Assessing Light Non-Aqueous Phase Liquids in the Subsurface Using the Soil Gas Rn Deficit Technique: A Literature Overview of Field Studies
Abstract
1. Introduction
2. LNAPL Detection
| Technique | Information Provided | |||||
|---|---|---|---|---|---|---|
| Mobile LNAPL Presence | Residual LNAPL Presence | LNAPL Saturation | LNAPL Composition | TPH Concentrations in Soil | TPH Concentrations in Water | |
| Soil sampling | ○ | ● | ○ | ○ | ● | |
| Groundwater sampling (monitoring wells) | ● | ● | ● | |||
| Direct push analytical technologies (MIP, LIF, OIP) | ● | ● | ○ | ○ | ○ | |
| Surface geophysical methods (ERT, IP, GPR) | ● | ● | ||||
| Soil gas sampling | ○ | ○ | ○ | ○ | ||
| Tracer techniques | ● | ● | ○ | ○ | ||
| Radon | ● | ● | ○ | ○ | ○ | |
3. Radon
- 219Rn, actinon, which belongs to the radioactive series of 235U, is formed by the decay of 223Ra and decays in 215Po. It is characterized by a half-life of 3.92 s and is naturally present in very low amounts;
- 220Rn, thoron, which belongs to the radioactive series of 232Th, is formed by the decay of 224Ra and decays in 216Po. Thoron has a half-life of 55.6 s, but the isotope that produces it is instead quite abundant in nature;
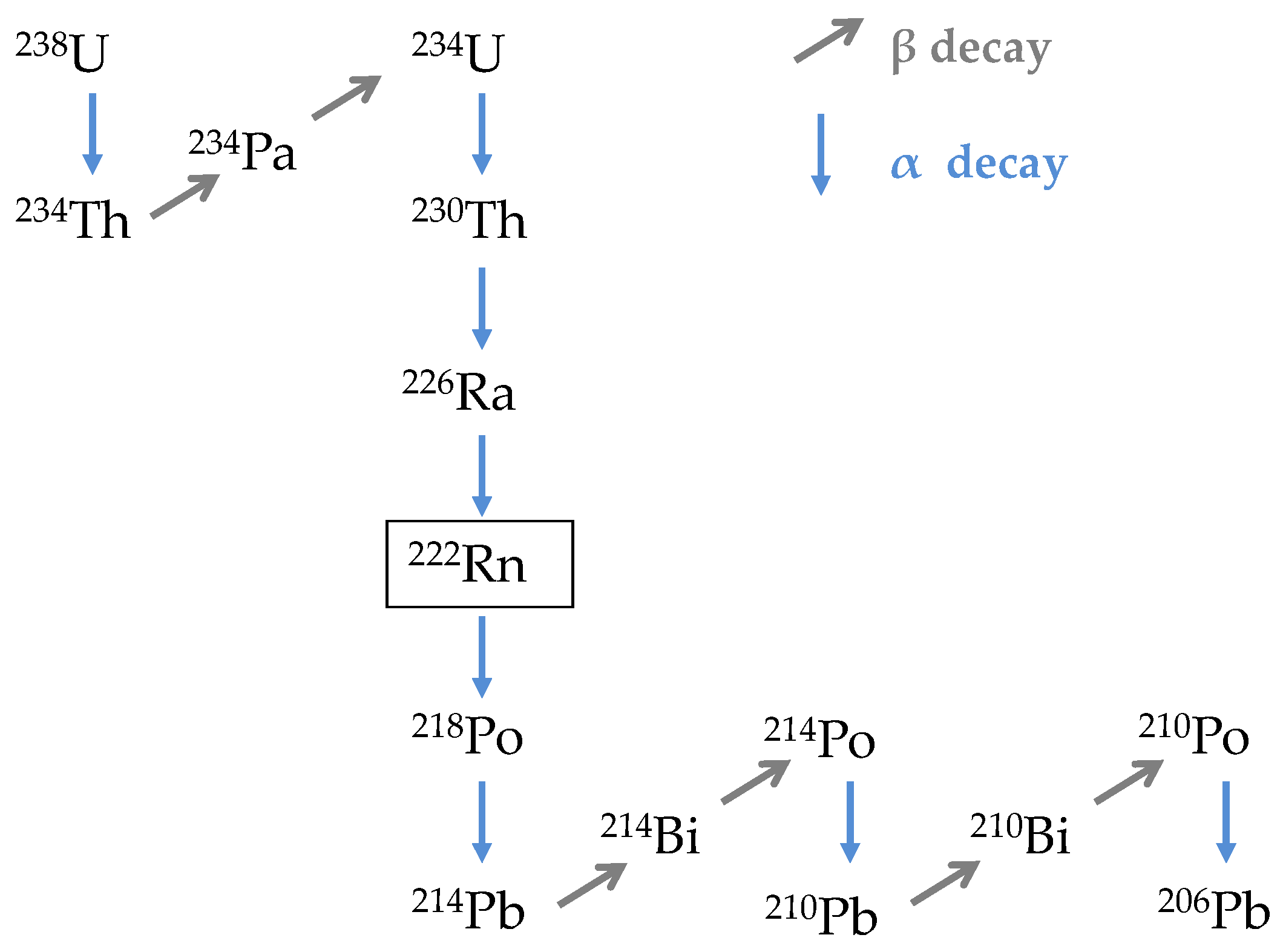
- 222Rn, radon, a more stable isotope, which belongs to the radioactive series of 238U, is formed by the decay of 226Ra and decays in 218Po (Figure 1). It has the highest half-life of all isotopes, at 3.825 days, and is present in almost all mineral grains in the soils and rocks of the earth’s crust.
3.1. Radon Migration Process in the Subsurface and Its Influence Factors
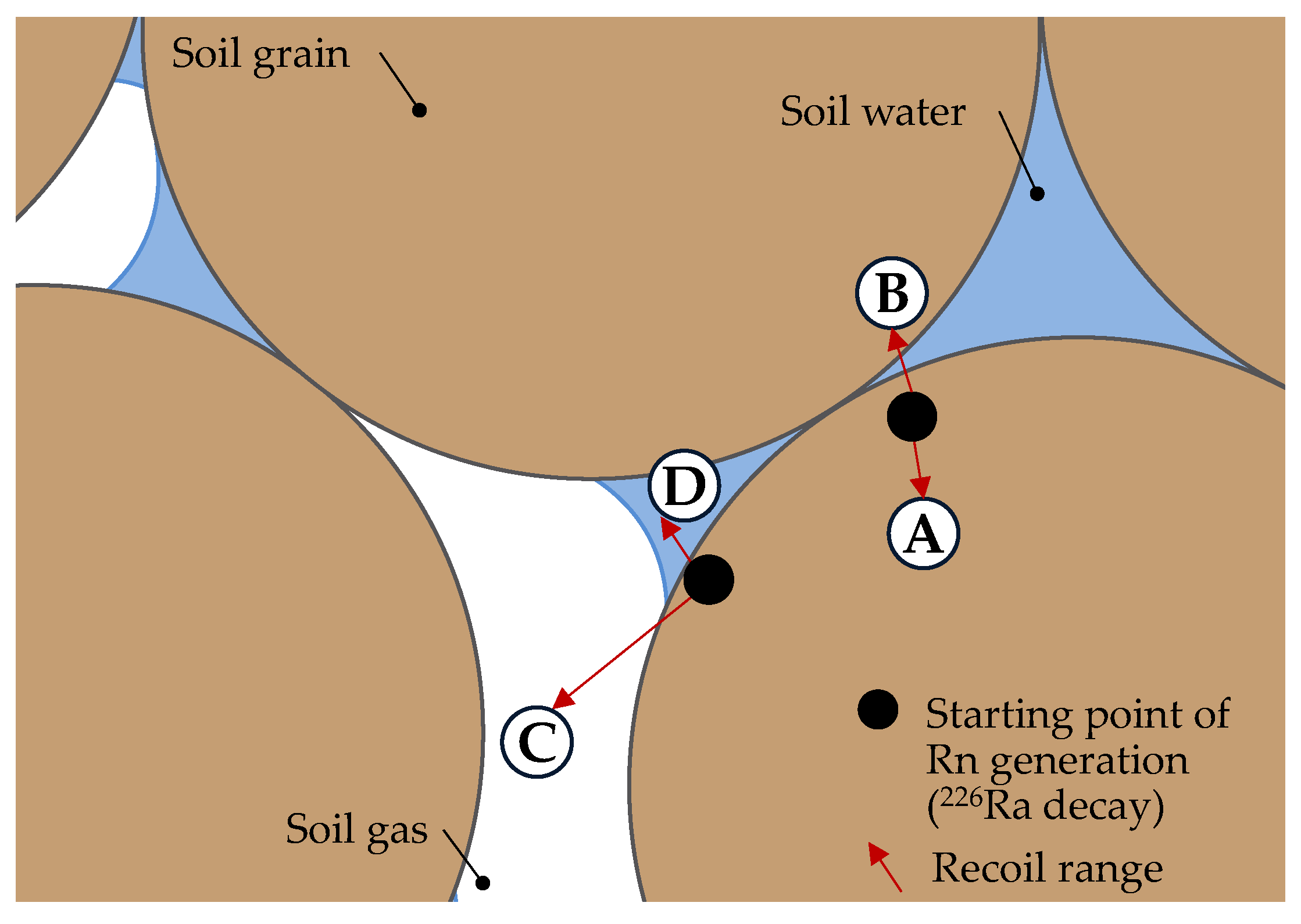
3.1.1. Emanation
3.1.2. Partitioning in Soil Pores
3.1.3. Transport
3.1.4. Exhalation
4. Radon as a Natural Tracer for the Identification of LNAPL Contamination
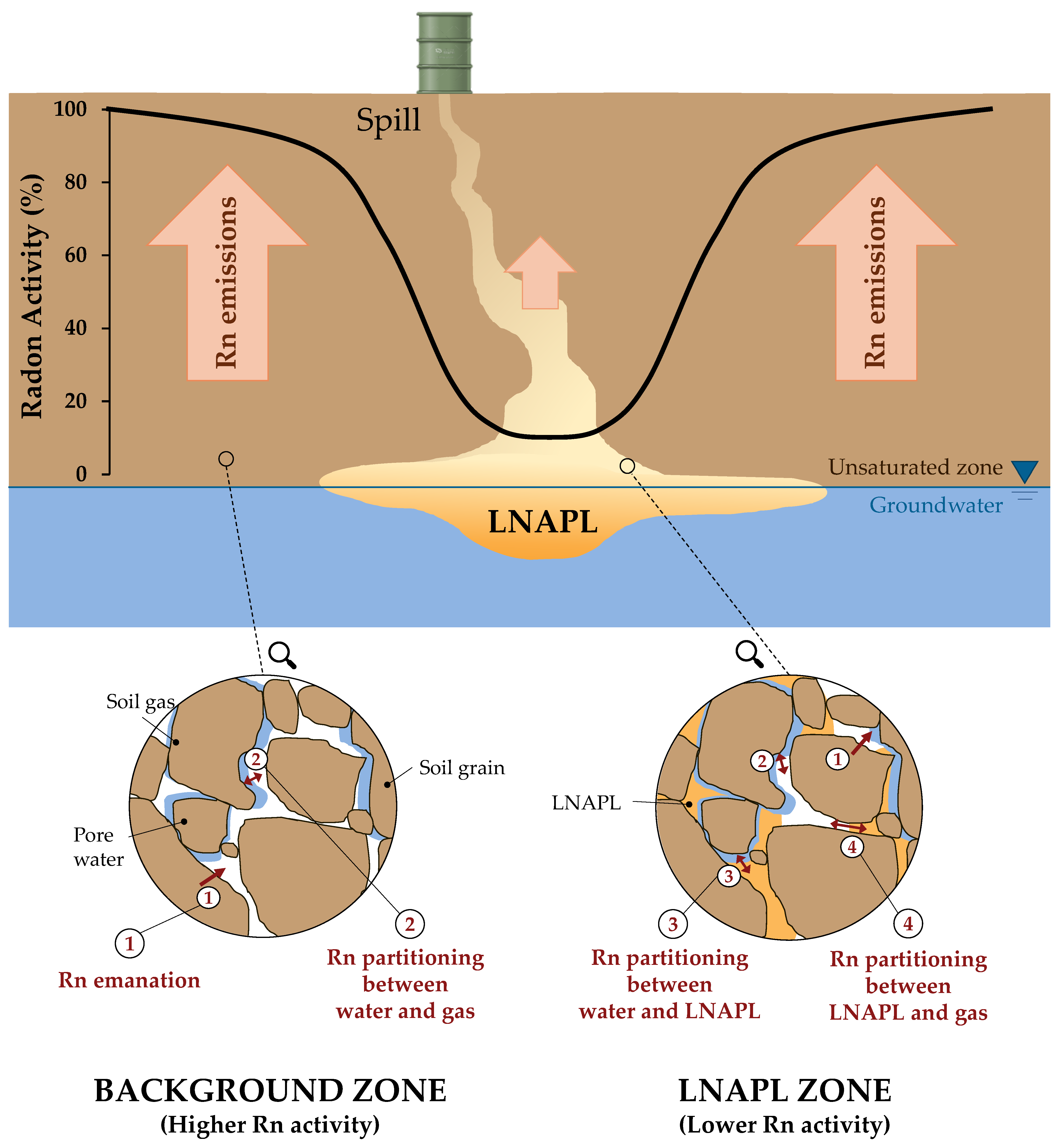
4.1. Radon Deficit in the Subsurface in Equilibrium Conditions
4.2. Soil Gas Radon Transport in the Presence of LNAPL—Modeling Approaches
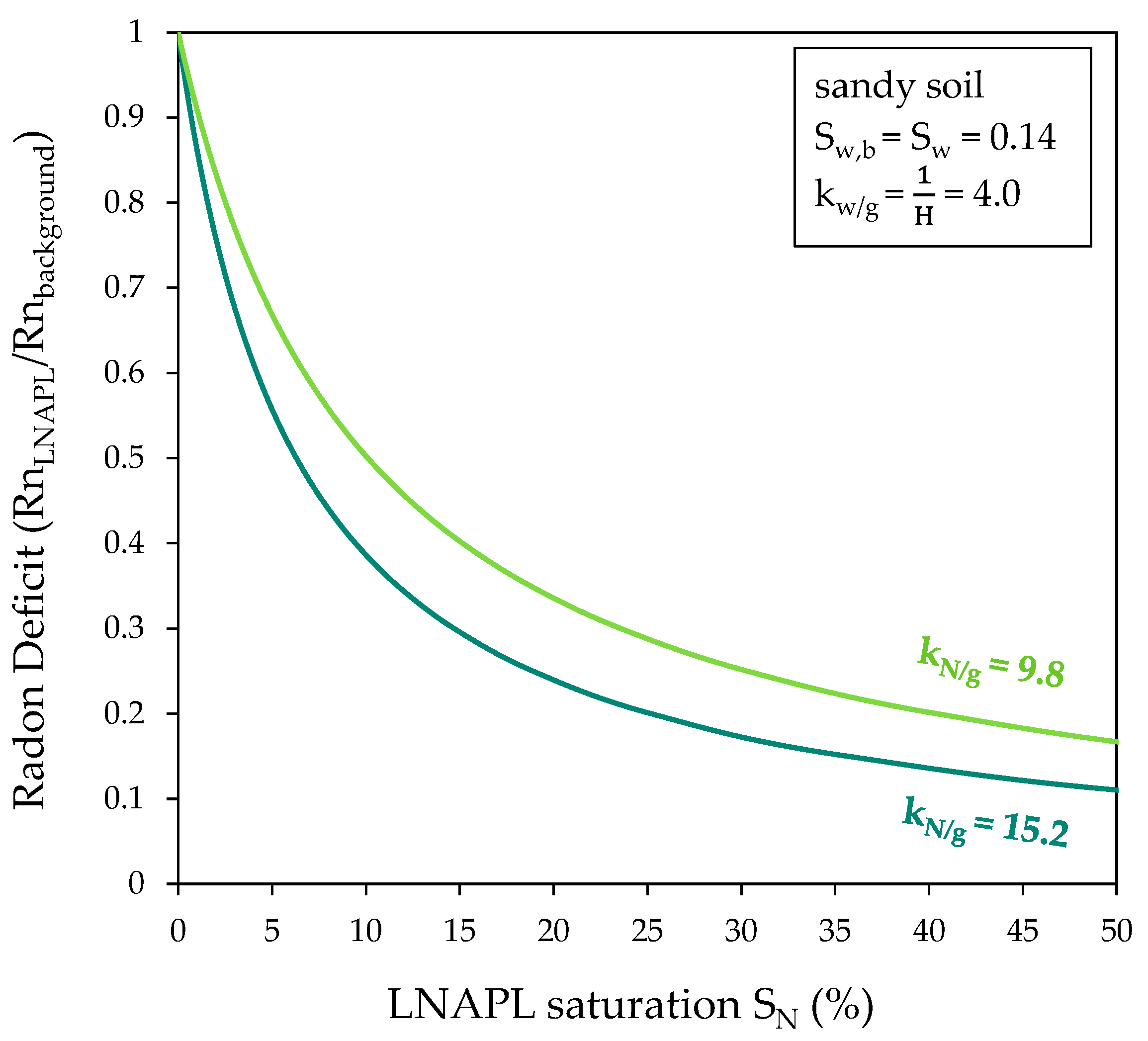
4.3. Rn Partition Coefficients
| Substance | kN/w (-) | kN/g (-) |
|---|---|---|
| Hexane | 57.2 ± 3.1 [53] | 14.45 ± 0.8 |
| Ethanol | 27.9 ± 0.4 [112] | 7.05 ± 0.1 |
| Benzene | 40.8 ± 5.7 [53] | 10.31 ± 1.4 |
| Toluene | 42 (10 °C) [52] | 14.66 (10 °C) |
| 46.8 ± 0.4 [53] | 11.82 ± 0.1 | |
| Gasoline | 30.8 ± 4.6 (evaporated) [110] | 7.78 ± 1.2 (evaporated) |
| 33 ± 4.9 (UV-degraded) [110] | 8.34 ± 1.2 (UV-degraded) | |
| 37.4 ± 5.6 (fresh) [110] | 9.45 ± 1.4 (fresh) | |
| 38.9 ± 0.9 [53] | 9.83 ± 0.2 | |
| 50.9 ± 5.8 [19] | 12.86 ± 1.5 | |
| Kerosene | 40.6 ± 8.3 [19] | 10.26 ± 2.1 |
| 47.4 ± 0.2 [53] | 11.98 ± 0.1 | |
| Diesel | 25.1 ± 2.5 (evaporated) [110] | 6.34 ± 0.6 (evaporated) |
| 40 ± 2.3 (12 °C) [52] | 13.03 ± 0.7 (12 °C) | |
| 43.8 ± 4.6 [19] | 11.07 ± 1.2 | |
| 47.7 [54] | 12.05 | |
| 60.0 ± 1.3 [53] | 15.16 ± 0.3 | |
| 60.7 ± 6.1 (fresh) [110] | 15.34 ± 1.5 (fresh) | |
| 74.8 ± 7.5 (UV-degraded) [110] | 18.90 ± 1.9 (UV-degraded) | |
| Crude oil | 38.5 ± 2.9 [113] | 9.73 ± 0.7 |
5. Applications of the Rn Deficit Technique
5.1. Groundwater Applications of the Rn Deficit Technique
5.2. Soil Gas Applications of the Technique
5.2.1. Soil Gas Radon Measurements
5.2.2. Soil Gas Applications Using Active Sampling
5.2.3. Soil Gas Applications Using Passive Sampling
6. Conclusions, Limitations, and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US EPA. Ground Water Issue: Light Nonaqueous Phase Liquids; US EPA: Washington, DC, USA, 1995. [Google Scholar]
- Alden, D.F.; García-Rincón, J.; Rivett, M.O.; Wealthall, G.P.; Thomson, N.R. Complexities of Petroleum Hydrocarbon Contaminated Sites. In Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R., Eds.; Environmental Contamination Remediation and Management; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–19. ISBN 978-3-031-34447-3. [Google Scholar]
- CL:AIRE. An Illustrated Handbook of LNAPL Transport and Fate in the Subsurface; CL:AIRE: London, UK, 2014. [Google Scholar]
- Sweeney, R.E.; Ririe, G.T. Small Purge Method to Sample Vapor from Groundwater Monitoring Wells Screened across the Water Table. Groundw. Monit. Remediat. 2017, 37, 51–59. [Google Scholar] [CrossRef]
- US EPA. A Decision-Making Framework for Cleanup of Sites Impacted with Light Non-Aqueous Phase Liquids (LNAPL); US EPA: Washington, DC, USA, 2004. [Google Scholar]
- ITRC. LNAPL Site Management: LCSM Evolution, Decision Process, and Remedial Technologies; ITRC: Washington, DC, USA, 2018. [Google Scholar]
- ITRC. Implementing Advanced Site Characterization Tools; ITRC: Washington, DC, USA, 2019. [Google Scholar]
- Bertolla, L.; Porsani, J.L.; Soldovieri, F.; Catapano, I. GPR-4D Monitoring a Controlled LNAPL Spill in a Masonry Tank at USP, Brazil. J. Appl. Geophys. 2014, 103, 237–244. [Google Scholar] [CrossRef]
- McCall, W.; Christy, T.M.; Pipp, D.A.; Jaster, B.; White, J.; Goodrich, J.; Fontana, J.; Doxtader, S. Evaluation and Application of the Optical Image Profiler (OIP) a Direct Push Probe for Photo-Logging UV-Induced Fluorescence of Petroleum Hydrocarbons. Environ. Earth Sci. 2018, 77, 374. [Google Scholar] [CrossRef]
- Shao, S.; Guo, X.; Gao, C. Fresh Underground Light Non-Aqueous Liquid (LNAPL) Pollution Source Zone Monitoring in an Outdoor Experiment Using Cross-Hole Electrical Resistivity Tomography. Environ. Sci. Pollut. Res. 2019, 26, 18316–18328. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, E.H.; Isler, E.; Polese, L.; Baessa, M.P.M.; Chang, H.K. LNAPL Saturation Derived from Laser Induced Fluorescence Method. Sci. Total Environ. 2019, 683, 762–772. [Google Scholar] [CrossRef] [PubMed]
- García-Rincón, J.; Gatsios, E.; Rayner, J.L.; McLaughlan, R.G.; Davis, G.B. Laser-Induced Fluorescence Logging as a High-Resolution Characterisation Tool to Assess LNAPL Mobility. Sci. Total Environ. 2020, 725, 138480. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wang, Y.; Li, H. GPR Attribute Analysis for the Detection of LNAPL Contaminated Soils. IOP Conf. Ser. Earth Environ. Sci. 2021, 660, 012033. [Google Scholar] [CrossRef]
- Meng, J.; Dong, Y.; Xia, T.; Ma, X.; Gao, C.; Mao, D. Detailed LNAPL Plume Mapping Using Electrical Resistivity Tomography inside an Industrial Building. Acta Geophys. 2022, 70, 1651–1663. [Google Scholar] [CrossRef]
- Mineo, S. Groundwater and Soil Contamination by LNAPL: State of the Art and Future Challenges. Sci. Total Environ. 2023, 874, 162394. [Google Scholar] [CrossRef] [PubMed]
- St. Germain, R. High-Resolution Delineation of Petroleum NAPLs. In Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R., Eds.; Environmental Contamination Remediation and Management; Springer International Publishing: Cham, Switzerland, 2024; pp. 213–286. ISBN 978-3-031-34447-3. [Google Scholar]
- Rao, P.S.C.; Annable, M.D.; Kim, H. NAPL Source Zone Characterization and Remediation Technology Performance Assessment: Recent Developments and Applications of Tracer Techniques. J. Contam. Hydrol. 2000, 45, 63–78. [Google Scholar] [CrossRef]
- Semprini, L.; Hopkins, O.S.; Tasker, B.R. Laboratory, Field and Modeling Studies of Radon-222 as a Natural Tracer for Monitoring NAPL Contamination. Transp. Porous Media 2000, 38, 223–240. [Google Scholar] [CrossRef]
- Schubert, M.; Freyer, K.; Treutler, H.-C.; Weiß, H. Using the Soil Gas Radon as an Indicator for Ground Contamination by Non-Aqueous Phase-Liquids. J. Soils Sediments 2001, 1, 217–222. [Google Scholar] [CrossRef]
- Davis, B.M.; Istok, J.D.; Semprini, L. Push–Pull Partitioning Tracer Tests Using Radon-222 to Quantify Non-Aqueous Phase Liquid Contamination. J. Contam. Hydrol. 2002, 58, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Höhener, P.; Surbeck, H. Radon-222 as a Tracer for Nonaqueous Phase Liquid in the Vadose Zone: Experiments and Analytical Model. Vadose Zone J. 2004, 3, 1276–1285. [Google Scholar] [CrossRef]
- Schubert, M. Using Radon as Environmental Tracer for the Assessment of Subsurface Non-Aqueous Phase Liquid (NAPL) Contamination—A Review. Eur. Phys. J. Spec. Top. 2015, 224, 717–730. [Google Scholar] [CrossRef]
- Castelluccio, M.; Agrahari, S.; De Simone, G.; Pompilj, F.; Lucchetti, C.; Sengupta, D.; Galli, G.; Friello, P.; Curatolo, P.; Giorgi, R.; et al. Using a Multi-Method Approach Based on Soil Radon Deficit, Resistivity, and Induced Polarization Measurements to Monitor Non-Aqueous Phase Liquid Contamination in Two Study Areas in Italy and India. Environ. Sci. Pollut. Res. 2018, 25, 12515–12527. [Google Scholar] [CrossRef]
- Briganti, A.; Tuccimei, P.; Voltaggio, M.; Carusi, C.; Galli, G.; Lucchetti, C. Assessing Methyl Tertiary Butyl Ether Residual Contamination in Groundwater Using Radon. Appl. Geochem. 2020, 116, 104583. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Díaz-Curiel, J.; De Miguel, E. Field Performance of the Radon-Deficit Technique to Detect and Delineate a Complex DNAPL Accumulation in a Multi-Layer Soil Profile. Environ. Pollut. 2021, 269, 116200. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, E.; Barrio-Parra, F.; Izquierdo-Díaz, M.; Fernández, J.; García-González, J.E.; Álvarez, R. Applicability and Limitations of the Radon-Deficit Technique for the Preliminary Assessment of Sites Contaminated with Complex Mixtures of Organic Chemicals: A Blind Field-Test. Environ. Int. 2020, 138, 105591. [Google Scholar] [CrossRef] [PubMed]
- Mattia, M.; Tuccimei, P.; Ciotoli, G.; Soligo, M.; Carusi, C.; Rainaldi, E.; Voltaggio, M. Combining Radon Deficit, NAPL Concentration, and Groundwater Table Dynamics to Assess Soil and Groundwater Contamination by NAPLs and Related Attenuation Processes. Appl. Sci. 2023, 13, 12813. [Google Scholar] [CrossRef]
- Fan, K.; Kuo, T.; Han, Y.; Chen, C.; Lin, C.; Lee, C. Radon Distribution in a Gasoline-Contaminated Aquifer. Radiat. Meas. 2007, 42, 479–485. [Google Scholar] [CrossRef]
- García-González, J.E.; Ortega, M.F.; Chacón, E.; Mazadiego, L.F.; Miguel, E.D. Field Validation of Radon Monitoring as a Screening Methodology for NAPL-Contaminated Sites. Appl. Geochem. 2008, 23, 2753–2758. [Google Scholar] [CrossRef][Green Version]
- Yoon, Y.Y.; Koh, D.C.; Lee, K.Y.; Cho, S.Y.; Yang, J.H.; Lee, K.K. Using 222Rn as a Naturally Occurring Tracer to Estimate NAPL Contamination in an Aquifer. Appl. Radiat. Isot. 2013, 81, 233–237. [Google Scholar] [CrossRef] [PubMed]
- CL:AIRE. Site Characterisation in Support of Monitored Natural Attenuation of Fuel Hydrocarbons and MTBE in a Chalk Aquifer in Southern England; CL:AIRE Case Study Bulletin, CSB 1; CL:AIRE: London, UK, 2002. [Google Scholar]
- Tsai, Y.-J.; Chou, Y.-C.; Wu, Y.-S.; Lee, C.-H. Noninvasive Survey Technology for LNAPL-Contaminated Site Investigation. J. Hydrol. 2020, 587, 125002. [Google Scholar] [CrossRef]
- Lenhard, R.J.; Parker, J.C. Estimation of Free Hydrocarbon Volume from Fluid Levels in Monitoring Wells. Groundwater 1990, 28, 57–67. [Google Scholar] [CrossRef]
- Kemblowski, M.W.; Chiang, C.Y. Hydrocarbon Thickness Fluctuations in Monitoring Wells. Groundwater 1990, 28, 244–252. [Google Scholar] [CrossRef]
- Atteia, O.; Palmier, C.; Schäfer, G. On the Influence of Groundwater Table Fluctuations on Oil Thickness in a Well Related to an LNAPL Contaminated Aquifer. J. Contam. Hydrol. 2019, 223, 103476. [Google Scholar] [CrossRef] [PubMed]
- Arato, A.; Wehrer, M.; Biró, B.; Godio, A. Integration of Geophysical, Geochemical and Microbiological Data for a Comprehensive Small-Scale Characterization of an Aged LNAPL-Contaminated Site. Environ. Sci. Pollut. Res. 2014, 21, 8948–8963. [Google Scholar] [CrossRef] [PubMed]
- Biosca, B.; Arévalo-Lomas, L.; Barrio-Parra, F.; Díaz-Curiel, J. Application and Limitations of Time Domain-Induced Polarization Tomography for the Detection of Hydrocarbon Pollutants in Soils with Electro-Metallic Components: A Case Study. Environ. Monit. Assess. 2020, 192, 115. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.W.; Cohen, R.M. A Review of Immiscible Fluids in the Subsurface: Properties, Models, Characterization and Remediation. J. Contam. Hydrol. 1990, 6, 107–163. [Google Scholar] [CrossRef]
- Karimi Askarani, K.; Sale, T.; Palaia, T. Natural Source Zone Depletion of Petroleum Hydrocarbon NAPL. In Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; García-Rincón, J., Gatsios, E., Lenhard, R.J., Atekwana, E.A., Naidu, R., Eds.; Environmental Contamination Remediation and Management; Springer International Publishing: Cham, Switzerland, 2024; pp. 113–138. ISBN 978-3-031-34447-3. [Google Scholar]
- Setarge, B.; Danzer, J.; Klein, R.; Grathwohl, P. Partitioning and Interfacial Tracers to Characterize Non-Aqueous Phase Liquids (NAPLs) in Natural Aquifer Material. Phys. Chem. Earth Part B Hydrol. Oceans Atmos. 1999, 24, 501–510. [Google Scholar] [CrossRef]
- Annable, M.D.; Rao, P.S.C.; Hatfield, K.; Graham, W.D.; Wood, A.L.; Enfield, C.G. Partitioning Tracers for Measuring Residual NAPL: Field-Scale Test Results. J. Environ. Eng. 1998, 124, 498–503. [Google Scholar] [CrossRef]
- US EPA. Cost and Performance Report for LNAPL Characterization and Remediation Partition Interwell Tracer Testing (PITT) and Rapid Optical Screening Tool (ROSTTM). In Characterization and Evaluation of the Feasibility of Surfactant Enhanced Aquifer Remediation (SEAR) at the Chevron Cincinnati Facility; US EPA: Hooven, OH, USA, 2005. [Google Scholar]
- Stacks, A.M. Radon: Geology, Environmental Impact and Toxicity Concerns; Nova Publishers: Hauppauge, NY, USA, 2015; p. 280. ISBN 978-1-63463-777-0. [Google Scholar]
- Rösch, F. The Basics of Nuclear Chemistry and Radiochemistry: An Introduction to Nuclear Transformations and Radioactive Emissions. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–61. ISBN 978-3-319-98947-1. [Google Scholar]
- Gutiérrez Villanueva, J.-L.G. Radon Concentrations in Air, Soil, and Water in a Granitic Area: Instrumental Development and Measurements. Doctoral Dissertation, Universidad de Valladolid, Valladolid, Spain, 2008. [Google Scholar]
- NRC. Risk Assessment of Radon in Drinking Water; National Research Council (US) Committee on Risk Assessment of Exposure to Radon in Drinking Water; National Academies Press (US): Washington, DC, USA, 1999; ISBN 978-0-309-06292-3. [Google Scholar]
- Nazaroff, W.; Moed, B.; Sextro, R.; Revzan, K.; Nero, A. Factors Influencing Soil as a Source of Indoor Radon: Framework for Assessing Radon Source Potential; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1989; p. LBL-20645. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- Baskaran, M. Radon: A Tracer for Geological, Geophysical and Geochemical Studies; Springer: Basel, Switzerland, 2016; ISBN 978-3-319-21329-3. [Google Scholar]
- Quindos Poncela, L.S.; Sainz Fernandez, C.; Fuente Merino, I.; Gutierrez Villanueva, J.L.; Gonzalez Diez, A. The Use of Radon as Tracer in Environmental Sciences. Acta Geophys. 2013, 61, 848–858. [Google Scholar] [CrossRef]
- Sukanya, S.; Noble, J.; Joseph, S. Application of Radon (222Rn) as an Environmental Tracer in Hydrogeological and Geological Investigations: An Overview. Chemosphere 2022, 303, 135141. [Google Scholar] [CrossRef] [PubMed]
- Hunkeler, D.; Hoehn, E.; Höhener, P.; Zeyer, J. 222Rn as a Partitioning Tracer To Detect Diesel Fuel Contamination in Aquifers: Laboratory Study and Field Observations. Environ. Sci. Technol. 1997, 31, 3180–3187. [Google Scholar] [CrossRef][Green Version]
- Schubert, M.; Lehmann, K.; Paschke, A. Determination of Radon Partition Coefficients between Water and Organic Liquids and Their Utilization for the Assessment of Subsurface NAPL Contamination. Sci. Total Environ. 2007, 376, 306–316. [Google Scholar] [CrossRef]
- Briganti, A.; Voltaggio, M.; Rainaldi, E.; Carusi, C. Vertical Light Non-Aqueous Phase Liquid (LNAPL) Distribution by Rn Prospecting in Monitoring Wells. Environ. Monit. Assess. 2024, 196, 19. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Pena, P.; Balcazar, M.; Meissner, R.; Lopez, A.; Flores, J.H. Determination of Radon Distribution Patterns in the Upper Soil as a Tool for the Localization of Subsurface NAPL Contamination. Radiat. Meas. 2005, 40, 633–637. [Google Scholar] [CrossRef]
- Barbosa, E.Q.; Galhardi, J.A.; Bonotto, D.M. The Use of Radon (Rn-222) and Volatile Organic Compounds in Monitoring Soil Gas to Localize NAPL Contamination at a Gas Station in Rio Claro, São Paulo State, Brazil. Radiat. Meas. 2014, 66, 1–4. [Google Scholar] [CrossRef]
- De Simone, G.; Galli, G.; Lucchetti, C.; Tuccimei, P. Using Natural Radon as a Tracer of Gasoline Contamination. Procedia Earth Planet. Sci. 2015, 13, 104–107. [Google Scholar] [CrossRef]
- Mateus, C.; Pecequilo, B.R.S. Preliminary Results of NAPL Contamination in a Disused Industry in the City of São Paulo, Brazil, by Radon Evaluation with CR-39 Detectors. In Proceedings of the INAC 2015: International Nuclear Atlantic Conference, Sao Paulo, SP, Brazil, 4–9 October 2015. [Google Scholar]
- Cohen, G.J.V.; Jousse, F.; Luze, N.; Höhener, P.; Atteia, O. LNAPL Source Zone Delineation Using Soil Gases in a Heterogeneous Silty-Sand Aquifer. J. Contam. Hydrol. 2016, 192, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.J.V.; Bernachot, I.; Su, D.; Höhener, P.; Mayer, K.U.; Atteia, O. Laboratory-Scale Experimental and Modelling Investigations of 222Rn Profiles in Chemically Heterogeneous LNAPL Contaminated Vadose Zones. Sci. Total Environ. 2019, 681, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, W.W. Radon Transport from Soil to Air. Rev. Geophys. 1992, 30, 137–160. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation; UNSCEAR: Vienna, Austria, 1977. [Google Scholar]
- Lombardi, S.; Voltattorni, N. Rn, He and CO2 Soil Gas Geochemistry for the Study of Active and Inactive Faults. Appl. Geochem. 2010, 25, 1206–1220. [Google Scholar] [CrossRef]
- Davidson, J.R.J.; Fairley, J.; Nicol, A.; Gravley, D.; Ring, U. The Origin of Radon Anomalies along Normal Faults in an Active Rift and Geothermal Area. Geosphere 2016, 12, 1656–1669. [Google Scholar] [CrossRef]
- Tanner, A.B. Radon Migration in the Ground: A Supplementary Review; US Geological Survey: Reston, VA, USA, 1980; pp. 5–56. [Google Scholar]
- Sakoda, A.; Ishimori, Y.; Yamaoka, K. A Comprehensive Review of Radon Emanation Measurements for Mineral, Rock, Soil, Mill Tailing and Fly Ash. Appl. Radiat. Isot. 2011, 69, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Hosoda, M.; Ishikawa, T.; Sorimachi, A.; Sahoo, S.K.; Tokonami, S.; Fukushi, M. Radon Migration Process and Its Influence Factors; Review. Jpn. J. Health Phys. 2009, 44, 218–231. [Google Scholar] [CrossRef]
- Krishnaswami, S.; Seidemann, D.E. Comparative Study of 222Rn, 40Ar, 39Ar and 37Ar Leakage from Rocks and Minerals: Implications for the Role of Nanopores in Gas Transport through Natural Silicates. Geochim. Cosmochim. Acta 1988, 52, 655–658. [Google Scholar] [CrossRef]
- Markkanen, M.; Arvela, H. Radon Emanation from Soils. Radiat. Prot. Dosim. 1992, 45, 269–272. [Google Scholar] [CrossRef]
- Stranden, E.; Kolstad, A.K.; Lind, B. The Influence of Moisture and Temperature on Radon Exhalation. Radiat. Prot. Dosim. 1984, 7, 55–58. [Google Scholar] [CrossRef]
- Iskandar, D.; Yamazawa, H.; Iida, T. Quantification of the Dependency of Radon Emanation Power on Soil Temperature. Appl. Radiat. Isot. 2004, 60, 971–973. [Google Scholar] [CrossRef] [PubMed]
- NCRP. Measurement of Radon and Radon Daughters in Air; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 1988. [Google Scholar]
- Perrier, F.; Richon, P.; Sabroux, J.-C. Temporal Variations of Radon Concentration in the Saturated Soil of Alpine Grassland: The Role of Groundwater Flow. Sci. Total Environ. 2009, 407, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Yoon, Y.Y.; Ko, K.S. Determination of the Emanation Coefficient and the Henry’s Law Constant for the Groundwater Radon. J. Radioanal. Nucl. Chem. 2010, 286, 381–385. [Google Scholar] [CrossRef]
- Schery, S.D.; Whittlestone, S. Desorption of Radon at the Earth’s Surface. J. Geophys. Res. 1989, 94, 18297–18304. [Google Scholar] [CrossRef]
- Penman, H.L. Gas and Vapour Movements in the Soil: I. The Diffusion of Vapours through Porous Solids. J. Agric. Sci. 1940, 30, 437–462. [Google Scholar] [CrossRef]
- Penman, H.L. Gas and Vapour Movements in the Soil: II. The Diffusion of Carbon Dioxide through Porous Solids. J. Agric. Sci. 1940, 30, 570–581. [Google Scholar] [CrossRef]
- Marshall, T.J. A Relation Between Permeability and Size Distribution of Pores. J. Soil Sci. 1958, 9, 1–8. [Google Scholar] [CrossRef]
- Millington, R.J.; Quirk, J.P. Permeability of Porous Solids. Trans. Faraday Soc. 1961, 57, 1200–1207. [Google Scholar] [CrossRef]
- Millington, R.J.; Shearer, R.C. Diffusion in Aggregated Porous Media. Soil Sci. 1971, 111, 372–378. [Google Scholar] [CrossRef]
- Strong, K.P.; Levins, D.M. Effect of Moisture Content on Radon Emanation from Uranium Ore and Tailings. Health Phys. 1982, 42, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.B. Radon Migration in the Ground: A Review. In Natural Radiation Environment; University of Chicago Press: Chicago, IL, USA, 1964; pp. 161–190. [Google Scholar]
- Broecker, W.S.; Peng, T.H. Gas Exchange Rates between Air and Sea1. Tellus 1974, 26, 21–35. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Nain, M.; Kant, K. Radon Diffusion Studies through Some Building Materials: Effect of Grain Size. Radiat. Meas. 2008, 43, S445–S448. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Soil Physics; Academic Press: Cambridge, MA, USA, 1982; ISBN 978-0-08-091869-3. [Google Scholar]
- Scanlon, B.R.; Nicot, J.P.; Massmann, J.W. Soil Gas Movement in Unsaturated Systems. Soil Phys. Companion 2002, 389, 297–341. [Google Scholar]
- Auer, L.H.; Rosenberg, N.D.; Birdsell, K.H.; Whitney, E.M. The Effects of Barometric Pumping on Contaminant Transport. J. Contam. Hydrol. 1996, 24, 145–166. [Google Scholar] [CrossRef]
- Forde, O.N.; Cahill, A.G.; Beckie, R.D.; Mayer, K.U. Barometric-Pumping Controls Fugitive Gas Emissions from a Vadose Zone Natural Gas Release. Sci. Rep. 2019, 9, 14080. [Google Scholar] [CrossRef] [PubMed]
- Etiope, G.; Lombardi, S. Evidence for Radon Transport by Carrier Gas through Faulted Clays in Italy. J. Radioanal. Nucl. Chem. 1995, 193, 291–300. [Google Scholar] [CrossRef]
- Čeliković, I.; Pantelić, G.; Vukanac, I.; Nikolić, J.K.; Živanović, M.; Cinelli, G.; Gruber, V.; Baumann, S.; Ciotoli, G.; Poncela, L.S.Q.; et al. Overview of Radon Flux Characteristics, Measurements, Models and Its Potential Use for the Estimation of Radon Priority Areas. Atmosphere 2022, 13, 2005. [Google Scholar] [CrossRef]
- Qi, S.; Luo, J.; O’Connor, D.; Wang, Y.; Hou, D. A Numerical Model to Optimize LNAPL Remediation by Multi-Phase Extraction. Sci. Total Environ. 2020, 718, 137309. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Wang, G.; Chen, Q.; Yao, Y. Investigating the Role of Vadose Zone Breathing in Vapor Intrusion from Contaminated Groundwater. J. Hazard. Mater. 2021, 416, 126272. [Google Scholar] [CrossRef] [PubMed]
- Cavelan, A.; Golfier, F.; Colombano, S.; Davarzani, H.; Deparis, J.; Faure, P. A Critical Review of the Influence of Groundwater Level Fluctuations and Temperature on LNAPL Contaminations in the Context of Climate Change. Sci. Total Environ. 2022, 806, 150412. [Google Scholar] [CrossRef]
- Cunningham, R.E.; Williams, R.J.J. Diffusion in Gases and Porous Media; Springer: Boston, MA, USA, 1980; ISBN 978-1-4757-4985-4. [Google Scholar]
- Yang, C.; Wang, Z.; Hollebone, B.P.; Brown, C.E.; Yang, Z.; Landriault, M. Chromatographic Fingerprinting Analysis of Crude Oils and Petroleum Products. In Handbook of Oil Spill Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 93–163. ISBN 978-1-118-98998-2. [Google Scholar]
- Kitto, M.E. Interrelationship of Indoor Radon Concentrations, Soil-Gas Flux, and Meteorological Parameters. J. Radioanal. Nucl. Chem. 2005, 264, 381–385. [Google Scholar] [CrossRef]
- Zhuo, W.; Chen, B.; Li, D.; Liu, H. Reconstruction of Database on Natural Radionuclide Contents in Soil in China. J. Nucl. Sci. Technol. 2008, 45, 180–184. [Google Scholar] [CrossRef]
- Hirao, S.; Yamazawa, H.; Moriizumi, J. Estimation of the Global 222Rn Flux Density from the Earth’s Surface. Jpn. J. Health Phys. 2010, 45, 161–171. [Google Scholar] [CrossRef]
- López-Coto, I.; Mas, J.L.; Bolivar, J.P. A 40-Year Retrospective European Radon Flux Inventory Including Climatological Variability. Atmos. Environ. 2013, 73, 22–33. [Google Scholar] [CrossRef]
- Andrews, J.N.; Wood, D.F. Mechanism of Radon Release in Rock Matrices and Entry into Groundwaters. Inst. Min. Met. Trans. Sect B 1972, 81, 198–209. [Google Scholar]
- Cecconi, A.; Verginelli, I.; Baciocchi, R.; Lanari, C.; Villani, F.; Bonfedi, G. Using Groundwater Monitoring Wells for Rapid Application of Soil Gas Radon Deficit Technique to Evaluate Residual LNAPL. J. Contam. Hydrol. 2023, 258, 104241. [Google Scholar] [CrossRef] [PubMed]
- Brost, E.J.; DeVaull, G.E. Non-Aqueous Phase Liquid (NAPL) Mobility Limits in Soil; American Petroleum Institute: Washington, DC, USA, 2000. [Google Scholar]
- Van Der Spoel, W.H.; Van Der Graaf, E.R.; De Meijer, R.J. Diffusive Transport of Radon in a Homogeneous Column of Dry Sand. Health Phys. 1997, 72, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, W.H.; Van Der Graaf, E.R.; De Meijer, R.J. Combined Diffusive and Advective Transport of Radon in a Homogeneous Column of Dry Sand. Health Phys. 1998, 74, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Höhener, P. Diffusive Partitioning Tracer Test for Nonaqueous Phase Liquid (NAPL) Detection in the Vadose Zone. Environ. Sci. Technol. 2002, 36, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, A.; Verginelli, I.; Barrio-Parra, F.; De Miguel, E.; Baciocchi, R. Influence of Advection on the Soil Gas Radon Deficit Technique for the Quantification of LNAPL. Sci. Total Environ. 2023, 875, 162619. [Google Scholar] [CrossRef]
- Cecconi, A.; Verginelli, I.; Baciocchi, R. Modeling of Soil Gas Radon as an in Situ Partitioning Tracer for Quantifying LNAPL Contamination. Sci. Total Environ. 2022, 806, 150593. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.U.; Frind, E.O.; Blowes, D.W. Multicomponent Reactive Transport Modeling in Variably Saturated Porous Media Using a Generalized Formulation for Kinetically Controlled Reactions. Water Resour. Res. 2002, 38, 13-1–13-21. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Hidalgo, A.; Izquierdo-Díaz, M.; Arévalo-Lomas, L.; De Miguel, E. 1D_RnDPM: A Freely Available 222Rn Production, Diffusion, and Partition Model to Evaluate Confounding Factors in the Radon-Deficit Technique. Sci. Total Environ. 2022, 807, 150815. [Google Scholar] [CrossRef] [PubMed]
- Le Meur, M.; Cohen, G.J.V.; Laurent, M.; Höhener, P.; Atteia, O. Effect of NAPL Mixture and Alteration on 222Rn Partitioning Coefficients: Implications for NAPL Subsurface Contamination Quantification. Sci. Total Environ. 2021, 791, 148210. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Available online: https://pubs.acs.org/doi/pdf/10.1021/cr60306a003 (accessed on 19 July 2023).
- Schubert, M.; Paschke, A.; Lau, S.; Geyer, W.; Knöller, K. Radon as a Naturally Occurring Tracer for the Assessment of Residual NAPL Contamination of Aquifers. Environ. Pollut. 2007, 145, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ponsin, V.; Chablais, A.; Dumont, J.; Radakovitch, O.; Höhener, P. 222Rn as Natural Tracer for LNAPL Recovery in a Crude Oil-Contaminated Aquifer. Groundw. Monit. Remediat. 2015, 35, 30–38. [Google Scholar] [CrossRef]
- De Simone, G.; Lucchetti, C.; Pompilj, F.; Galli, G.; Tuccimei, P.; Curatolo, P.; Giorgi, R. Soil Radon Survey to Assess NAPL Contamination from an Ancient Spill. Do Kerosene Vapors Affect Radon Partition? J. Environ. Radioact. 2017, 171, 138–147. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, E.; Barrio-Parra, F.; Elío, J.; Izquierdo-Díaz, M.; García-González, J.E.; Mazadiego, L.F.; Medina, R. Applicability of Radon Emanometry in Lithologically Discontinuous Sites Contaminated by Organic Chemicals. Environ. Sci. Pollut. Res. 2018, 25, 20255–20263. [Google Scholar] [CrossRef] [PubMed]
- Mattia, M.; Tuccimei, P.; Soligo, M.; Carusi, C. Radon as a Natural Tracer for Monitoring NAPL Groundwater Contamination. Water 2020, 12, 3327. [Google Scholar] [CrossRef]
- Schubert, M.; Freyer, K.; Treutler, H.C.; Weiss, H. Using Radon-222 in Soil Gas as an Indicator of Subsurface Contamination by Non-Aqueous Phase-Liquids (NAPLs). Geofísica Int. 2002, 41, 433–437. [Google Scholar]
- Davis, B.M.; Istok, J.D.; Semprini, L. Numerical Simulations of Radon as an in Situ Partitioning Tracer for Quantifying NAPL Contamination Using Push–Pull Tests. J. Contam. Hydrol. 2005, 78, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Balcázar, M.; Lopez, A.; Peña, P.; Flores, J.H.; Knöller, K. Combination of Radon and Stable Isotope Analysis as a Tool for Decision Support Concerning the Remediation of NAPL-Contaminated Sites. Isot. Environ. Health Stud. 2007, 43, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Galhardi, J.A.; Bonotto, D.M. Radon in Groundwater Contaminated by Dissolved Hydrocarbons in Santa Bárbara d’Oeste, São Paulo State, Brazil. Appl. Radiat. Isot. 2012, 70, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Ju, Y.; Lee, S.-S.; Kaown, D.; Lee, K.-K. Characterization of a NAPL-Contaminated Site Using the Partitioning Behavior of Noble Gases. J. Contam. Hydrol. 2020, 235, 103733. [Google Scholar] [CrossRef] [PubMed]
- Semprini, L.; Broholm, K.; McDonald, M. Radon-222 Deficit Method for Locating and Quantifying NAPL Contamination in the Subsurface. EOS Trans Am. Geophys Union 1993, 74, 299. [Google Scholar]
- Semprini, L.; Hopkins, O.S.; Gottipati, S.; Tasker, B.R. Field, Laboratory and, Modeling Studies of Radon-222 as a Natural Tracer for Detecting NAPL Contamination in the Subsurface and Monitoring the Progress of Remediation. EOS Trans. Am. Geophys. Union 1995, 76, F276. [Google Scholar]
- Wang, X.; Li, H.; Zhang, Y.; Qu, W.; Schubert, M. Submarine Groundwater Discharge Revealed by 222Rn: Comparison of Two Continuous on-Site 222Rn-in-Water Measurement Methods. Hydrogeol. J. 2019, 27, 1879–1887. [Google Scholar] [CrossRef]
- Janik, M.; Ishikawa, T.; Omori, Y.; Kavasi, N. Invited Article: Radon and Thoron Intercomparison Experiments for Integrated Monitors at NIRS, Japan. Rev. Sci. Instrum. 2014, 85, 022001. [Google Scholar] [CrossRef] [PubMed]
- Sumesh, C.G.; Jha, S.K.; Patra, A.C.; Aswal, D.K. Evolution of Analytical Methods for Radon Measurement in India. MAPAN 2024, 39, 181–192. [Google Scholar] [CrossRef]
- George, A.C. An Overview of Instrumentation for Measuring Environmental Radon and Radon Progeny. IEEE Trans. Nucl. Sci. 1990, 37, 892–901. [Google Scholar] [CrossRef]
- Papastefanou, C. Measuring Radon in Soil Gas and Groundwaters: A Review. 2007. Available online: http://hdl.handle.net/2122/3870 (accessed on 19 July 2023).
- Lucas, H.F. Improved Low-Level Alpha-Scintillation Counter for Radon. Rev. Sci. Instrum. 1957, 28, 680–683. [Google Scholar] [CrossRef]
- Sethy, N.K.; Jha, V.N.; Ravi, P.M.; Tripathi, R.M. A Simple Method for Calibration of Lucas Scintillation Cell Counting System for Measurement of 226Ra and 222Rn. J. Radiat. Res. Appl. Sci. 2014, 7, 472–477. [Google Scholar] [CrossRef]
- Fortmann, R.C. Measurement Methods and Instrumentation. In Radon; ASTM International: West Conshohocken, PA, USA, 1993. [Google Scholar]
- Nunes, L.J.R.; Curado, A.; Lopes, S.I. The Relationship between Radon and Geology: Sources, Transport and Indoor Accumulation. Appl. Sci. 2023, 13, 7460. [Google Scholar] [CrossRef]
- Dalla Betta, G.-F.; Ye, J. Silicon Radiation Detector Technologies: From Planar to 3D. Chips 2023, 2, 83–101. [Google Scholar] [CrossRef]
- Nikezic, D.; Yu, K.N. Formation and Growth of Tracks in Nuclear Track Materials. Mater. Sci. Eng. R Rep. 2004, 46, 51–123. [Google Scholar] [CrossRef]
- Miles, J.C.H.; Sinnaeve, J. The Performance of Different Types of Etched-Track Radon Dosemeters in Two International Intercomparisons. Int. J. Radiat. Appl. Instrum. Part Nucl. Tracks Radiat. Meas. 1986, 12, 735–738. [Google Scholar] [CrossRef]
- Gewali, J.P.; Sheron, P.; Thakur, A.; Jaishy, B. Study of Radiation Interactions in Makrofol-E and LR-115 Detectors Using SSNTD Technique. In Selected Progresses in Modern Physics; Sengupta, S., Dey, S., Das, S., Saikia, D.J., Panda, S., Podila, R., Eds.; Springer: Singapore, 2021; pp. 375–385. [Google Scholar]
- Kotrappa, P.; Brubaker, T.; Dempsey, J.C.; Stieff, L.R. Electret Ion Chamber System for Measurement of Environmental Radon and Environmental Gamma Radiation. Radiat. Prot. Dosim. 1992, 45, 107–110. [Google Scholar] [CrossRef]
- ISO 11665-1:2019; Measurement of Radioactivity in the Environment—Air: Radon-222—Part 1: Origins of Radon and Its Short-Lived Decay Products and Associated Measurement Methods. ISO: Geneva, Switzerland, 2019.
- L’Annunziata, M.F. Handbook of Radioactivity Analysis; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-384874-1. [Google Scholar]
- Neznal, M.; Matolín, M.; Just, G.; Turek, K. Short-Term Temporal Variations of Soil Gas Radon Concentration and Comparison of Measurement Techniques. Radiat. Prot. Dosim. 2004, 108, 55–63. [Google Scholar] [CrossRef] [PubMed]
- ITRC. The Use of Direct Push Well Technology for Long-Term Environmental Monitoring in Groundwater Investigations; ITRC: Washington, DC, USA, 2006. [Google Scholar]
- CalEPA, (California Environmental Protection Agency Department of Toxic Substances Control). Advisory: Active Soil Gas Investigations; CalEPA: Sacramento, CA, USA, 2015. [Google Scholar]
- Voltaggio, M.; Spadoni, M. Determination of 222Rn in Water by Absorption in Polydimethylsiloxane Mixed with Activated Carbon and Gamma-Ray Spectrometry: An Example Application in the Radon Budget of Paterno Submerged Sinkhole (Central Italy). Appl. Geochem. 2013, 34, 65–74. [Google Scholar] [CrossRef]
- Schubert, M.; Schulz, H. Diurnal Radon Variations in the Upper Soil Layers and at the Soil-Air Interface Related to Meteorological Parameters. Health Phys. 2002, 83, 91–96. [Google Scholar] [CrossRef] [PubMed]
- King, C.-Y.; Minissale, A. Seasonal Variability of Soil-Gas Radon Concentration in Central California. Radiat. Meas. 1994, 23, 683–692. [Google Scholar] [CrossRef]
- Hutter, A.R. Spatial and Temporal Variations of Soil Gas 220Rn and 222Rn at Two Sites in New Jersey. Environ. Int. 1996, 22, 455–469. [Google Scholar] [CrossRef]
- Castelluccio, M.; De Simone, G.; Lucchetti, C.; Moroni, M.; Salvati, F.; Tuccimei, P. A New Technique to Measure in Situ Soil Gas Permeability. J. Geochem. Explor. 2015, 148, 56–59. [Google Scholar] [CrossRef]
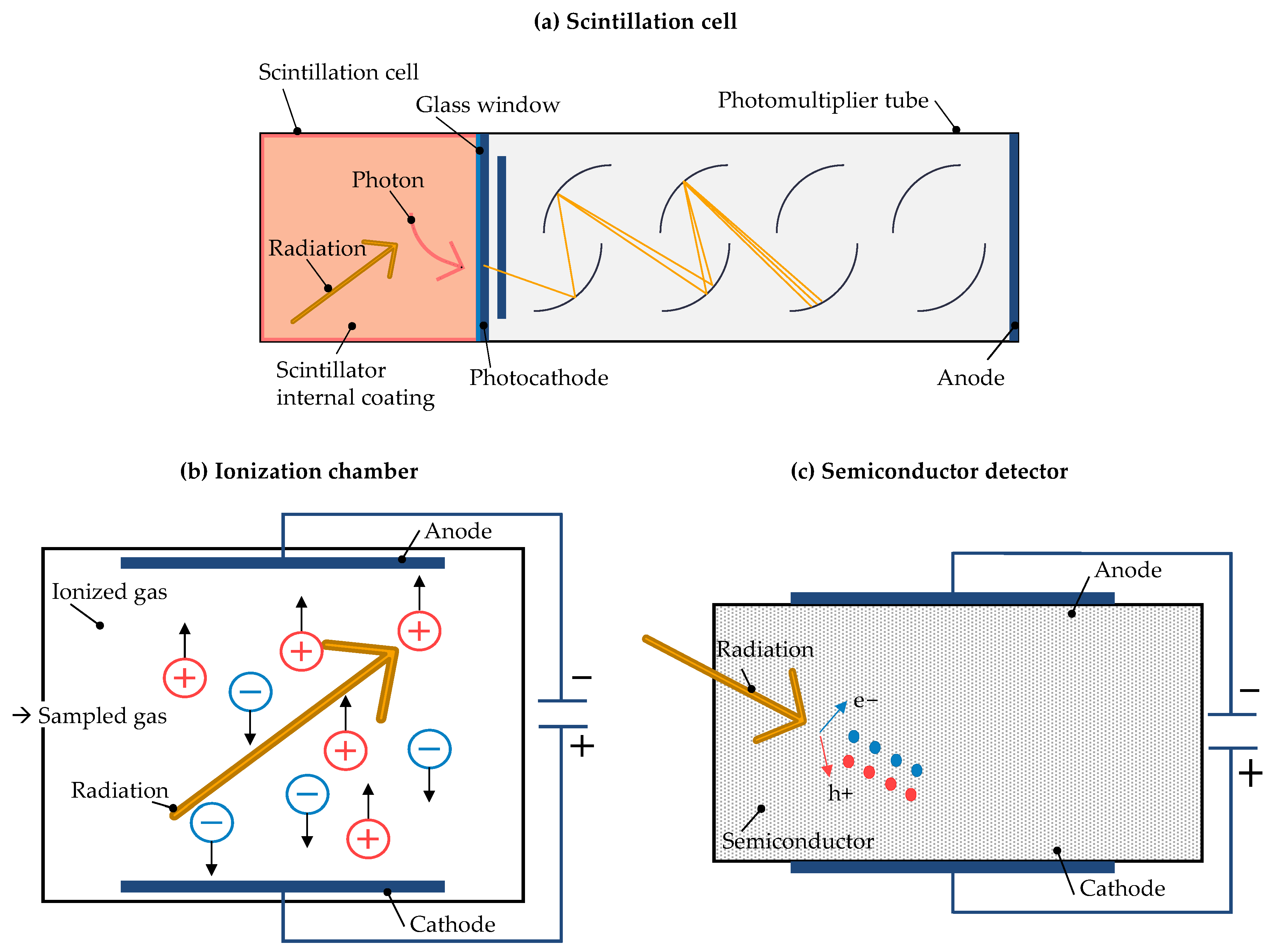
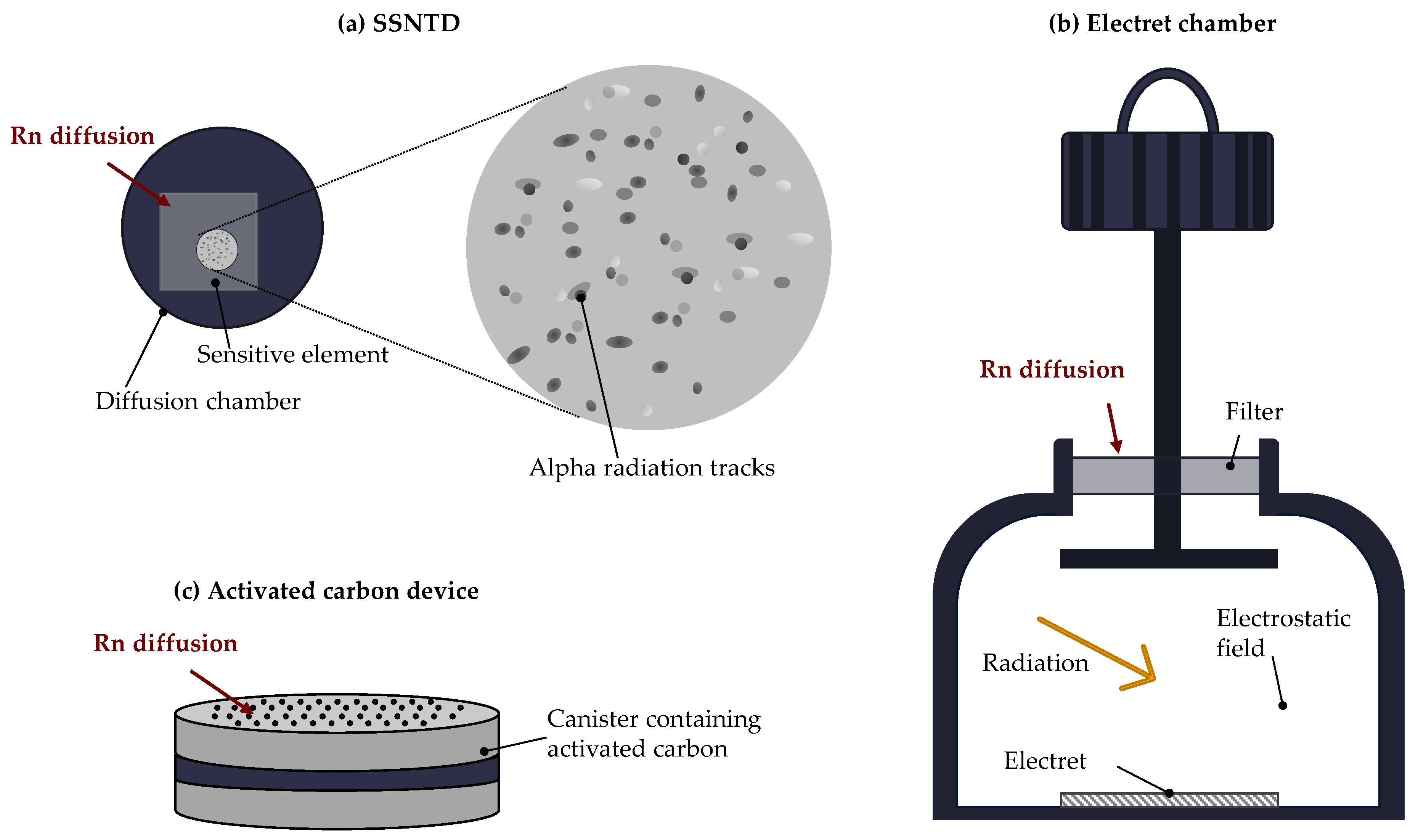
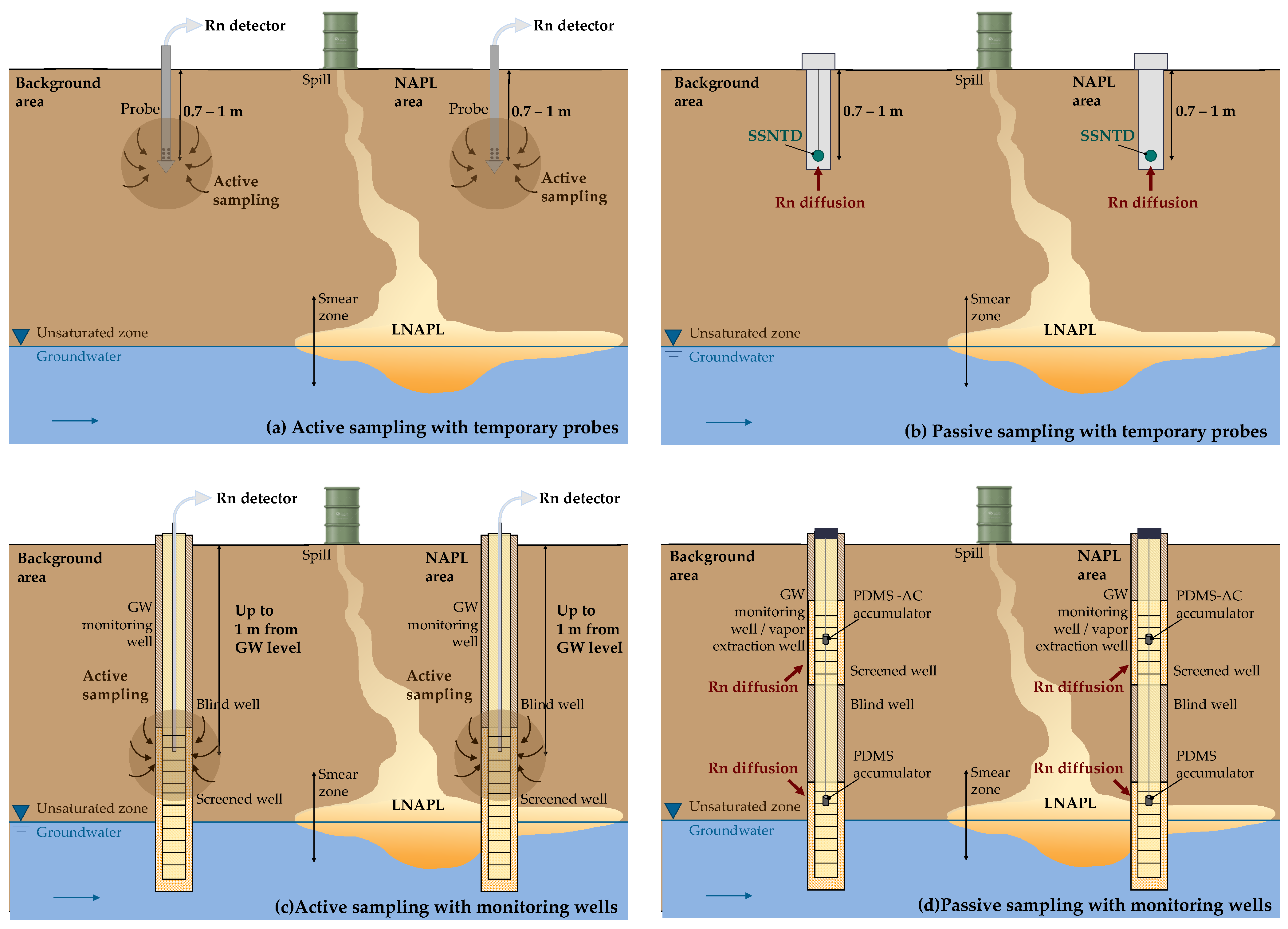
| Rock Type | 238U (Bq kg−1) |
|---|---|
| Igneous—Acidic (e.g., granite) | 59.2 |
| Igneous—Intermediate (e.g., diorite) | 22.9 |
| Igneous—Mafic (e.g., basalt) | 11.5 |
| Igneous—Ultrabasic | 0.4 |
| Sedimentary—Limestone | 27.7 |
| Sedimentary—Carbonate | 26.6 |
| Sedimentary—Sandstones | 18.5 |
| Sedimentary—Shale | 44.4 |
| Temperature °C | Dimensionless Henry’s Constant (H) |
|---|---|
| 0 | 1.875 ± 0.155 |
| 10 | 2.365 ± 0.255 |
| 20 | 3.65 ± 0.27 |
| 30 | 5.27 ± 0.34 |
| Parameter | Value | Reference |
|---|---|---|
| Rn diffusion coefficient in air Dmg | 1.2 × 10−5 m2 s−1 | [82] |
| Rn diffusion coefficient in water Dmw | 1.16 × 10−9 m2 s−1 | [83] |
| Authors | Application | Sampling | NAPL | Soil | Rn Measurement | Main Results |
|---|---|---|---|---|---|---|
| [52] | GW—FS | GW-MW | LNAPL | Coarse | LSC | Rn deficit (ranging between 0.54 and 0.69) in MW within LNAPL zone, and with dissolved HC. No quantitative correlations. |
| [18] | GW—Controlled test site | GW-MW | DNAPL | Coarse | LSC | Rn deficit (ranging between 0.3 and 0.5) in the NAPL zone (with a saturation of 4.5%). Rn downgradient re-equilibrated within a few meters to the upgradient value. |
| [19,117] | UZ—FS | T-SGP | LNAPL | Fine | IC | Area of Rn deficit (down to 0.2) closely matches the LNAPL zone. |
| [19,117] | UZ—Military site | T-SGP | LNAPL | Coarse | IC | Area of Rn deficit (down to 0.3) closely matches the LNAPL zone. |
| [20,118] | GW—Former IS | In-well push–pull tests with tracers | LNAPL | Coarse | LSC | Higher Rn retardation in test in the LNAPL zone. Uncertainty in estimated saturations. Research is needed to investigate the differences between injection and extraction-phase retardation factors. |
| [55] | UZ—Former airfield | T-SGP | LNAPL | Coarse | IC | Rn deficit (down to 0.1) area closely matches the LNAPL zone. |
| [28] | GW—IS | GW-MW | LNAPL | Heterogeneous | LSC | Rn deficit (around 0.31) found in MW within the LNAPL zone. LNAPL saturation (around 5.18%) from Rn deficit agrees with soil chemical analysis. To effectively use the technique, Rn emanation rate must be virtually homogeneous in the aquifer. |
| [112] | GW—Former FS | GW-MW | LNAPL | Heterogeneous; coarse aquifer | On-site stripping from GW samples + IC + LSC | Estimated LNAPL saturation (around 1.5%) from Rn deficit (around 0.55) agrees with chemical analysis. No Rn deficit in areas with only dissolved BTEX. |
| [119] | GW—IS | GW-MW | LNAPL | Heterogeneous; coarse aquifer | On-site stripping from GW samples + IC | Rn data allowed differentiation between LNAPL source zone (deficit of 0.18–0.4) and LNAPL plume (not possible by GW samples alone). LNAPL quantification (saturations of 8.1–2.7%) was possible but complicated by mineralogical heterogeneities. |
| [29] | UZ—FS | T-SGP | LNAPL | Heterogeneous | SC | Rn deficit (ranging between 0.1 and 0.2) in soil gas above accumulations of HC but with uncertainty in Rn background value. |
| [120] | GW—FS | GW-MW | LNAPL | Heterogeneous | Laboratory stripping from GW samples + IC | Dissolved HC tends to increase Rn concentration in water, due to the preferential partition. Rn can indicate the dissolved plume, but it did not provide information on residual concentrations. |
| [30] | GW—IS | GW-MW | DNAPL | Heterogeneous | LSC | Low correlation between TCE concentration in GW and Rn, reflecting the local heterogeneity of the aquifer. |
| [56] | UZ—FS | T-SGP | LNAPL | Fine | IC | No significant correlation between Rn and VOCs, but the ratio between the minimum and maximum Rn values indicates that Rn near the fuel leak the Rn deficit is around 0.15 with respect to upstream areas. |
| [95] | GW—IS | GW-MW | DNAPL | Backfill soil over bedrock | SC in closed-loop mode | The variation in the Rn activities with respect to the associated contaminant concentrations can be a helpful tool for tracing residual contamination but may give ambiguous results in case of heterogeneity of the subsurface matrix. |
| [58] | UZ—Former IS | SSNTD | LNAPL | Heterogeneous | SSNTD | Rn deficit (down to 0.02) found within the LNAPL zone. No correlation for the second contaminated location. |
| [113] | GW | GW-MW | LNAPL | Coarse over fine | SC in closed-loop mode | Rn was measured successfully when a pump-and-skim system was active. Low Rn activities suggest zones of good recovery, while high activities were not always related to poor recovery. Rn deficit was down to 0.03 with estimated LNAPL saturations up to 68% but with large uncertainties in quantification of residual contamination based on Rn |
| [59] | UZ—Railway site | T-SGP | LNAPL | Heterogeneous | Scintillation + photomultiplier + scale count | Low correlation between Rn and LNAPL lens, due to the heterogeneity of the sediment. Also, for VOC, O2, CO2, and CH4, diffusive gas transfer is locally restricted |
| [114] | UZ | T-SGP | LNAPL | Coarse | Two SCs in series | Residual LNAPL saturation (1.7–24.1%) from Rn deficit (0.65–0.06) agreed with direct determination of HC concentrations. Estimated saturations from very high deficits can be affected by large uncertainties (high errors from Rn measurements). |
| [23] | UZ | T-SGP | LNAPL | Coarse | SC | Direct correlation between Rn deficit and the highest electrical resistivity but no correlation with induced polarization. VOC concentrations were not linked with Rn reduction (old spillage). |
| [23] | UZ | T-SGP | LNAPL | Heterogeneous | SC | Rn deficit (about 0.61 and 0.16 for two areas) found within a contaminated zone, but the LNAPL saturation (about 0.71 and 11.6% for two areas) was influenced by seasonal soil conditions. Direct correlation between Rn deficit at shallow depth and the highest electrical resistivity at greater depth. |
| [115] | UZ—IS | T-SGP | LNAPL | Fractured system (active fault) | Portable α monitors | Rn reduction in one section responds to a discontinuity in the subsurface (not to LNAPL). Rn deficit (0.5–0.7) in the other section suggests possible LNAPL contamination. Rn emanation above background levels due to co-advective transport in the fractured system. |
| [24] | GW—Former FS | GW-MW | LNAPL | Backfill soil over bedrock | Laboratory stripping from GW samples + SC | Rn deficit (ranging from 0.21 to 0.75) allowed for the identification of areas with residual LNAPL after 15 years of the spill. No realistic evaluation of saturation was possible because the required assumptions of aquifer homogeneity were not respected. |
| [121] | GW—IS | GW-MW | DNAPL | Backfill soil over fractured bedrock | Laboratory stripping from GW samples | Rn measurements were not successful in characterizing residual TCE because of the heterogeneity of the fractured bedrock. Other noble gases (Ne, Ar, and Xe) could be useful for the characterization of the TCE-contaminated site. |
| [26] | UZ—Former IS | P-SGP | DNAPL | Heterogeneous | Pulse IC | Rn deficits correspond with contamination hotspots obtained using standard direct and indirect prospecting techniques and are coherent with the location of historical pollution sources. |
| [116] | GW—FS | GW-MW | LNAPL | Backfill soil over bedrock | Laboratory stripping from GW samples + SC | Rn deficit (0.49–0.88) areas corresponded to the highest dissolved concentrations areas and described the residual source zone. Low Rn levels were detected downstream of the recharge wells, possibly due to the injection of treated GW, depleted in Rn. |
| [25] | UZ—Former IS | T-SGP | DNAPL | Heterogeneous | Pulse IC | Rn measurements dependent on atmospheric temperature. Negative spatial correlation of rescaled Rn and contaminant load in the upper layers. Inability to detect deep DNAPL. |
| [27] | UZ—FS | P-SGP | LNAPL | Backfill soil over ignimbrites | Laboratory SC | Inverse correlation between Rn and VOC agrees with the Rn deficit. Rn deficit in SG (down to 0.01) allowed for the identification of areas with residual LNAPL. |
| [27] | GW—FS | GW-MW | LNAPL | Alluvial deposits | Laboratory stripping from GW samples + SC | Rn deficit validated with multi-parameter monitoring (Rn, LNAPL, and GW levels) and chemical analysis. Rn deficit in GW (down to 0.01) allowed for the identification of areas with residual LNAPL. LNAPL saturation (up to 54%) estimated based on GW Rn deficit values. |
| [101] | UZ—Active FS | SG-MW | LNAPL | Heterogeneous | SC | Rn measures in the headspace of MW are a promising approach for detecting mobile and residual LNAPL. Rn deficits in MW ranged from 0.05 to 0.82 for different sites. The quantitative assessment is still to be evaluated. |
| [54] | GW—Active FS | PDMS-AC in MW in GW | LNAPL | Heterogeneous | Passive accumulators | PDMS-AC passive accumulators can be useful to determine Rn vertical variations in the soil profile. For GW, quantitative estimations (LNAPL saturations of 1.2–2.5%) were also possible. |
| [54] | UZ—Active FS | PDMS-AC in MW in UZ | LNAPL | Heterogeneous fractured system | Passive accumulators | PDMS-AC passive accumulators can be useful to determine Rn vertical variations in the soil profile. For UZ, quantitative estimations were considered not feasible. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecconi, A.; Verginelli, I.; Baciocchi, R. Assessing Light Non-Aqueous Phase Liquids in the Subsurface Using the Soil Gas Rn Deficit Technique: A Literature Overview of Field Studies. Sustainability 2024, 16, 3317. https://doi.org/10.3390/su16083317
Cecconi A, Verginelli I, Baciocchi R. Assessing Light Non-Aqueous Phase Liquids in the Subsurface Using the Soil Gas Rn Deficit Technique: A Literature Overview of Field Studies. Sustainability. 2024; 16(8):3317. https://doi.org/10.3390/su16083317
Chicago/Turabian StyleCecconi, Alessandra, Iason Verginelli, and Renato Baciocchi. 2024. "Assessing Light Non-Aqueous Phase Liquids in the Subsurface Using the Soil Gas Rn Deficit Technique: A Literature Overview of Field Studies" Sustainability 16, no. 8: 3317. https://doi.org/10.3390/su16083317
APA StyleCecconi, A., Verginelli, I., & Baciocchi, R. (2024). Assessing Light Non-Aqueous Phase Liquids in the Subsurface Using the Soil Gas Rn Deficit Technique: A Literature Overview of Field Studies. Sustainability, 16(8), 3317. https://doi.org/10.3390/su16083317







