Towards a Sustainable Mining: Reuse of Slate Stone Cutting Sludges for New Geopolymer Binders
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Slate Stone Cutting Sludge from La Cabrera Baja (Leon, Spain)
2.1.2. Alkali Activation Solution: Sodium Hydroxide and Sodium Silicate
2.1.3. Methodology
2.1.4. Chemical and Physical Characterization of SSCS from La Cabrera
2.1.5. Preparation of SSCS–Alkali Activation Solution Geopolymer Samples
3. Results
3.1. Physical and Chemical Characterization Test Results of the SSCS
3.1.1. Elemental Analysis
3.1.2. X-ray Fluorescence
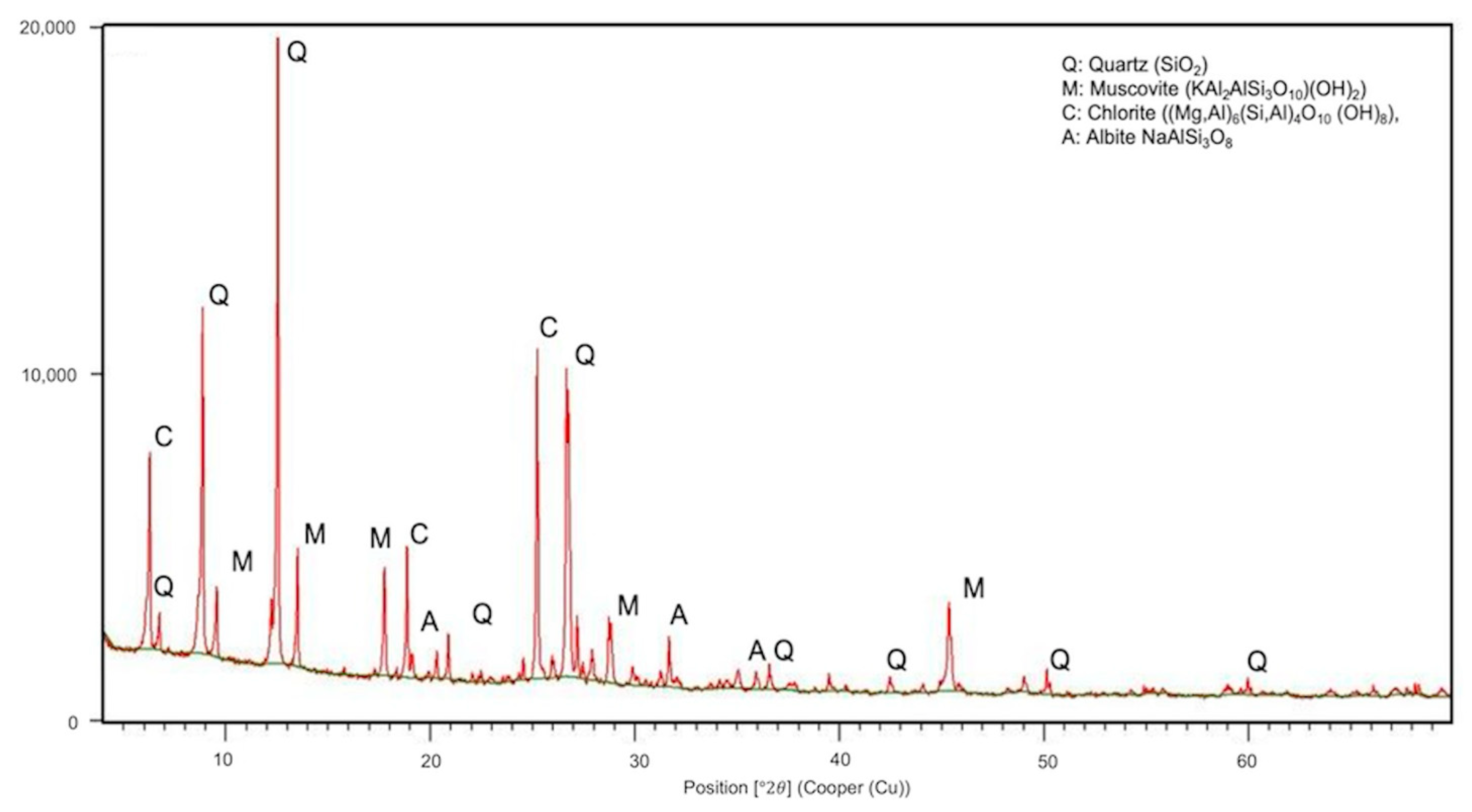
3.1.3. X-ray Diffraction
3.1.4. Particle Size Distribution
3.1.5. Density
3.1.6. pH Determination
3.1.7. Moisture Determination Test
3.2. Physical and Mechanical Characterization Tests of Geopolymer Samples Using SSCS
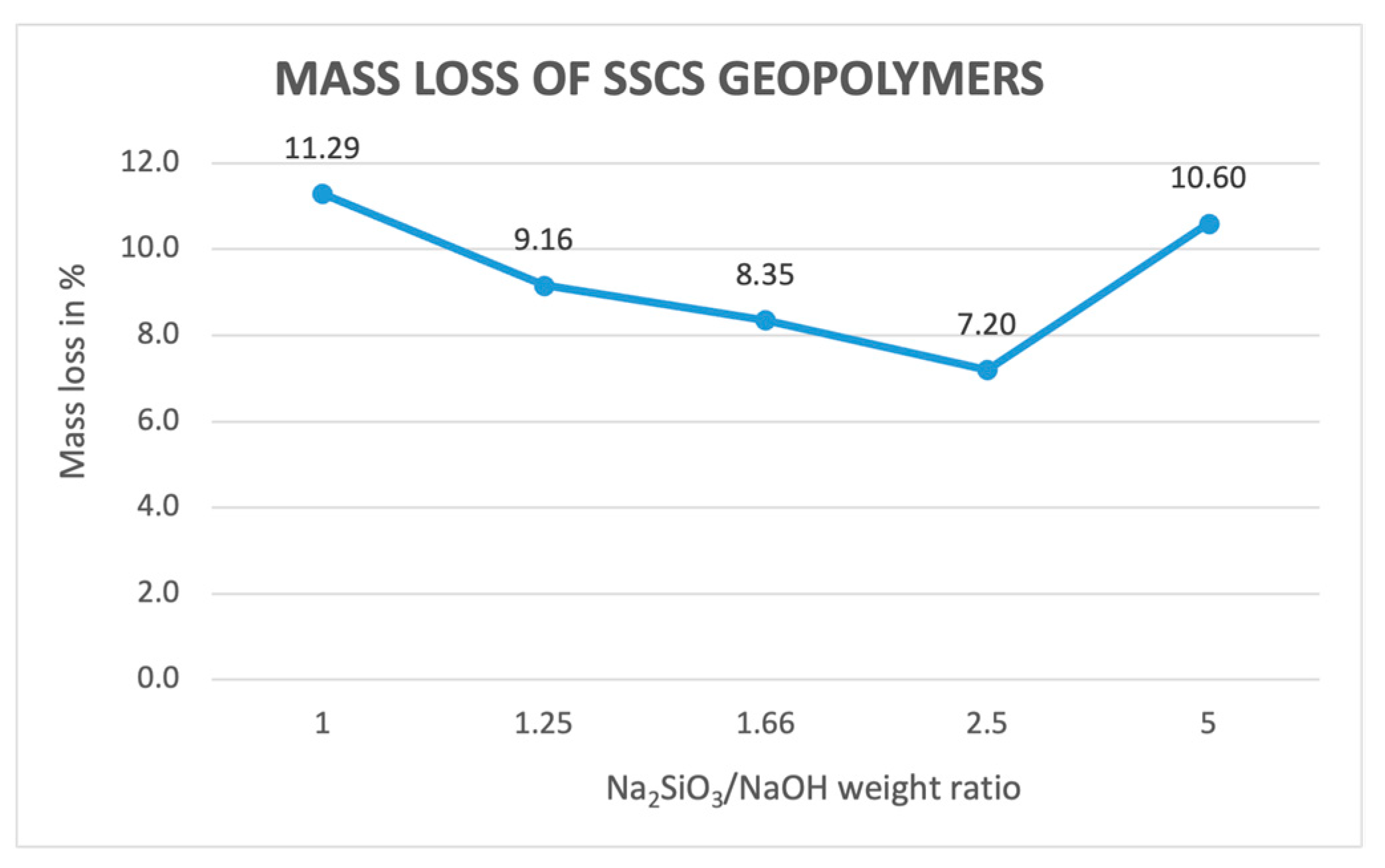
3.2.1. Mass Loss Determination
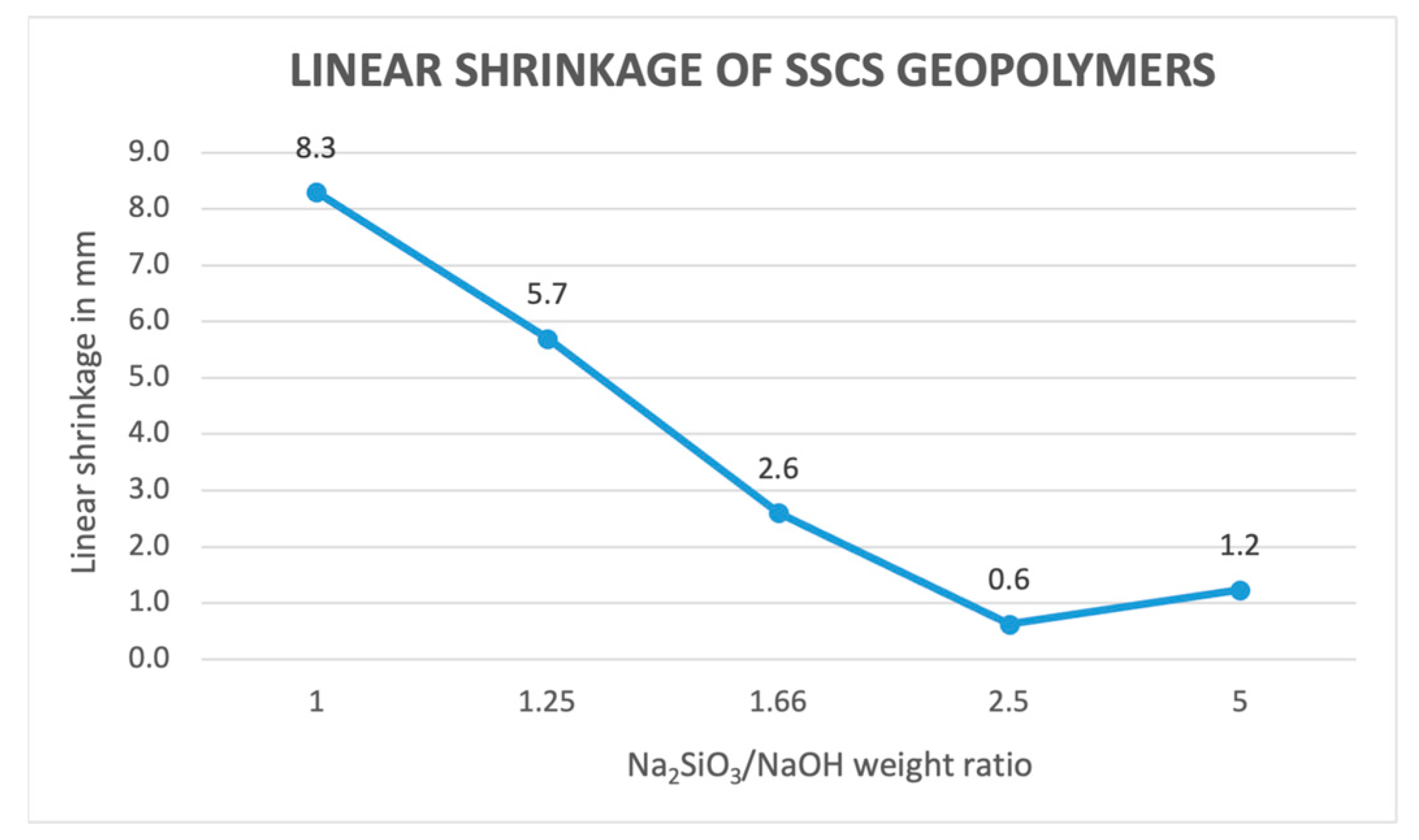
3.2.2. Linear Shrinkage Determination
3.2.3. Capillary Water Absorption Determination
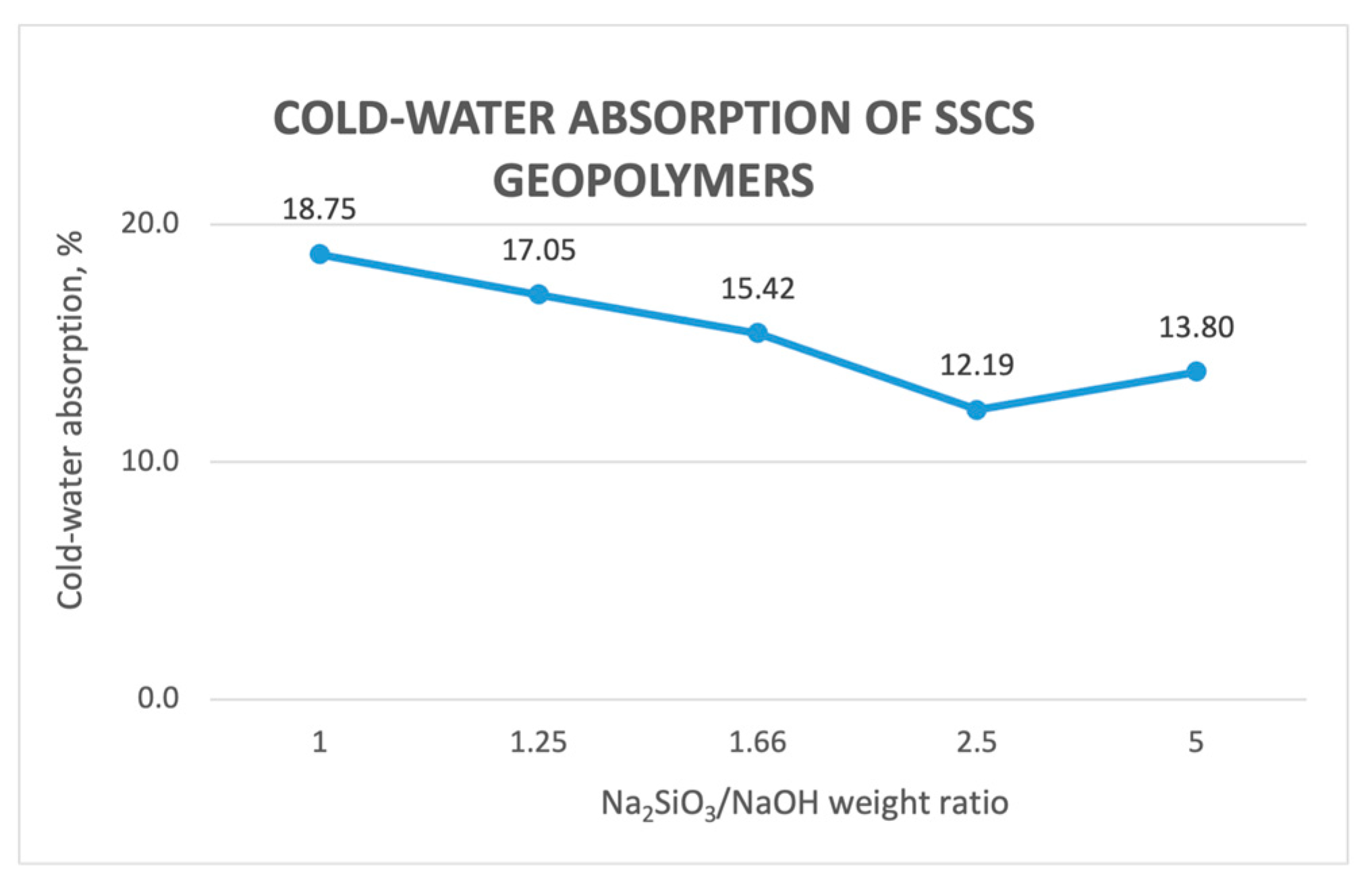
3.2.4. Cold Water Absorption of the SSCS Geopolymers
3.2.5. Open Porosity of the SSCS Geopolymers
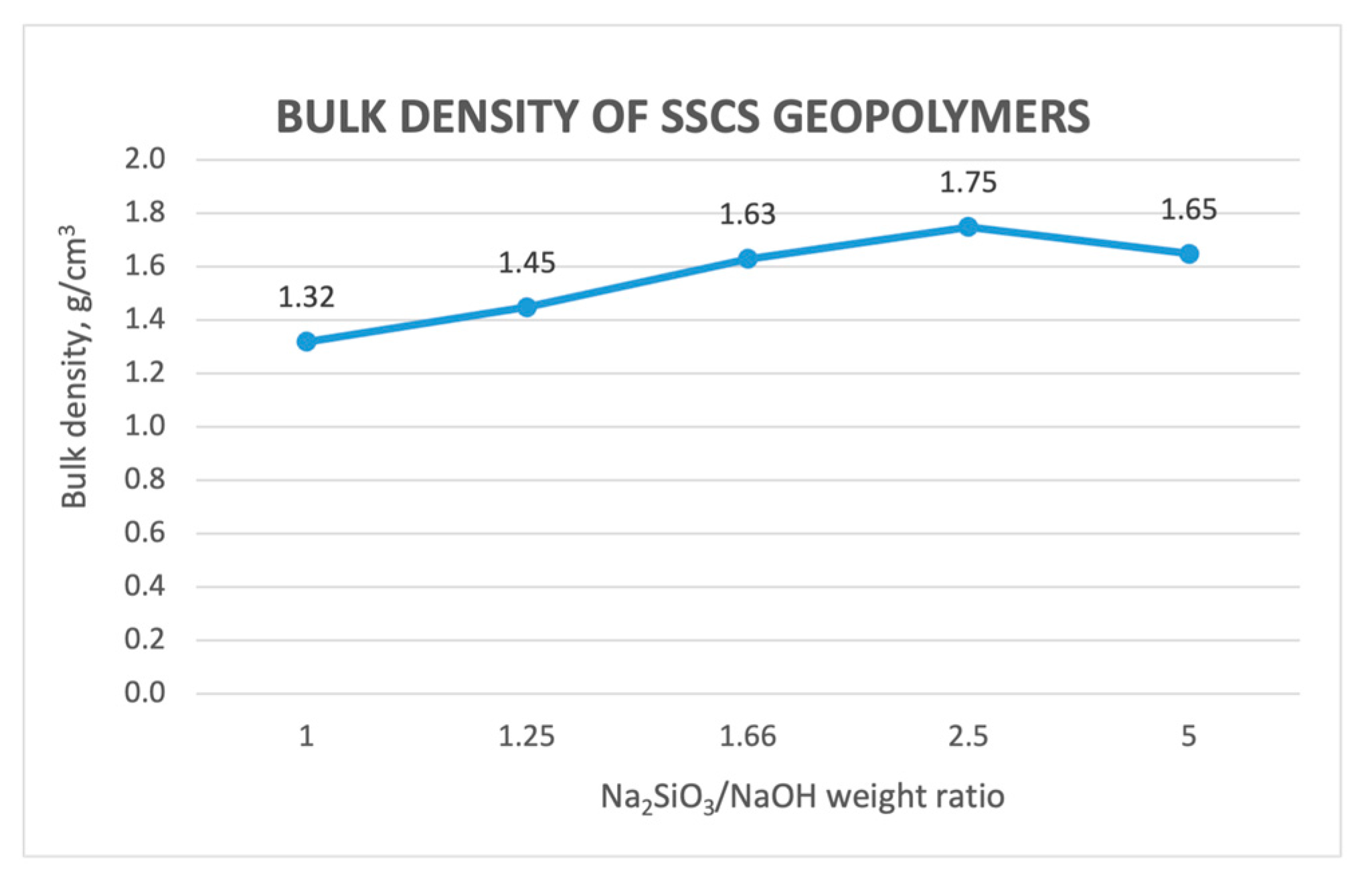
3.2.6. Bulk Density Determination
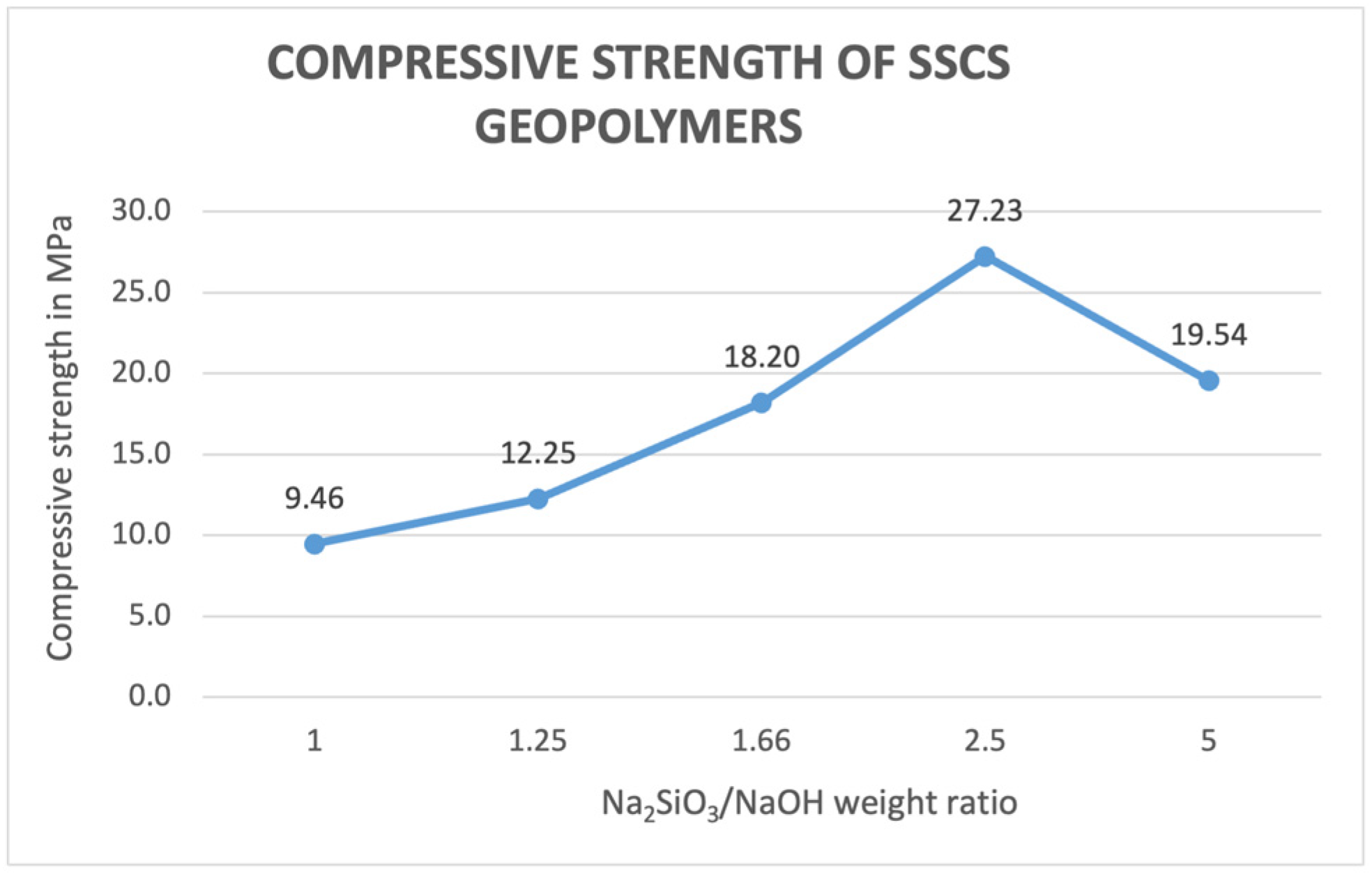
3.2.7. Compression Strength Test Results of the SSCS Geopolymer
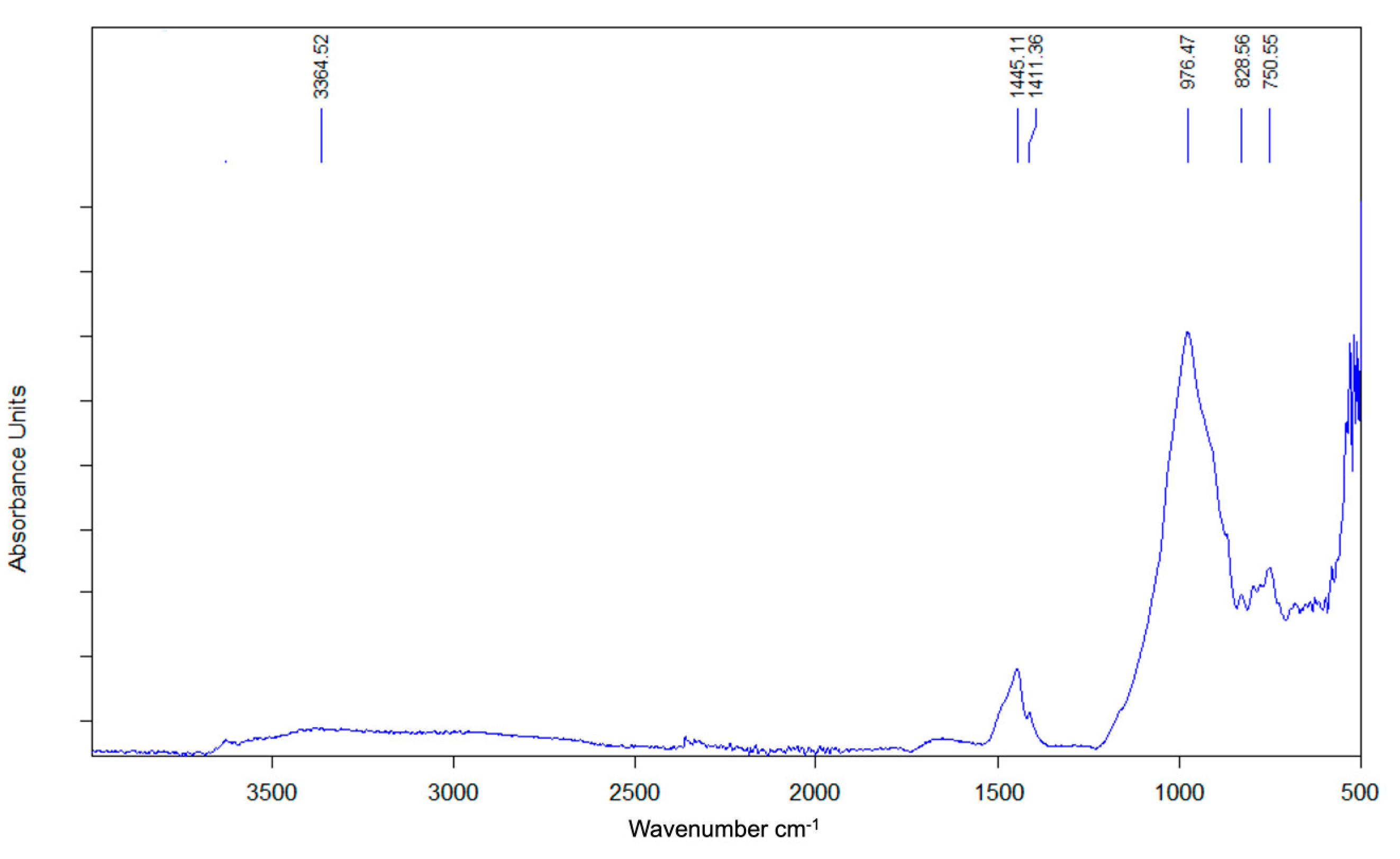
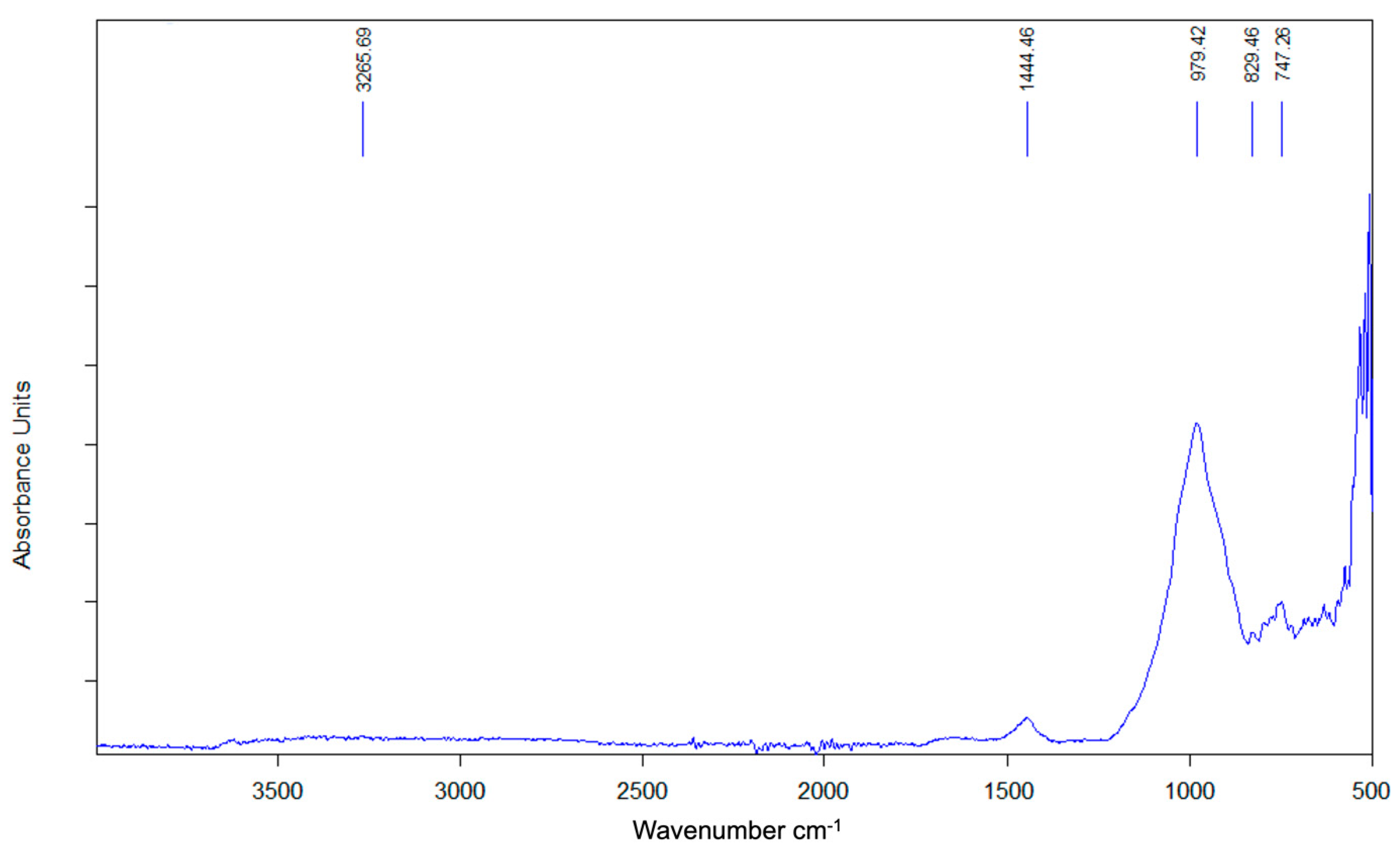
3.2.8. FTIR of the SSCS Geopolymer
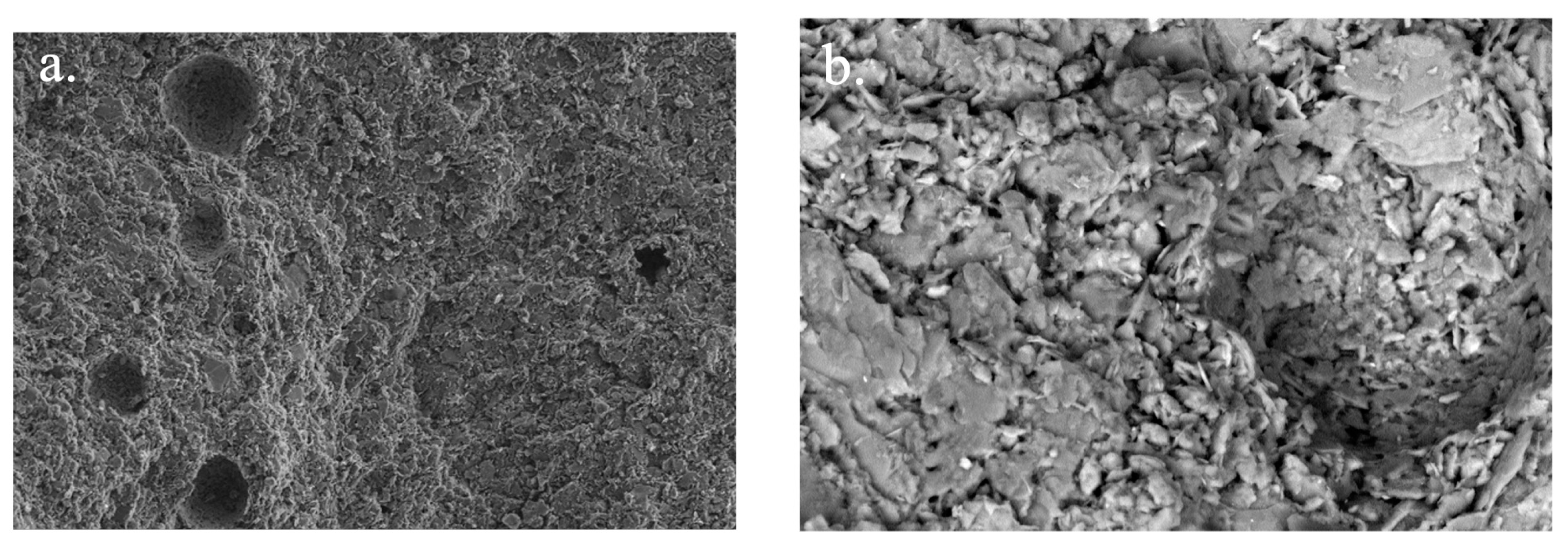
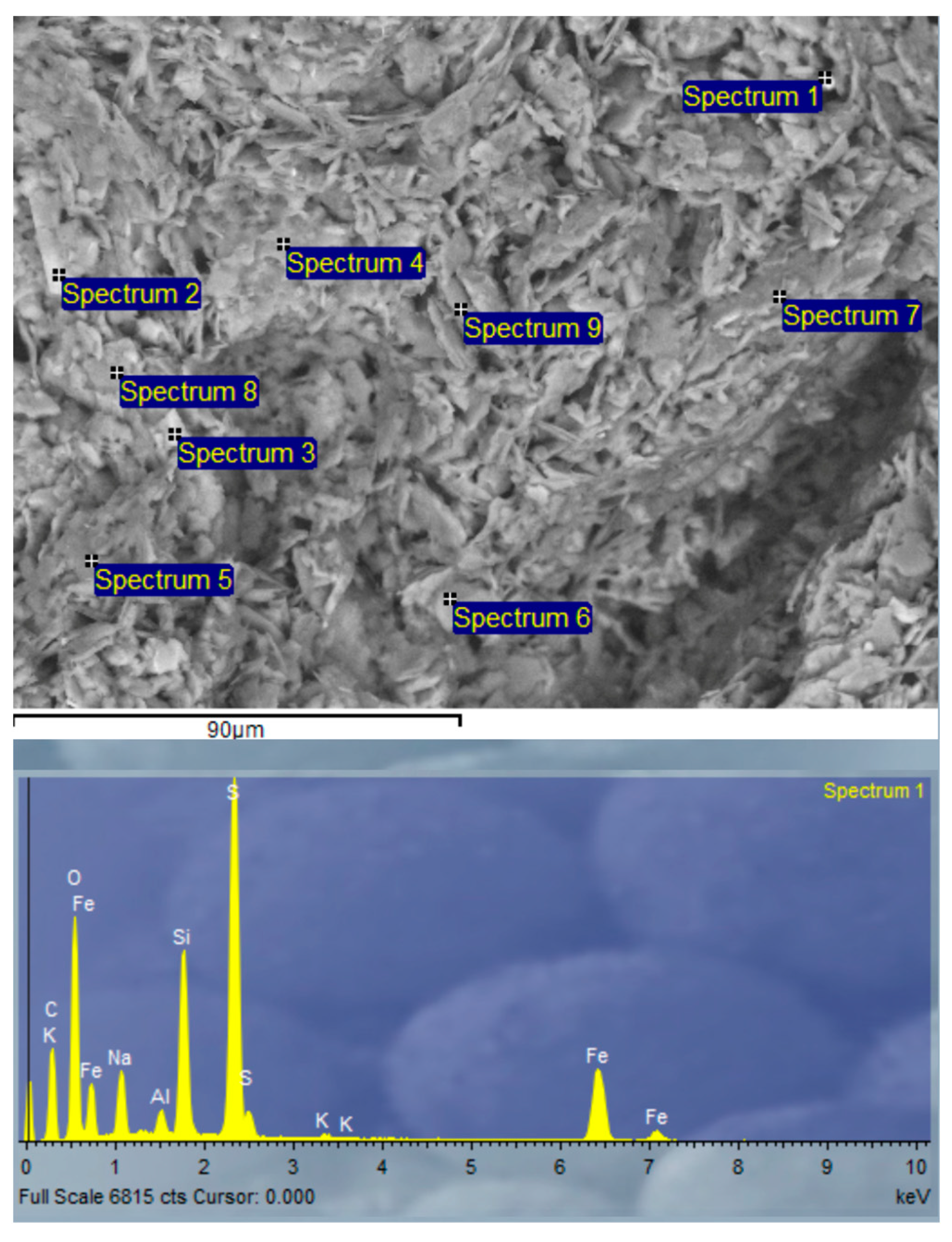
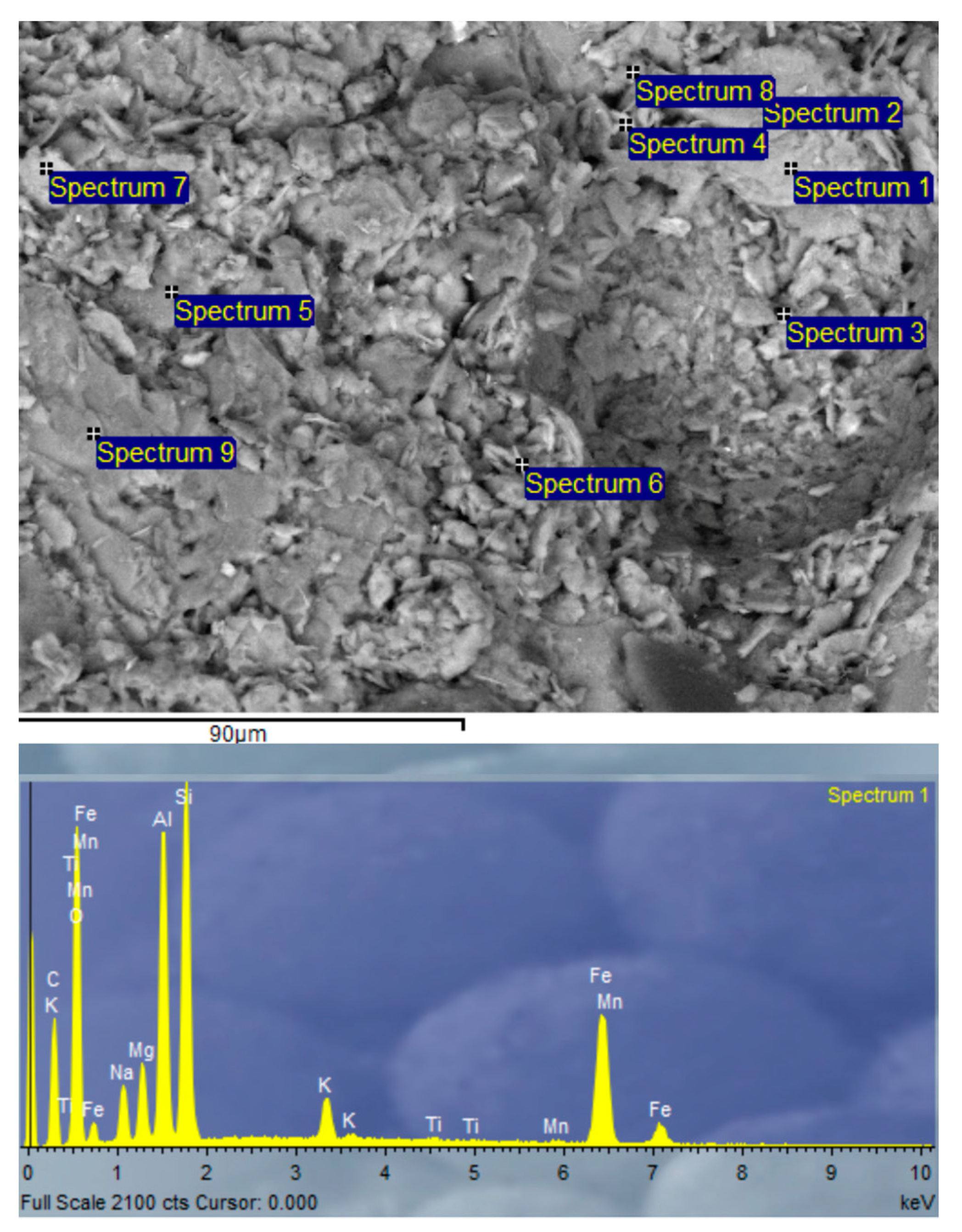
3.2.9. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis of SSCS
4. Conclusions
- The particle size distribution of SSCS shows that this mining sludge is composed of finer particles; therefore, crushing operations are not required for this experiment. This particle size distribution guarantees the geopolymerization because smaller particle size increases the specific surface, and therefore its reactivity.
- The chemical composition of SSCS demonstrated low values of carbon, hydrogen, and nitrogen, as well as no presence of organic material that could jeopardize the geopolymerization process.
- The X-ray fluorescence showed percentages of silica and alumina, 50.75 and 22.18 wt.%, respectively, and low content of carbonates, less than 1.81 wt.%, making this mining raw material a potential source of aluminosilicates.
- SSCS material, with a density of 2.12 g/cm3 and pH value of 8.53, did not show rejection with alkali activator solution during the geopolymer conformation phase.
- After the completion of physical tests performed on the different SSCS geopolymer samples with alkali activator ratios (1, 1.25, 1.66, 2.5, and 5), we observed that the ratio of alkali activation solution constitutes an important role in the final properties of the geopolymer.
- The obtained values of mass loss, linear shrinkage, capillary water absorption, cold water absorption, and open porosity of the different geopolymer samples conformed in the experiment showed that that geopolymers with a lower ratio of alkali solution led to greater values up to a 2.5 ratio. This is caused by a better geopolymerization process, leading to a denser internal structure because of a less interconnection between pores.
- Bulk density and compressive strength values showed that when the Na2SiO3/NaOH ratio increases, both values increase up to a maximum value of 2.5. From this ratio, compressive strength and density tend to decrease due to an excess of alkali activation solution, producing a retardation of the geopolymerization process and leading to a geopolymer that is not fully reacted.
- The compressive strength test served to show that an increase in alkaline activation ratio in the SSCS geopolymers increased the compressive strength of the geopolymer up to a maximum of 2.5 ratio, with a value of 27.23 MPa, after which it decreased. Higher proportions of NaOH in the alkali activator ratio resulted in lower compressive strength values.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Liu, B.; Du, J.; Liu, C.; Wang, S. CO2 Emission Linkage Analysis in Global Construction Sectors: Alarming Trends from 1995 to 2009 and Possible Repercussions. J. Clean. Prod. 2019, 221, 863–877. [Google Scholar] [CrossRef]
- International Energy Agency. 2019 Global Status Report for Buildings and Construction: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector. 2019. Available online: https://www.Iea.Org/Reports/Global-Status-Report-for-Buildings-and-Construction-2019 (accessed on 15 January 2024).
- Carmo, F.F.; Lanchotti, A.O.; Kamino, L.H.Y. Mining Waste Challenges: Environmental Risks of Gigatons of Mud, Dust and Sediment in Megadiverse Regions in Brazil. Sustainability 2020, 12, 8466. [Google Scholar] [CrossRef]
- Carvalho, F.P. Mining Industry and Sustainable Development: Time for Change. Food Energy Secur. 2017, 6, 61–77. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.T.; Yang, W.; Zhang, Y. The Environmental Impact of Mining and Its Countermeasures. MATEC Web Conf. 2016, 63, 04010. [Google Scholar] [CrossRef]
- Khobragade, K. Impact of Mining Activity on Environment: An Overview. Int. J. Sci. Res. Publ. 2020, 10, 784–791. [Google Scholar] [CrossRef]
- Jain, R.K.; Cui, Z.C.; Domen, J.K. Environmental Impacts of Mining. In Environmental Impact of Mining and Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 53–157. ISBN 978-0-12-804040-9. [Google Scholar]
- Ma, M.; Tam, V.W.; Le, K.N.; Butera, A.; Li, W.; Wang, X. Comparative Analysis on International Construction and Demolition Waste Management Policies and Laws for Policy Makers in China. J. Civ. Eng. Manag. 2023, 29, 107–130. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Li, Y.; Wang, H.; Gao, Y.; Sun, Y.; Wang, B.; Bian, R.; Li, W.; Zhan, M. Impact of Incineration Slag Co-Disposed with Municipal Solid Waste on Methane Production and Methanogens Ecology in Landfills. Bioresour. Technol. 2023, 377, 128978. [Google Scholar] [CrossRef] [PubMed]
- Estadística Minera. Ministerio de Transición Ecológica y Reto Demográfico. Available online: https://www.miteco.gob.es/es/energia/mineria-explosivos.html (accessed on 15 December 2023).
- Cárdenes, V.; Rubio, A.; Argandoña, V.G.R.D. Roofing Slate from Bernardos, Spain: A Potential Candidate for Global Heritage Stone. Episodes 2021, 44, 3–9. [Google Scholar] [CrossRef]
- Cluster de La Piedra Natural. Informe Sectorial Piedra Natural 2021. 2022. Available online: https://Clusterpiedra.Com/Wp-Content/Uploads/2022/04/Informe-Sectorial-CLUSTER-PIEDRA-2021.Pdf. (accessed on 4 December 2023).
- Lamghari, Z. Process Mining: Basic Definitions and Concepts. Int. J. Syst. Innov. 2022, 7, 35–45. [Google Scholar] [CrossRef]
- Lee, W.-H.; Lin, K.-L.; Chang, T.-H.; Ding, Y.-C.; Cheng, T.-W. Sustainable Development and Performance Evaluation of Marble-Waste-Based Geopolymer Concrete. Polymers 2020, 12, 1924. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; De Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Recycling of Mine Tailings for the Geopolymers Production: A Systematic Review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Simão, L.; Hotza, D.; Ribeiro, M.J.; Novais, R.M.; Montedo, O.R.K.; Raupp-Pereira, F. Development of New Geopolymers Based on Stone Cutting Waste. Constr. Build. Mater. 2020, 257, 119525. [Google Scholar] [CrossRef]
- Salihoglu, N.K.; Salihoglu, G. Marble Sludge Recycling by Using Geopolymerization Technology. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018019. [Google Scholar] [CrossRef]
- Gier Della Rocca, D.; Santos E Sousa, F.A.; Domingos Ardisson, J.; Peralta, R.A.; Rodríguez-Castellón, E.; Peralta Muniz Moreira, R.D.F. Magnetic Mining Waste Based-Geopolymers Applied to Catalytic Reactions with Ozone. Heliyon 2023, 9, e17097. [Google Scholar] [CrossRef] [PubMed]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Castañón, A.M.; Gómez-Fernández, F.; Corpas-Iglesias, F.A. Retention of Pollutants Elements from Mine Tailings of Lead in Geopolymers for Construction. Materials 2021, 14, 6184. [Google Scholar] [CrossRef] [PubMed]
- Cobîrzan, N.; Muntean, R.; Thalmaier, G.; Felseghi, R.-A. Recycling of Mining Waste in the Production of Masonry Units. Materials 2022, 15, 594. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and Geopolymeric Materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Amin, M.; Elsakhawy, Y.; Abu El-Hassan, K.; Abdelsalam, B.A. Behavior Evaluation of Sustainable High Strength Geopolymer Concrete Based on Fly Ash, Metakaolin, and Slag. Case Stud. Constr. Mater. 2022, 16, e00976. [Google Scholar] [CrossRef]
- Krotov, O.; Gromyko, P.; Gravit, M.; Belyaeva, S.; Sultanov, S. Thermal Conductivity of Geopolymer Concrete with Different Types of Aggregate. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1030, 012018. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sust. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar, S.; Szczotok, A.M.; Rodríguez, J.F.; Valentini, L.; Lanzón, M.; Pamies, R.; Kjøniksen, A.-L. Effect of Freeze-Thaw Cycles on the Mechanical Behavior of Geopolymer Concrete and Portland Cement Concrete Containing Micro-Encapsulated Phase Change Materials. Constr. Build. Mater. 2019, 200, 94–103. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Pang, B.; Zheng, H.; Jin, Z.; Hou, D.; Zhang, Y.; Song, X.; Sun, Y.; Liu, Z.; She, W.; Yang, L.; et al. Inner Superhydrophobic Materials Based on Waste Fly Ash: Microstructural Morphology of Microetching Effects. Compos. Part B Eng. 2024, 268, 111089. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; Van Deventer, J.S.J. The Effect of Alkali and Si/Al Ratio on the Development of Mechanical Properties of Metakaolin-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Palhares, L.B.; Dos Santos, C.G.; Binda, F.; Hunter, T.N. Characterization of Slate Powder Wastes from Minas Gerais—Brazil. Key Eng. Mater. 2020, 848, 10–19. [Google Scholar] [CrossRef]
- Claudio, F.-D.M.; Reyes, A.; Samuel, R.; Noguel, O.; José, A. Caracterización preliminar de las pizarras del depósito Tchihingue (Angola) con fines de uso como roca industrial. Min. Geol. 2020, 36, 253–267. [Google Scholar]
- Astariani, N.K.; Salain, I.M.A.K.; Sutarja, I.N.; Widiarsa, I.B.R. Setting Time of Geopolymer Binder Based on Umeanyar Slate Stone Powder. IOP Conf. Ser. Earth Environ. Sci. 2021, 871, 012002. [Google Scholar] [CrossRef]
- Marta, G.-G. Reciclado de lodos de Pizarra y Granito para la Fabricación de Cerámicos Tradicionales de Interés en el Sector de los Materiales de Construcción. Doctoral Dissertation, University of Extremadura, Badajoz, Spain, 2015. [Google Scholar]
- García, F. La Industria de la Pizarra en España. Universidad de Vigo, Vigo. 1997. Available online: http://webs.Uvigo.Es/Bastante/PDF/INDUSTRIA.Pdf (accessed on 5 December 2023).
- Soria, A.B. Manual de Rocas Ornamentales: Prospección, Explotación, Elaboración y Colocación; Jimeno, C.L., Ed.; Entorno Gráfico: Madrid, Spain, 1995; ISBN 84-605-2681-X. [Google Scholar]
- UNE-EN 10381-1:2007; Soil Quality—Sampling—Part 1: Guidance on the Design of Sampling Programmes. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0039566 (accessed on 6 November 2023).
- Cong, P.; Cheng, Y. Advances in Geopolymer Materials: A Comprehensive Review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Jiang, X.; Li, W.; Zhu, X.; Huang, B. Effect of Particle Size and Curing Temperature on Mechanical and Microstructural Properties of Waste Glass-Slag-Based and Waste Glass-Fly Ash-Based Geopolymers. J. Clean. Prod. 2020, 273, 122970. [Google Scholar] [CrossRef]
- Neupane, K.; Hadigheh, S.A. Sodium Hydroxide-Free Geopolymer Binder for Prestressed Concrete Applications. Constr. Build. Mater. 2021, 293, 123397. [Google Scholar] [CrossRef]
- Kai, M.-F.; Dai, J.-G. Understanding Geopolymer Binder-Aggregate Interfacial Characteristics at Molecular Level. Cem. Concr. Res. 2021, 149, 106582. [Google Scholar] [CrossRef]
- Dong, T.; Sun, T.; Xu, F.; Ouyang, G.; Wang, H.; Yang, F.; Wang, Z. Effect of Solid Sodium Silicate on Workability, Hydration and Strength of Alkali-Activated GGBS/Fly Ash Paste. Coatings 2023, 13, 696. [Google Scholar] [CrossRef]
- UNE-EN 13581:2003; Products and Systems for the Protection and Repair of Concrete Structures—Test Method—Determination of Loss of Mass of Hydrophobic Impregnated Concrete after Freeze-Thaw Salt Stress. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0028760 (accessed on 17 January 2024).
- UNE-EN 13872:2004; Method of Test for Smoothing and/or Levelling Compounds—Determination of Shrinkage. Asociación Española de Normalización (UNE). Available online: https://tienda.aenor.com/norma-une-en-13872-2004-n0031776 (accessed on 16 January 2024).
- UNE-EN 1015-18:2003; Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0029220 (accessed on 16 January 2024).
- UNE-EN 1015-10:2000; Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0022409 (accessed on 16 January 2024).
- UNE-EN 1015-11:2020; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0063703 (accessed on 17 January 2024).
- De Oliveira, L.B.; De Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of Geopolymers with Industrial Waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- França, S.; Sousa, L.N.; Silva, M.V.D.M.S.; Borges, P.H.R.; Bezerra, A.C.D.S. Preliminary Reactivity Test for Precursors of Alkali-Activated Materials. Buildings 2023, 13, 693. [Google Scholar] [CrossRef]
- UNE-EN 933-1:2012; Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0049638 (accessed on 7 January 2024).
- UNE-EN 1097-07:2009; Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0042553 (accessed on 15 January 2024).
- UNE-EN 10390:2022; Soil, Treated Biowaste and Sludge—Determination of pH (ISO 10390:2021). Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0068663 (accessed on 16 January 2024).
- Mermerdaş, K.; Algın, Z.; Ekmen, Ş. Experimental Assessment and Optimization of Mix Parameters of Fly Ash-Based Lightweight Geopolymer Mortar with Respect to Shrinkage and Strength. J. Build. Eng. 2020, 31, 101351. [Google Scholar] [CrossRef]
- Bhagath Singh, G.V.P.; Subramaniam, K.V.L. Evaluation of Sodium Content and Sodium Hydroxide Molarity on Compressive Strength of Alkali Activated Low-Calcium Fly Ash. Cem. Concr. Compos. 2017, 81, 122–132. [Google Scholar] [CrossRef]
- Srinivasa Reddy, V.; Vamsi Krishna, K.; Seshagiri Rao, M.V.; Shrihari, S. Effect of Molarity of Sodium Hydroxide and Molar Ratio of Alkaline Activator Solution on the Strength Development of Geopolymer Concrete. E3S Web Conf. 2021, 309, 01058. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H.; Cheah, C.B. Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer. Materials 2021, 14, 4253. [Google Scholar] [CrossRef]
- Szabó, R.; Mucsi, G. Effect of SiO2, Al2O3 and Na2O Content and Fly Ash Fineness on the Structure and Mechanical Properties of Fly Ash Based Geopolymer. Recycl. Sustain. Dev. 2019, 12, 61–68. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Dinh, H.L.; Ong, D.E.L.; Zi, G.; You, I. Effect of Si/Al Molar Ratio on the Strength Behavior of Geopolymer Derived from Various Industrial Waste: A Current State of the Art Review. Constr. Build. Mater. 2022, 329, 127134. [Google Scholar] [CrossRef]
- UNE-EN 80220:2012; Cement Test Methods. Chemical Analysis. Moisture Determination. Asociación Española de Normalización (UNE). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0023281 (accessed on 16 January 2024).
- Nath, S.K.; Kumar, S. Reaction Kinetics of Fly Ash Geopolymerization: Role of Particle Size Controlled by Using Ball Mill. Adv. Powder Technol. 2019, 30, 1079–1088. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Chemistry & Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; ISBN 978-2-9544531-1-8. [Google Scholar]
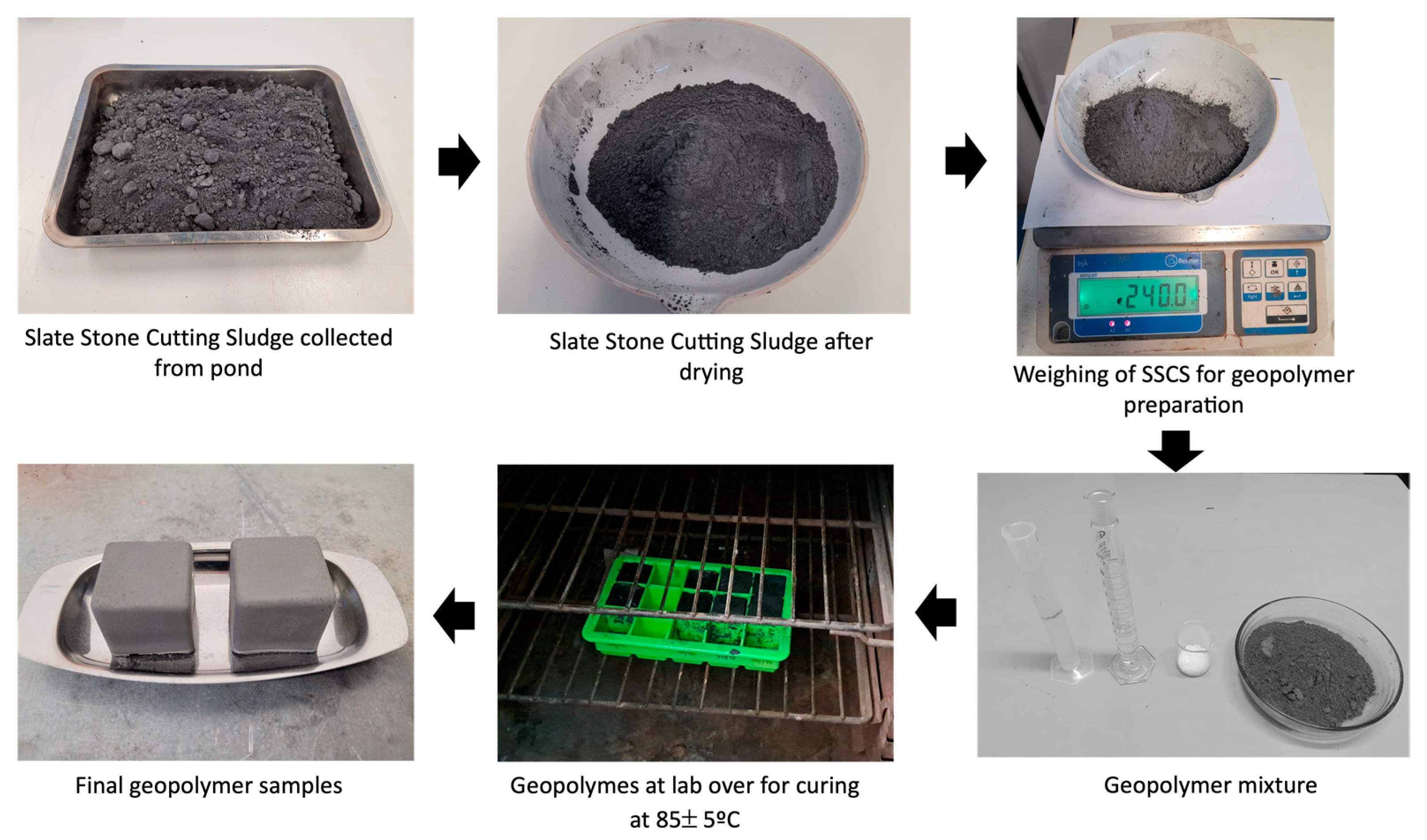







| Sample Series Code | Solid (g) | Alkaline Activator Solution (g) | Na2SiO3/NaOH Weight Ratio |
|---|---|---|---|
| G50/10 | 240 | 192 | 5.00 |
| G50/20 | 240 | 168 | 2.50 |
| G50/30 | 240 | 144 | 1.66 |
| G50/40 | 240 | 120 | 1.25 |
| G50/50 | 240 | 96 | 1.00 |
| Sample | Nitrogen, % | Carbon, % | Hydrogen, % |
|---|---|---|---|
| SSCS * | 0.104 ± 0.003 | 1.003 ± 0.057 | 0.337 ± 0.002 |
| Element | Wt.% | Est. Error |
|---|---|---|
| SiO2 | 50.75 | 0.38 |
| Al2O3 | 22.18 | 0.18 |
| CaO | 0.456 | 0.03 |
| Fe2O3 | 10.68 | 0.22 |
| K2O | 4.63 | 0.17 |
| MgO | 2.68 | 0.18 |
| TiO2 | 1.28 | 1.28 |
| Na2O | 1.24 | 0.12 |
| SO3 | 0.50 | 0.03 |
| P2O5 | 0.28 | 0.01 |
| LOI 1 | 5.02 | - |
| Phase | Formula | wt.% |
|---|---|---|
| Q: Quartz | SiO2 | 16.5 ± 0.5 |
| M: Muscovite | Kal2 (AlSi3O10)(OH)2 | 25.4 ± 0.8 |
| C: Chlorite | (Mg,Al)6(Si,Al)4º10(OH)8 | 26.7 ± 0.9 |
| A: Albite | NaAlSiO3 | 7.8 ± 0.6 |
| Sample | pH | Temp. (°C) |
|---|---|---|
| SSCS 1 | 8.55 | 24.8 |
| SSCS 2 | 8.53 | 24.5 |
| SSCS 3 | 8.51 | 24.4 |
| Average | 8.53 | 24.5 |
| Sample | WM (%) |
|---|---|
| SSCS 1 | 0.89 |
| SSCS 2 | 0.93 |
| SSCS 3 | 0.87 |
| Function Group | Wavenumber Range (cm−1) | FTIR Peaks (cm−1) | ||
|---|---|---|---|---|
| Alkaline activator ratio | 1.25 | 1.66 | 2.50 | |
| Stretching vibration O-H | 3300–3700 | 3370 | 3364 | 3265 |
| Bending vibration H-O-H | 1622–1658 | 1654 | 1652 | 1654 |
| Asymmetric stretching vibration O-C-O | 1432–1412 | 1442 | 1445 | 1444 |
| Asymmetric stretching vibration Si-O-T | 1200–830 | 827 | 826 | 829 |
| Bending symmetric stretching vibration Si-O-Si | 800–700 | 750 | 747 | 750 |
| Bending symmetrical Si-O of SiO4 | 694 | 670 | 672 | 672 |
| Bending vibration Si-O-Si | 400–500 | 467 | 468 | 464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo Beltrán, R.; Picazo Camilo, E.; Perea Toledo, G.; Corpas Iglesias, F.A. Towards a Sustainable Mining: Reuse of Slate Stone Cutting Sludges for New Geopolymer Binders. Sustainability 2024, 16, 3322. https://doi.org/10.3390/su16083322
Carrillo Beltrán R, Picazo Camilo E, Perea Toledo G, Corpas Iglesias FA. Towards a Sustainable Mining: Reuse of Slate Stone Cutting Sludges for New Geopolymer Binders. Sustainability. 2024; 16(8):3322. https://doi.org/10.3390/su16083322
Chicago/Turabian StyleCarrillo Beltrán, Raúl, Elena Picazo Camilo, Griselda Perea Toledo, and Francisco Antonio Corpas Iglesias. 2024. "Towards a Sustainable Mining: Reuse of Slate Stone Cutting Sludges for New Geopolymer Binders" Sustainability 16, no. 8: 3322. https://doi.org/10.3390/su16083322
APA StyleCarrillo Beltrán, R., Picazo Camilo, E., Perea Toledo, G., & Corpas Iglesias, F. A. (2024). Towards a Sustainable Mining: Reuse of Slate Stone Cutting Sludges for New Geopolymer Binders. Sustainability, 16(8), 3322. https://doi.org/10.3390/su16083322







