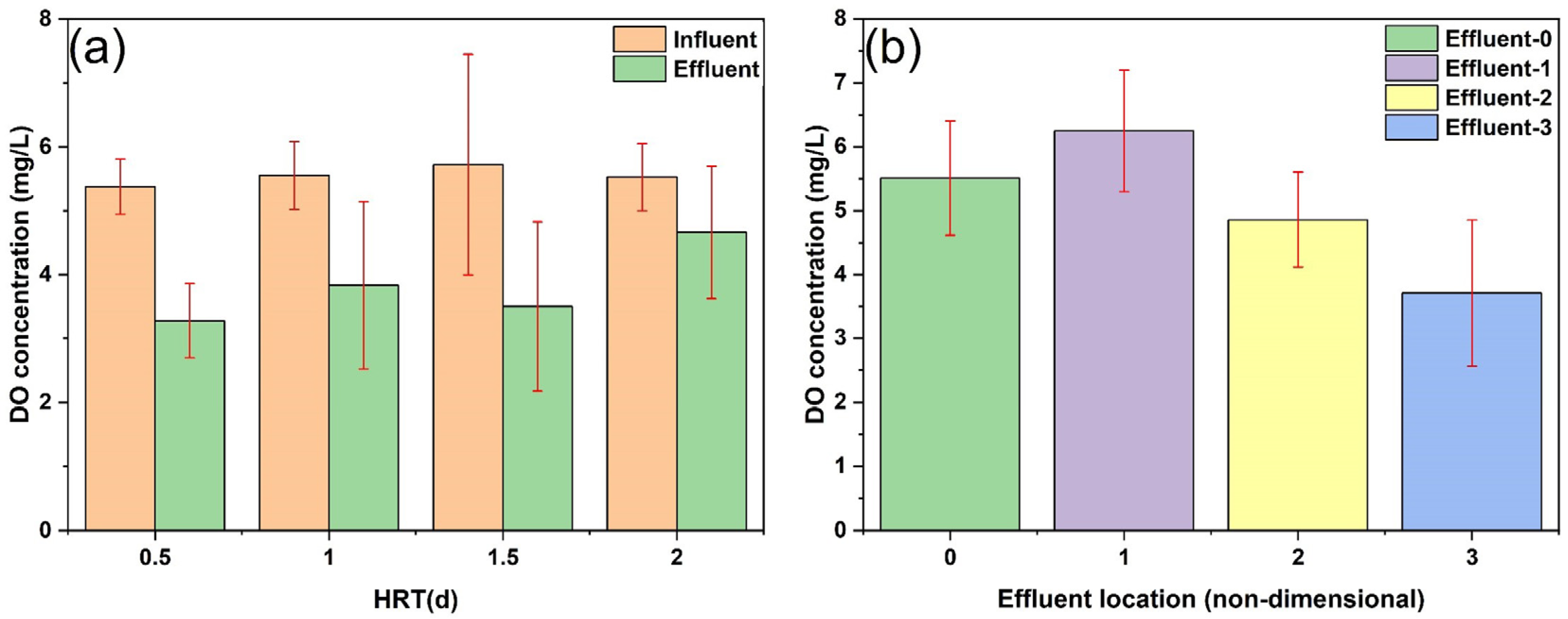
3.2.1. Changes in DO
3.2.2. Changes in pH
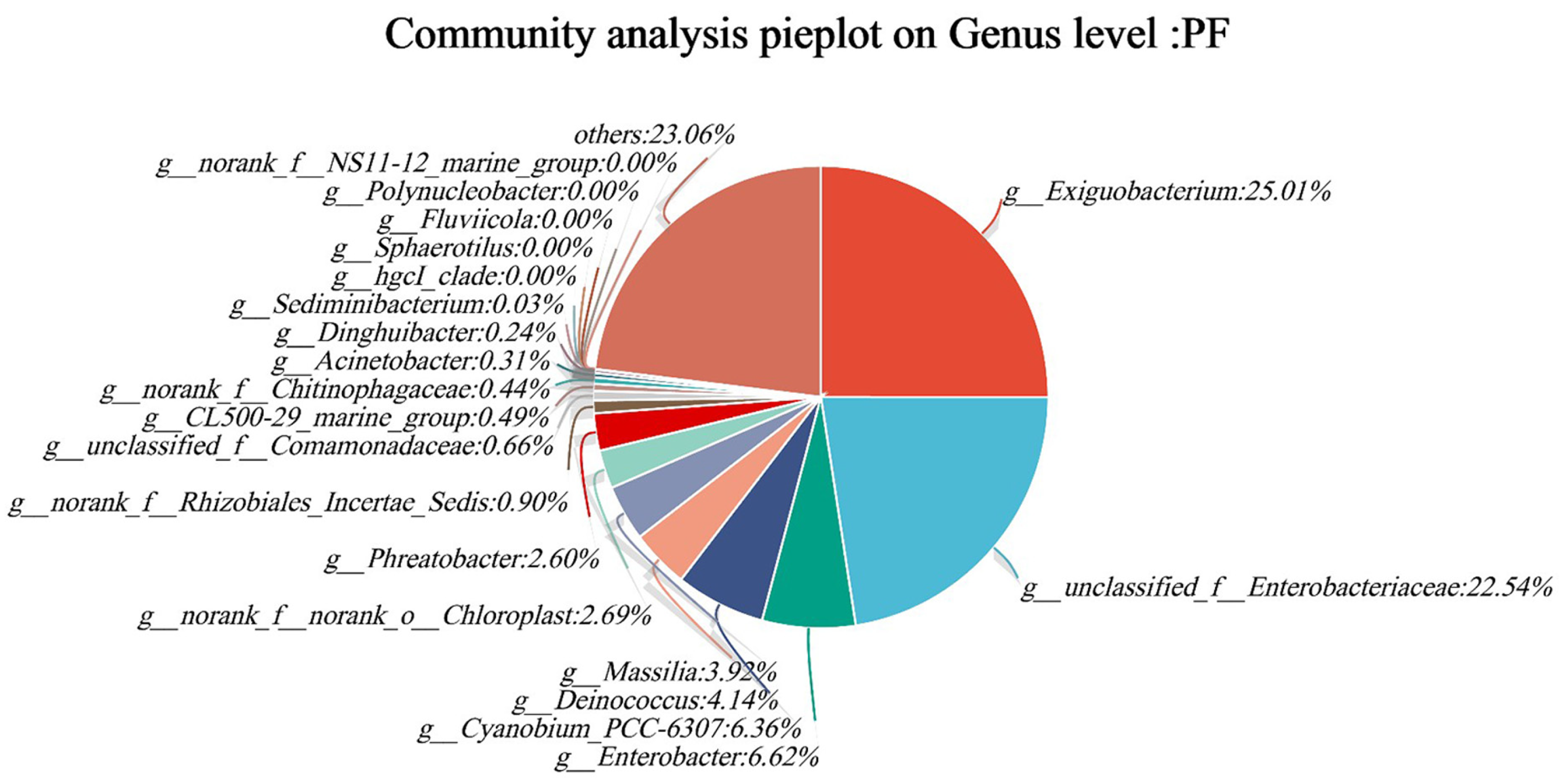
3.3. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez-Fernandez, M.I.; Vega, P.T.M.D.L.; Jaramillo-Morán, M.A.; Garrido, M. Hybrid Constructed Wetland to Improve Organic Matter and Nutrient Removal. Water 2020, 12, 2023. [Google Scholar] [CrossRef]
- Lu, J.; Guo, Z.; Kang, Y.; Fan, J.; Zhang, J. Recent advances in the enhanced nitrogen removal by oxygen-increasing technology in constructed wetlands. Ecotox. Environ. Saf. 2020, 205, 111330. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.D.; Davidson, T.A.; Jones, J.I. Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: A eutrophication-driven pathway from plants to plankton? Freshw. Biol. 2010, 55, 500–513. [Google Scholar] [CrossRef]
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 2012, 691, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, J.; Henriksen, P.; Heiskanen, A.-S. Summer algal blooms in shallow estuaries: Definition, mechanisms, and link to eutrophication. Limnol. Oceanogr. 2007, 52, 370–384. [Google Scholar] [CrossRef]
- Zhang, C.; Quan, B.; Tang, J.; Cheng, K.; Tang, Y.; Shen, W.; Su, P.; Zhang, C. China’s wastewater treatment: Status quo and sustainability perspectives. J. Water Process Eng. 2023, 53, 103708. [Google Scholar] [CrossRef]
- Lakusic, S. Constructed wetlands for wastewater treatment. J. Croat. Assoc. Civ. Eng. 2017, 69, 639–652. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Wu, S.; Yang, C.; Zeng, G. Microalgal and duckweed based constructed wetlands for swine wastewater treatment: A review. Bioresour. Technol. 2020, 318, 123858. [Google Scholar] [CrossRef]
- Singh, K.K.; Vaishya, R.C. Municipal Wastewater Treatment uses Vertical Flow Followed by Horizontal Flow in a Two-Stage Hybrid-Constructed Wetland Planted with Calibanus hookeri and Canna indica (Cannaceae). Water Air Soil Pollut. 2022, 233, 510–521. [Google Scholar] [CrossRef]
- Liang, M.-Y.; Han, Y.-C.; Easa, S.M.; Chu, P.-P.; Wang, Y.-L.; Zhou, X.-Y. New solution to build constructed wetland in cold climatic region. Sci. Total Environ. 2020, 719, 137124. [Google Scholar] [CrossRef]
- Yi, X.; Lin, D.; Li, J.; Zeng, J.; Wang, D.; Yang, F. Ecological treatment technology for agricultural non-point source pollution in remote rural areas of China. Environ. Sci. Pollut. Res. Int. 2021, 28, 40075. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Pei, F.F.; Song, X.K.; Guo, Z. Study on the Purification Effects of Constructed Wetland Plants in TP Disposal in Living Wastewater. Appl. Mech. Mat. 2012, 137, 357–361. [Google Scholar] [CrossRef]
- Shao, L.; Chen, G.Q. Embodied water accounting and renewability assessment for ecological wastewater treatment. J. Clean. Prod. 2016, 112, 4628–4635. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, C. Study on Phosphorus Removal Pathway in Constructed Wetlands with Thermally Modified Sepiolite. Sustainability 2022, 14, 12535. [Google Scholar] [CrossRef]
- Lu, S.; Pei, L.; Bai, X. Study on method of domestic wastewater treatment through new-type multi-layer artificial wetland. Int. J. Hydrogen Energy 2015, 40, 11207. [Google Scholar] [CrossRef]
- Greenway, M. Constructed Wetlands for Water Pollution Control—Processes, Parameters and Performance. Dev. Chem. Eng. Miner. Process. 2004, 12, 491–504. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jing, Z.; Tao, M.; Kong, Y.; Guan, L.; Jia, Q. A novel filter-type constructed wetland for secondary effluent treatment: Performance and its microbial mechanism. Bioresour. Technol. 2023, 380, 129075. [Google Scholar] [CrossRef]
- Tao, Z.; Jing, Z.; Tao, M.; Chen, R. Recycled utilization of ryegrass litter in constructed wetland coupled microbial fuel cell for carbon-limited wastewater treatment. Chemosphere 2022, 302, 134882. [Google Scholar] [CrossRef]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive Effects of Hypoxia and Temperature on Coastal Pelagic Zooplankton and Fish. Front. Mar. Sci. 2019, 6, 139–156. [Google Scholar] [CrossRef]
- Politikos, D.V.; Petasis, G.; Katselis, G. Interpretable machine learning to forecast hypoxia in a lagoon. Ecol. Inform. 2021, 66, 101480. [Google Scholar] [CrossRef]
- Breitburg, D. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuar. Coast. 2002, 25, 767–781. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Do Laboratory Scale Experiments Improve Constructed Wetland Treatment Technology? Environ. Sci. Technol. 2018, 52, 12956–12957. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-G.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Janssen, F.; Aleynik, D.; Bange, H.W.; Boltacheva, N.; Çagatay, M.N.; Dale, A.W.; Etiope, G.; Erdem, Z.; Geraga, M.; et al. Investigating hypoxia in aquatic environments: Diverse approaches to addressing a complex phenomenon. Biogeosciences 2014, 11, 1215–1259. [Google Scholar] [CrossRef]
- Mallin, M.A.; Johnson, V.L.; Ensign, S.H.; MacPherson, T.A. Factors contributing to hypoxia in rivers, lakes, and streams. Limnol. Oceanogr. 2006, 51 Part 2, 690–701. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Qin, Y.; Zhou, M.; Sun, J. Iron-carbon microelectrolysis for wastewater remediation: Preparation, performance and interaction mechanisms. Chemosphere 2021, 278, 130483. [Google Scholar] [CrossRef]
- Jia, L.; Liu, H.; Kong, Q.; Li, M.; Wu, S.; Wu, H. Interactions of high-rate nitrate reduction and heavy metal mitigation in iron-carbon-based constructed wetlands for purifying contaminated groundwater. Water Res. 2020, 169, 115285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Ammar, S.H.; Sabit, D.A.; Najim, A.A.; Radeef, A.Y.; Taher, A.G. The latest progress in the design and application of semiconductor photocatalysis systems for degradation of environmental pollutants in wastewater: Mechanism insight and theoretical calculations. Mater. Sci. Semicon. Process. 2024, 173, 108153. [Google Scholar] [CrossRef]
- Ye, M.; Pan, W.; Dai, W. Composite iron-carbon constructed wetland combined with photocatalytic film to restore eutrophic water body and the hydraulic performance of constructed wetland. J. Water Process Eng. 2023, 53, 103590. [Google Scholar] [CrossRef]
- Cui, X. Study on the Application of Photocatalytic Synergistic Microalgae in the Treatment of Aquaculture Wastewater. Master’s Thesis, Fuzhou University, Fuzhou City, China, 2020. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Pu, J.; Feng, C.; Liu, Y.; Li, R.; Kong, Z.; Chen, N.; Tong, S.; Hao, C.; Liu, Y. Pyrite-based autotrophic denitrification for remediation of nitrate contaminated groundwater. Bioresour. Technol. 2014, 173, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Zhu, S.; Xing, W. Advanced low carbon-to-nitrogen ratio wastewater treatment by electrochemical and biological coupling process. Environ. Sci. Pollut. Res. 2016, 23, 5361–5373. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, W.; Zheng, P.; Zheng, X.; He, S.; Zhao, M. Iron scraps enhance simultaneous nitrogen and phosphorus removal in subsurface flow constructed wetlands. J. Hazard. Mater. 2020, 395, 122612. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Li, J.; Ren, J.; Chi, L.; Cheng, X. Iron-carbon could enhance nitrogen removal in Sesuvium portulacastrum constructed wetlands for treating mariculture effluents. Bioresour. Technol. 2021, 325, 124602. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Shi, J.; Cui, F.; Shen, J.; Li, H. Fe(2+)/H2O2-Strengite method with the enhanced settlement for phosphorus removal and recovery from pharmaceutical effluents. Chemosphere 2021, 277, 130343. [Google Scholar] [CrossRef]
- Leite, S.T.; do Nascimento, F.H.; Masini, J.C. Fe(III)-polyhydroxy cations supported onto K10 montmorillonite for removal of phosphate from waters. Heliyon 2020, 6, e03868. [Google Scholar] [CrossRef]
- Dong, C.; Li, M.; Zhuang, L.-L.; Zhang, J.; Shen, Y.; Li, X. The Improvement of Pollutant Removal in the Ferric-Carbon Micro-Electrolysis Constructed Wetland by Partial Aeration. Water 2020, 12, 389. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, M.; Zhou, X.; Chen, W.; Lu, D.; Zhang, Y.; Shao, X. Enhanced removal mechanism of iron carbon micro-electrolysis constructed wetland on C, N, and P in salty permitted effluent of wastewater treatment plant. Sci. Total Environ. 2019, 649, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwak, S.-Y. Functional mesoporous silica with controlled pore size for selective adsorption of free fatty acid and chlorophyll. Micropor. Mesopor. Mater. 2020, 306, 110410. [Google Scholar] [CrossRef]
- Hocaoglu, S.M.; Insel, G.; Cokgor, E.U.; Orhon, D. Effect of low dissolved oxygen on simultaneous nitrification and denitrification in a membrane bioreactor treating black water. Bioresour. Technol. 2011, 102, 4333–4340. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Zendehzaban, M.; Mirghavami, M.L.; Kakehazar, S. Application of solar light for degradation of ammonia in petrochemical wastewater by a floating TiO2/LECA photocatalyst. J. Ind. Eng. Chem. 2014, 20, 2806–2813. [Google Scholar] [CrossRef]
- Fan, J.; Wang, W.; Zhang, B.; Guo, Y.; Ngo, H.H.; Guo, W.; Zhang, J.; Wu, H. Nitrogen removal in intermittently aerated vertical flow constructed wetlands: Impact of influent COD/N ratios. Bioresour. Technol. 2013, 143, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, J.H.; Rossi, V.; Renault, L.; Haynes, P.; Morel, Y.; Garçon, V. Effects of upwelling duration and phytoplankton growth regime on dissolved-oxygen levels in an idealized Iberian Peninsula upwelling system. Nonlinear Process. Geophys. 2020, 27, 277–294. [Google Scholar] [CrossRef]
- Song, Q.; Sun, Z.; Chang, Y.; Zhang, W.; Lv, Y.; Wang, J.; Sun, F.; Ma, Y.; Li, Y.; Wang, F.; et al. Efficient degradation of polyacrylate containing wastewater by combined anaerobic–aerobic fluidized bed bioreactors. Bioresour. Technol. 2021, 332, 125108. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Y.; Zhang, Y.; Huang, T.; Zhou, Z.; Li, Y.; Li, Z. Pollutant removal performance and microbial enhancement mechanism by water-lifting and aeration technology in a drinking water reservoir ecosystem. Sci. Total Environ. 2020, 709, 135848. [Google Scholar] [CrossRef]
- Chao, J.; Li, J.; Kong, M.; Shao, K.; Tang, X. Bacterioplankton diversity and potential health risks in volcanic lakes: A study from Arxan Geopark, China. Environ. Pollut. 2024, 342, 123058. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, M.; Li, X.; Lu, W.; Li, J. River bacterial community structure and co-occurrence patterns under the influence of different domestic sewage types. J. Environ. Manag. 2020, 266, 110590. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kulshreshtha, N.; Kumar, R.; Begum, Z.; Shivaji, S.; Kumar, A. Exiguobacterium alkaliphilum sp. nov. isolated from alkaline wastewater drained sludge of a beverage factory. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 12, 4374–4379. [Google Scholar] [CrossRef] [PubMed]
- Zharikova, N.V.; Markusheva, T.V.; Galkin, E.G.; Korobov, V.V.; Zhurenko, E.Y.; Sitdikova, L.R.; Kolganova, T.V.; Kuznetsov, B.B.; Turova, T.P. Raoultella planticola, a new strain degrading 2,4,5-trichlorophenoxyacetic acid. Appl. Biochem. Microbiol. 2006, 42, 258–262. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Chang, F.; Xie, P.; Zhang, Y.; Wu, H.; Zhang, X.; Peng, W.; Liu, F. eDNA revealed in situ microbial community changes in response to Trapa japonica in Lake Qionghai and Lake Erhai, southwestern China. Chemosphere 2022, 288, 132605. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yang, C.; Tian, Y.-Y.; Liu, J.-X. Regulation of Chloroplast Development and Function at Adverse Temperatures in Plants. Plant Cell Physiol. 2022, 63, 580–591. [Google Scholar] [CrossRef] [PubMed]





| Index | Concentration |
|---|---|
| (mg/L) | 0.36~0.61 |
| TN (mg/L) | 1.76~2.11 |
| TP (mg/L) | 0.021~0.034 |
| COD (mg/L) | 42.32~55.90 |
| Chlorophyll a (μg/L) | 8.15~13.37 |
| DO (mg/L) | 3.70~7.64 |
| pH (non-dimensional) | 7.75~8.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ye, M.; Chen, N.; Pan, W.; Dai, W. Study of a New Photocatalytic Film Process Combined with a Constructed Wetland and an Analysis of Reoxygenation Pathways in a Water Body. Sustainability 2024, 16, 3123. https://doi.org/10.3390/su16083123
Chen S, Ye M, Chen N, Pan W, Dai W. Study of a New Photocatalytic Film Process Combined with a Constructed Wetland and an Analysis of Reoxygenation Pathways in a Water Body. Sustainability. 2024; 16(8):3123. https://doi.org/10.3390/su16083123
Chicago/Turabian StyleChen, Shihao, Ming Ye, Nuo Chen, Wenbin Pan, and Wenxin Dai. 2024. "Study of a New Photocatalytic Film Process Combined with a Constructed Wetland and an Analysis of Reoxygenation Pathways in a Water Body" Sustainability 16, no. 8: 3123. https://doi.org/10.3390/su16083123
APA StyleChen, S., Ye, M., Chen, N., Pan, W., & Dai, W. (2024). Study of a New Photocatalytic Film Process Combined with a Constructed Wetland and an Analysis of Reoxygenation Pathways in a Water Body. Sustainability, 16(8), 3123. https://doi.org/10.3390/su16083123






