Design and Simulation of the Biodiesel Process Plant for Sustainable Fuel Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Analytical Design and Calculations
- Flange = 0.2 • Bend = 0.3;
- Sudden entrance = 0.5 • Valve = 0.2; the valve is assumed to be completely open.
| Design Item | Description | Design Value | Calculation |
|---|---|---|---|
| Pipe network | Pipe material | Commercial Steel ASTM/ASME A53/SA53-Seamless and welded standard steel pipes Grade B [52] | Bursting pressure = = |
| Pipe dia (inner and outer) | Di = 0.10 m Do = 0.11 m As per standard, Sch 40S | ||
| Pipe wall thickness | t = 0.006 m | ||
| Busting pressure | 49.68 MPa | ||
| Fitting loss factor | 51.3 | The calculation is shown in Table 3 | |
| Friction factor (f) |
|
| Oil & KOH & Methanol Tank | Reactor’s Tank | Methanol Recovery Tank | Water Washing Tank | Reactor Tank for Glycerol Removal | FAME/Glycerol Storage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In | Out | In | Out | In | Out | In | Out | In | Out | In | Out | |
| Flanges | 0.8 | 0.6 | 2 | 0.8 | 0.4 | 0.4 | 0.6 | 0.4 | 0.8 | 0.6 | 0.4 | 0.4 |
| Bends | 0 | 1.8 | 6 | 1.8 | 1.2 | 3.6 | 2.4 | 4.8 | 3.6 | 3 | 2.4 | 1.2 |
| Sudden entrance | 1.5 | 0 | 1.5 | 0 | 0.5 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Valve | 0.6 | 0 | 2 | 0.8 | 0 | 0 | 0 | 0.6 | 0.4 | 0 | 0 | 0.4 |
| Total | 2.9 | 2.4 | 11.5 | 3.4 | 2.1 | 4 | 4 | 5.8 | 5.8 | 3.6 | 3.8 | 2 |
| 51.3 | ||||||||||||
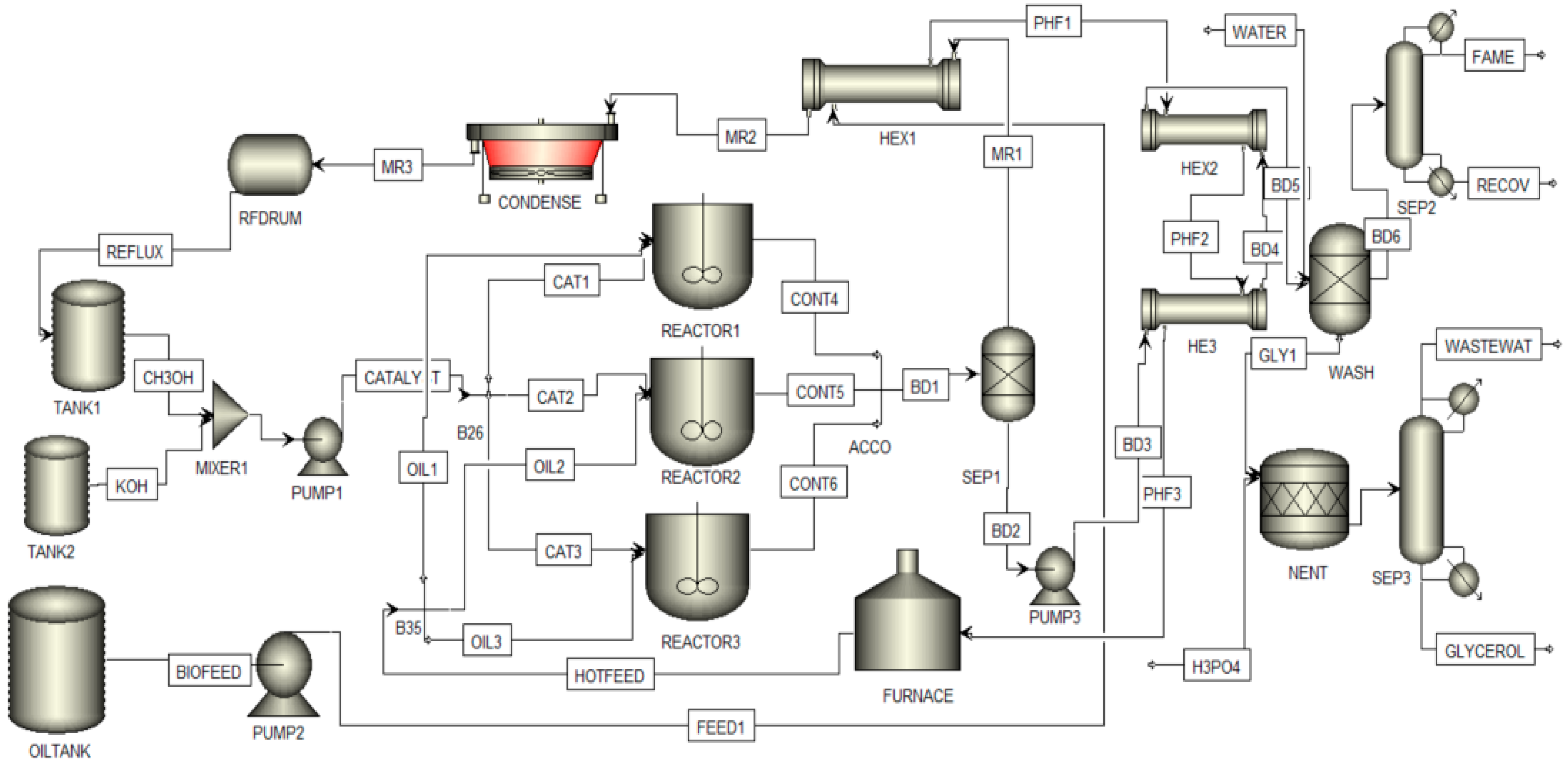
3.2. Biodiesel Plant Model
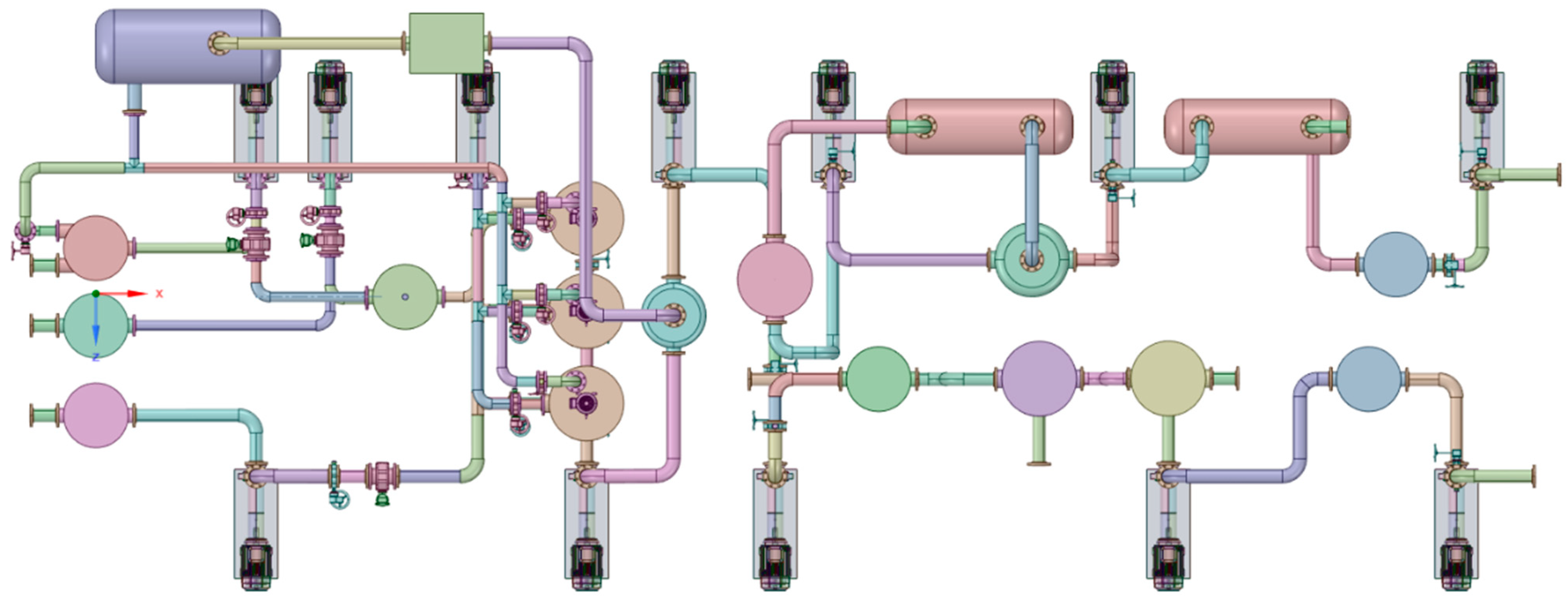
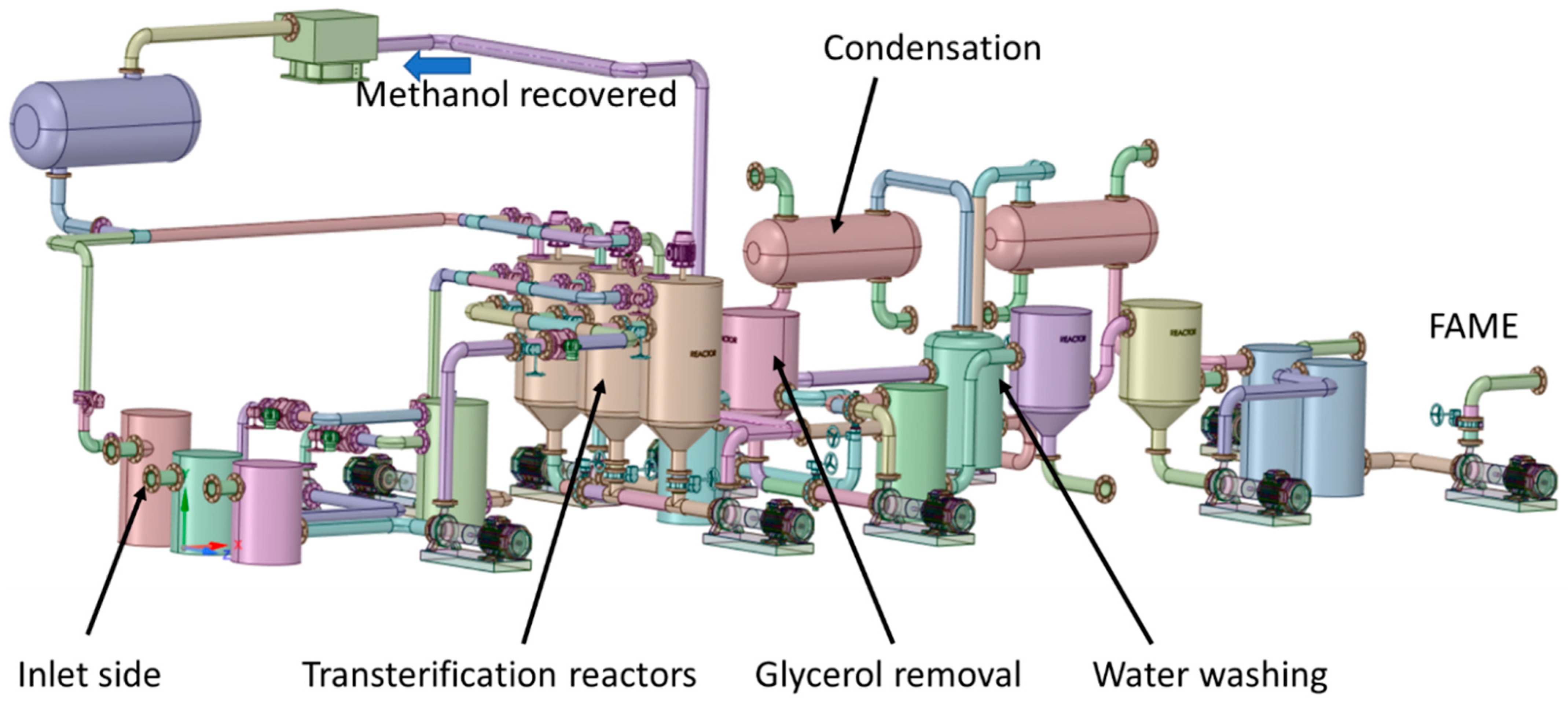
3.3. Simulations Model
4. Conclusions and Recommendations
- (1)
- For the proposed plant model, the bursting pressure of steel pipe is 49.68 MPa for the chosen diameter and thickness, which must withstand max pressure of 4 bar and turbulent flow behavior with Reynold number of 2.3 × 106; thus, the system was found to be designed safely. Also, the friction factor of the pipe is 0.0165, which is a fairly smooth pipe with smooth flow and less resistance;
- (2)
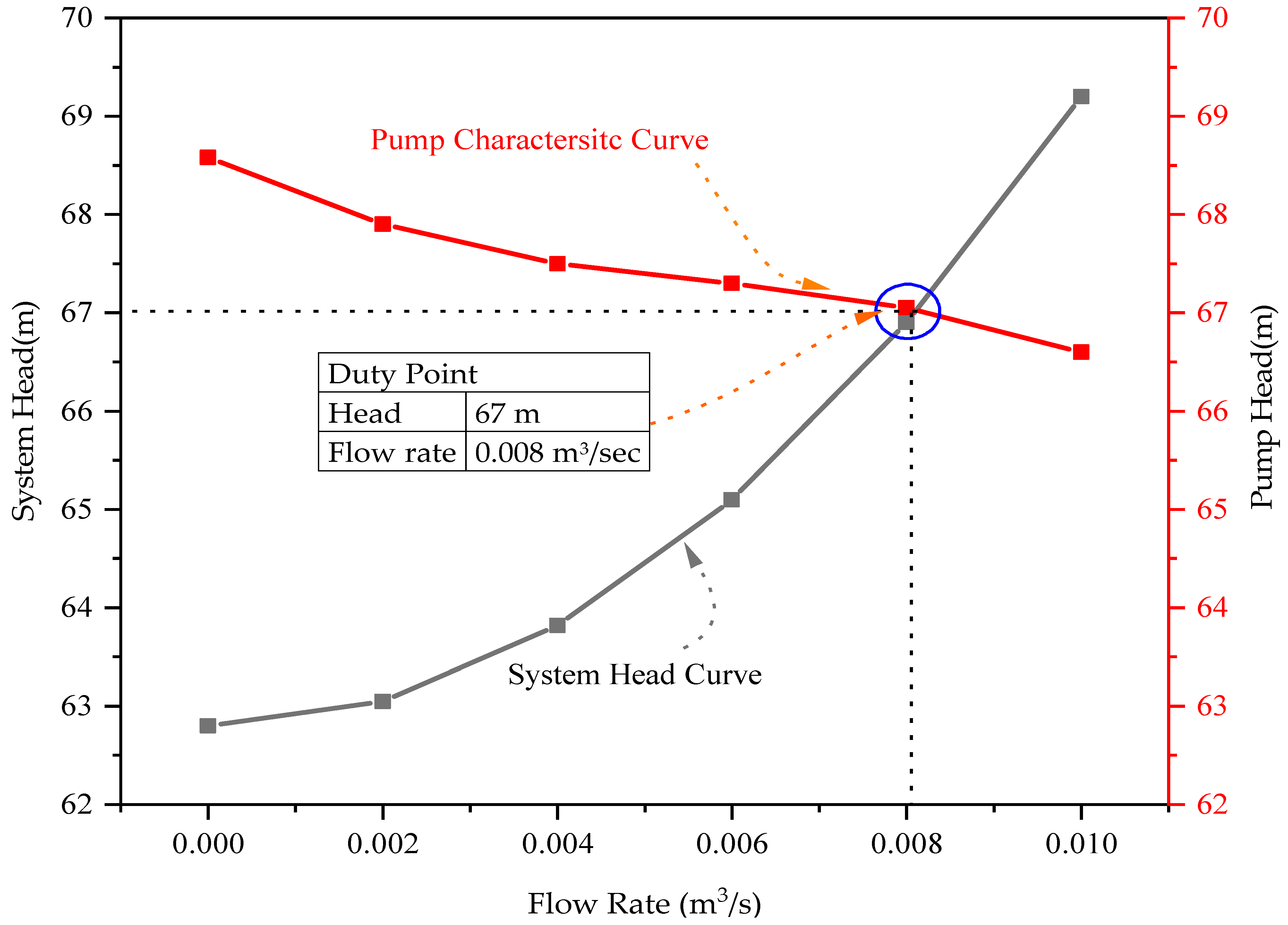
- The required head and flow rates are 0.008 m3/s and 66.9 m, and the duty point obtained from the results shows that the selected pump gives the desired output;
- (3)
- The simulation results closely match the experimental biodiesel composition, and biodiesel yield was recorded as 88.8%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ASME | American Society of Mechanical Engineers |
| ASTM | American Society for Testing and Materials |
| EIA | Energy Information Administration |
| FAME | Fatty Acid Methyl Ester |
| KOH | Potassium Hydroxide |
| NPSHA | Net Positive Suction Head |
| NPSHR | Net Positive Suction Head Required |
| NRTL | Non-Random Two-Liquid |
| TAG | Triacyl Glycerides |
References
- Hezam, I.M.; Vedala, N.R.; Kumar, B.R.; Mishra, A.R.; Cavallaro, F. Assessment of Biofuel Industry Sustainability Factors Based on the Intuitionistic Fuzzy Symmetry Point of Criterion and Rank-Sum-Based MAIRCA Method. Sustainability 2023, 15, 6749. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- EIA. EIA Projects Nearly 50% Increase in World Energy Use by 2050, Led by Growth in Renewables. 2021. Available online: https://www.eia.gov/todayinenergy/detail.php?id=49876 (accessed on 21 February 2024).
- Raju, V.D.; Veza, I.; Venu, H.; Soudagar, M.E.M.; Kalam, M.A.; Ahamad, T.; Appavu, P.; Nair, J.N.; Rahman, S.M.A. Comprehensive Analysis of Compression Ratio, Exhaust Gas Recirculation, and Pilot Fuel Injection in a Diesel Engine Fuelled with Tamarind Biodiesel. Sustainability 2023, 15, 5222. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, P.; Yu, Y.; Chen, S.-H.; Wang, C.-X.; He, L.; Nie, H.-T.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; et al. Regeneration mechanism of a novel high-performance biochar mercury adsorbent directionally modified by multimetal multilayer loading. J. Environ. Manag. 2023, 326, 116790. [Google Scholar] [CrossRef] [PubMed]
- Rattanaphra, D.; Tawkaew, S.; Chuichulcherm, S.; Kingkam, W.; Nuchdang, S.; Kitpakornsanti, K.; Suwanmanee, U. Evaluation of Life Cycle Assessment of Jatropha Biodiesel Processed by Esterification of Thai Domestic Rare Earth Oxide Catalysts. Sustainability 2024, 16, 100. [Google Scholar] [CrossRef]
- Musharavati, F.; Sajid, K.; Anwer, I.; Nizami, A.-S.; Javed, M.H.; Ahmad, A.; Naqvi, M. Advancing Biodiesel Production System from Mixed Vegetable Oil Waste: A Life Cycle Assessment of Environmental and Economic Outcomes. Sustainability 2023, 15, 16550. [Google Scholar] [CrossRef]
- Zailani, S.; Iranmanesh, M.; Sean Hyun, S.; Ali, M.H. Barriers of Biodiesel Adoption by Transportation Companies: A Case of Malaysian Transportation Industry. Sustainability 2019, 11, 931. [Google Scholar] [CrossRef]
- Dey, S.; Singh, A.P.; Gajghate, S.S.; Pal, S.; Saha, B.B.; Deb, M.; Das, P.K. Optimization of CI Engine Performance and Emissions Using Alcohol–Biodiesel Blends: A Regression Analysis Approach. Sustainability 2023, 15, 14667. [Google Scholar] [CrossRef]
- Etim, A.O.; Betiku, E.; Ajala, S.O.; Olaniyi, P.J.; Ojumu, T.V. Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability 2018, 10, 707. [Google Scholar] [CrossRef]
- Ahmad, G.; Imran, S.; Farooq, M.; Shah, A.N.; Anwar, Z.; Rehman, A.U.; Imran, M. Biodiesel Production from Waste Cooking Oil Using Extracted Catalyst from Plantain Banana Stem via RSM and ANN Optimization for Sustainable Development. Sustainability 2023, 15, 3599. [Google Scholar] [CrossRef]
- Azad, A.K.; Halder, P.; Wu, Q.; Rasul, M.G.; Hassan, N.M.S.; Karthickeyan, V. Experimental investigation of ternary biodiesel blends combustion in a diesel engine to reduce emissions. Energy Convers. Manag. X 2023, 20, 100499. [Google Scholar] [CrossRef]
- Doppalapudi, A.T.; Azad, A.; Khan, M. Combustion chamber modifications to improve diesel engine performance and reduce emissions: A review. Renew. Sustain. Energy Rev. 2021, 152, 111683. [Google Scholar] [CrossRef]
- López-Zapata, B.Y.; Adam-Medina, M.; Álvarez-Gutiérrez, P.E.; Castillo-González, J.P.; León, H.R.H.-d.; Vela-Valdés, L.G. Virtual Sensors for Biodiesel Production in a Batch Reactor. Sustainability 2017, 9, 455. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kamil, M.; Bakar, R.A. Engine performance and optimum injection timing for 4-cylinder direct injection hydrogen fueled engine. Simul. Model. Pract. Theory 2011, 19, 734–751. [Google Scholar] [CrossRef]
- ASTM D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2023.
- Almuhayawi, M.S.; Hassan, E.A.; Almasaudi, S.; Zabermawi, N.; Azhar, E.I.; Najjar, A.; Alkuwaity, K.; Abujamel, T.S.; Alamri, T.; Harakeh, S. Biodiesel Production through Rhodotorula toruloides Lipids and Utilization of De-Oiled Biomass for Congo Red Removal. Sustainability 2023, 15, 13412. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the time finally come for green oleochemicals and biodiesel production using large-scale enzyme technologies? Current status and new developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Azad, A.K.; Doppalapudi, A.T.; Khan, M.M.K.; Hassan, N.M.S.; Gudimetla, P. A landscape review on biodiesel combustion strategies to reduce emission. Energy Rep. 2023, 9, 4413–4436. [Google Scholar] [CrossRef]
- Doppalapudi, A.T.; Azad, A.K.; Khan, M.M.K. Advanced strategies to reduce harmful nitrogen-oxide emissions from biodiesel fueled engine. Renew. Sustain. Energy Rev. 2023, 174, 113123. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Alnefaie, K.A. A comparative study of almond biodiesel-diesel blends for diesel engine in terms of performance and emissions. BioMed Res. Int. 2015, 2015, 529808. [Google Scholar] [CrossRef]
- Antony Miraculas, G.; Bose, N.; Edwin Raj, R. Optimization of process parameters for biodiesel extraction from tamanu oil using design of experiments. J. Renew. Sustain. Energy 2014, 6, 033120. [Google Scholar] [CrossRef]
- Azad, A.K.; Rasul, M.G.; Khan, M.M.K.; Sharma, S.C.; Mofijur, M.; Bhuiya, M.M.K. Prospects, feedstocks and challenges of biodiesel production from beauty leaf oil and castor oil: A nonedible oil sources in Australia. Renew. Sustain. Energy Rev. 2016, 61, 302–318. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M.; Lim, H.C. A study on the performance and emission of a diesel engine fueled with Karanja biodiesel and its blends. Energy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Liaquat, A.M.; Masjuki, H.H.; Kalam, M.; Fattah, I.R.; Hazrat, M.; Varman, M.; Mofijur, M.; Shahabuddin, M. Effect of coconut biodiesel blended fuels on engine performance and emission characteristics. Procedia Eng. 2013, 56, 583–590. [Google Scholar] [CrossRef]
- Moreira, K.S.; Moura Junior, L.S.; Monteiro, R.R.; de Oliveira, A.L.; Valle, C.P.; Freire, T.M.; Fechine, P.B.; de Souza, M.C.; Fernandez-Lorente, G.; Guisan, J.M. Optimization of the production of enzymatic biodiesel from residual babassu oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Oniya, O.O.; Bamgboye, A.I. Production of biodiesel from groundnut (Arachis hypogea, L.) oil. Agric. Eng. Int. CIGR J. 2014, 16, 143–150. [Google Scholar]
- Otamiri, F.O.; Ogugua, V.N.; Joshua, P.E.; Odiba, A.S.; Ukegbu, C.Y. Physicochemical characterization of coconut copra (Dry Flesh) oil and production of biodiesel from coconut copra oil. Jökull J. Univ. Niger. Nsukka 2014, 64, 201–236. [Google Scholar]
- Patel, R.L.; Sankhavara, C. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Azad, A.K. Biodiesel from Mandarin Seed Oil: A Surprising Source of Alternative Fuel. Energies 2017, 10, 1689. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresour. Technol. 2006, 97, 379–384. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Ghedini, E.; Taghavi, S.; Menegazzo, F.; Signoretto, M. A Review on the Efficient Catalysts for Algae Transesterification to Biodiesel. Sustainability 2021, 13, 10479. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Techno-economic study of different alternatives for biodiesel production. Fuel Process. Technol. 2008, 89, 740–748. [Google Scholar] [CrossRef]
- Samuel, O.D.; Aigba, P.A.; Tran, T.K.; Fayaz, H.; Pastore, C.; Der, O.; Erçetin, A.; Enweremadu, C.C.; Mustafa, A. Comparison of the Techno-Economic and Environmental Assessment of Hydrodynamic Cavitation and Mechanical Stirring Reactors for the Production of Sustainable Hevea brasiliensis Ethyl Ester. Sustainability 2023, 15, 16287. [Google Scholar] [CrossRef]
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef]
- Lee, S.; Posarac, D.; Ellis, N. Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol. Chem. Eng. Res. Des. 2011, 89, 2626–2642. [Google Scholar] [CrossRef]
- Poddar, T.; Jagannath, A.; Almansoori, A. Biodiesel Production using Reactive Distillation: A Comparative Simulation Study. Energy Procedia 2015, 75, 17–22. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Adeoye, A.S.; Onifade, D.V. Modelling and optimisation of biodiesel production from Euphorbia lathyris using ASPEN Hysys. SN Appl. Sci. 2019, 1, 1452. [Google Scholar] [CrossRef]
- Giwa, A.; Giwa, S.O.; Olugbade, E.A. Application of Aspen HYSYS process simulator in green energy revolution: A case study of biodiesel production. ARPN J. Eng. Appl. Sci 2018, 13, 569–581. [Google Scholar]
- Salehi, A.; Karbassi, A.; Ghobadian, B.; Ghasemi, A.; Doustgani, A. Simulation process of biodiesel production plant. Environ. Prog. Sustain. Energy 2019, 38, e13264. [Google Scholar] [CrossRef]
- Apostolakou, A.; Kookos, I.; Marazioti, C.; Angelopoulos, K. Techno-economic analysis of a biodiesel production process from vegetable oils. Fuel Process. Technol. 2009, 90, 1023–1031. [Google Scholar] [CrossRef]
- Avinash, A.; Murugesan, A. Economic analysis of biodiesel production from waste cooking oil. Energy Sources Part B Econ. Plan. Policy 2017, 12, 890–894. [Google Scholar] [CrossRef]
- Glisic, S.B.; Pajnik, J.M.; Orlović, A.M. Process and techno-economic analysis of green diesel production from waste vegetable oil and the comparison with ester type biodiesel production. Appl. Energy 2016, 170, 176–185. [Google Scholar] [CrossRef]
- Nagarajan, S.; Chou, S.K.; Cao, S.; Wu, C.; Zhou, Z. An updated comprehensive techno-economic analysis of algae biodiesel. Bioresour. Technol. 2013, 145, 150–156. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae biodiesel production in China: A preliminary economic analysis. Renew. Sustain. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. A techno-economic analysis of biodiesel biorefineries: Assessment of integrated designs for the co-production of fuels and chemicals. Energy 2011, 36, 4671–4683. [Google Scholar] [CrossRef]
- Gerhart, P.M.; Gerhart, A.L.; Hochstein, J.I. Munson, Young and Okiishi’s Fundamentals of Fluid Mechanics; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- McDonald, A.G.; Magande, H. Introduction to Thermo-Fluids Systems Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Coronado, M.; Montero, G.; Garcia, C.; Schorr, M.; Valdez, B.; Eliezer, A. Equipment, Materials, and Corrosion in the Biodiesel Industry. 2020. Available online: https://www.materialsperformance.com/articles/material-selection-design/2019/06/equipment-materials-and-corrosion-in-the-biodiesel-industry (accessed on 12 March 2024).
- ToolBox, T.E. Young’s Modulus, Tensile Strength and Yield Strength Values for Some Materials. 2003. Available online: https://www.engineeringtoolbox.com/young-modulus-d_417.html (accessed on 12 March 2024).
- ASTM/ASME A53/SA53; Standard Specification for Pipe, Steel, Black and Hot-Dipped, Zinc-Coated, Welded and Seamless. ASTM International: West Conshohocken, PA, USA, 2012.
- Chiavola, O.; Palmieri, F. Investigating the Fuel Type Influence on Diesel CR Pump Performance. Energy Procedia 2018, 148, 908–915. [Google Scholar] [CrossRef]
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochemistry 2005, 66, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; César, A.d.S. Calophyllum inophyllum L.—A prospective non-edible biodiesel feedstock. Study of biodiesel production, properties, fatty acid composition, blending and engine performance. Renew. Sustain. Energy Rev. 2014, 37, 644–655. [Google Scholar] [CrossRef]
- Atabani, A. A Comprehensive Analysis of Edible and Non-Edible Biodiesel Feedstocks, in Mechanical Engineering; University of Malaya: Kuala Lumpur, Malaysia, 2013; Volume 188. [Google Scholar]
- Atabania, A.; Silitonga, A.; Mahliaa, T.; Masjukia, H.; Badruddina, I. Calophyllum inophyllum L. as a potential feedstock for bio-diesel production. Seed 2011, 40, 40–50. [Google Scholar]
- Sahoo, P.; Das, L. Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Fattah, I.R.; Kalam, M.; Masjuki, H.; Wakil, M. Biodiesel production, characterization, engine performance, and emission characteristics of Malaysian Alexandrian laurel oil. RSC Adv. 2014, 4, 17787–17796. [Google Scholar] [CrossRef]
- Serrano-Bermúdez, L.M.; Monroy-Peña, C.A.; Moreno, D.; Abril, A.; Imbachi Niño, A.D.; Martínez Riascos, C.A.; Buitrago Hurtado, G.; Narváez Rincón, P.C. Kinetic model for the esterification of free fatty acids from palm oil with methanol using sulfuric acid as catalyst and sensitivity analysis in tubular reactors. J. Am. Oil Chem. Soc. 2022, 99, 253–263. [Google Scholar] [CrossRef]
- Zulqarnain; Yusoff, M.H.M.; Ayoub, M.; Nazir, M.H.; Sher, F.; Zahid, I.; Ameen, M. Solvent extraction and performance analysis of residual palm oil for biodiesel production: Experimental and simulation study. J. Environ. Chem. Eng. 2021, 9, 105519. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Catalyst-Free Biodiesel Production Methods: A Comparative Technical and Environmental Evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef]
- Yancy-Caballero, D.M.; Guirardello, R. Modeling and parameters fitting of chemical and phase equilibria in reactive systems for biodiesel production. Biomass Bioenergy 2015, 81, 544–555. [Google Scholar] [CrossRef]
- Rabelo Silva, G.C.; Caño de Andrade, M.H. Simulation and optimization of CSTR reactor of a biodiesel plant by various plant sources using Aspen Plus. Int. J. Chem. React. Eng. 2020, 18, 20200085. [Google Scholar] [CrossRef]
- Narváez, P.C.; Rincon, S.; Sanchez, F. Kinetics of palm oil methanolysis. J. Am. Oil Chem. Soc. 2007, 84, 971–977. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Wakil, M.A.; Ashraful, A.M.; Shahir, S.A. Experimental investigation of performance and regulated emissions of a diesel engine with Calophyllum inophyllum biodiesel blends accompanied by oxidation inhibitors. Energy Convers. Manag. 2014, 83, 232–240. [Google Scholar] [CrossRef]
- Albuquerque, A.A.; Ng, F.T.T.; Danielski, L.; Stragevitch, L. Phase equilibrium modeling in biodiesel production by reactive distillation. Fuel 2020, 271, 117688. [Google Scholar] [CrossRef]
- Granjo, J.F.O.; Duarte, B.P.M.; Oliveira, N.M.C. Integrated production of biodiesel in a soybean biorefinery: Modeling, simulation and economical assessment. Energy 2017, 129, 273–291. [Google Scholar] [CrossRef]
- Osmieri, L.; Alipour Moghadam Esfahani, R.; Recasens, F. Continuous biodiesel production in supercritical two-step process: Phase equilibrium and process design. J. Supercrit. Fluids 2017, 124, 57–71. [Google Scholar] [CrossRef]
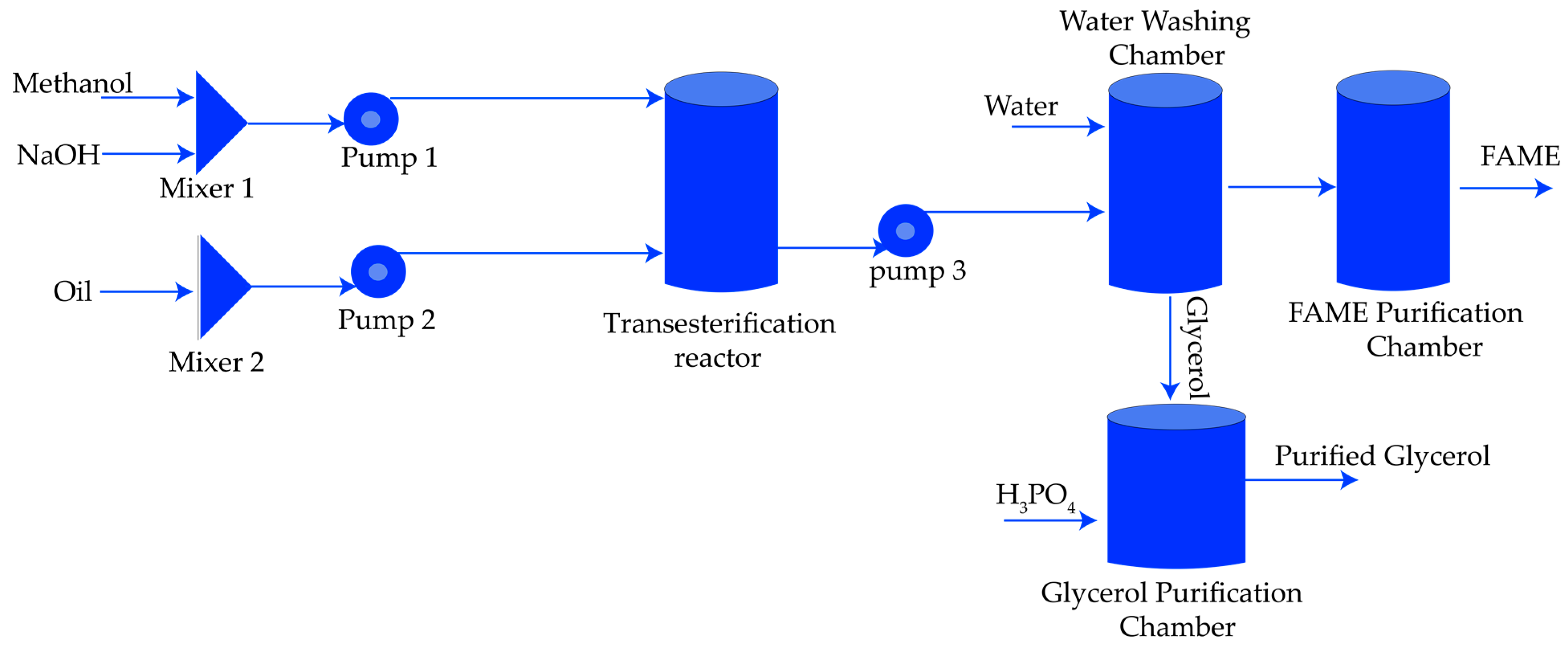




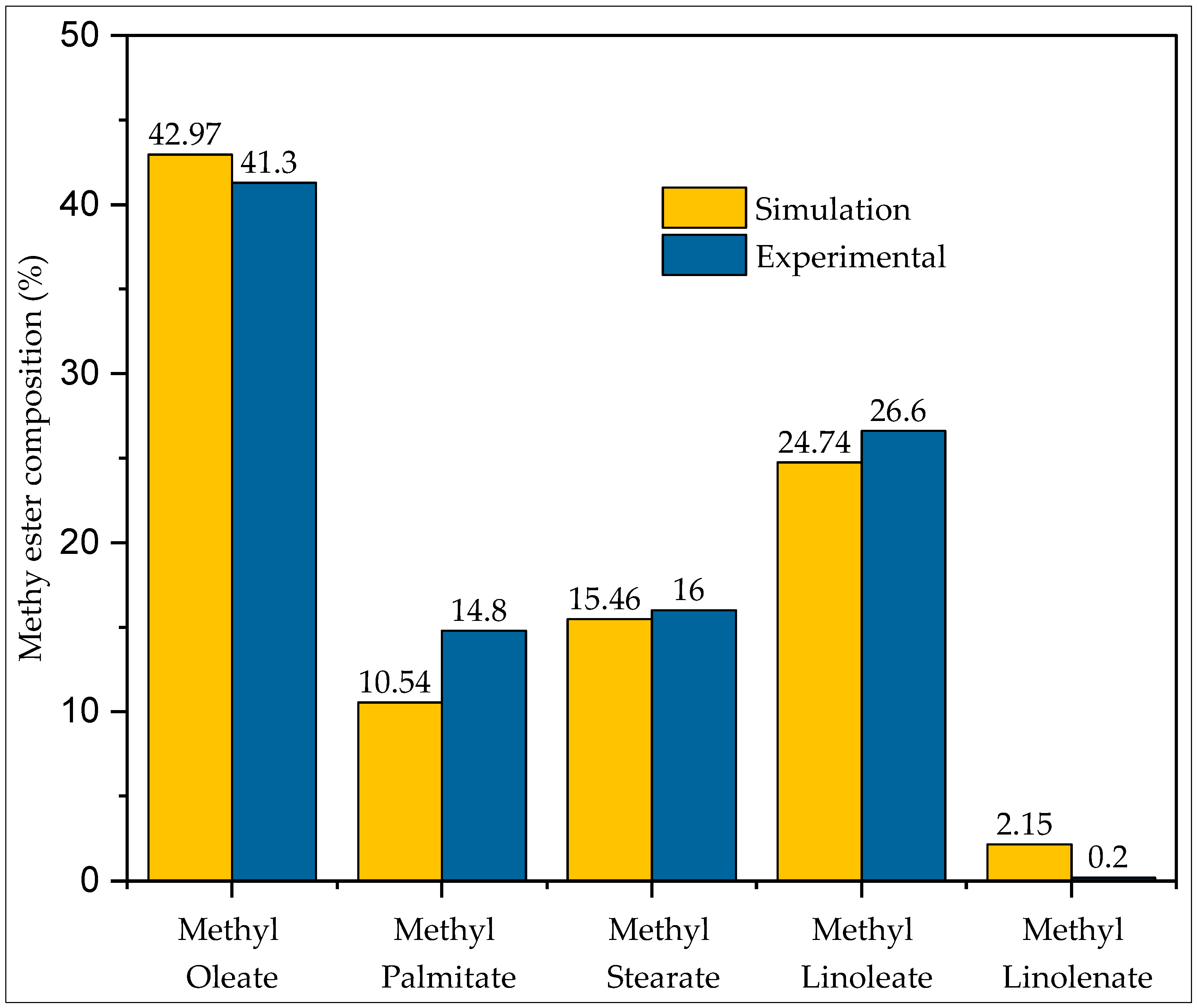
| Name | Block | Process | Purpose | Comment |
|---|---|---|---|---|
| RCSTR |  | Transesterification | Oil reacts with alcohol in the presence of the catalyst to separate FAME and glycerol | Simplified simulation with stoichiometric reactions |
| RadFrac |  | Methanol recovery | Recover the excess and unreacted methanol | Rigorous multi-stage distillation model with seven theoretical stages. |
| L/L Sep |  | Water washing | Remove unreacted catalyst and other impurities | Rigorous multi-stage liquid-liquid extractor model with six theoretical stages. |
| RedFrac |  | FAME purification | Purify FAME and recover bio-oil | Rigorous multi-stage liquid-liquid extractor model with six theoretical stages. |
| RStoic & L/L Sep |  | Catalyst removal | Remove excess catalyst | Simplified simulation with stoichiometric reactions and solid removal. |
| RedFrac |  | Glycerol purification | Purify glycerol | Rigorous multi-stage liquid-liquid extractor model. |
| Design Item | Description | Value | Calculation |
|---|---|---|---|
| Pump | System head equation = Static Head + Dynamic Head |
|
| Pump Specifications | |
|---|---|
| Pump Type | Centrifugal |
| Pump Model | MP Pumps, Petroleum 40 |
| Suction and Discharge Size | 4-inch × 4-inch NPT |
| Maximum Capacity | 0.047 m3/s |
| Maximum Head | 69 m |
| Speed | 3500 rpm |
| Power | 37.28 kW |
| Component | Description | Notes |
|---|---|---|
| Reactors |
|
|
| Methanol recovery |
| Assumption: Condensed methanol from the distillation block. A blower and heat exchangers are used to condense the methanol on an industrial scale. |
| Water washing |
| Assumption: 20 min time is allowed for water washing. |
| Block | Total Stages | Recovery Stage | Distillate Rate | Reflux Ratio | Bottoms Rate | Condenser Pressure |
|---|---|---|---|---|---|---|
| SEP1 | 7 | 4 | 111 kg/hr | 2 | - | 0.2 |
| SEP2 | 6 | 4 | - | 1 | 100 kg/h | 0.1 |
| SEP3 | 6 | 3 | - | 2 | 103 kg/h | 0.4 |
| Input | Output | ||
|---|---|---|---|
| material feed | Flow rate | Recorded results | Flow rate |
| Oil | 890 kg/h (1000 lits of oil) | FAME | 800.9 kg/h |
| Methanol | 199 kg/h | Glycerol | 76.68 kg/h |
| KOH | 8.9 kg/h | Oil recovered | 220 kg/h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azad, A.K.; Jadeja, A.C.; Doppalapudi, A.T.; Hassan, N.M.S.; Nabi, M.N.; Rauniyar, R. Design and Simulation of the Biodiesel Process Plant for Sustainable Fuel Production. Sustainability 2024, 16, 3291. https://doi.org/10.3390/su16083291
Azad AK, Jadeja AC, Doppalapudi AT, Hassan NMS, Nabi MN, Rauniyar R. Design and Simulation of the Biodiesel Process Plant for Sustainable Fuel Production. Sustainability. 2024; 16(8):3291. https://doi.org/10.3390/su16083291
Chicago/Turabian StyleAzad, Abul Kalam, Abhijaysinh Chandrasinh Jadeja, Arun Teja Doppalapudi, Nur Md Sayeed Hassan, Md Nurun Nabi, and Roshan Rauniyar. 2024. "Design and Simulation of the Biodiesel Process Plant for Sustainable Fuel Production" Sustainability 16, no. 8: 3291. https://doi.org/10.3390/su16083291
APA StyleAzad, A. K., Jadeja, A. C., Doppalapudi, A. T., Hassan, N. M. S., Nabi, M. N., & Rauniyar, R. (2024). Design and Simulation of the Biodiesel Process Plant for Sustainable Fuel Production. Sustainability, 16(8), 3291. https://doi.org/10.3390/su16083291








