Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Cheesemaking
2.3. Nutritional Analyses
2.3.1. Chemicals and Reagents
2.3.2. Moisture and Protein Contents of OC and Provola
2.3.3. Polyphenols Content
2.4. Lipids Analysis of OC and Provola
2.4.1. Extraction of Lipids
2.4.2. Preparation of FAMEs
2.4.3. Chromatographic Analysis
2.4.4. Lipid Quality Indices of Provola
2.5. Statistical Analysis
3. Results and Discussion
3.1. Olive Cake Composition
3.2. Provola Cheese Composition
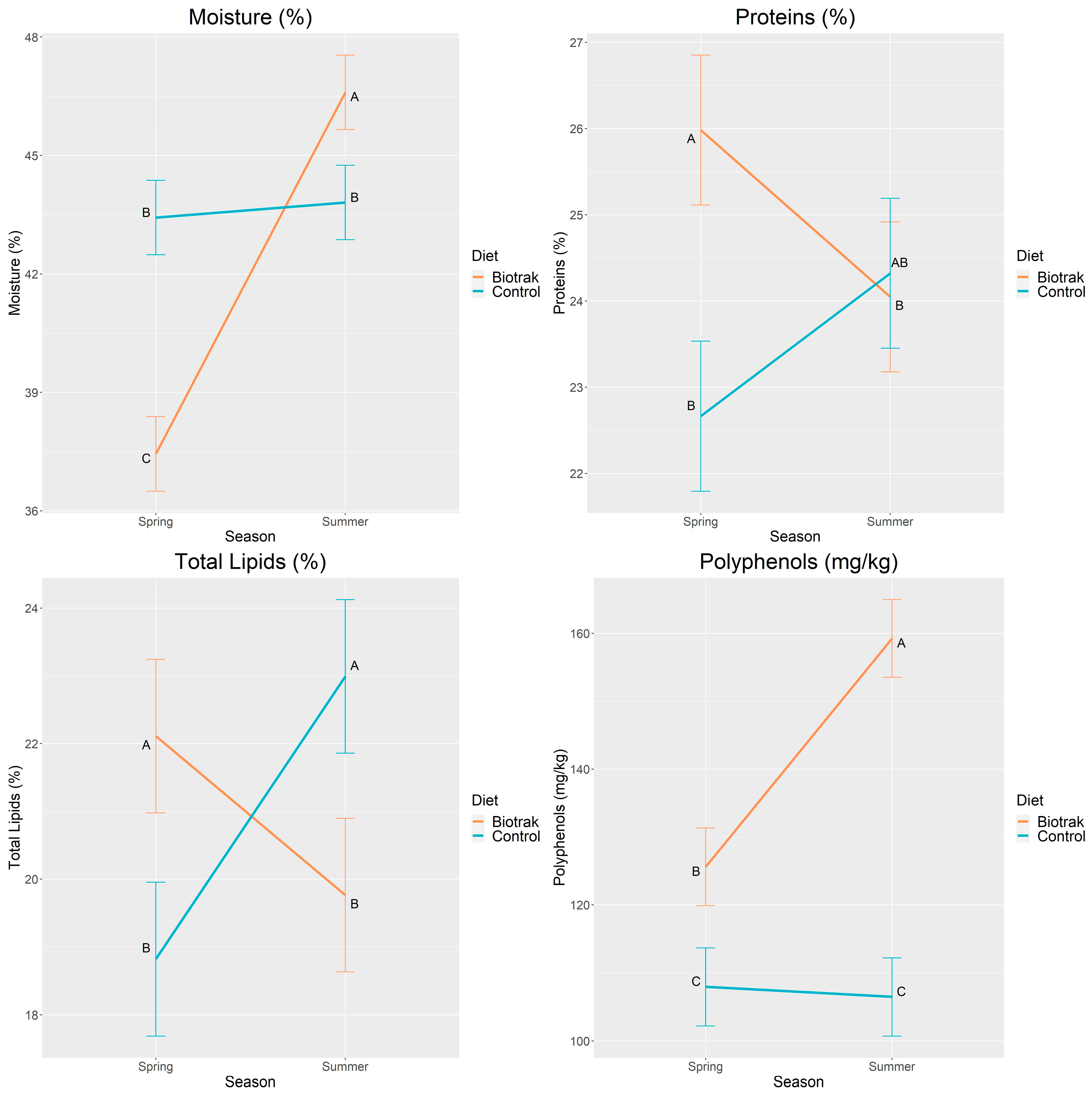
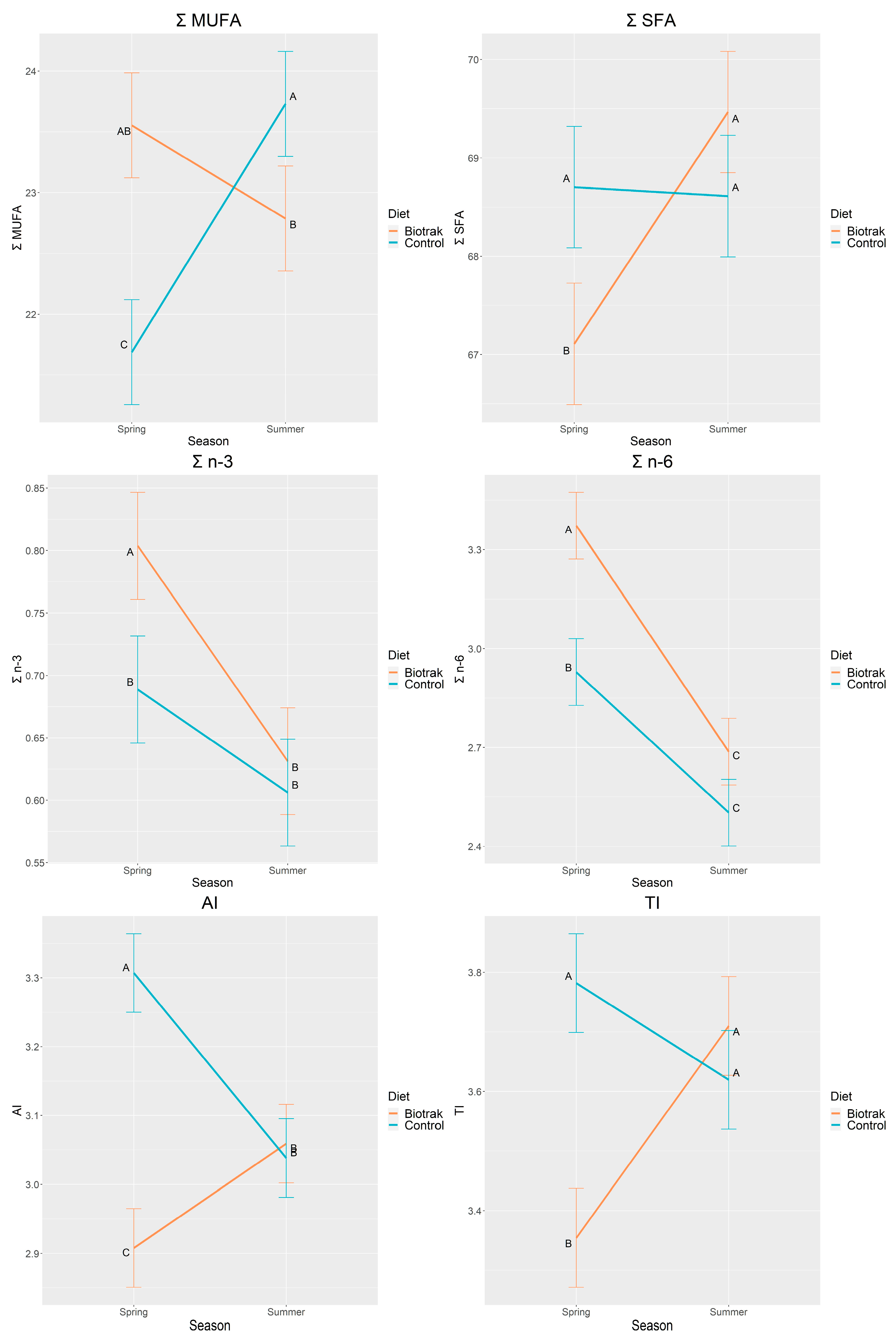
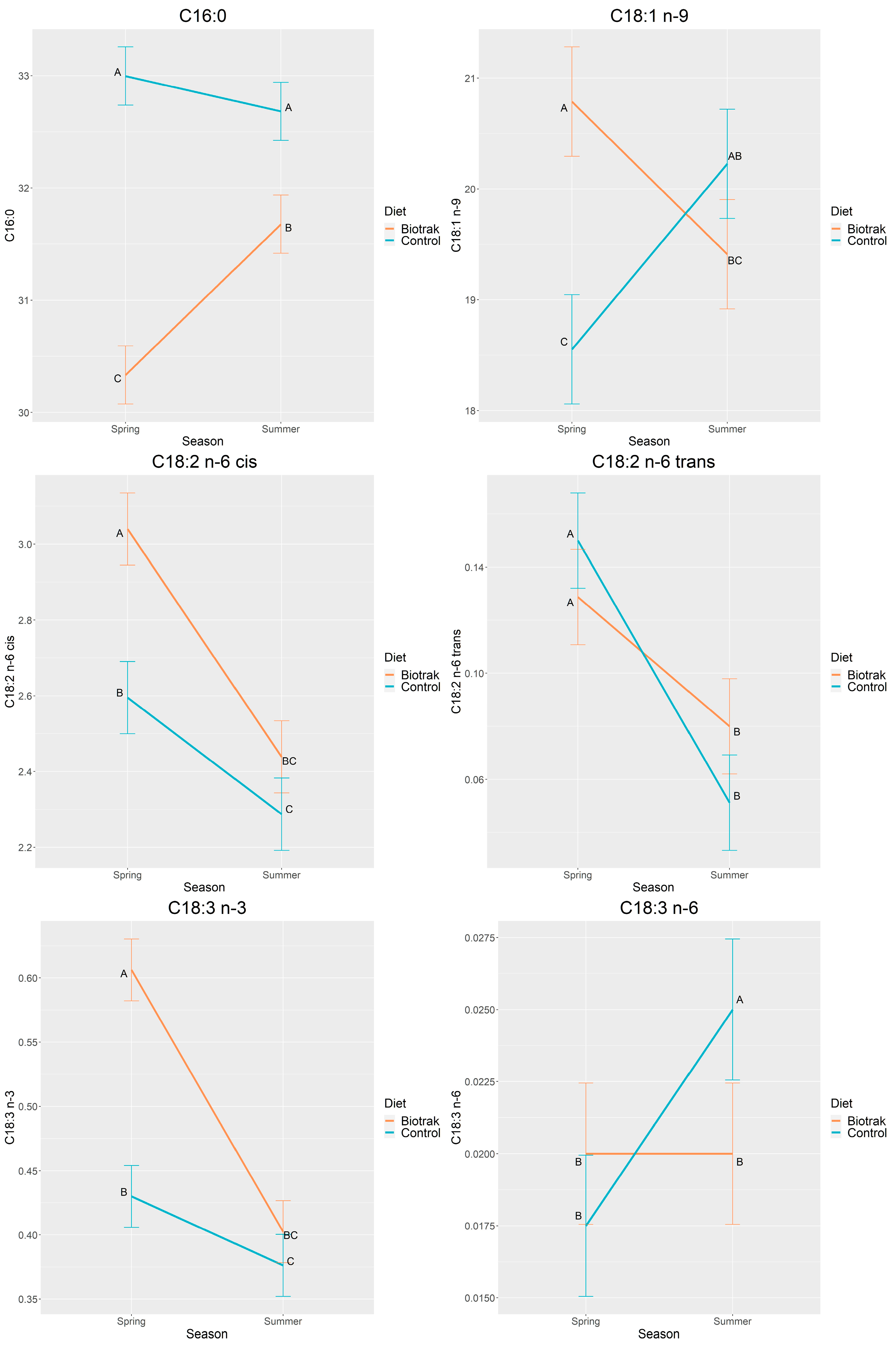
3.3. Season and Diet×Season Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Radulescu, C.; Buruleanu, L.C.; Olteanu, R.L.; Nicolescu, C.M.; Bumbac, M.; Gorghiu, L.M.; Nechifor, M.D. Grape by-Products: Potential Sources of Phenolic Compounds for Novel Functional Foods; Intech Open: London, UK, 2023; Available online: https://www.intechopen.com/online-first/88679 (accessed on 4 April 2024).
- Fogliano, V.; Vitaglione, P. Functional foods: Planning and development. Mol. Nutr. Food Res. 2005, 49, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods–a review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Tsala, A.; Mpekelis, V.; Karvelis, G.; Tsikakis, P.; Goliomytis, M.; Simitzis, P. Effects of dried olive pulp dietary supplementation on quality characteristics and antioxidant capacity of pig meat. Foods 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, V.; Liotta, L.; Lo Presti, V.; Gresta, F.; Di Rosa, A.R.; Chiofalo, B. Effect of dietary olive cake supplementation on performance, carcass characteristics, and meat quality of beef cattle. Animals 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food byproducts as sustainable ingredients for innovative and healthy dairy foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Lo Turco, V.; Potortì, A.G.; Luppino, R.R.; Fotia, V.; Conte, F.; Dugo, G.M. Classification of the geographical origin of Italian donkey’s milk based on differences in inorganic anions. Food Addit. Contam. Part A 2012, 29, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Potortì, A.G.; Turco, V.L.; Licata, P.; Rastrelli, L.; Dugo, G. Donkey’s milk safety: POCs and PCBs levels and infant daily intake. Food Control 2014, 46, 210–216. [Google Scholar] [CrossRef]
- Robert, K.W.; Parris, T.M.; Leiserowitz, A.A. What is sustainable development? Goals, indicators, values, and practice. Environ. Sci. Policy Sustain. Dev. 2005, 47, 8–21. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Russo, N.; Celano, G.; Pino, A.; Lopreiato, V.; Litrenta, F.; Randazzo, C.L. Effect of olive by-products feed supplementation on physicochemical and microbiological profile of Provola cheese. Front. Microbiol. 2023, 14, 1112328. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Fatty acids act on vascular endothelial cells and influence the development of cardiovascular disease. Prostaglandins Other Lipid Mediat. 2023, 165, 106704. [Google Scholar] [CrossRef]
- Lo Turco, V.; Potortì, A.G.; Rando, R.; Ravenda, P.; Dugo, G.; Di Bella, G. Functional properties and fatty acids profile of different beans varieties. Nat. Product Res. 2016, 30, 2243–2248. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Cardinali, R.; Mourvaki, E.; Moscati, L.; Battistacci, L.; Taticchi, A. Olive cake dietary supplementation in rabbit: Immune and oxidative status. Italian J. Anim. Sci. 2007, 6 (Suppl. S1), 713–715. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Vera, R.R.; Aguilar, C.; Lira, R.; Peña, I.; Fernández, J. Feeding olive cake to ewes improves fatty acid profile of milk and cheese. Anim. Feed Sci. Technol. 2013, 184, 94–99. [Google Scholar] [CrossRef]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of olive cake for ewe feeding: Effect on milk yield and composition. Small Rumin. Res. 2004, 55, 169–176. [Google Scholar] [CrossRef]
- Cibik, M.; Keles, G. Effect of stoned olive cake on milk yield and composition of dairy cows. Cellulose 2016, 15, 27–28. [Google Scholar]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Russo, N.; Floridia, V.; D’Alessandro, E.; Lopreiato, V.; Pino, A.; Chiofalo, V.; Randazzo, C.L. Influence of olive cake dietary supplementation on fecal microbiota of dairy cows. Front. Microbiol. 2023, 14, 1137452. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of olive by-products in livestock with emphasis on small ruminants: Implications on rumen function, growth performance, milk and meat quality. Animals 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, L.; Fan, J.; Sun, X.; Yin, Y. n-6: N-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 2014, 111, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Gandy, J. (Ed.) Manual of Dietetic Practice; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Todd, J. FAO/WHO consultation on dietary recommendations on total fat and fatty Acids. Food N. Z. 2010, 10, 12–13. [Google Scholar]
- Kelley, N.S.; Hubbard, N.E.; Erickson, K.L. Conjugated linoleic acid isomers and cancer. J. Nutr. 2007, 137, 2599–2607. [Google Scholar] [CrossRef]
- McLeod, R.S.; LeBlanc, A.M.; Langille, M.A.; Mitchell, P.L.; Currie, D.L. Conjugated linoleic acids, atherosclerosis, and hepatic very-low-density lipoprotein metabolism. Am. J. Clin. Nutr. 2004, 79, 1169S–1174S. [Google Scholar] [CrossRef]
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of conjugated linoleic acid for reducing fat mass: A meta-analysis in humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. [Google Scholar] [CrossRef]
- Kaur, K.K.; Allahbadia, G.; Singh, M. Synthesis and functional significance of poly unsaturated fatty acids (PUFA’s) in body. Acta Sci. Nutr. Health 2018, 2, 8. [Google Scholar]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef]
- Pawar, M.M.; Srivastaba, A.K.; Chauhan, H.D.; Damor, S.V. Nutritional strategies to alleviate heat stress in dairy animals–A Review. Int. J. Livest. Res. 2018, 8, 8–18. [Google Scholar] [CrossRef]
- Gorniak, T.; Meyer, U.; Südekum, K.H.; Dänicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Summer, A.; Lora, I.; Formaggioni, P.; Gottardo, F. Impact of heat stress on milk and meat production. Anim. Front. 2019, 9, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Basiricò, L.; Morera, P.; Dipasquale, D.; Vitali, A.; Cappelli, F.P.; Calamari, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, R.; Lahmar, M.; Majdoub, A.; Belyea, R. The relationship of temperature-humidity index with milk production of dairy cows in a Mediterranean climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef]
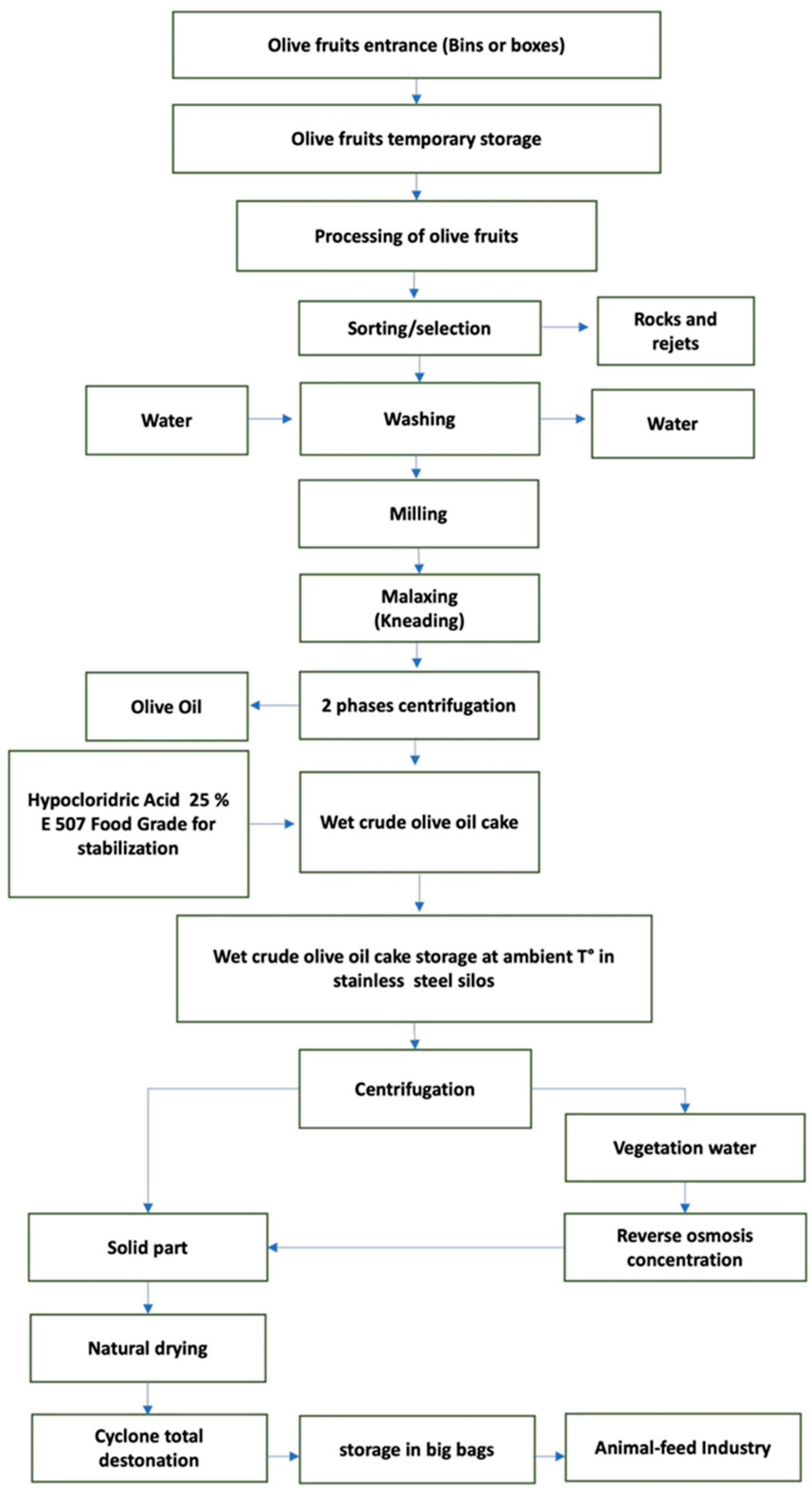
| Diet Chemical Composition (g/kg of DM) | CTR | TEST |
|---|---|---|
| Moisture | 109.0 | 107.0 |
| Starch | 407.0 | 407.0 |
| Crude protein | 194.0 | 196.0 |
| Ether extract | 45.8 | 51.1 |
| Non-fibre carbohydrates | 465.0 | 440.0 |
| Crude fibre | 60.0 | 72.0 |
| Acid detergent fibre | 78.2 | 105 |
| Ash | 64.1 | 70.2 |
| NEL (milk UFL/kf of DM) * | 1.09 | 1.07 |
| Olive Cake | |
|---|---|
| Chemical composition (g/kg dry matter) | |
| Moisture | 36.47 ± 0.57 |
| Ashes | 34.13 ± 0.81 |
| Crude fat | 180.80 ± 0.26 |
| Energy (ME) (Kcal/Kg) | 3535.1 |
| Crude protein (total N × 6.25) | 61.00 ± 1.73 |
| Neutral detergent fibre | 410.33 ± 0.20 |
| Acid detergent fibre | 320.53 ± 0.34 |
| Total polyphenols | 10.18 ± 1.59 |
| Fatty acids (g/100 g fat) | |
| C14:0 | 0.02 ± 0.01 |
| C16:0 | 16.14 ± 0.17 |
| C16:1 | 0.64 ± 0.07 |
| C17:0 | 0.32 ± 0.07 |
| C17:1 | 0.32 ± 0.02 |
| C18:0 | 3.80 ± 0.16 |
| C18:1 n-9 | 66.63 ± 0.19 |
| C18:2 | 10.66 ± 0.16 |
| C18:3 | 0.57 ± 0.05 |
| C20:0 | 0.53 ± 0.05 |
| C20:1 | 0.33 ± 0.03 |
| C22:0 | 0.03 ± 0.01 |
| C24:0 | 0.01 ± 0.01 |
| TEST | CTR | SEM | Diet | Season | Diet×Season | |
|---|---|---|---|---|---|---|
| Moisture (%) | 42.02 | 43.62 | 0.34 | 0.0025 | <0.0001 | <0.0001 |
| Proteins (%) | 25.02 | 23.49 | 0.31 | 0.0019 | 0.7570 | 0.0004 |
| Total lipids (%) | 20.94 | 20.91 | 0.41 | 0.9598 | 0.1259 | <0.0001 |
| Total polyphenols (mg/kg) | 142.42 | 107.19 | 2.01 | <0.0001 | <0.0001 | <0.0001 |
| Σ FA | 95.34 | 95.02 | 0.18 | 0.2206 | 0.0102 | 0.4615 |
| Σ MUFA | 23.17 | 22.71 | 0.16 | 0.0460 | 0.0073 | <0.0001 |
| Σ PUFA | 3.88 | 3.66 | 0.05 | 0.0028 | <0.0001 | 0.9269 |
| Σ SFA | 68.29 | 68.66 | 0.22 | 0.2517 | 0.0012 | 0.0006 |
| Σ n-3 | 0.72 | 0.65 | 0.02 | 0.0034 | <0.0001 | 0.0489 |
| Σ n-6 | 3.03 | 2.72 | 0.04 | <0.0001 | <0.0001 | 0.0181 |
| Σ n-6/Σ n-3 | 4.26 | 4.20 | 0.09 | 0.6950 | 0.5710 | 0.5962 |
| Undefined FA | 4.66 | 4.98 | 0.18 | 0.2206 | 0.0102 | 0.4615 |
| Atherogenic Index | 2.98 | 3.17 | 0.02 | <0.0001 | 0.0535 | <0.0001 |
| Thrombogenic Index | 3.53 | 3.70 | 0.03 | 0.0004 | 0.0303 | <0.0001 |
| C12:0 | 3.60 | 3.62 | 0.04 | 0.7454 | <0.0001 | 0.6527 |
| C14:0 | 11.40 | 11.54 | 0.06 | 0.1115 | <0.0001 | 0.8423 |
| C16:0 | 31.01 | 32.84 | 0.09 | <0.0001 | 0.0006 | 0.0000 |
| C18:0 | 12.17 | 10.71 | 0.30 | 0.0016 | 0.4862 | 0.8813 |
| C18:1 n-9 | 20.10 | 19.39 | 0.18 | 0.0087 | 0.5608 | <0.0001 |
| C18:2 n-6 cis | 2.74 | 2.44 | 0.03 | <0.0001 | <0.0001 | 0.0053 |
| C18:2 n-6 trans | 0.10 | 0.10 | 0.01 | 0.6852 | <0.0001 | 0.0108 |
| C18:3 n-3 | 0.50 | 0.40 | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| C20:4 n-6 | 0.02 | 0.01 | 0.002 | 0.0759 | 0.0154 | 0.4670 |
| C20:5 n-3 | 0.04 | 0.04 | 0.003 | 0.2410 | <0.0001 | 0.1347 |
| TEST | CTR | SEM | p-Values | |||
|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | |||
| Moisture (%) | 37.44 | 46.60 | 43.43 | 43.81 | 0.481 | <0.0001 |
| Protein (%) | 25.98 | 24.05 | 22.66 | 24.32 | 0.444 | 0.0004 |
| Total lipids (%) | 22.11 | 19.76 | 18.82 | 22.99 | 0.578 | <0.0001 |
| Total polyphenols (ppm) | 125.61 | 159.24 | 107.94 | 106.45 | 2.922 | <0.0001 |
| C16:0 | 30.33 | 31.68 | 33.00 | 32.68 | 0.132 | <0.0001 |
| C18:1 n-9 | 20.79 | 19.77 | 18.43 | 19.91 | 0.252 | <0.0001 |
| C18:2 n-6 cis | 3.04 | 2.44 | 2.60 | 2.29 | 0.049 | 0.0053 |
| C18:2 n-6 trans | 0.129 | 0.080 | 0.150 | 0.051 | 0.009 | 0.0108 |
| C18:3 n-3 | 0.606 | 0.403 | 0.430 | 0.376 | 0.012 | <0.0001 |
| Σ MUFA | 23.55 | 22.79 | 21.69 | 23.73 | 0.220 | <0.0001 |
| Σ n-3 | 0.80 | 0.63 | 0.69 | 0.61 | 0.022 | 0.0489 |
| Σ n-6 | 3.37 | 2.69 | 2.93 | 2.50 | 0.052 | 0.0181 |
| Σ SFA | 67.11 | 69.47 | 68.70 | 68.61 | 0.315 | 0.0006 |
| Atherogenic Index | 2.91 | 3.06 | 3.31 | 3.04 | 0.029 | <0.0001 |
| Thrombogenic Index | 3.35 | 3.71 | 3.78 | 3.62 | 0.042 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attard, G.; Bionda, A.; Litrenta, F.; Lopreiato, V.; Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Liotta, L. Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese. Sustainability 2024, 16, 3306. https://doi.org/10.3390/su16083306
Attard G, Bionda A, Litrenta F, Lopreiato V, Di Bella G, Potortì AG, Lo Turco V, Liotta L. Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese. Sustainability. 2024; 16(8):3306. https://doi.org/10.3390/su16083306
Chicago/Turabian StyleAttard, George, Arianna Bionda, Federica Litrenta, Vincenzo Lopreiato, Giuseppa Di Bella, Angela Giorgia Potortì, Vincenzo Lo Turco, and Luigi Liotta. 2024. "Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese" Sustainability 16, no. 8: 3306. https://doi.org/10.3390/su16083306
APA StyleAttard, G., Bionda, A., Litrenta, F., Lopreiato, V., Di Bella, G., Potortì, A. G., Lo Turco, V., & Liotta, L. (2024). Using Olive Cake as a Sustainable Ingredient in Diets of Lactating Dairy Cows: Effects on Nutritional Characteristics of Cheese. Sustainability, 16(8), 3306. https://doi.org/10.3390/su16083306










