Abstract
A plastic-type flat panel photobioreactor (PTFPP) prototype was designed for microalgae cultivation as biodiesel feedstock. The growth, biomass, and lipid production of the oleaginous microalga Scenedesmus obliquus were optimized through the enhanced design and cultivation conditions in the PTFPP. The optimization conditions include cultivation of the microalga in a flat panel photobioreactor manufactured from a 10 µm-thick plastic sheet with dimensions of 40 cm in width and 60 cm in height. The width of the designed plastic bags was adjusted by “4 ports” of circular adhesion points which make the volumetric cultural capacity 5 L. Cultivation of the microalga was optimized through the replacement of the sodium nitrate of the BBM medium with urea as a nitrogen source. Cultivation bags were subjected to continuous illumination with 3000 lux white, fluorescent lamps and aerated with 1.5 L air/min (equal to 0.3 VVM). Biomass production from the designed PTFPP reached 3 g/L with around 40% lipid content (on a dry weight basis). Based on a GC-MS analysis of the produced fatty acid methyl ester (biodiesel) from S. obliquus, the percentage of C16 and C18 fatty acids reached more than 90% of the defined fatty acids. Out of this percentage, 66.6% were unsaturated fatty acids. The produced fatty acid profile of the S. obliquus biomass cultivated in the designed PTFPP prototype could be considered a suitable feedstock for biodiesel production.
1. Introduction
Due to the increase in industrialization and population, there is a constant need for energy, especially renewable energy, which helps sustain resources, preserves the environment, and reduces carbon emissions and global warming [1,2]. Biodiesel (fatty acid methyl ester), in particular, is one of the most widely used and in-demand types of biofuel. Commercial biodiesel is currently produced from animal fats, waste frying oil, and vegetable oils, whose competition with edible vegetable oils on agricultural land remains a controversial issue. Microalgae are one of the renewable feedstocks currently used (mostly on a research scale) for biodiesel production. The advantage of producing biodiesel from microalgae compared to other plant sources is that it does not compete with humans for food or arable land. Algae can also be produced for another purpose, such as treating wastewater, and then used to produce biofuel. Algae production continues throughout the year, unlike oil crops that grow in specific seasons and compete for land and water that is suitable for irrigation or drinking [3,4]. Despite the success and promise of producing biodiesel from microalgae (especially oleaginous), its commercial use is still restricted due to the high cost of production and harvesting [5]. Microalgae are a huge and diverse group of aquatic organisms that can be found in a variety of environments and under challenging conditions [1]. Microalgae can grow rapidly in fresh and salt water, or even in partially treated wastewater, converting solar energy into biomass by fixing carbon dioxide without competing with food crops on land or water. Some species of microalgae can accumulate a high percentage of lipids, up to 60% of their dry mass, making them good candidates for biodiesel production [6]. Microalgae can be grown in four different types of conditions: phototrophic, heterotrophic, and mixotrophic [7]. Temperature, carbon dioxide, dissolved oxygen concentration, light intensity, pH, salinity, and nutrient availability are among the factors affecting the growth of microalgae [8].
Microalgae can be grown in open pond systems or closed photobioreactors (PBRs), and, although they are all designed to meet the requirements of algal growth, each has its own advantages and disadvantages [9,10]. Although open systems for microalgae cultivation are easier to construct and operate than closed systems, they face several operational problems, including evaporation, pollution, exposure to weather conditions, and large land requirements. On the other hand, closed systems are expensive to build and complex to operate; however, they provide the ability to better control temperature and pH, distribute light effectively, and reduce pollution from the outside. Such controlled conditions in closed systems make their biomass productivity and quality much higher than that of closed systems [8,11,12]. For instance, the biomass productivity of the microalga Chlorella sorokiniana for the stirred tank, bubble column, and airlift PBRs were 0.064, 0.097, and 0.072 gdw/L·day, respectively [13]. While the “estimated annual productivity average productivity” of the same microalga in raceway ponds was estimated to be 0.040 gdw/L·day (taking into account that one square meter contains around 400 L) [14].
PBRs are often constructed as flat or tubular reactors, which are usually made of glass, plastic, or other transparent materials that allow light to penetrate the microalgae to photosynthesize [15,16]. The closed PBRs, known as flat panel photobioreactors (FP-PBRs), have a large illuminated surface-to-volume ratio and a restricted light channel [17]. Glass, plexiglass, or polycarbonate are just a few examples of transparent or translucent materials used to make flat panels. It has been reported that the high photosynthetic productivity of microalgae grown in FP-PBRs is a direct result of the enormous lighting surface area in such PBRs [16,18]. It is worth noting that providing the appropriate light in terms of duration and intensity to microalgae stimulates photosynthesis processes. It then increases algal biomass productivity and stimulates the formation and accumulation of some economically important secondary metabolites, such as lipids used in biodiesel production [19]. Flat plate reactors are among the most widely used closed photobioreactor systems for growing microalgae for both research studies and commercial applications. As the commercial use of these plate photobioreactors expands, efforts have been made to develop their design and reduce their construction cost by selecting suitable cheap materials such as plastic bags/sheets [20]. Polyethylene bags are commonly used as photobioreactors because of their affordability, transparency, and ability to maintain sterility at the high temperature of film extrusion during the initial stages of cultivation. These photobioreactors resemble vertical bags that are suspended either singly or in a row in parallel and fixed to a support (rack) [21]. However, the design and dimensions of the plastic bag PBRs, along with the raw materials from which they are made, are expected to play a major role in the microalgal growth efficiency and biomass productivity. The ideal design of a microalgae PBR should take into account the appropriate use of light, provision of aeration and agitation, and optimization of unit space. In addition, microalgae productivity must be maximized by providing the appropriate nitrogen source, appropriate lighting duration and intensity, and appropriate air and agitation ratio to the target algae [13,22].
Therefore, the main objective of the present study was to explore and develop flatbed photobioreactors for microalgae cultivation based on plastic bags/sheets as cheap and locally available materials for the purpose of biofuel production. The optimization of Scenedesmus obliquus cultivation parameters in the designed plastic-type photobioreactor as a model lipid-rich microalga for biodiesel production was considered in this work.
2. Materials and Methods
2.1. Microalgal Strain and Growth Medium
All experiments were conducted using the freshwater oleaginous microalga S. obliquus commonly used in biofuel production (30% lipids from its dry biomass) [23]. The microalgal isolate was kindly provided by the Department of Agricultural Microbiology, National Research Centre as a model microalgae for biodiesel production (S. obliquus NRCIbr1 accession No.: KY621475). The microalga was cultivated and maintained on Bold Basal Medium (BBM) with the following composition (per liter): 175 mg KH2PO4, 25 mg CaCl2·2H2O, 75 mg MgSO4·7H2O, 250 mg NaNO3, 75 mg K2HPO4, 25 mg NaCl, 11.42 mg H3BO3, and 1 mL from Microelement stock solution containing 8.82 g; ZnSO4·7H2O; 1.44 g MnCl2·4H2O, 0.71 g MoO3, 1.57 g, CuSO4·5H2O, and 0.49 g Co(NO3)2·6H2O, per liter, 1 mL of Solution 1 containing 50 g Na2EDTA and 3.1 g KOH per liter, and 1 mL of Solution 2 containing 4.98 g FeSO4 and 1 mL concentrated H2SO4 per liter and a final pH of 6.8.
Unless otherwise mentioned, S. obliquus cultivation was performed under continuous illumination using white fluorescence (2000 Lux) for two weeks and incubated under an ambient temperature.
2.2. Growth Parameters
The evaluation of microalgal growth was regularly performed using the measurements of the cultural optical density, and chlorophyll a content at two-day intervals and at the end of the cultivation period using dry biomass evaluation. The cultural optical density (OD) reflects the density of cells under different cultivation conditions, while chlorophyll content indicates the photosynthetic capacity of microalgal cultures and reflects their vitality [24].
2.2.1. Optical Density
Using a spectrophotometer (SHIMADZU UV-2401PC, Kyoto, Japan), the optical density of the samples at 680 nm was regularly used to estimate the microalga’s growth rate, as reported by [23]. Appropriate dilutions of dense microalgal cultures were carried out using deionized water before measuring the culture’s optical density.
2.2.2. Chlorophyll Content
The chlorophyll a content was determined according to Eida et al. [23]. In brief, 2 mL of microalgal culture was centrifuged at 10,000 rpm for 10 min (Centurion Scientific K3 series, Irvine, CA, USA) and the cell-free supernatant was discarded. The collected cells were re-suspended in 1 mL of 90% methanol, vortexed for 1 min, and subjected to sonication in an ultrasonic water bath (Ultrasonic Cleaner, SH80, USA) at maximum power for 15 min. After being vortexed for five minutes, the liquid was centrifuged, and the supernatant was poured into a fresh tube. To remove nearly all of the chlorophyll from the cells, additional extraction procedures were carried out using 1 mL of 90% methanol. The mixture was centrifuged once more following the second extraction, and the supernatant was added to the first portion of the extract. Finally, the volume was raised to 2 mL with 90% methanol, and the color intensity was measured at 650 and 665 nm. The chlorophyll a content was calculated according to the following formula:
Chlorophyll a (mgL−1) = (16.5 × A665) − (8.3 × A650)
2.2.3. Biomass Production
At the end of the experiments, the dry weight of the microalga per liter (gL−1) was gravimetrically calculated. Briefly, cells of S. obliquus from a 50 mL volume culture were collected by centrifugation at 8000 rpm for 10 min (Herarus Megafuge; Thermo ScientificsTM, Langensel Bold, Germany). The supernatant was discarded, and the harvested biomass was subjected to washing twice with deionized water and oven-drying overnight at 105 °C [25].
2.3. Lipid Contents
The total lipids were determined according to the common Bligh and Dyer method described by Eida et al. [23] with the following modifications according to: 100 mL of microalgae culture was harvested by centrifugation at 6000 rpm for 20 min and re-suspended in 1 mL distilled water. The sample was then mixed in a screw-capped glass tube with 2.5 mL chloroform and 5 mL methanol (1: 2 v/v) and then sonicated for 30 min at the maximum power. After sonication, the tubes were shaken with the extraction solvents overnight. The next day, an additional portion of chloroform (2.5 mL) was added to each tube and the mixture was sonicated again for 30 min and vortexed. Then, 2.5 mL of distilled water was added to separate the chloroform and aqueous methanol layers by centrifugation at 4000 rpm for 10 min (Herarus Megafuge; Thermo ScientificsTM, Langensel Bold, Germany). The chloroform layer was gently removed, and then the suspension was vortexed. The chloroform portions were collected and washed with 5 mL of 5% NaCl solution, after which the chloroform was evaporated in the oven at 50 °C. The total lipids were measured gravimetrically.
2.4. GC Characterization of Lipid Profile for Microalgae Samples
The fatty acid methyl esters were prepared for the GC analysis according to the methods adopted by Ichihara and Fukubayashi [26]. In brief, the lipid samples were dissolved in toluene (0.20 mL), placed in screw-capped glass test tubes, and mixed with 1.8 mL of 8.0% methanolic HCl (w/v) followed by heating at 100 °C for 1 h. After cooling to room temperature, 1 mL of hexane was vortexed with the mixture for the extraction of fatty acid methyl esters (FAMEs) followed by the addition of 1 mL of water to enable layer separation. The hexane layer was pipetted out and purified through a membrane filter for the GC analysis. The methyl esters of the fatty acids were analyzed by a gas chromatography system (Hewlett Packard, HP 6890 series, Palo Alto, CA, USA) equipped with an Alltech BPX70 Capillary Column (60 m × 320 μm × 0.25 μm) coated with 70% poly silphenyl-siloxane supplied with a flame ionizing detector. Fatty acid content was expressed as a percentage of the total fatty acids identified in the oil.
2.5. Materials and Design of Used Cultivation Systems
The main objective of this study was to explore and develop a microalgae cultivation system for the purpose of biofuel production, using common, economical, and locally available materials in Egypt. Thus, some common inexpensive materials were included for investigation as photobioreactor materials for culturing lipid-rich microalgae for biofuel production. Plastic bottles, glass basins, and plastic bags were used to evaluate their suitability for microalgae cultivation in comparison with the commonly used glass tubular photobioreactor. Aeration was provided by installing a cylindrical air sparger at the bottom of the examined photobioreactor design (glass tank, plastic bags, column, or plastic bottles). The dimensions of the air sparger that was connected with an air tube at the bottom of the reactor were 5 mL in length and 2 mL in diameter. Unless otherwise stated, illumination was provided by two side-mounted white fluorescent lamps located 15 cm from the microalgae containers.
2.5.1. Glass Tanks as Flat-Panel Photobioreactor (FPP)
The tanks were made of transparent flat glass panels with a thickness of 6 mm and dimensions of 10, 20, and 40 cm for width, length, and height, respectively. The culture capacity of the tanks was 6 L and the aeration and illumination were installed as previously described in glass tanks.
2.5.2. Plastic Bags as Flat Panel Photobioreactors (FPPs)
The plastic bags used in this study were transparent polypropylene sheets designed using a heat-sealing machine. Three sheet thicknesses, i.e., 7.5, 10, and 15 microns, were investigated, and the bags were manufactured with dimensions of 40 cm in width and 60 cm in height. The bags were welded from the top to facilitate their hanging on a stand made of plastic pipes and installed against the lighting lamps installed on the sides. The culture working volume capacity of the bags was 6 L, and the aeration and illumination were installed as previously described in glass tanks.
2.5.3. 6 L Plastic Bottles
Two types of domestically popular commercial polyethylene drinking water plastic bottles were investigated for microalgae cultivation. The first was 6-L transparent bottles in which the illumination was provided from outside as previously described. The second type was 18-L bottles in which illumination was provided by installing a white fluorescent lamp tube in a glass column sealed from the bottom vertically inside the bottle from the middle.
2.6. Optimization of Cultivation Conditions
Different cultivation conditions were studied to improve the biomass and lipid content of S. obliquus microalga as feedstock for biofuel production. The optimization conditions included the types of photobioreactor materials, thickness and design of plastic bags as flat-panel photobioreactors (FPPs), as well as the nitrogen sources for cultivation, aeration ratio, and light intensity.
2.6.1. Types of Photobioreactors Materials
As common and locally available materials in Egypt (and many countries), glass tanks, plastic sheets, and plastic bottles have been investigated as photobioreactor materials for microalgae cultivation. The material and dimensions of applied photobioreactors were previously described. S. obliquus microalga was inoculated in a sufficient volume of BBM medium and then divided into the different investigated photobioreactors. The cultivation in the investigated 6 L glass tanks, 6 L plastic bottles, 18 L plastic bottles, and plastic bags was compared with that of 1.5 L tubular glass photobioreactors. Aeration was provided, as previously described, at a rate of 1 L/min, and samples were tested at three-day intervals during the 15-day incubation period for OD, chlorophyll, and pH, while lipids and biomass were determined at the end of the incubation days.
2.6.2. Thickness of Plastic Sheets as FPP
Three different thicknesses of plastic sheets were used to construct the FPP algae plastic bags. The thicknesses were 7.5, 10, and 15 μm of transparent PVC and the dimensions of the bags, aeration, and lighting have been described before. The capacity of the applied bags was 6 L, and, thus, the depth of the FPP in the same bag differed, as it increased in the middle of the bags and decreased at the edges, and the different bag thicknesses had an effect on light absorption and differences in growth and biomass.
2.6.3. Plastic Bags Design
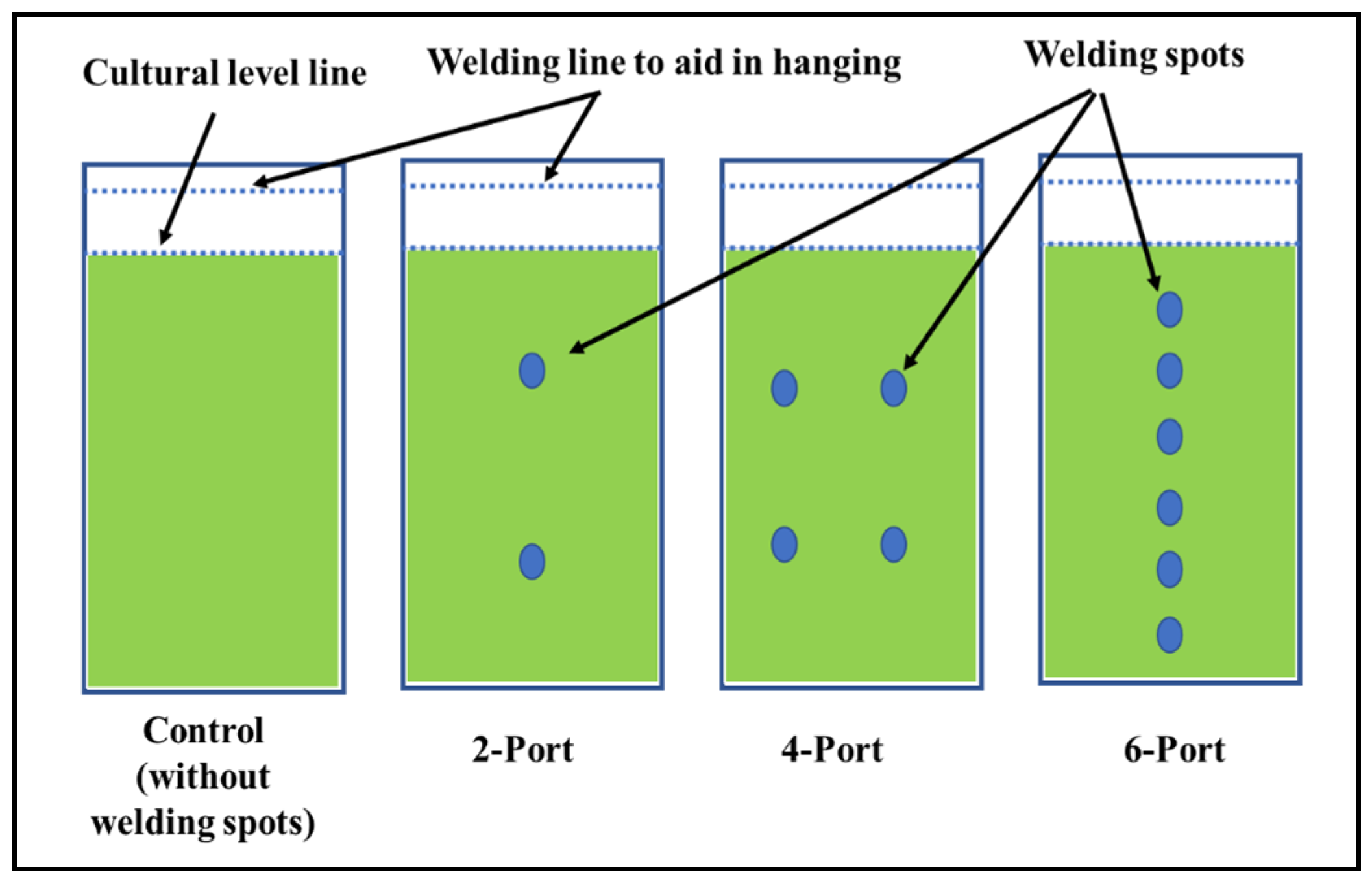
In addition to the thickness of the plastic material, the light penetration in a plastic-type FPP depends on the distance between the two bag layers. This distance can be controlled by making adhesion points between the two layers of the plastic bags at different distances. In this experiment, three different designs were applied in the plastic bags of 10 μm thickness to control the width between the two layers of the bags, as illustrated by Figure 1. Plastic bags without any adhesion points were used for cultivation as a control. According to the number of adhesion points (welding ports), the final volume of the differently designed bags were 10, 5, 4.5, and 4 L for the control, “2-port”, “4-port”, and “6-port” designs, respectively. Aeration, illumination, and other cultivation condition were performed as previously described for the freshwater microalga S. obliquus.
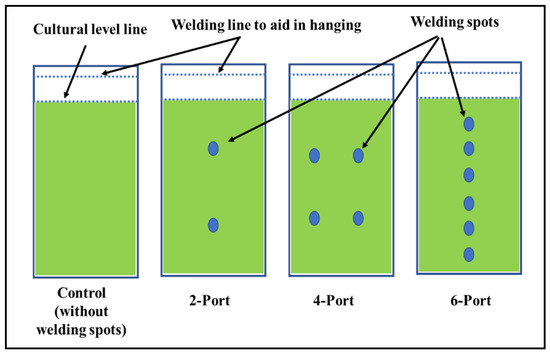
Figure 1.
Plastic bag designs as flat-panel photobioreactor(s).
2.6.4. Nitrogen Source
The influence of the type of nitrogen source on the biomass and lipid production of Scenedesmus sp. was investigated. Different nitrogen sources, i.e., NaNO3, (NH4)2SO4, NH4NO3, KNO3, (NH4)2 CO3, and urea, were individually added to the BBM medium. The main nitrogen source of the BBM medium (NaNO3) was replaced with equal amounts of N from different sources. The cultivation was performed in the plastic-type FPPBRs under continuous illumination and aeration for 15 days. Samples for chlorophyll and pH measurements were obtained regularly, while the biomass and total lipid contents were determined at the end of the incubation period.
2.6.5. Light Intensity
The influence of light intensity was investigated as an important parameter for microalgae growth. Four different light intensities, i.e., 1000, 2000, 3000, and 4000 lux, were applied on the surface of the plastic-type FPPBRs for the cultivation of Scenedesmus sp. The distance between the plastic-type FPPBRs and the light source (two white fluorescent lamps) was adjusted in order to control the light intensity. Cultivation was performed in triplicate, and other growth conditions and sampling was performed as previously described.
2.6.6. Optimization of Air Flow
The effect of airflow was studied as an important parameter for microalgae growth. Four different airflows were applied to the designed plastic-type flat panel photobioreactor to cultivate the microalga S. obliquus. Air was pumped into the examined bioreactor at different flow rates, i.e., 0.5, 1, 1.5, and 2 L per minute, and ventilation was provided by installing a cylindrical air exchanger below the photobioreactor. The dimensions of the air diffuser (or sparger) used were 5 mL in length and 2 mL in diameter connected to the air ducting tube at the bottom of the reactor. Unless otherwise noted, illumination was provided by two side-mounted white fluorescent lamps located 15 cm from the microalgae containers.
2.7. Biodiesel Production
The microalga S. obliquus was cultivated under optimized conditions (from the previously examined experiments). The biomass was harvested by centrifugation, and the total lipids were extracted. The extracted lipids were converted to biodiesel (FAME) according to the method described before. The characteristics of the produced biodiesel were determined according to the fatty acid profile of the GC analysis.
2.8. Statistical Analysis
All experiments were performed in triplicate and the results were presented as average ± standard error. The averages of data for each experiment were statistically analyzed using analysis of variance using IBM Corp, released 2011, IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA: IBM Corp., and the means were compared using the Duncan Multiple Range Test at 0.05% probability.
3. Results and Discussion
3.1. Screening Different Cultivation Containers for Microalgal Growth
Growing microalgae in closed systems has many requirements, including the provision of light and ventilation with an adequate carbon dioxide percentage. These closed systems for growing algae, or so-called photobioreactors, are built from many materials, which include glass, plastic, and polymers, that must provide the property of light transmittance [27,28]. Some common materials for microalgae cultivation were screened, in this study, for their suitability to cultivate the oleaginous microalga S. obliquus to be used as biodiesel feedstock. Different types of containers, i.e., plastic bottles, glass basins, and plastic bags with three different thicknesses (7.5, 10, and 15 µm), were evaluated compared to the commonly used glass tube photobioreactor.
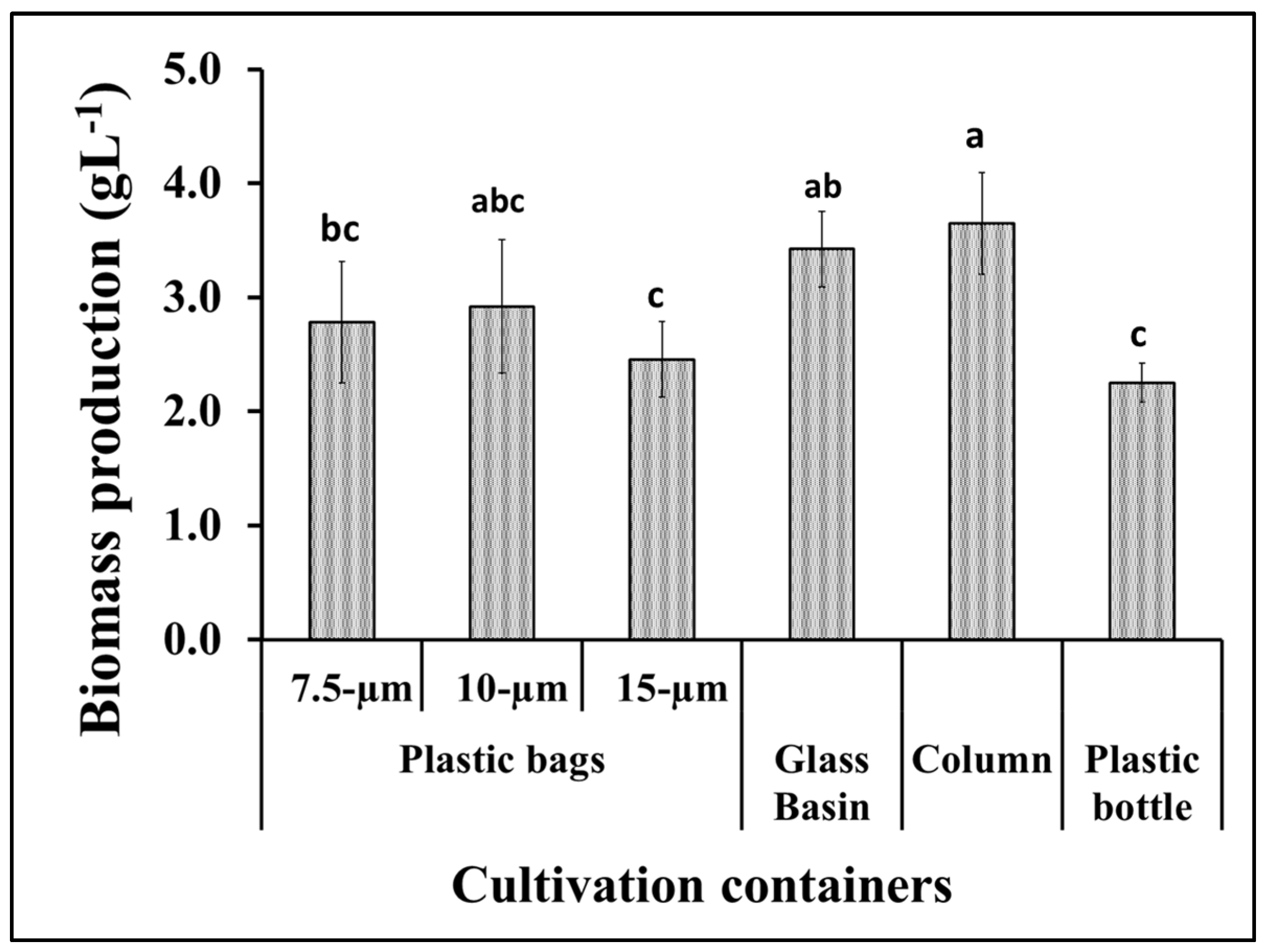
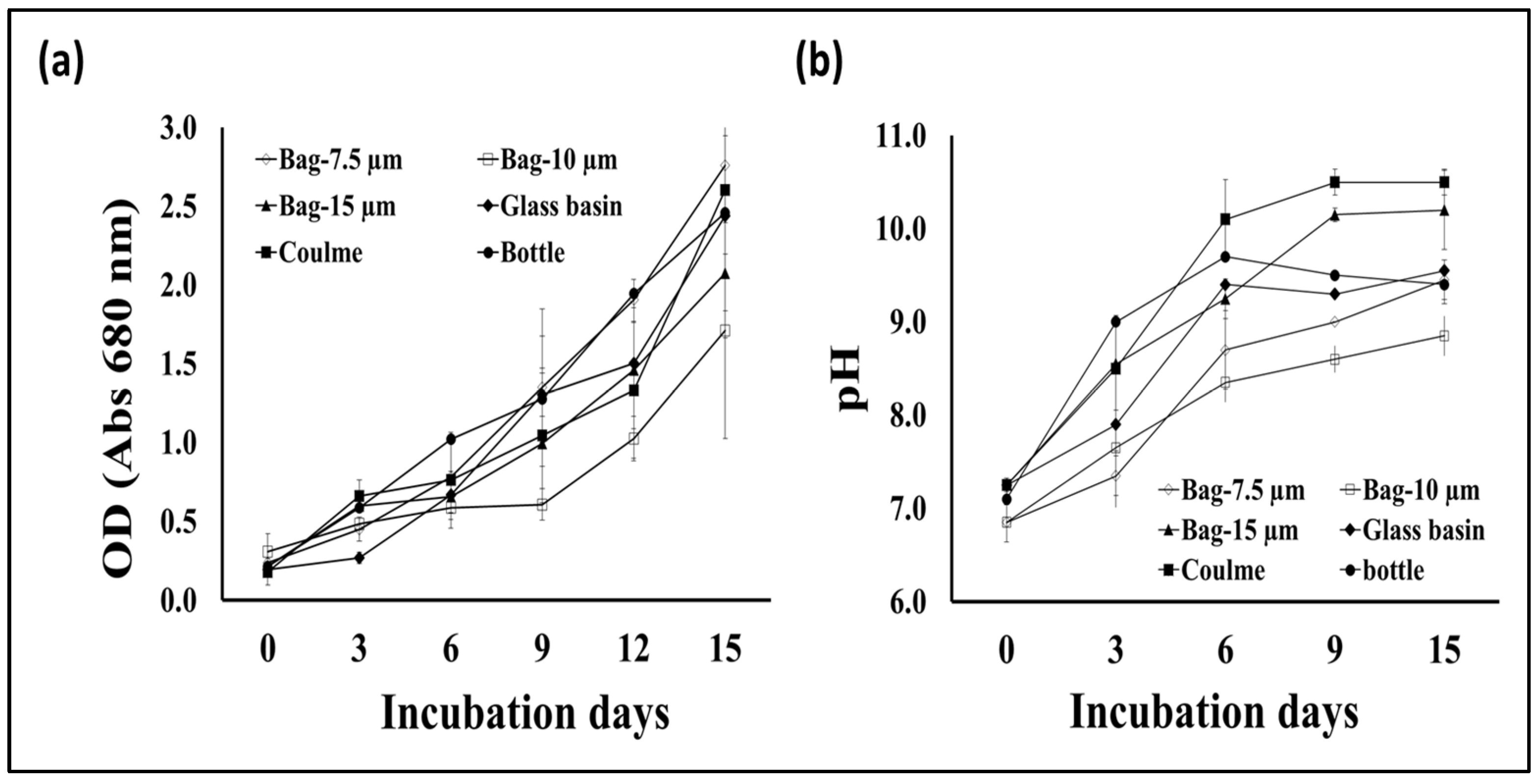
S. obliquus growth was represented as the total dry biomass per liter of culture in addition to the optical density of the culture in the different cultivation containers. According to the data in Figure 2, the algal biomass production from the tubular glass column was much higher than the microalgae production from the plastic bottles as well as the 7.5 and 15 μm thick plastic bags. However, the biomass production from the glass basin was slightly lower than the amount produced from the glass column and slightly higher than the productivity obtained using the 10 μm thick plastic bags. The light intensity of S. obliquus cultures during the 15-day growth period in the different culture containers was also variable in a manner similar to the biomass productivity of the same cultures. However, the optical densities of the 10 μm thick plastic bags recorded the lowest values during the period of greatest cultivation (Figure 3a). On the other hand, the pH values of the algae cultures grown in different containers showed different values, but, in general, these values increased as the incubation period passed. The pH values increased from around 7 at the beginning of incubation to 10.5 at the end of the incubation period when grown in glass tubes, while they rose to only 8.5 when grown in 10-micron plastic bags (Figure 3b). It is worth noting that, although the design and dimensions of the plastic bags used to grow the algae were close to the dimensions of the glass tanks used in the same experiment, the growth and biomass results were clearly different. The obtained results point out the importance of the container type for the vigorous growth and biomass production of microalgae. This could be due to the direct impact of the container’s material on the light penetration into the photosynthetic microalgal cells. Light is a critical factor that affects the activity of photosynthetic microorganisms which, in turn, affects their biomass productivity as well as their cellular contents [29]. The difference in growth and biomass production of photosynthetic microalgae when using cultivation containers of different designs or materials may be due to the difference in the degree of light transmittance to the cells inside [17]. When choosing the materials used in the implementation of microalgae photobioreactors, in addition to the light transmittance factor, the cost factor of the material used, and the possibility of its repeated use, must be taken into account [29].
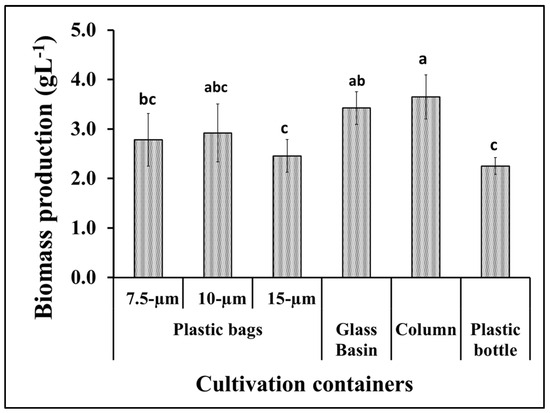
Figure 2.
Biomass production of S. obliquus under different cultivation containers. Different lowercase letters in the figure represent significant differences (p < 0.05).
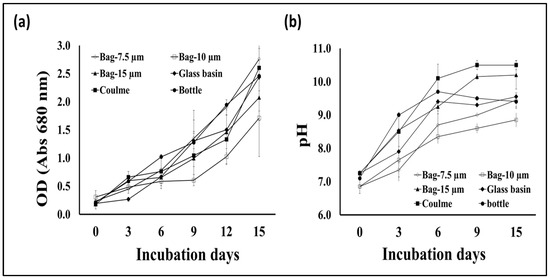
Figure 3.
Optical density at 680 nm (a) and cultural pH (b) of S. obliquus cultivated in different containers.
It is worth noting that the results of biomass and growth in plastic bags with thicknesses of 10 microns and 7.5 microns were not significantly different from their counterparts in glass basins or glass tubes (Figure 2). A similar study that compared plastic bags, glass columns, and glass tanks as closed photobioreactors for microalgae also recommended the use of plastic bags on the basis of their appropriate biomass productivity and economic cost [30]. Hence, based on the obtained results and taking into account the price of plastic bags, the rest of the experiments were performed using plastic bags with a thickness of 10 microns as cultivation containers for the oleaginous microalga S. obliquus.
3.2. Optimization of Plastic Bag Design on Microalgal Growth
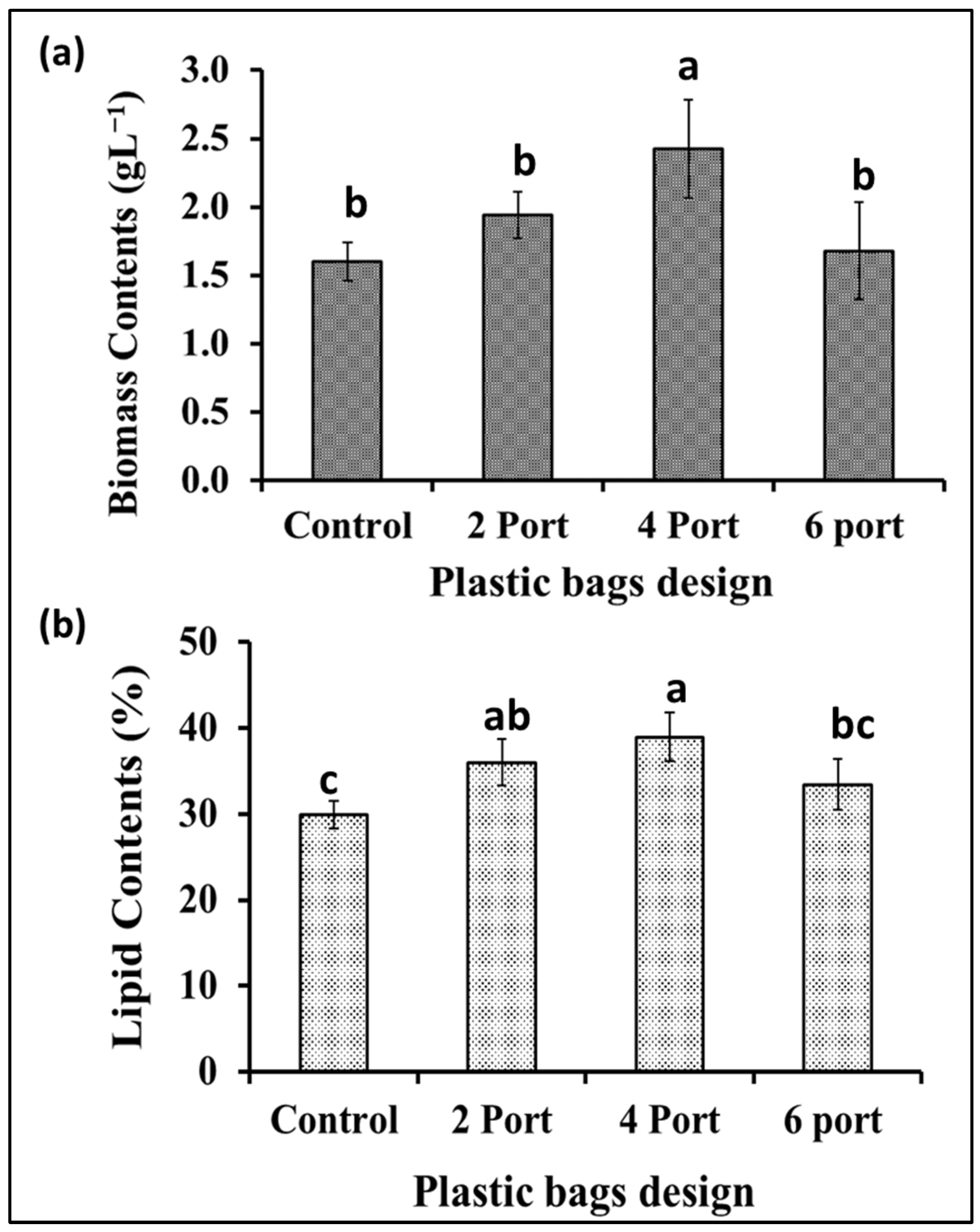
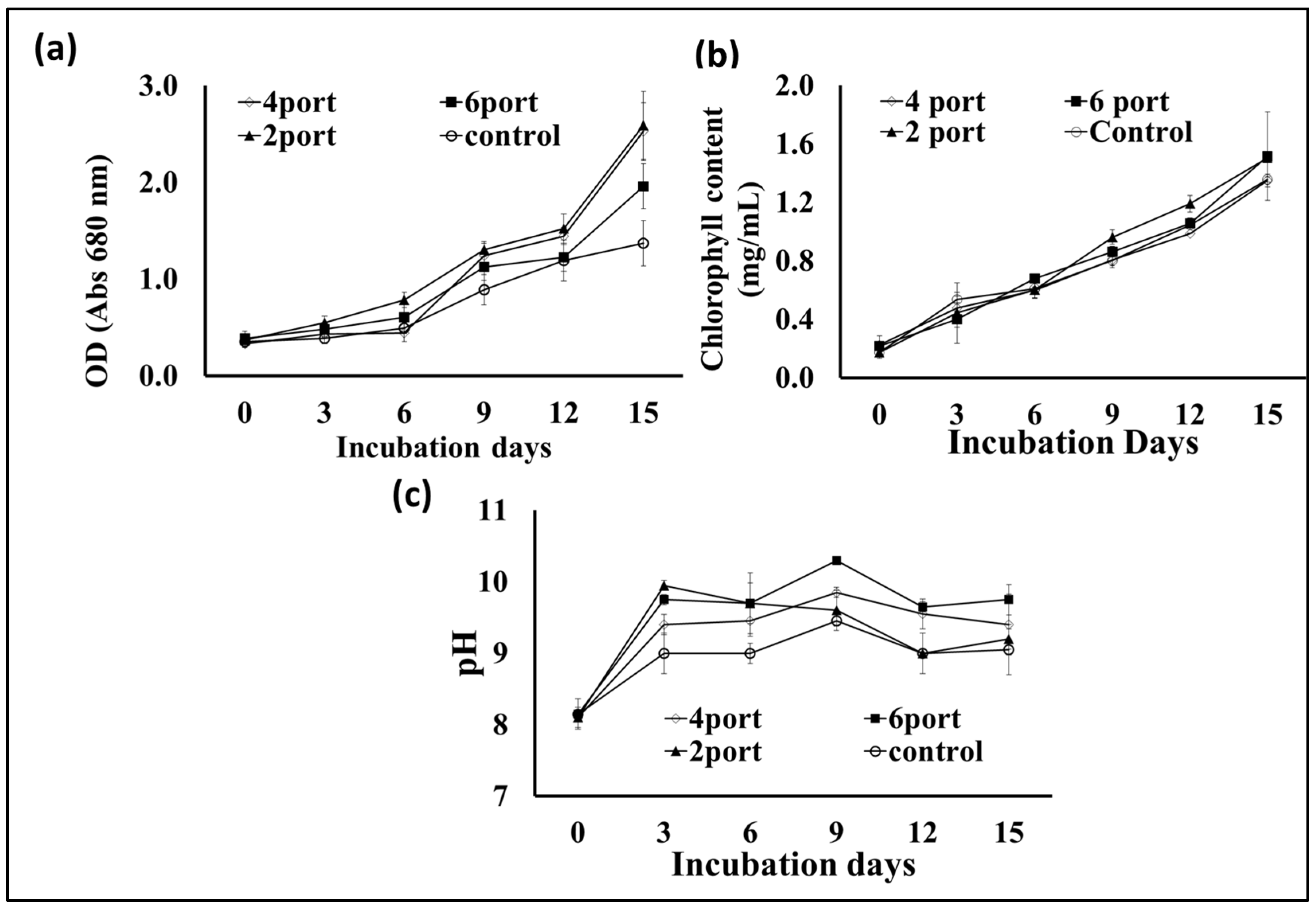
Based on the data obtained from the previous experiment, plastic bags with a thickness of 10 microns were selected for designing a microalgal flat panel photobioreactor. Hence, the plastic bags were designed for microalgae cultivation by controlling the distance between their two layers by controlling the distribution of the adhesion points (welding ports) (the control bag did not receive any ports of adhesion, hence, it contained more cultural volume (10 L) as well as a wider distance between its two sides). The “2-port”, “4-port”, and “6-port” designs resulted in lower culture volumes, i.e., 5, 4.5, and 4 L. According to the results presented in Figure 4a, the S. obliquus biomass production from the “4-port” design was significantly higher than that of all other designed reactors including the control design. Based on the distribution of circular attachment points for plastic bag photobioreactors, the “4-port” design provides the lowest lateral thickness of the culture. This design is supposed to provide better light distribution and penetration through the microalgae culture. Although the “6-port” design has the largest number of adhesion points, their arrangement in one vertical line (in the middle of the bags) makes them less efficient in distributing the points and, thus, thicker in terms of light penetration and less efficient in distributing and penetrating light. Likewise, the efficiency of air distribution and gas exchange of algal cells is affected based on the distribution of side attachment points (ports) of photobioreactors in plastic bags. The distribution of these points affects the thickness of the culture and, at the same time, represents barriers that slow down the passage of air and thus increase gas exchange. Therefore, the “4-port” design is supposed to provide better air distribution through the microalgae culture, supporting the higher results obtained regarding biomass and chlorophyll. Regarding lipid content (Figure 4b), the highest percentage of lipid content in S. obliquus dry biomass, i.e., 39%, was recorded in the case of the “4-port” design followed by the “2-port” design (36%), without significant difference. However, while the lipid percentage of the “6-port” (33.4%) design was lower than that of the “2-port” and “4-port” designs, it was higher than that produced by the control treatment (29.9%). On the other hand, all growth parameters represented as optical densities (ODs) and total chlorophyll (Figure 5a,b) revealed good growth for all cultivations in all the plastic bags used. However, the highest OD values were recorded for the “2-port” and “4-port” designs, which were compatible with the higher biomass productivity of the plastic-type flat panel photobioreactors. In addition, the cultural pH values (Figure 5c) for all treatments were increased during the first three days of incubation, from 8 to 9 in the case of the control treatment, while this increase was higher for the rest of the treatments. According to the designs of the “2-port” and “4-port” plastic bags, the light penetration and air distribution could be higher than that of the “6-port” and control bags. This might be ascribed to the fact that light and aeration are crucial for the highest microalgal biomass and lipid production in such FPPBRs. A similar conclusion was reached by Chen et al. [6] based on their experiments on the cultivation of Chlorella sp. in plastic bags as FPPBRs. The industry productivity of microalgae for different applications is significantly influenced by the light and aeration efficiency. Although artificial light and aeration can increase the cost of microalgal production, their sufficient supply is important for efficient productivity [29]. In general, the FPPBR as a microalgal growing system provides a broad surface area for light radiation, resulting in great light-consumption efficiency and the ability to avoid dark zones in the microalgae suspension [28].
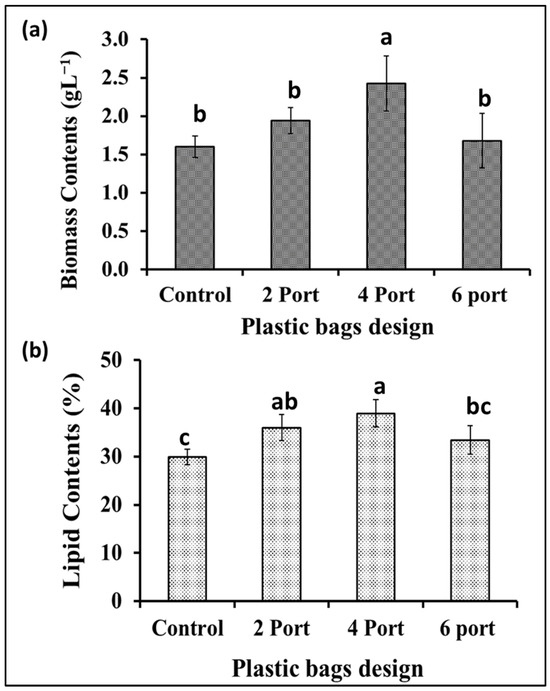
Figure 4.
Biomass production (a) and lipid contents (b) of S. obliquus cultivated in different plastic bag FPPBRs with different designs. Different lowercase letters in the figure represent significant differences (p < 0.05).
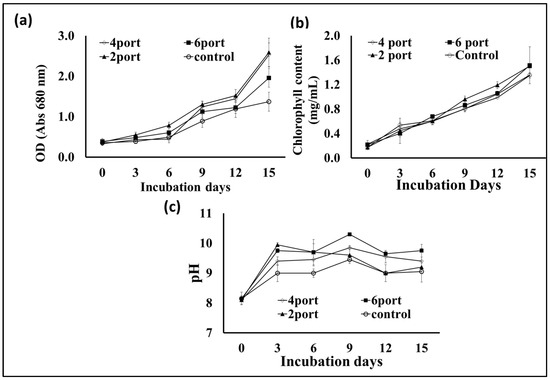
Figure 5.
Optical density at 680 nm (a) and chlorophyll contents (b) and cultural pH (c) of S. obliquus cultivated in different plastic bag FPPBRs with different designs.
3.3. Optimization of N-Source
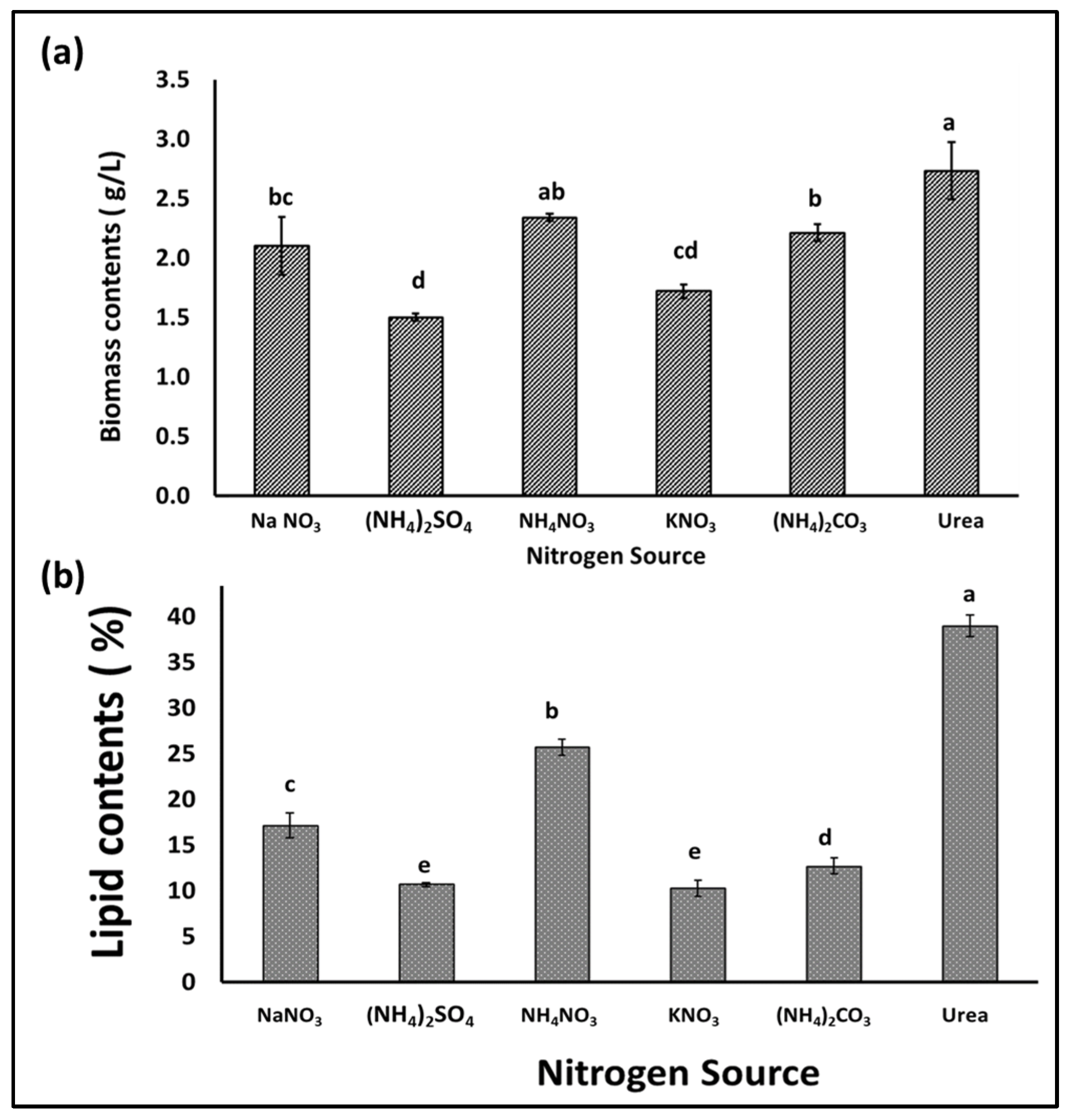
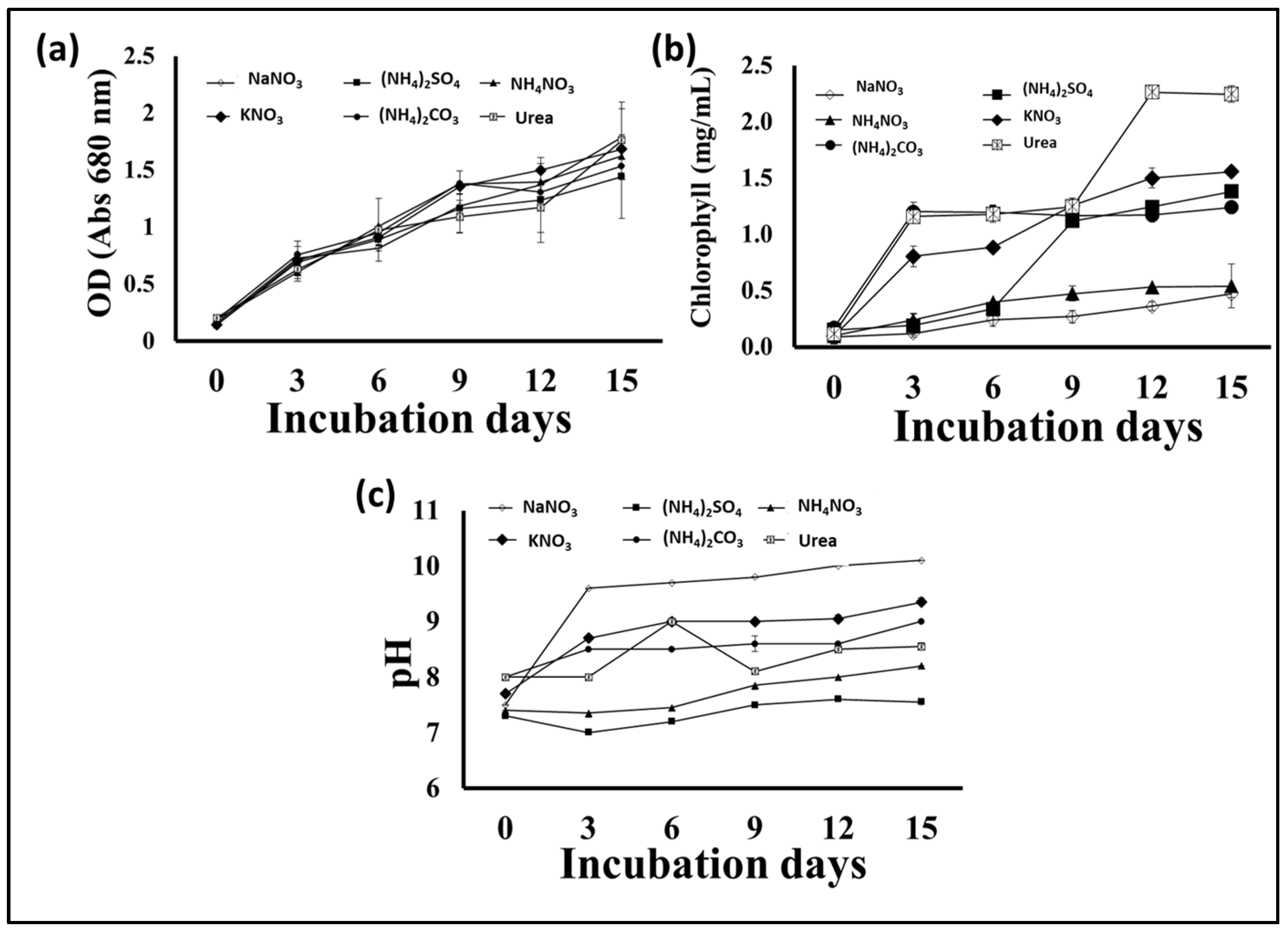
Nitrogen is an important nutrient for microalgae growth, and its presence in the appropriate form and concentration is essential for rapid growth and biomass production, especially during the early growth stages. In addition, the form and concentration of the nitrogen supplement in the growth medium has an effect on the quantity and types of fatty acids produced by microalgae, which affects the biodiesel extracted from the biomass [31]. Hence, in this experiment, different nitrogen sources were individually supplied to an S. obliquus culture medium in order to optimize its growth, biomass, and lipid quantity and quality for biodiesel production. According to the obtained results, the application of the different N sources in the BBM medium had different impacts on the growth and production of algal biomass, as well as the lipid content of the produced S. obliquus biomass. Figure 6a indicates that the highest biomass production was achieved in the growth medium containing urea, followed by that produced using ammonium nitrate as the N supplement (2.7 and 2.3 g/L, respectively). However, the biomass produced in the urea growth medium was 30% and 82% higher than those produced using sodium nitrate and ammonium sulfate as N supplements, respectively. Moreover, the percentage of lipids in the algal biomass (Figure 6b) harvested from the urea growth medium (39%) was higher than those obtained from all the examined media. The lipid content of the S. obliquus grown with urea as a nitrogen source was 2.25 and 3.8 times higher than that of the samples grown with sodium nitrate and ammonium sulfate, respectively. Although the optical density of microalgae in cultures grown with different nitrogen sources did not show obvious differences, the difference was evident in the case of chlorophyll content (Figure 7a,b). The percentage of chlorophyll increased in the different cultures, but the highest increase was reached by applying urea as an N source. This higher chlorophyll content points to higher biomass production in the medium containing urea as an N source. On the other hand, the pH of the culture increased by increasing the cultivation period, with a maximum pH increase of 10 recorded in the culture medium containing sodium nitrate as an N source (Figure 7c). Microalgae can mainly use many inorganic N sources, while some can use organic N sources, such as urea, yeast extracts, amino acids, etc. [32]. Although many studies have shown that sodium nitrate is the best form of nitrogen for the growth of many microalgae [2,33], many studies have reported other N forms. Urea, ammonium carbonate, and ammonium nitrate were recommended for obtaining the highest biomass and lipid productivity from many oleaginous microalgae for biodiesel production [34,35,36]. Because urease-producing microalgae may utilize urea as a nitrogen (N) source, urea has been associated with microalgae growth. Many microalgae including Scenedesmus spp. have the ability to utilize urea by converting it into ammonia (and CO2) by the action of urease enzymes [37]. The produced ammonia is more readily absorbed by the cell membrane and converted into amino acids during protein translation than NO2 and NO3 [38]. In addition, urea may serve as a source of carbon (C) to meet additional growth requirements when dense microalgae growth creates alkaline conditions and the dissolved CO2 concentrations are vanishingly low [39]. In addition to the high biomass and lipid productivity of microalgae when using urea as an N source, the relatively low price of urea represents an additional economic advantage [40]. The production of biodiesel from microalgae biomass requires reducing the production cost to make this process less expensive and scalable on a commercial and economic scale.
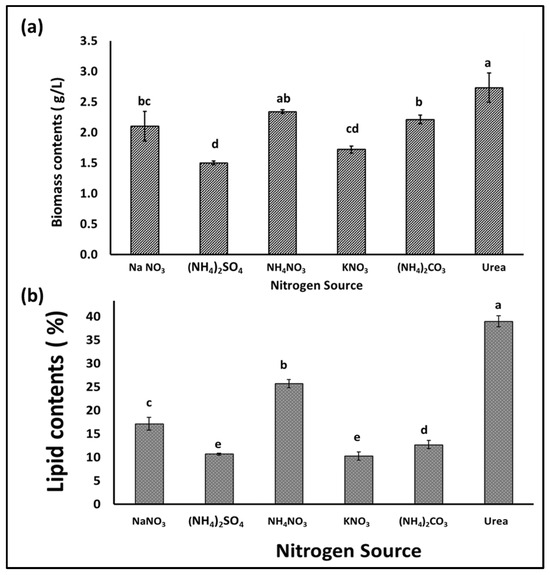
Figure 6.
Biomass (a) and lipid content (b) of S. obliquus cultivated using different nitrogen sources. Different lowercase letters in the figure represent significant differences (p < 0.05).
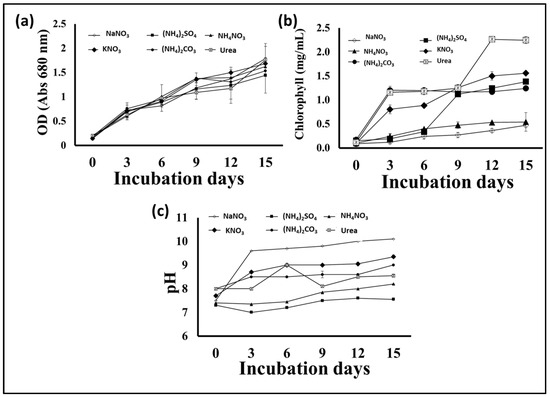
Figure 7.
Optical density at 680 nm (a), chlorophyll contents (b), and cultural pH (c) of S. obliquus cultivated under different nitrogen sources.
3.4. Optimization of Light Intensity
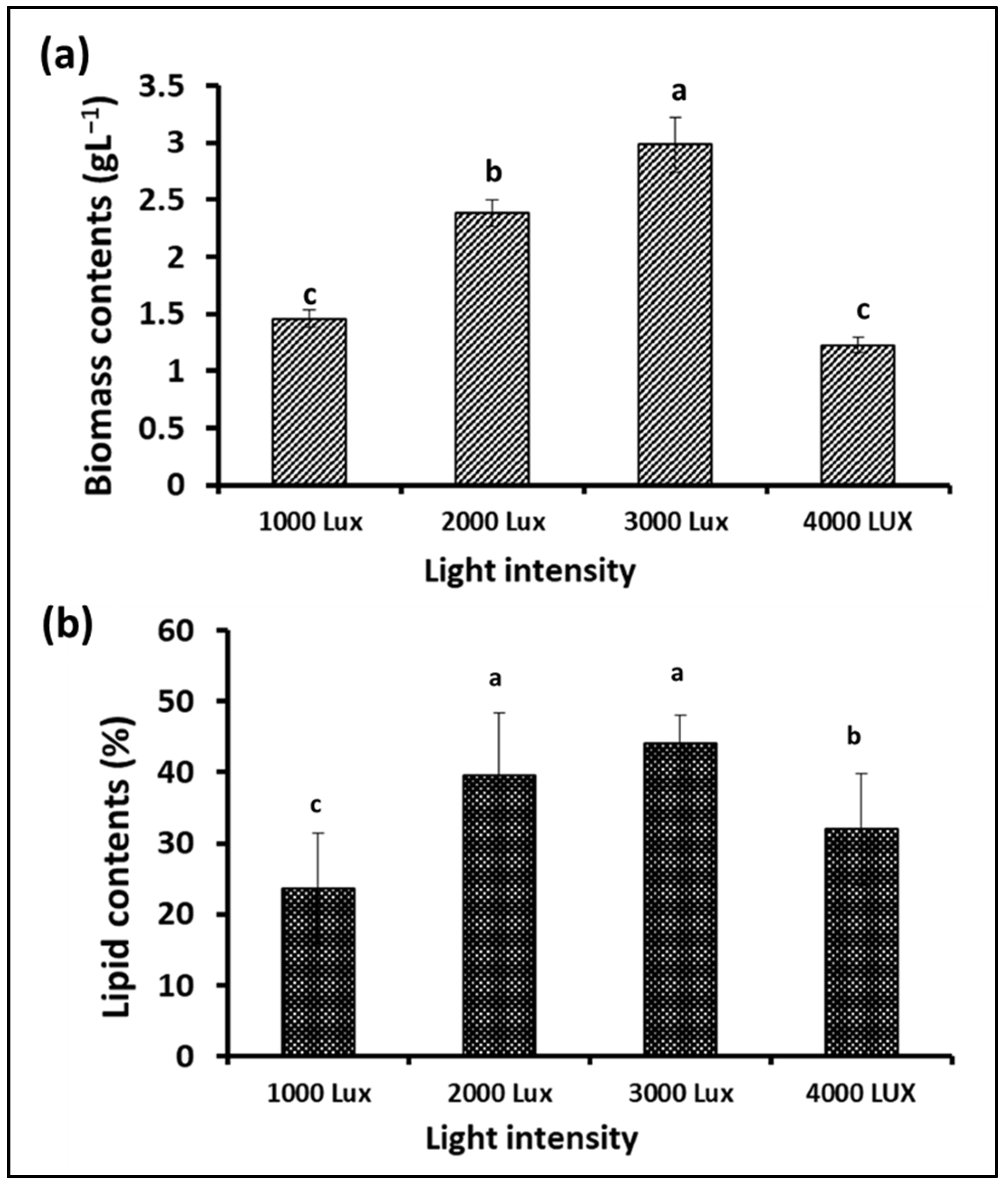
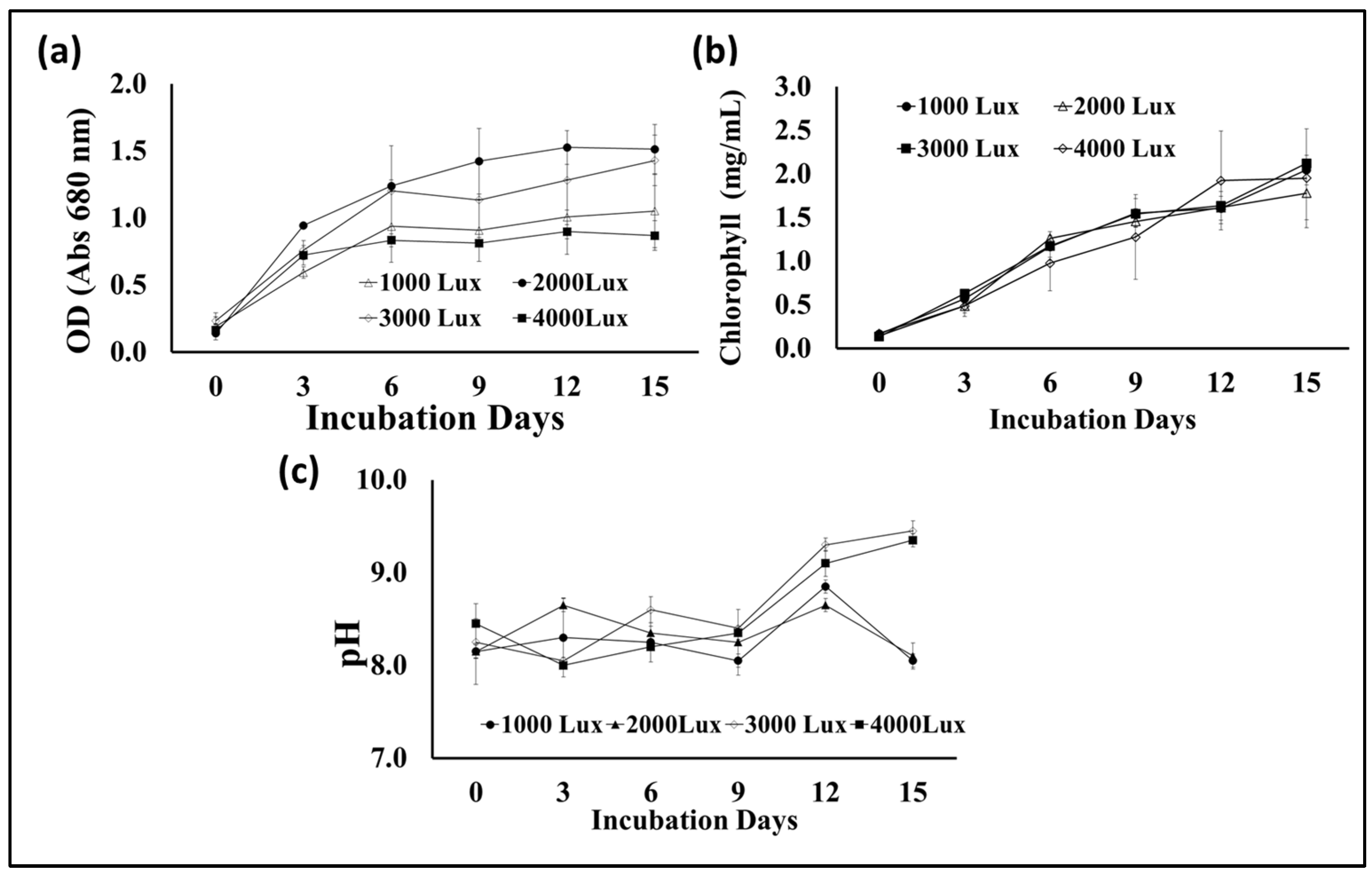
Photosynthesis is one of the most important metabolic processes in microalgae, which affects their growth and the types and quantities of their biological metabolites. In addition to carbon dioxide, water, and mineral elements, the process of photosynthesis depends mainly on light, which can be provided to microalgae cultures through sunlight or artificial lighting [41]. Light intensity is an important factor in determining the photosynthesis efficiency of microalgae cells during growth and, thus, biomass yield and lipid content. Therefore, this experiment was designed to test the growth of S. obliquus under different light intensities to maximize biomass and lipid production. Plastic bags were used as flat photobioreactors, and fluorescent lamps were placed at a distance from them to give the required lighting intensities, i.e., 1000, 2000, 3000, and 4000 lux. According to the data illustrated in Figure 8a, the maximum S. obliquus biomass (around 3 g/L) was collected from the culture subjected to continuous 3000 lux white illumination. This value was significantly higher than the other lighting intensities, with 25, 105.5, and 142% of the biomass resulting from microalgal cultures exposed to 2000, 1000, and 4000 lux, respectively. Regarding lipid contents, the microalgal culture exposed to 3000 lux of illumination produced insignificantly higher lipids than the culture supplied with 2000 lux (11.6% difference). In contrast, the percentage of lipid content was higher with a significant difference of 38% and 87% for the cultures exposed to 4000 lux and 1000 lux, respectively. Regarding the cell density, expressed as optical density (Figure 9a) in the microalgae cultures under different light intensities, it was more or less representative of the result of the biomass produced from the same cultures. The percentage of chlorophyll in the cells under different lighting treatments (Figure 9b) was very similar, despite the difference in the percentage of biomass in each of them. On the other hand, the pH levels of the S. obliquus cultures (Figure 9c) during the growth periods were close and almost constant until the end of day 12. After that, the pH values in the 3000 and 4000 lux treatments increased slightly (about 9.3 for both treatments). The appropriate light intensity levels for microalgae growth vary from one species to another and also vary according to the byproduct that will be produced from the algae [42,43]. Vélez-Landa et al. [44] studied three levels of light intensity, i.e., 1000, 2000, and 3000 lux, in cultivating the microalga Verrucodesmus verrucosus (in a three-liter photobioreactor) as a biodiesel feedstock. Based on the microalgal biomass (through the cell count), lipid content, and fatty acid profile of the extracted lipids, the light intensity of 2000 lux was reported as the optimum illumination condition. Another study by Gao et al. [41] reported that the best light intensity for the growth of the marine microalga C. vulgaris was 5000 lux. However, 2000 lux has been recommended as the optimal light intensity for microalgal growth in several related studies [45,46], while 4000 lux has been reported in other studies [47]. It is worth noting that providing adequate illumination for microalgae to obtain the highest yield of lipids and biomass as biofuel feedstock must also be taken into consideration from an economic perspective. The use of an artificial illumination source increases the operational cost of photobioreactors. Thus, in order to minimize artificial lighting and thereby lower the financial cost, efforts must be made to replace artificial lighting sources with sustainable lighting sources, such as sunshine in photobioreactors.
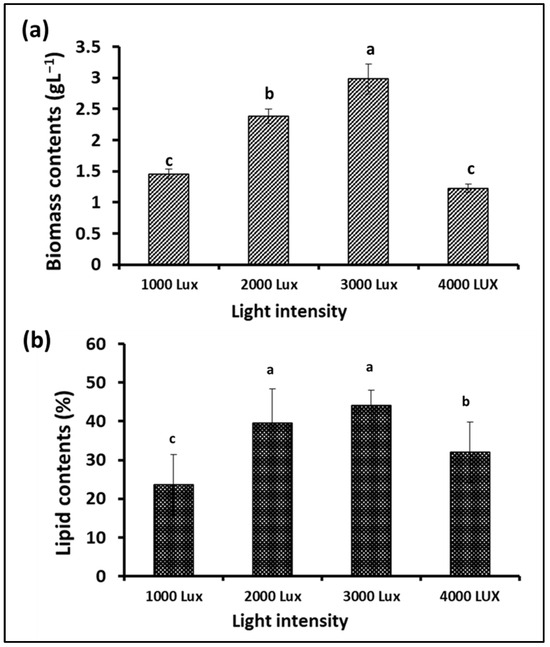
Figure 8.
Biomass (a) and lipid content (b) of S. obliquus cultivated under different light intensities. Different lowercase letters in the figure represent significant differences (p < 0.05).
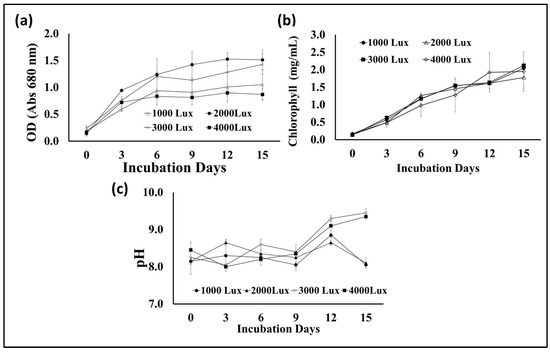
Figure 9.
Optical density at 680 nm (a), chlorophyll contents (b), and cultural pH (c) of S. obliquus cultivated under different light intensities.
3.5. Optimization of Air Flow
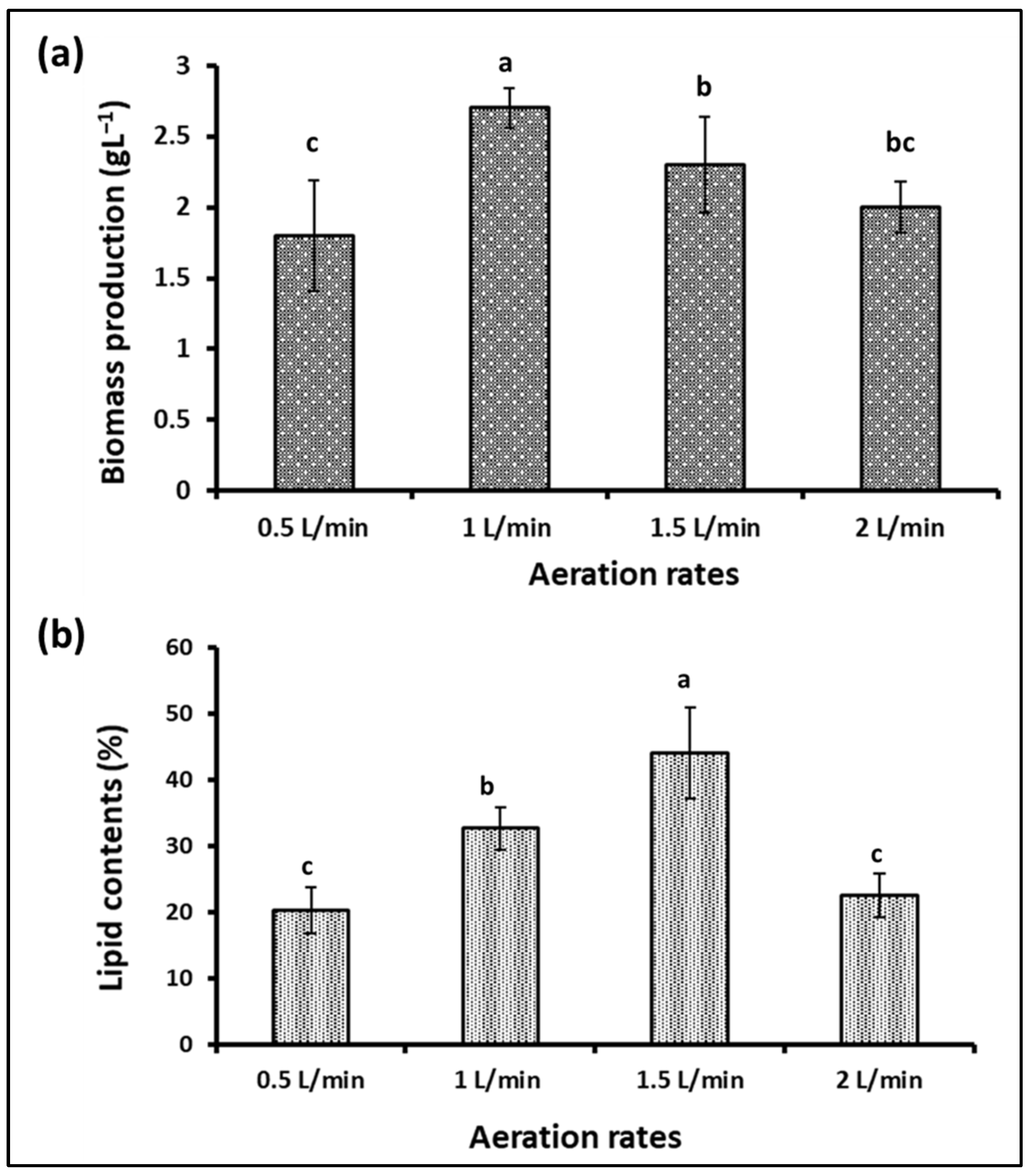
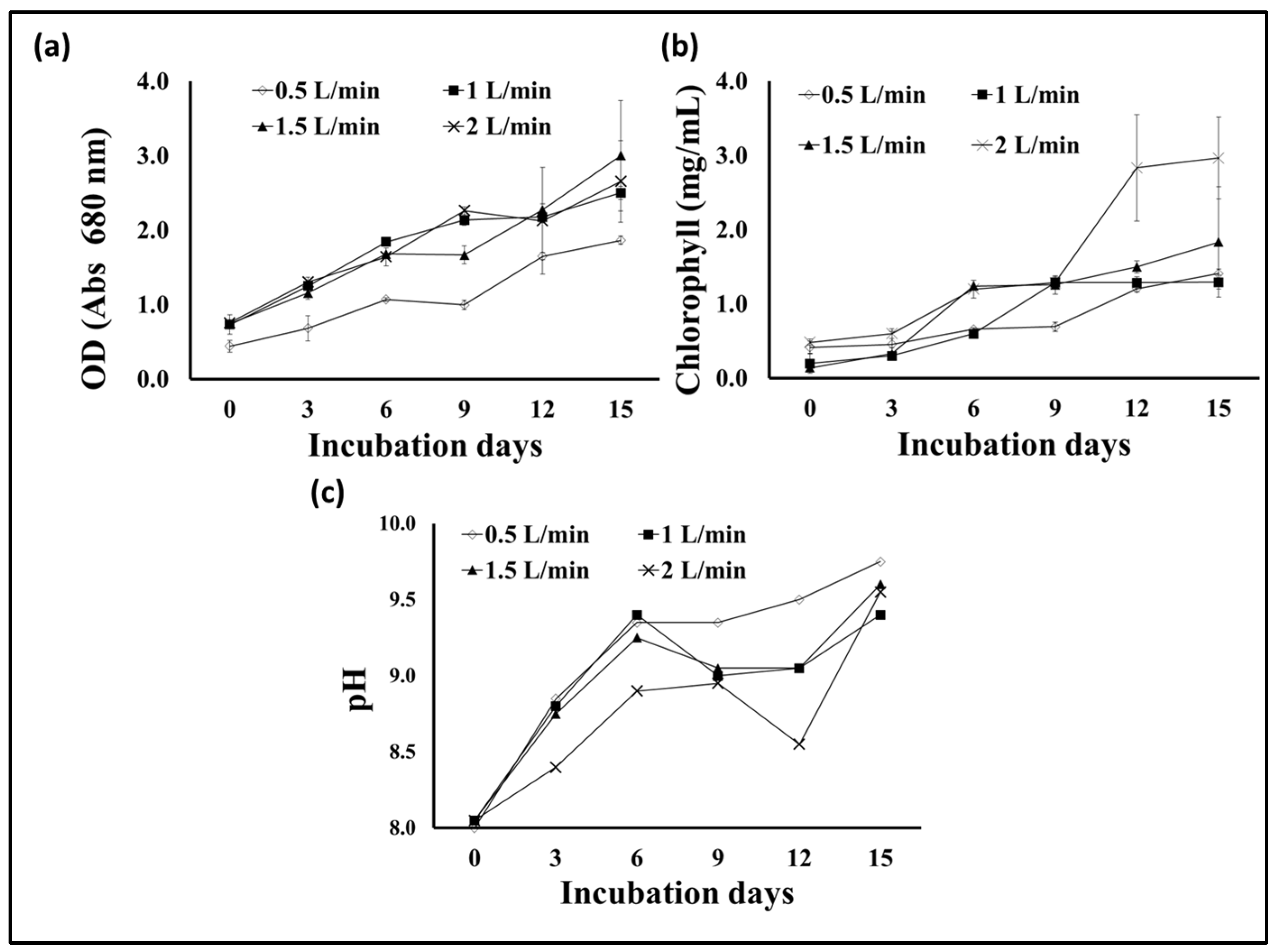
Aeration and agitation are important factors in photobioreactors and algal growth. Algae usually obtain the carbon dioxide they need from the atmosphere, where it dissolves in the algae’s environment, and they benefit from it. Pumping air into the algae culture at an appropriate rate provides sufficient carbon dioxide and the necessary circulation for the algal cells. However, excessive stirring and aeration may cause shearing effects and damage to the cells and reduce growth. Additionally, the excessive dissolved oxygen in the photosynthetic algal cultures is considered a suppressive growth agent [48,49]. Therefore, controlling the aeration rate in microalgae fermenters is considered one of the factors determining their growth and production of active biological materials, in addition to determining the economics of algae production. To determine the appropriate level of S. obliquus during cultivation in the plastic bag bioreactor designed in our study, four levels of aeration were tested, i.e., 0.5, 1, 1.5, and 2 L/min. According to the results obtained (Figure 10a), the S. obliquus biomass produced from the 1 L/min aerated bags was significantly higher than that produced under the rest of the aeration rate treatments. The dry biomass resulting from this treatment (2.7 g/L) was 17.4, 35, and 50% higher than that resulting from the 1.5, 2, and 0.5 L/min treatments, respectively. It should be noted that there was no significant difference between the biomass produced from the 0.5 and 2 L/min treatments, as was the case between the biomass produced from the 0.5 and 2 L/min treatments. Regarding the percentage of lipids in the algal biomass (Figure 10b), the highest percentage resulted from the 1.5 L air/min treatment (44%), followed by the 1 L/min treatment (32.7%). The lipid levels in the aeration treatments with 0.5 and 2 L air/min were significantly lower than those of the 1 and 1.5 L air/min treatments. On the other hand, the culture optical density, chlorophyll contents, and pH (Figure 11a–c) of the S. obliquus cultures exposed to different aeration rates were relatively similar. It was noted that the optical density and chlorophyll were lower and the pH was higher for the farm exposed to a rate of half a liter of air per minute than for the rest of the treatments. The decrease in biomass and lipid production as well as chlorophyll content in the case of the 0.5 L air/min treatment indicates that this rate is insufficient to provide CO2 and sufficient agitation to the microalga. In addition to aiding gas exchange and providing the carbon dioxide needed for algae growth, aeration of PBRs helps stimulate the culture, helping each cell to obtain enough nutrients, carbon dioxide, and light exposure [50]. However, the relatively low lipid content of the treatment using 2L air/min could be a result of excessive aeration and mixing rates which may damage the microalgal cells. According to the results of growth, biomass, and lipids, an air injection rate of 1.5 L/min (under experimental conditions) can be considered the best. It is worth noting that this aeration rate (1.5 L of air/min) is equivalent to 0.3 VVM (volume per volume per minute) of algae culture, taking into account that the capacity of the designed plastic-type photobioreactor is five liters. The same aeration rate was recommended by Thanh et al. [51] through their study on the effect of the air injection rate on cell biomass and lipid content of Scenedesmus quadricauda in a flat plate photobioreactor. They reported that “the best air injection rate for saving injection energy among 5 to 65 L/min was 15 L/min”, which is equal to 0.33 VVM (taking into account the final volume of the culture, i.e., 45 L). Likewise, in another study, Thanh et al. [52] confirmed the increase in growth and vital mass for S. quadricauda (cultivated in a column photobioreactor) with an increase in the ventilation rate. However, the same study also confirmed that an aeration rate higher than 0.6 VVM caused a decrease in both the growth rate and the concentration of biomass.
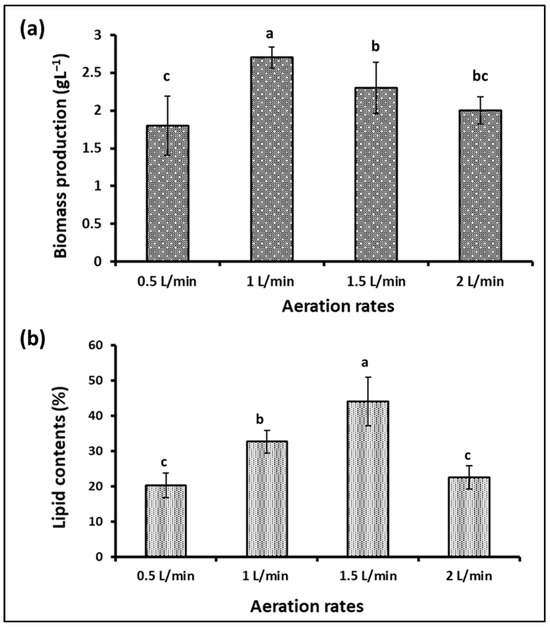
Figure 10.
Biomass (a) and lipid content (b) of S. obliquus cultivated under different aeration rates using air. Different lowercase letters in the figure represent significant differences (p < 0.05).
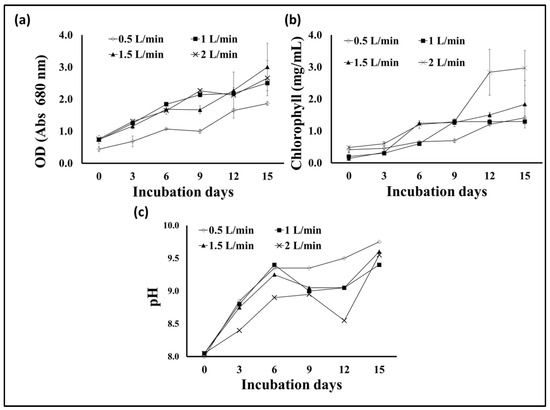
Figure 11.
Optical density at 680 nm (a), chlorophyll contents (b), and cultural pH (c) of S. obliquus cultivated under different aeration rates using air.
3.6. Biodiesel Production from S. obliquus under Optimized Conditions
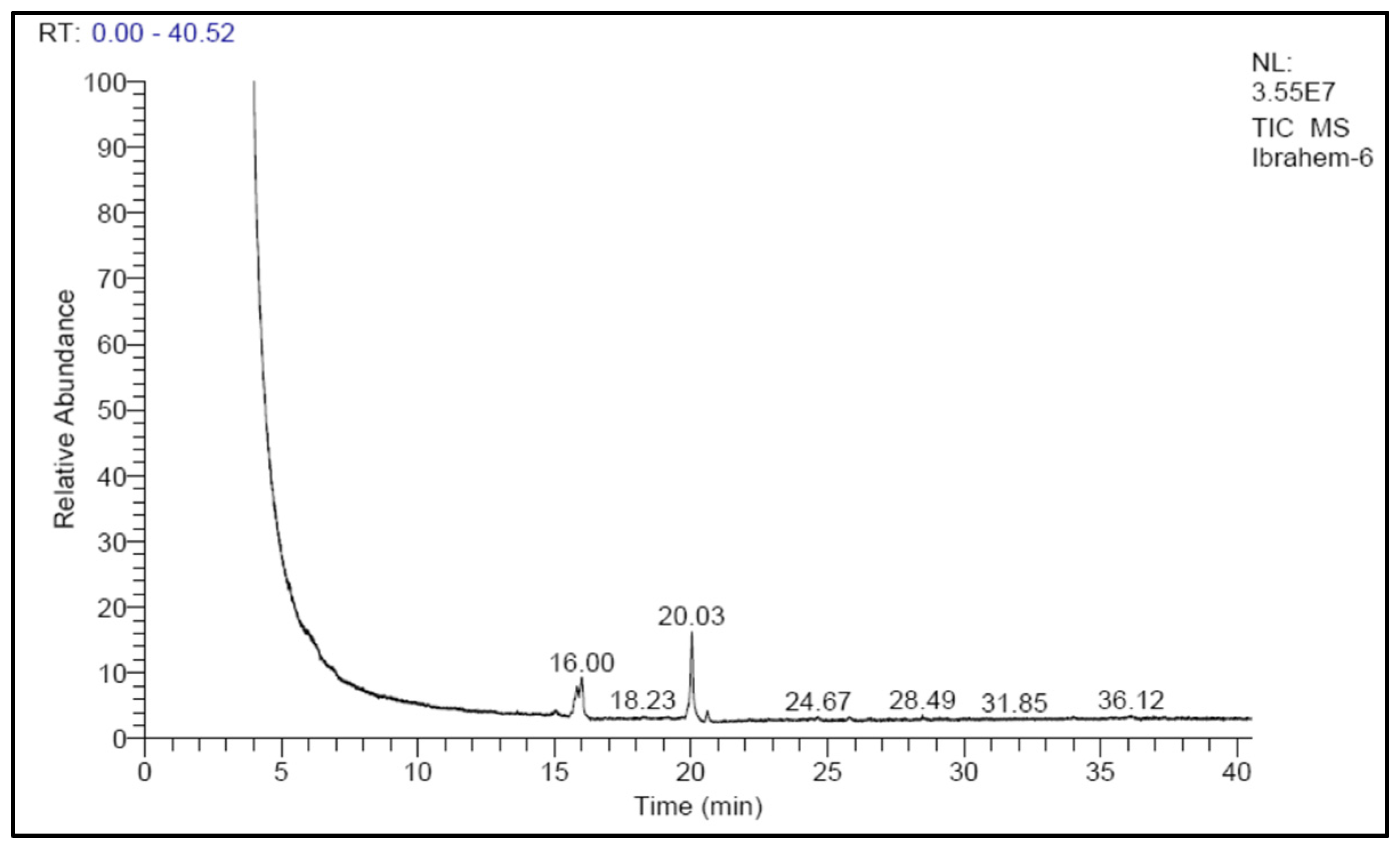
Cultivation conditions optimized for biomass and lipid contents were applied from previous experiments to grow the freshwater oleaginous microalga S. obliquus for use as biodiesel feedstock. The optimization conditions included cultivation of the microalga in a flat panel photobioreactor manufactured from a 10 µm-thick plastic sheet with dimensions of 40 cm in width and 60 cm in height. The width of the designed plastic bags was adjusted by the addition of “4 ports” of adhesion points which made the volumetric cultural capacity 5 L. Cultivation of the microalga was optimized through the replacement of the sodium nitrate of the BBM medium with urea as a nitrogen source. Cultivation bags were subjected to continuous illumination with 3000 lux white fluorescent lamps and aerated with 1.5 L air/min (equal to 0.3 VVM). After an incubation period of 15 days, the harvested biomass (3 g/L) was extracted with a chloroform/methanol solvent mixture and the extracted lipid (40% based on dry weight) was converted to fatty acid methyl ester (biodiesel) through acid esterification. The produced biodiesel was analyzed by GC-Ms to identify its fatty acid content, which reflects the suitability of this biodiesel as biofuel (Figure 12). According to the obtained results of the produced biodiesel fatty acid profile (Table 1), the percentage of C16 and C18 fatty acids was higher than 90% of the defined fatty acids, and out of this percentage, 66.6% were unsaturated fatty acids. The relatively high percentage of palmitoleic acid as well as palmitic acid in the S. obliquus under the study conditions indicates its suitability as a feedstock for the production of biodiesel. A similar conclusion was stated by Touliabah et al. [53]; according to their lipid analysis, more than 70% of the lipids were composed of C16:0 and C16:1 palmitic and palmitoleic acids. There was a high percentage of low-density fatty acids, which are thought to be the best fatty acids for producing biodiesel with a very high technical grade [23]. Accordingly, S. obliquus is considered a suitable candidate to produce biodiesel feedstock using the designed plastic-type photobioreactor after controlled cultivation. Although the high growth rate of microalgae and their lipid content make them suitable as a biodiesel feedstock, their use in biofuel production is still uneconomical [54]. Extending laboratory-scale PBRs to commercial applications requires modification and optimization of all production conditions, including economic and technical aspects. The materials used in the manufacture of microalgal PBRs, as well as the cultivation conditions, can play an important role in determining the amount of biomass and quality of lipids obtained. In addition, such parameters are considered as the base stone determining the economics of commercial-scale production. Further research could improve the economics of biomass and lipid production from microalgae. The dual use of algae in wastewater treatment/biodiesel production, for example, can offset the high cost of producing biofuel by adding an environmental dimension to the production process.
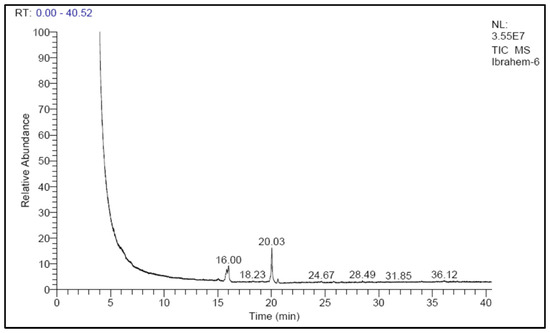
Figure 12.
GC-MS graph of biodiesel produced from S. obliquus cultivated in the designed plastic-type flat panel photobioreactor under optimized conditions.

Table 1.
Fatty acid profile of biodiesel produced from S. obliquus cultivated in the designed plastic-type flat panel photobioreactor under optimized conditions.
4. Conclusions
Enhancing the biomass and lipid productivity of microalgae is an essential step towards expanding their commercial use for the production of many useful products, such as biofuels and especially biodiesel. This enhancement of algal productivity can be achieved by using cultivation systems suitable for intensive production, using available and economical raw materials, and with appropriate designs such as flat plastic photobioreactors. Providing suitable cultivation conditions, such as nitrogen source, airflow, and light intensity, plays an important role in maximizing the biomass and lipid productivity of microalgae. In addition, the material and design of plastic flat-type photobioreactors also play an important role by enabling light penetration and diffusion, and aeration distribution for the bulk microalgal culture. The fatty acid profile of the lipids produced by S. obliquus through the designed system and under optimized conditions is suitable for biodiesel production. Such findings are considered a promising step in extending research efforts in this field for a sustainable environment.
Author Contributions
Conceptualization, I.A.M. and M.A.A.; methodology, I.A.M. and A.A.-B.; software, I.A.M. and A.A.-B.; validation, I.A.M., A.A.-B. and M.A.A.; formal analysis, I.A.M. and A.A.-B.; investigation, I.A.M. and A.A.-B.; data curation, I.A.M. and A.A.-B.; writing—original draft preparation, I.A.M. and A.A.-B.; writing—review and editing, I.A.M. and M.A.A.; visualization, I.A.M.; supervision, I.A.M. and M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to the National Research Centre, Cairo, Egypt, for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abomohra, A.E.F.; El-Sheekh, M.; Hanelt, D. Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenergy 2014, 64, 237–244. [Google Scholar] [CrossRef]
- Bisht, T.S.; Pandey, M.; Pande, V. Impact of different nitrogen sources on biomass growth and lipid productivity of Scenedesmus sp. for biodiesel production. J. Algal Biomass Utln. 2016, 7, 8–36. [Google Scholar]
- Ayad, H.I.; Matter, I.A.; Gharieb, M.M.; Darwesh, O.M. Bioflocculation harvesting of oleaginous microalga Chlorella sp. using novel lipid-rich cellulolytic fungus Aspergillus terreus (MD1) for biodiesel production. Biomass Convers. Biorefin. 2023, 1–13. [Google Scholar] [CrossRef]
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.E.; Lee, Y.C.; Oh, Y.K. Flocculation harvesting techniques for microalgae: A review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef]
- Amaral, M.S.; Loures, C.C.; Naves, F.L.; Samanamud, G.L.; Silva, M.B.; Prata, A.M. Microalgae cultivation in photobioreactors aiming at biodiesel production. In Biotechnological Applications of Biomass; Peixoto Basso, T., Olitta Basso, T., Carlos Basso, L., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Chen, Y.P.; Huang, Y.H.; Huang, H.C. Different plastic-bag type photobioreactor for biomass production of Chlorella species. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Johor, Malaysia, 16–17 January 2021; IOP Publishing: Bristol, UK, 2021; Volume 1113, p. 012004. [Google Scholar]
- Chen, C.Y.; Chang, J.S.; Chang, H.Y.; Chen, T.Y.; Wu, J.H.; Lee, W.L. Enhancing microalgal oil/lipid production from Chlorella sorokiniana CY1 using deep-sea water supplemented cultivation medium. Biochem. Eng. J. 2013, 77, 74–81. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nurun, N.; Deb, U.K. The growth factors involved in microalgae cultivation for biofuel production: A review. Comput. Water Eng. Environ. Eng. 2020, 9, 185–215. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, N.; Koji, I.; Yuzir, A.; Muhammad, S.E. Evolution of photobioreactors: A review based on microalgal perspective. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Johor, Malaysia, 16–17 January 2021; IOP Publishing: Bristol, UK, 2021; Volume 1142, p. 012004. [Google Scholar]
- Hijazi, R.; Mounsef, J.R.; Kanaan, H.Y. Design considerations for photo-bioreactors: A review. In Proceedings of the 2020 5th International Conference on Renewable Energies for Developing Countries (REDEC), Marrakech, Morocco, 29–30 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Ambati, R.R. (Eds.) Handbook of Algal Technologies and Phytochemicals: Phycoremediation, Biofuels and Global Biomass Production; CRC Press: Boca Raton, FL, USA, 2019; Volume 2. [Google Scholar] [CrossRef]
- Al-Dailami, A.; Koji, I.; Ahmad, I.; Goto, M. Potential of photobioreactors (PBRs) in cultivation of microalgae. J. Adv. Res. Appl. Sci. Eng. Technol. 2022, 27, 32–44. [Google Scholar] [CrossRef]
- Uyar, B.; Ali, M.D.; Uyar, G.E.O. Design parameters comparison of bubble column, airlift and stirred tank photobioreactors for microalgae production. Bioprocess Biosyst. Eng. 2024, 47, 195–209. [Google Scholar] [CrossRef]
- Hirayama, A.; Sueyoshi, M.N.; Nakano, T.; Ota, Y.; Kurita, H.; Tasaki, M.; Kuroiwa, Y.; Kato, T.; Serizawa, S.; Kojima, K.; et al. Development of large-scale microalgae production in the Middle East. Bioresour. Technol. 2022, 343, 126036. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Bhattacharya, S.; Reesh, I.M.A.; Bhuvanesh, S.; Sreekrishnan, T.R. Challenges in the design and operation of an efficient photobioreactor for microalgae cultivation and hydrogen production. In Biohydrogen Production: Sustainability of Current Technology and Future Perspective; Singh, A., Rathore, D., Eds.; Springer: New Delhi, India, 2017; pp. 147–162. [Google Scholar] [CrossRef]
- Ihsan, E.K.İ.N. Types of microalgae cultivation photobioreactors and production process of microalgal biodiesel as alternative fuel. Acta Biol. Turc. 2020, 33, 114–131. [Google Scholar]
- Wang, X.; Zhou, Y.; Peng, Q.; Han, Y.; Yang, J.; Xu, H.; Li, C.; Li, L.; Dou, S.; Yang, M.; et al. Development of plastic flatbed-based algal culture system deployable on non-arable land. Algal Res. 2022, 66, 102814. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R.; Jonathan, E. Biophotonics for improving algal photobioreactor performance: A review. Int. J. Energy Res. 2020, 44, 5071–5092. [Google Scholar] [CrossRef]
- Płaczek, M.; Patyna, A.; Witczak, S. Technical evaluation of photobioreactors for microalgae cultivation. E3S Web Conf. 2017, 19, 02032. [Google Scholar] [CrossRef]
- Norsker, N.H.; Cuaresma, M.; de Vree, J.; Ruiz-Domínguez, M.C.; García, M.C.M.; Uronen, P.; Barbosa, M.J.; Wijffels, R. Neochloris oleoabundans oil production in an outdoor tubular photobioreactor at pilot scale. J. Appl. Phycol. 2021, 33, 1327–1339. [Google Scholar] [CrossRef]
- Eida, M.F.; Darwesh, O.M.; Matter, I.A. Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. J. Ecol. Eng. 2018, 19, 38–51. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, T.; Wang, C. Simultaneous detection of viability and concentration of microalgae cells based on chlorophyll fluorescence and bright field dual imaging. Micromachines 2021, 12, 896. [Google Scholar] [CrossRef]
- de Freitas, C.; Carmona, E.; Brienzo, M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. 2019, 18, 100184. [Google Scholar] [CrossRef]
- Ichihara, K.I.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography [S]. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Monteiro, C.M.; Malcata, F.X. Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri. J. Appl. Phycol. 2009, 21, 543–552. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Sirohi, P.; Verma, H.; Singh, S.K.; Singh, V.K.; Pandey, J.; Khusharia, S.; Kumar, D.; Kaushalendra; Teotia, P.; Kumar, A. Microalgal carotenoids: Therapeutic application and latest approaches to enhance the production. Curr. Issues Mol. Biol. 2022, 44, 6257–6279. [Google Scholar] [CrossRef]
- Pahlavanyali, M.; Jalili, H.; Noroozi, M.; Morady, Y.; Hallajisani, A. The effect of temperature and different carbon and nitrogen sources on the growth and fatty acid profile of a newly isolated microorganism Aurantiochytrium sp. strain SHY. Iran. J. Fish. Sci. 2020, 19, 3112–3126. [Google Scholar]
- Chi, Y.; Chen, F.; Takiguchi, Y. Effect of nitrogen source on biomass and lipid production of a marine microalga, Nannochloropsis oceanica IMET1. Green Sustain. Chem. 2015, 5, 101. [Google Scholar] [CrossRef]
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lin, J. Effects of nitrogen source and concentration on biomass and oil production of a Scenedesmus rubescens like microalga. Bioresour. Technol. 2011, 102, 1615–1621. [Google Scholar] [CrossRef]
- Amin, N.F.; Khalafallah, M.A.; Ali, M.A.; Abou-Sdera, S.A.; Matter, I.A. Effect of some nitrogen sources on growth and lipid of microalgae Chlorella sp. for biodiesel production. J. Appl. Sci. Res. 2013, 9, 4845–4855. [Google Scholar]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.K. Influence of nitrogen source and growth phase on extracellular biosynthesis of silver nanoparticles using cultural filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Dhup, S.; Kannan, D.C.; Dhawan, V. Understanding urea assimilation and its effect on lipid production and fatty acid composition of Scenedesmus sp. SOJ Biochem. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Ruatpuia, J.V.; Ao, S.; Chetia, A.C.; Satyan, K.B.; Rokhum, S.L. Microalgae as a sustainable feedstock for biodiesel and other production industries: Prospects and challenges. Energy Nexus 2023, 12, 100255. [Google Scholar] [CrossRef]
- Krausfeldt, L.E.; Farmer, A.T.; Castro Gonzalez, H.F.; Zepernick, B.N.; Campagna, S.R.; Wilhelm, S.W. Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front. Microbiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Zhang, Z.; Zhang, C.; Merkens, H.; Tan, R.; Wong, A.A.; Uribe, C.F.; Bénard, F.; Lin, K.S. Lys-urea-Aad, Lys-urea-Cmc and Lys-urea-Cms as potential pharmacophores for the design of PSMA-targeted radioligands to reduce off-target uptake in kidneys and salivary glands. Theranostics 2023, 13, 4559. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Guo, L.; Gao, M.; Zhao, Y.; Jin, C.; She, Z. Regulation of carbon source metabolism in mixotrophic microalgae cultivation in response to light intensity variation. J. Environ. Manag. 2022, 302, 114095. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Landa, L.; Hernández-De León, H.R.; Pérez-Luna, Y.D.C.; Velázquez-Trujillo, S.; Moreira-Acosta, J.; Berrones-Hernández, R.; Sánchez-Roque, Y. Influence of light intensity and photoperiod on the photoautotrophic growth and lipid content of the microalgae Verrucodesmus verrucosus in a photobioreactor. Sustainability 2021, 13, 6606. [Google Scholar] [CrossRef]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and biopigment accumulation of cyanobacterium Spirulina platensis at different light intensities and temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, W.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour. Technol. 2014, 152, 292–298. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Ding, W.; Lu, S.; Song, K.; Chen, C.; Qin, C.; Chen, Y.; Tu, R.; Zhou, X. Effects of nutrient composition, lighting conditions, and metal ions on the growth and lipid yield of the high-lipid-yielding microalgae (Chlorella pyrenoidosa) cultivated in municipal wastewater. J. Environ. Chem. Eng. 2021, 9, 106491. [Google Scholar] [CrossRef]
- Kazbar, A.; Cogne, G.; Urbain, B.; Marec, H.; Le-Gouic, B.; Tallec, J.; Takache, H.; Ismail, A.; Pruvost, J. Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Res. 2014, 39, 101432. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for cultivation and synthesis: Specifications, challenges, and perspectives. Eng. Life Sci. 2022, 22, 712–724. [Google Scholar] [CrossRef]
- Amzah, N.F.; Mohamad, S.E.B.; Akhir, F.N.M.; Othman, F.S.; Suzuki, I.; Maeda, Y. Effect of agitation on growth and fatty acid composition of green microalgae Acutodesmus obliquus Q2-12E. Chem. Eng. Trans. 2023, 106, 1231–1236. [Google Scholar] [CrossRef]
- Thanh, N.T.; Uemura, Y.; Krishnan, V.; Ismail, L. The effect of air injection rate and medium nitrogen concentration on cell biomass and lipid content of Scenedesmus quadricauda in flat plate photobioreactor. Procedia Eng. 2016, 148, 538–545. [Google Scholar] [CrossRef]
- Thanh, N.T.; Uemura, Y.; Osman, N.; Ismail, L. The effect of aeration rate on the growth of Scenedesmus quadricauda in column photobioreactor. J. Jpn. Inst. Energy 2015, 94, 177–180. [Google Scholar] [CrossRef][Green Version]
- Touliabah, H.E.; Abdel-Hamid, M.I.; Almutairi, A.W. Long-term monitoring of the biomass and production of lipids by Nitzschia palea for biodiesel production. Saudi J. Biol. Sci. 2020, 27, 2038–2046. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.M.; Martins, A.A.; Freitas, M.A.V.; Caetano, N.S. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).