An Approach to CO2 Emission Reduction in the Iron and Steel Industry: Research Status and Development Trends of Integrated Absorption-Mineralization Technologies
Abstract
:1. Introductory
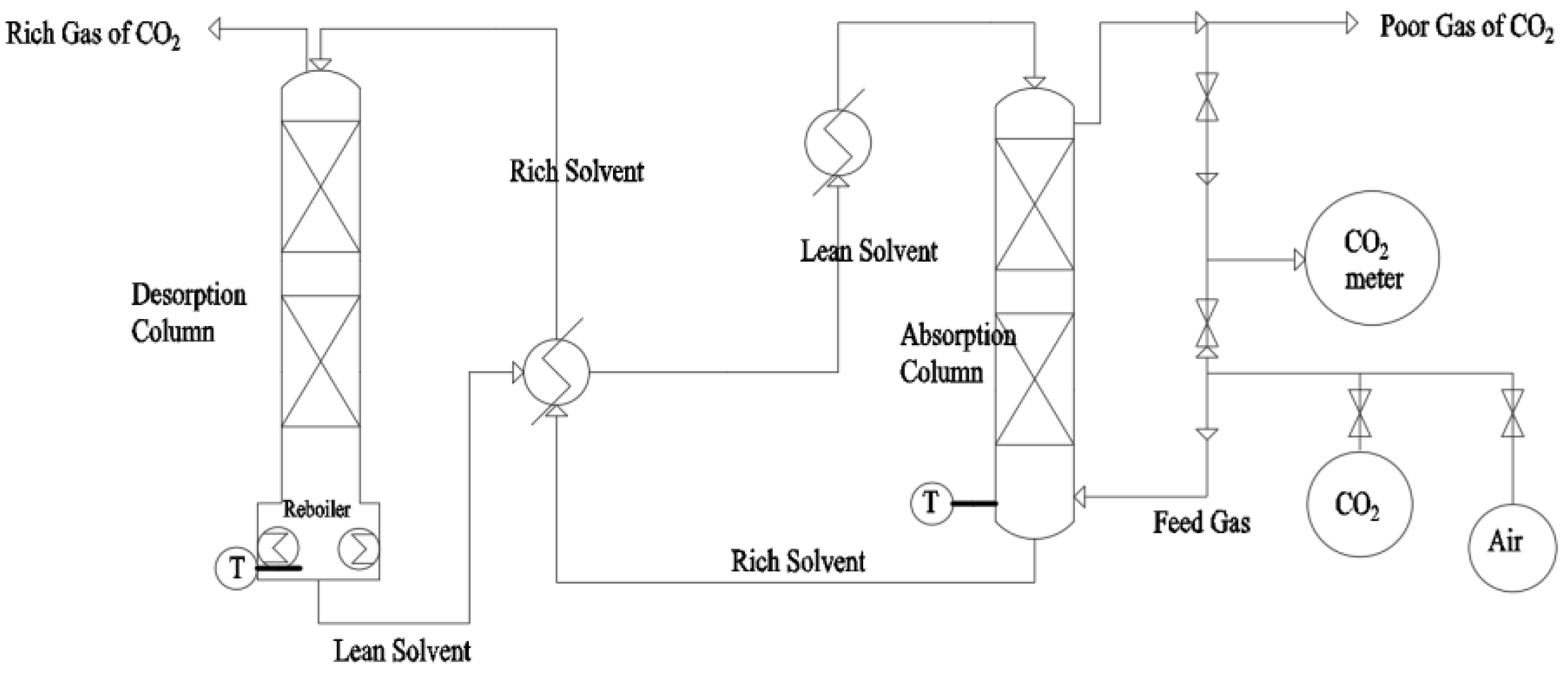
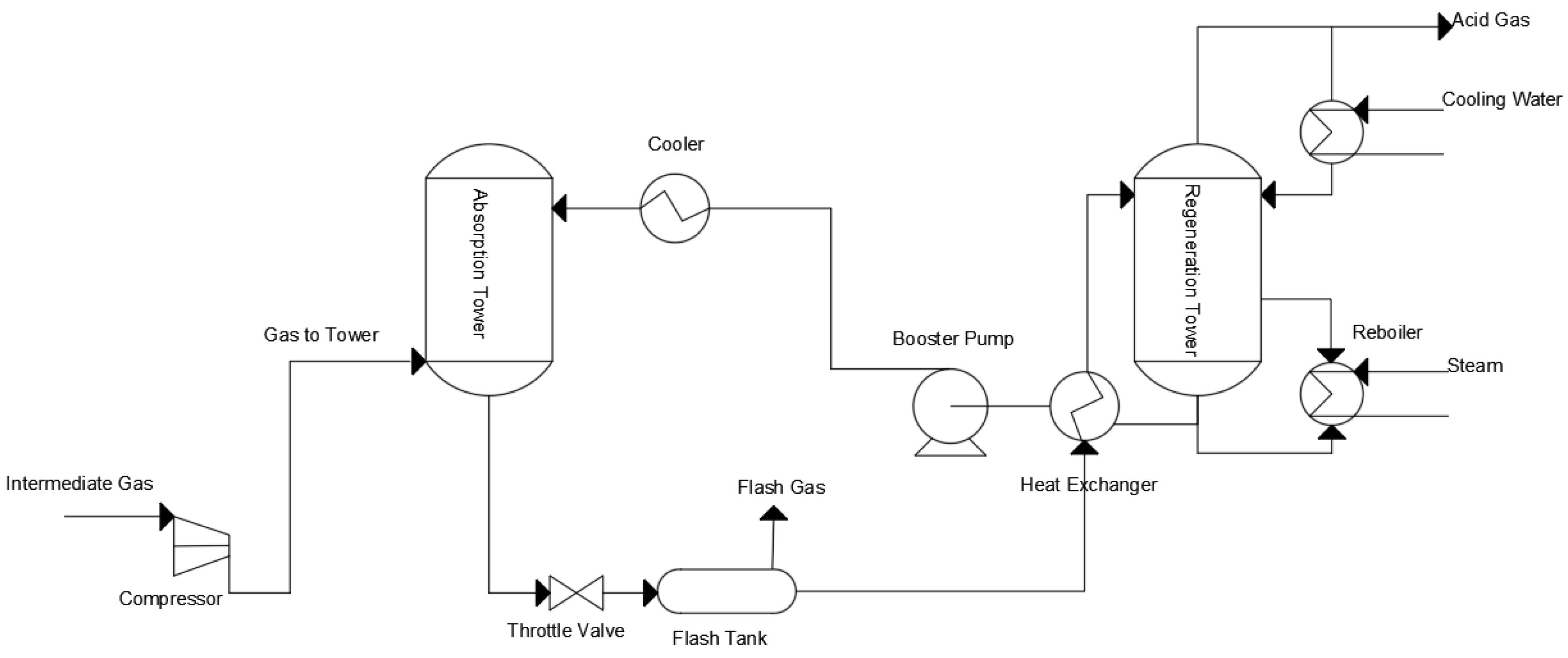
2. Status of CO2 Absorbents Research
2.1. Monoamine CO2 Absorbents
2.2. Mixed Amine CO2 Absorbents
| Absorbents | Regeneration Energy (GJ/t CO2) | Viscosity/cp | CO2 Absorption/ (mol/kg) | ||
|---|---|---|---|---|---|
| Name | Distribution Ratio | ||||
| Activated mixed amines absorbents | DEEA-MAPA [12] | 2 mol/L MAPA + 5 mol/L DEEA | 0.75 | 2.4 | / |
| BDA-DEEA [13] | 4 mol/L DEEA + 2 mol/L BDA | 0.505 | / | / | |
| Synergistic mixed amines absorbents | DEEA-HMDA [20] | V(DMSO):V(PMDETA) = 1:4 | / | 0.841 | |
| DEEA-AEEA [27] | 25 wt.% + AEEA25 wt.%DEEA+ | 0.64 | 2.58 | / | |
| DETA-DEEA [30] | 3 mol/L DEEA + 2 mol/L DETA | 1.94 | 2.14 | 13.5 | |
| Functional mixed amines absorbents | DMSO-PMDETA [34] | V(DMSO):V(PMDETA) = 4:6 | 2.5 | 0.97 | 13.92 |
| MEA-1-propanol-H2O [37] | V(MEA):V(1-propanol):V(H2O) = 3:4:3 | 2.4 | 2.4 | 16 | |
| MEA-Sulfolane [45] | M(MEA):M(Sulfolane) = 5:4 | 2.67 | 2.67 | 8 | |
| DETA-1-propanol-H2O [52] | 30 wt%DETA + 50 wt%1-propanol | 2.12 | 2.12 | 8 | |
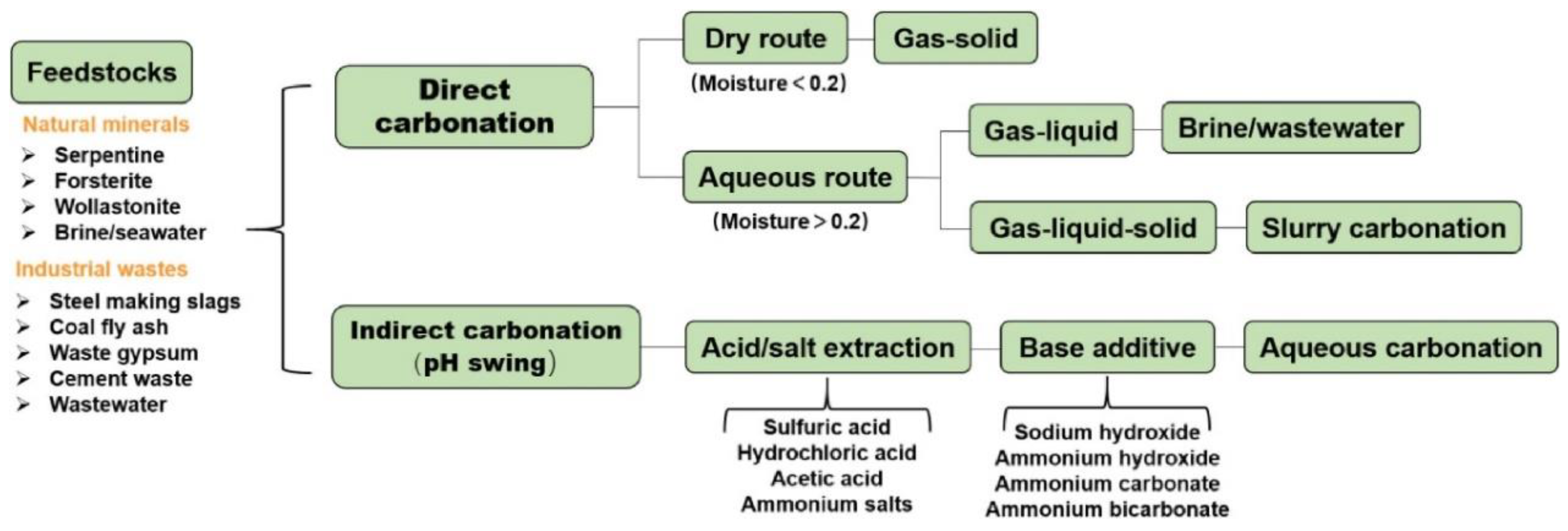
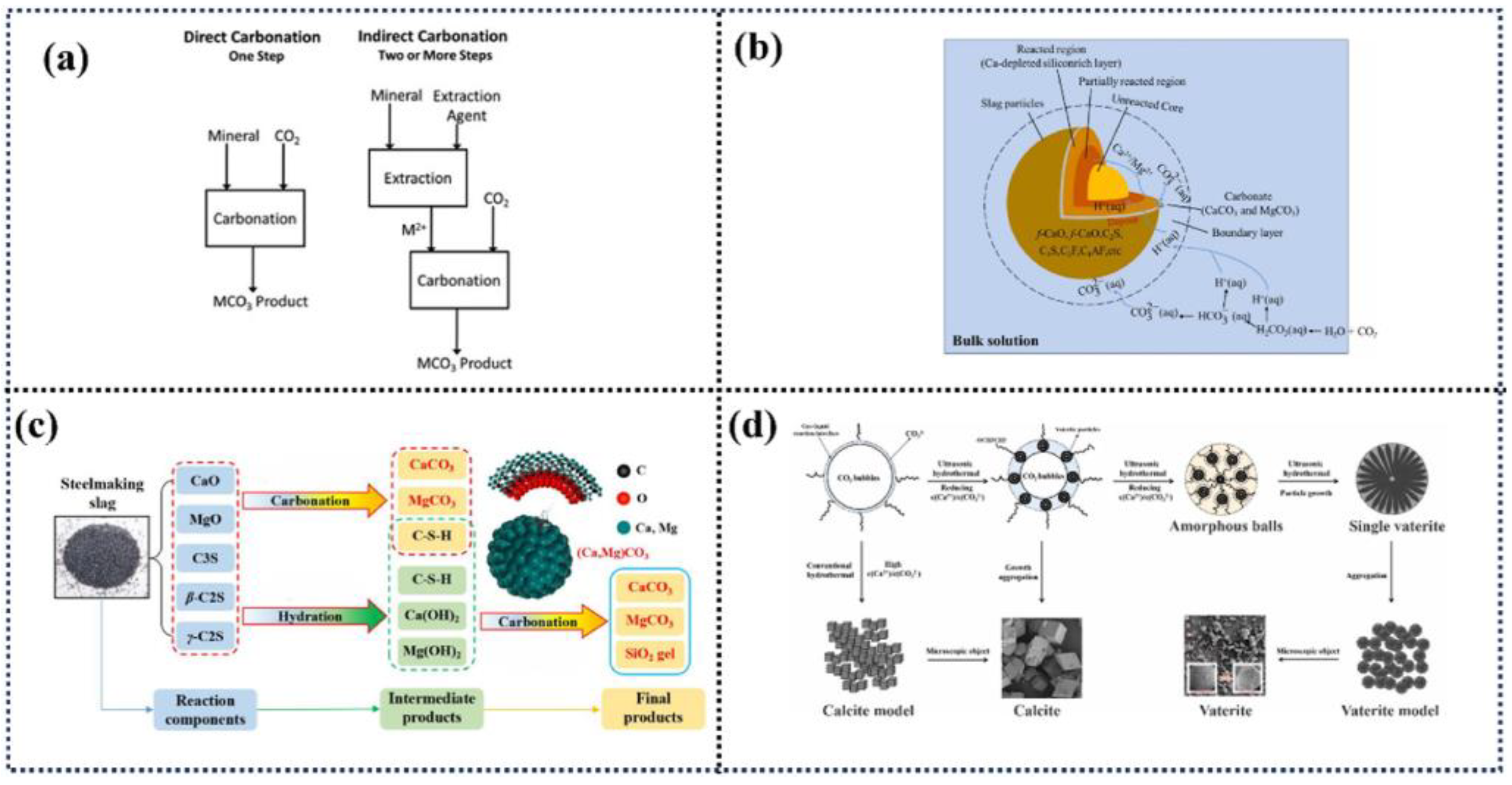
3. Current Status of CO2 Mineralization Research
3.1. Direct Mineralization
3.2. Indirect Mineralization
3.3. Multi-Scale Theoretical Calculation Methods for Studying Mineralization Mechanisms
- Electronic Scale Calculation: Represented by first-principles calculations based on fundamental principles of quantum mechanics, methods like Density Functional Theory (DFT) are employed to accurately describe the electronic structure and microscopic interactions of the system. This level primarily focuses on the microscopic features of electronic energy levels, electron density distributions, and chemical bonding.
- Atomic/Molecular Scale Simulation: This includes Ab Initio Molecular Dynamics and Classical Molecular Dynamics. Through tracking atomic/molecular trajectories, this level reveals the dynamic processes at the atomic/molecular level, including atomic/molecular migration as well as local structural evolution.
- Mesoscopic and Macroscopic Scale Simulation: Utilizing methods such as coarse-grained molecular dynamics or dissipative particle dynamics, this level studies the overall behavior of larger-scale systems. It explores thermodynamic properties and structural evolution at the macroscopic scale.
4. Feasibility Analysis of CO2 Absorption-Mineralization for Steel Industry Applications
5. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, J.L.; Lv, W.Q. Crop Production and Agricultural Carbon Emissions: Relationship Diagnosis and Decomposition Analysis. Int. J. Environ. Res. Public Health 2021, 18, 8219. [Google Scholar] [CrossRef]
- Aponte, C.; Kasel, S.; Nitschke, C.R.; Tanase, M.A.; Vickers, H.; Parker, L.; Fedrigo, M.; Kohout, M.; Ruiz-Benito, P.; Zavala, M.A.; et al. Structural diversity underpins carbon storage in Australian temperate forests. Glob. Ecol. Biogeogr. 2020, 29, 789–802. [Google Scholar] [CrossRef]
- Ryan, N.A.; Miller, S.A.; Skerlos, S.J.; Cooper, D.R. Reducing CO2 Emissions from US Steel Consumption by 70% by 2050. Environ. Sci. Technol. 2020, 54, 14598–14608. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Rehman, Z.U.; Ghasem, N.; Al-Marzouqi, M.; Abdullatif, N.; Nakhjiri, A.T.; Ghadiri, M.; Rezakazemi, M.; Marjani, A.; Pishnamazi, M.; et al. Intensification of CO2 absorption using MDEA-based nanofluid in a hollow fibre membrane contactor. Sci. Rep. 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, H.; Mashhadimoslem, H.; Ghaemi, A. Modeling and optimization of CO2 mass transfer flux into Pz-KOH-CO2 system using RSM and ANN. Sci. Rep. 2023, 13, 4011. [Google Scholar] [CrossRef]
- Chen, X.Y.; Cui, Y.K.; Wang, S.J. Corrosion Behavior of Carbon Steel in Diethylenetriamine Solution for Post-combustion CO2 Capture. ACS Omega 2024, 9, 13067–13080. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, P.; Lin, Y.; Du, H.; Liu, H.; Zhu, W.; Ren, H. Fight for carbon neutrality with state-of-the-art negative carbon emission technologies. Eco-Environ. Health 2022, 1, 259–279. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zheng, Y.; Li, G.L.; Gao, J.J.; Li, R.; Yue, T. Research progress on phase change absorbents for CO2 capture in industrial flue gas: Principles and application prospects. Sep. Purif. Technol. 2025, 354, 129296. [Google Scholar] [CrossRef]
- Ghani, S.M.M.; Rabat, N.E.; Rahim, A.R.A.; Johari, K.; Siyal, A.A.; Kumeresen, R. Amine Infused Fly Ash Grafted Acrylic Acid/Acrylamide Hydrogel for Carbon Dioxide (CO2) Adsorption and Its Kinetic Analysis. Gels 2023, 9, 229. [Google Scholar] [CrossRef]
- Kadirkhan, F.; Goh, P.S.; Ismail, A.F.; Mustapa, W.; Halim, M.H.M.; Soh, W.K.; Yeo, S.Y. Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications-A Review. Membranes 2022, 12, 71. [Google Scholar] [CrossRef]
- Zhou, X.W.; Shen, Y.; Liu, F.; Ye, J.X.; Wang, X.Y.; Zhao, J.K.; Zhang, S.A.; Wang, L.D.; Li, S.J.; Chen, J.M. A Novel Dual-Stage Phase Separation Process for CO2 Absorption into a Biphasic Solvent with Low Energy Penalty. Environ. Sci. Technol. 2021, 55, 15313–15322. [Google Scholar] [CrossRef]
- Xu, Z.C.; Wang, S.J.; Chen, C.H. CO2 absorption by biphasic solvents: Mixtures of 1,4-Butanediamine and 2-(Diethylamino)-ethanol. Int. J. Greenh. Gas Control 2013, 16, 107–115. [Google Scholar] [CrossRef]
- Ribeiro, F.R.C.; Modolo, R.C.E.; Kulakowski, M.P.; Brehm, F.A.; Moraes, C.A.M.; Ferreira, V.M.; Mesquita, E.F.T.; de Azevedo, A.R.G.; Monteiro, S.N. Production of Belite Based Clinker from Ornamental Stone Processing Sludge and Calcium Carbonate Sludge with Lower CO2 Emissions. Materials 2022, 15, 2352. [Google Scholar] [CrossRef] [PubMed]
- Fones, E.M.; Colman, D.R.; Kraus, E.A.; Nothaft, D.B.; Poudel, S.; Rempfert, K.R.; Spear, J.R.; Templeton, A.S.; Boyd, E.S. Physiological adaptations to serpentinization in the Samail Ophiolite, Oman. ISME J. 2019, 13, 1750–1762. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wang, H.Y.; Zhou, B.Y.; Gao, H.; Lin, Y.H. Resistance of Soda Residue-Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials 2021, 14, 785. [Google Scholar] [CrossRef]
- Ozkan, M.; Narappa, A.B.; Namboodiri, T.; Chai, Y.J.; Babu, M.; Jennings, J.S.E.; Gao, Y.F.; Tasneem, S.; Lam, J.; Talluri, K.R.; et al. Forging a sustainable sky: Unveiling the pillars of aviation e-fuel production for carbon emission circularity. iScience 2024, 27, 109154. [Google Scholar] [CrossRef]
- Jia, K.G.; Zeng, S.J.; Li, G.L.; Liu, W.; Bai, Y.G.; Zhang, X.P.; Wang, T.; Fang, M.X. Impurities effect on CO2 capture from flue gas by energy-efficient diazole-functionalized ionic liquid solvents. Sep. Purif. Technol. 2025, 358, 130270. [Google Scholar] [CrossRef]
- Shirazizadeh, H.A.; Haghtalab, A. Measurement and modeling of CO2 solubility in binary aqueous DMSO and MDEA and their ternary mixtures at different temperatures and compositions. Fluid Phase Equilib. 2021, 528, 112845. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. A Comparative Study of the CO2 Absorption in Some Solvent-Free Alkanolamines and in Aqueous Monoethanolamine (MEA). Environ. Sci. Technol. 2016, 50, 7239–7246. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Mumford, K.; Stevens, G.W.; Fei, W.; Wang, Y. Recent advances in carbon dioxide capture and utilization with amines and ionic liquids. Green Chem. Eng. 2020, 1, 16–32. [Google Scholar] [CrossRef]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon Dioxide Postcombustion Capture: A Novel Screening Study of the Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T.; Demirel, Y. Impact of thermodynamics and kinetics on the carbon capture performance of the amine-based CO2 capture system. Environ. Sci. Pollut. Res. Int. 2024, 31, 39350–39371. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Brandoni, C.; Martinez, J.; Snape, C.; Kaldis, S.; Rolfe, A.; Santos, A.; Lysiak, B.; Lappas, A.; Hewitt, N.; et al. Comparative techno-economic analysis of the integration of MEA-based scrubbing and silica PEI adsorbent-based CO2 capture processes into cement plants. J. Clean. Prod. 2023, 414, 137666. [Google Scholar] [CrossRef]
- Benamor, A.; Ali, B.S.; Aroua, M.K. Kinetic of CO2 absorption and carbamate formation in aqueous solutions of diethanolamine. Korean J. Chem. Eng. 2008, 25, 451–460. [Google Scholar] [CrossRef]
- Liu, F.; Fang, M.X.; Dong, W.F.; Wang, T.; Xia, Z.X.; Wang, Q.H.; Luo, Z.Y. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation. Appl. Energy 2019, 233, 468–477. [Google Scholar] [CrossRef]
- Afkhamipour, M.; Mofarahi, M. Sensitivity analysis of the rate-based CO2 absorber model using amine solutions (MEA, MDEA and AMP) in packed columns. Int. J. Greenh. Gas Control 2014, 25, 9–22. [Google Scholar] [CrossRef]
- Azarpour, A.; Zendehboudi, S. Hybrid Smart Strategies to Predict Amine Thermal Degradation in Industrial CO2 Capture Processes. ACS Omega 2023, 8, 26850–26870. [Google Scholar] [CrossRef] [PubMed]
- An, S.L.; Huang, X.; Li, N.; Li, Q.W.; Wang, R.J.; Qi, T.Y.; Wang, L.D. Comprehensive performance of a diethylenetriamine/2-diethylaminoethanol biphasic absorbent for CO2 capture. Fuel 2023, 353, 129178. [Google Scholar] [CrossRef]
- Nie, Y.M.; Li, Y.; Wang, H.J.; Guo, D.F.; Liu, L.B.; Fu, Y.H. Metal Oxides Enhance the Absorption Performance of N-Methyldiethanolamine Solution during the Carbon Dioxide Capture Process. ACS Omega 2023, 8, 11813–11823. [Google Scholar] [CrossRef]
- Polat, H.M.; van der Geest, C.; de Meyer, F.; Houriez, C.; Vlugt, T.J.H.; Moultos, O.A. Densities, viscosities, and diffusivities of loaded and unloaded aqueous CO2/H2S/MDEA mixtures: A molecular dynamics simulation study. Fluid Phase Equilib. 2023, 575, 113913. [Google Scholar] [CrossRef]
- Jaafari, L.; Jaffary, B.; Idem, R. Screening study for selecting new activators for activating MDEA for natural gas sweetening. Sep. Purif. Technol. 2018, 199, 320–330. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, X.B.; Wei, J.W.; Fan, Y.M.; Liao, L.; Wang, H.Q. Reducing the energy penalty and corrosion of carbon dioxide capture using a novel nonaqueous monoethanolamine-based biphasic solvent. Sep. Purif. Technol. 2021, 265, 118481. [Google Scholar] [CrossRef]
- Abdrabou, H.K.; AlNashef, I.; Abu Zahra, M.; Mokraoui, S.; Ali, E.; Hadj-Kali, M.K. Experimental investigation of novel ternary amine-based deep eutectic solvents for CO2 capture. PLoS ONE 2023, 18, e0286960. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.D.; Chen, H.; Wang, Y.B.; Zhu, X.B.; Yang, G.H. Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture. Molecules 2023, 28, 2661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Jin, X.H.; Tu, W.W.; Ma, Q.; Mao, M.L.; Cui, C.H. Development of MEA-based CO2 phase change absorbent. Appl. Energy 2017, 195, 316–323. [Google Scholar] [CrossRef]
- Rashidi, H.; Sahraie, S. Enhancing carbon dioxide absorption performance using the hybrid solvent: Diethanolamine-methanol. Energy 2021, 221, 119799. [Google Scholar] [CrossRef]
- Carrier, J.; Lai, C.-Y.; Radu, D. Lignin-Based Platform as a Potential Low-Cost Sorbent for the Direct Air Capture of CO2. ACS Environ. Au 2024, 4, 196–203. [Google Scholar] [CrossRef]
- Xiao, L.Y.; Qiu, Z.J.; Feng, S.; Duan, X.X.; Zhao, Z.S.; Liu, Y.C.; Ma, L. Carbon dioxide absorption and desorption experiments based on MDEA. Chem. Eng. Process. 2024, 204, 109931. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, N.; Hong, Y.; Wang, R.; Li, Q.; Li, M.; Wang, L. Tertiary amine based-biphasic solvent using ionic liquids as an activator to advance the energy-efficient CO2 capture. Sep. Purif. Technol. 2025, 355, 129671. [Google Scholar] [CrossRef]
- Kruszczak, E.; Kierzkowska-Pawlak, H. CO2 absorption into aqueous solutions of N-methyl-1,3-propane-diamine and its blends with N,N-diethylethanolamine-New kinetic data. Int. J. Energy Res. 2021, 45, 4098–4111. [Google Scholar] [CrossRef]
- Palomar, J.; Lemus, J.; Navarro, P.; Moya, C.; Santiago, R.; Hospital-Benito, D.; Hernández, E. Process Simulation and Optimization on Ionic Liquids. Chem. Rev. 2024, 124, 1649–1737. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, Y.; Sodiq, A.; Mir, N.; Bicer, Y.; Al-Ansari, T.; El-Naas, M.H.; Amhamed, A.I. Emerging trends in direct air capture of CO2: A review of technology options targeting net-zero emissions. RSC Adv. 2023, 13, 5687–5722. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Sung, H.L.; Noh, H.W.; Mazari, S.A.; Moon, J.H.; Kim, K.M. Synergistic effect of blended amines on carbon dioxide absorption: Thermodynamic modeling and analysis of regeneration energy. Renew. Sustain. Energy Rev. 2024, 197, 114362. [Google Scholar] [CrossRef]
- Du, J.X.; Yang, W.; Xu, L.L.; Bei, L.; Lei, S.Y.; Li, W.; Liu, H.T.; Wang, B.; Sun, L.S. Review on post-combustion CO2 capture by amine blended solvents and aqueous ammonia. Chem. Eng. J. 2024, 488, 150954. [Google Scholar] [CrossRef]
- Li, M.R.; Irtem, E.; Van Montfort, H.P.I.; Abdinejad, M.; Burdyny, T. Energy comparison of sequential and integrated CO2 capture and electrochemical conversion. Nat. Commun. 2022, 13, 5398. [Google Scholar] [CrossRef]
- Wang, L.D.; Liu, S.S.; Wang, R.J.; Li, Q.W.; Zhang, S.H. Regulating Phase Separation Behavior of a DEEA-TETA Biphasic Solvent Using Sulfolane for Energy-Saving CO2 Capture. Environ. Sci. Technol. 2019, 53, 12873–12881. [Google Scholar] [CrossRef]
- Ho, C.D.; Chang, H.; Tu, J.W.; Lim, J.W.; Chiou, C.P.; Chen, Y.J. Theoretical and Experimental Studies of CO2 Absorption in Double-Unit Flat-Plate Membrane Contactors. Membranes 2022, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.G.; Yu, S.A.; Ma, K.; Liang, B.; Tang, S.Y.; Liu, C.J.; Yue, H.R. Amine-functionalized mesoporous monolithic adsorbents for post-combustion carbon dioxide capture. Chem. Eng. J. 2021, 413, 127675. [Google Scholar] [CrossRef]
- Fassach, T.A.; Sommer, F.O.; Vorholt, A.J. Hydroaminomethylation in Aqueous Solvent Systems—An Efficient Pathway to Highly Functionalized Amines. Adv. Synth. Catal. 2018, 360, 1473–1482. [Google Scholar] [CrossRef]
- Yousefzadeh, H.; Güler, C.; Erkey, C.; Uzunlar, E. CO2 absorption into primary and secondary amine aqueous solutions with and without copper ions in a bubble column. Turk. J. Chem. 2022, 46, 999–1010. [Google Scholar] [CrossRef]
- Wang, R.J.; Yang, Y.Y.; Wang, M.F.; Lin, J.S.; Zhang, S.A.; An, S.L.; Wang, L.D. Energy efficient diethylenetriamine-1-propanol biphasic solvent for CO2 capture: Experimental and theoretical study. Appl. Energy 2021, 290, 116768. [Google Scholar] [CrossRef]
- Huan, Q.; Wibowo, H.; Yan, M.; Song, M. A review of CO2 mineral storage: Current processes, typical applications, and life cycle assessment. J. Environ. Chem. Eng. 2024, 12, 114785. [Google Scholar] [CrossRef]
- Karo, N.; Itov, G.; Mayraz, O.; Vogt, C. Carbon dioxide sequestration through mineralization from seawater: The interplay of alkalinity, pH, and dissolved inorganic carbon. Chem. Eng. J. 2024, 500, 156380. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, Y.; Xing, L.; Zhan, G.; Deng, Y.; Chen, Z.; Li, J. Carbon dioxide reduction through mineral carbonation by steel slag. J. Environ. Sci. 2025, 152, 664–684. [Google Scholar] [CrossRef]
- Li, Z.L.; Du, Y.H.; Duan, Y.G.; Peng, Y.; Li, J.Y.; Ma, S.B.; Sepehrnoori, K. Microscopic experimental study on the reaction of shale and carbon dioxide based on dual energy CT mineral recognition method. Fuel 2024, 371, 131874. [Google Scholar] [CrossRef]
- Gong, J.P.; Zhang, T.L.; Wang, J.D.; Jin, Y.; Li, J.; Wang, Y.B. Simultaneous rare earth sulfate transformation and carbon dioxide mineralization. Sep. Purif. Technol. 2025, 356, 129805. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Yuan, Q.; Chen, W.S.; Wan, J.H.; Guo, J.; Cai, J.Q. Effect of carbon dioxide mineralization curing on mechanical properties and microstructure of Portland cement-steel slag-granulated blast furnace slag. Constr. Build. Mater. 2024, 431, 136553. [Google Scholar] [CrossRef]
- Chang, Y.C.; Yang, S.Y.; Lin, J.Y.; Hanh, N.T.D.; Srinophakun, P.; Chiu, C.Y.; Liu, B.L.; Ng, I.S.; Chen, K.H.; Chang, Y.K. Scaling down recombinant carbonic anhydrase isolation with immobilized metal ion chromatography (IMAC): Harnessing enzymatic carbon dioxide capture and mineralization. J. Taiwan Inst. Chem. Eng. 2024, 165, 105727. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Xuan, T.; Zhang, X.; Wang, L.; Tian, Y.; Zhu, J. Simulation design and optimization of reactors for carbon dioxide mineralization. Energy Storage Sav. 2024, 3, 209–217. [Google Scholar] [CrossRef]
- Rodrigues, I.N.; de Medeiros, J.L.; Poblete, I.B.S.; Constantino, A.M.; Araújo, O.D.F. Landfill-gas-to-biomethane via a new carbon capture and utilization technology: One-pot sodium chloride batch mineralization. J. Clean. Prod. 2024, 478, 143979. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, S.; Wang, M. Biomimetic mineralization for carbon capture and sequestration. Carbon Capture Sci. Technol. 2024, 13, 100257. [Google Scholar] [CrossRef]
- Madhav, D.; Coppitters, T.; Ji, Y.K.; Thielemans, W.; Desplentere, F.; Moldenaers, P.; Vandeginste, V. Amino acid promoted single-step carbon dioxide capture and mineralization integrated with polymer-mediated crystallization of carbonates. J. Clean. Prod. 2023, 415, 137845. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.Y.; Liu, H.W.; Boczkaj, G.; Cao, Y.J.; Wang, C.Q. A review on carbon storage via mineral carbonation: Bibliometric analysis, research advances, challenges, and perspectives. Sep. Purif. Technol. 2024, 338, 126558. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.Z.; Liu, H.L.; Yang, Q.L.; Zhou, C.A.; Song, L.; Ma, K.; Yue, H.R. Numerical analysis of CO2 absorption characteristics in industrial flue gas mineralization spray tower reactor. Sep. Purif. Technol. 2025, 354, 129051. [Google Scholar] [CrossRef]
- Kumari, P.; Yahmadi, R.; Mumtaz, F.; Vega, L.F.; Ceriani, A.; Tribuzio, R.; Dumée, L.F.; Decarlis, A. CO2 capture via subsurface mineralization geological settings and engineering perspectives towards long-term storage and decarbonization in the Middle East. Carbon Capture Sci. Technol. 2024, 13, 100293. [Google Scholar] [CrossRef]
- Tao, H.Y.; Qian, X.; Zhou, Y.; Cheng, H.F. Research progress of clay minerals in carbon dioxide capture. Renew. Sustain. Energy Rev. 2022, 164, 112536. [Google Scholar] [CrossRef]
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Fayaz, H.; Saleel, C.A. Developments in mineral carbonation for Carbon sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, F.X.; Zou, L.; Wang, S.Q.; Liu, Y.F.; Hou, J.L.; Li, C.H.; Song, K.T.; Kong, L.Z.; Cui, L.P.; et al. CO2 mineralization by typical industrial solid wastes for preparing ultrafine CaCO3: A review. Green Energy Environ. 2024, 9, 1679–1697. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Na, Y.S.; Song, Y.S.; Wang, J.H. A review of carbon mineralization mechanism during geological CO2 storage. Heliyon 2023, 9, e23135. [Google Scholar] [CrossRef]
- Li, J.J.; Hitch, M. Structural and chemical changes in mine waste mechanically-activated in various milling environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilisation and storage. Appl. Energy 2021, 287, 116510. [Google Scholar] [CrossRef]
- Zhang, Z.E.; Pan, S.Y.; Li, H.; Cai, J.C.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sust. Energ. Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- del Pozo, C.A.; Cloete, S.; Cloete, J.H.; Alvaro, A.J.; Amini, S. The oxygen production pre-combustion (OPPC) IGCC plant for efficient power production with CO2 capture. Energy Conv. Manag. 2019, 201, 112109. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Wang, J.X.; Morsi, B.; Li, B.Y. Carbon Dioxide Conversion to Nanomaterials: Methods, Applications, and Challenges. Energy Fuels 2021, 35, 11820–11834. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, P.; Shen, X.Y.; Lu, J.Y.; Ye, S.X.; Wang, H.C.; Ling, T.C.; Ran, Q.P. Utilization of solid wastes to sequestrate carbon dioxide in cement-based materials and methods to improve carbonation degree: A review. J. CO2 Util. 2023, 72, 102502. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.Y.; Turvey, C.C.; Dipple, G.M.; Ni, W. Evaluation of the carbon sequestration potential of steel slag in China based on theoretical and experimental labile Ca. Resour. Conserv. Recycl. 2022, 186, 106590. [Google Scholar] [CrossRef]
- Sim, S.R.; Ryu, D.W. Effect of the Concrete Slurry Waste Ratio on Supercritical CO2 Sequestration. Materials 2023, 16, 742. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kim, J.; Qin, T.C. Mineral carbonation of iron and steel by-products: State-of-the-art techniques and economic, environmental, and health implications. J. CO2 Util. 2024, 81, 102707. [Google Scholar] [CrossRef]
- Marín, O.; Valderrama, J.O.; Kraslawski, A.; Cisternas, L.A. Potential of Tailing Deposits in Chile for the Sequestration of Carbon Dioxide Produced by Power Plants Using Ex-Situ Mineral Carbonation. Minerals 2021, 11, 320. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.X.; Xu, Z.H.; Zeng, H.B. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Ragipani, R.; Escobar, E.; Prentice, D.; Bustillos, S.; Simonetti, D.; Sant, G.; Wang, B. Selective sulfur removal from semi-dry flue gas desulfurization coal fly ash for concrete and carbon dioxide capture applications. Waste Manag. 2021, 121, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Woodall, C.M.; Lu, X.Y.; Dipple, G.; Wilcox, J. Carbon Mineralization with North American PGM Mine Tailings-Characterization and Reactivity Analysis. Minerals 2021, 11, 844. [Google Scholar] [CrossRef]
- Sandalow, D.; Aines, R.; Friedmann, J.; Kelemen, P.; McCormick, C.; Power, I.; Schmidt, B.; Wilson, S. Carbon Mineralization Roadmap Draft October 2021. In Proceedings of the Eighth Innovation for Cool Earth Forum, Tokyo, Japan, 4–8 October 2021; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2021. [Google Scholar]
- Miller, Q.R.S.; Schaef, H.T.; Kaszuba, J.P.; Gadikota, G.; McGrail, B.P.; Rosso, K.M. Quantitative Review of Olivine Carbonation Kinetics: Reactivity Trends, Mechanistic Insights, and Research Frontiers. Environ. Sci. Technol. Lett. 2019, 6, 431–442. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, J.; Zhang, W. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration. Chem. Ind. Eng. Prog. 2023, 42, 1572–1582. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Zhou, S.; Dao, V.; Chen, M.; Ji, L.; Benhelal, E. Activation and utilization of tailings as CO2 mineralization feedstock and supplementary cementitious materials: A critical review. Mater. Today Sustain. 2023, 24, 100530. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Anderberg, L.; Farhang, F.; Oliver, T.; Rayson, M.S.; Stockenhuber, M. Aqueous carbonation of peridotites for carbon utilisation: A critical review. Environ. Sci. Pollut. Res. 2022, 29, 75161–75183. [Google Scholar] [CrossRef]
- Kusin, F.M.; Hasan, S.; Molahid, V.L.M.; Yusuff, F.M.; Jusop, S. Carbon dioxide sequestration of iron ore mining waste under low-reaction condition of a direct mineral carbonation process. Environ. Sci. Pollut. Res. 2023, 30, 22188–22210. [Google Scholar] [CrossRef]
- Nie, K.; Liu, Y.; Jiao, W. Integrated CO2 absorption-mineralization process by the MEA + MDEA system coupled with Ba(OH)2: Absorption kinetics and mechanisms. Chem. Eng. J. 2024, 502, 158102. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Cheng, C.; Xie, Y.; Lan, C.; Jiang, W.; Zhao, Z.; Zhai, S.; Li, Y.; Wu, Y.; et al. High-efficiency metal-free CO2 mineralization battery using organic redox catalysts. Chem. Eng. J. 2024, 496, 154008. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Cai, Y.; Yu, X.; Yu, C.; Ran, Q. Carbonation behavior of calcium silicate hydrate (C-S-H): Its potential for CO2 capture. Chem. Eng. J. 2022, 431, 134243. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Abdolhosseini Qomi, M.J.; Miller, Q.R.S.; Zare, S.; Schaef, H.T.; Kaszuba, J.P.; Rosso, K.M. Molecular-scale mechanisms of CO2 mineralization in nanoscale interfacial water films. Nat. Rev. Chem. 2022, 6, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, G.; Chen, X.; Xu, X.; Liu, Y.; Yang, J.; Zhang, D. Unlocking the Potential of CO2 Capture: A Synergistic Hybridization Strategy for Polymeric Hydrogels with Tunable Physicochemical Properties. Small 2024, 20, 2402529. [Google Scholar] [CrossRef]
- Dai, X.; Wei, C.; Wang, M.; Song, Y.; Chen, R.; Wang, X.; Shi, X.; Vandeginste, V. Mineralization mechanism of carbon dioxide with illite interlayer cations using molecular dynamics simulation and experiments. J. CO2 Util. 2022, 64, 102161. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, S.S.; Kang, S.-P.; Lee, W.; Park, K.T.; Chun, D.H.; Rhim, G.B.; Hwang, S.-M.; Youn, M.H.; Jeong, S.K. Determination of kinetic factors of CO2 mineralization reaction for reducing CO2 emissions in cement industry and verification using CFD modeling. Chem. Eng. J. 2021, 420, 129420. [Google Scholar] [CrossRef]
- Mehdipour, I.; Falzone, G.; Prentice, D.; Neithalath, N.; Simonetti, D.; Sant, G. The role of gas flow distributions on CO2 mineralization within monolithic cemented composites: Coupled CFD-factorial design approach. React. Chem. Eng. 2021, 6, 494–504. [Google Scholar] [CrossRef]
- Bhardwaj, R.; van Ommen, J.R.; Nugteren, H.W.; Geerlings, H. Accelerating Natural CO2 Mineralization in a Fluidized Bed. Ind. Eng. Chem. Res. 2016, 55, 2946–2951. [Google Scholar] [CrossRef]
- Peng, J.; Xia, B. Multi-field and multi-scale dynamic numerical modeling of supercritical CO2 and fly ash mineralization. Appl. Therm. Eng. 2024, 257, 124195. [Google Scholar] [CrossRef]
- Li, S.; Pan, Y.; Yang, S.; Li, Z. A molecular insight into the mechanism of organic molecule detachment by supercritical CO2 from a water invasion calcite surface: Effect of water film and molecular absorbability. Geoenergy Sci. Eng. 2023, 231, 212290. [Google Scholar] [CrossRef]
- Meng, J.L.; Liao, W.J.; Zhang, G.Q. Emerging CO2-Mineralization Technologies for Co-Utilization of Industrial Solid Waste and Carbon Resources in China. Minerals 2021, 11, 274. [Google Scholar] [CrossRef]
- Khudhur, F.W.K.; MacDonald, J.M.; Macente, A.; Daly, L. The utilization of alkaline wastes in passive carbon capture and sequestration: Promises, challenges and environmental aspects. Sci. Total Environ. 2022, 823, 153553. [Google Scholar] [CrossRef] [PubMed]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Tang, L.; Xue, X.D.; Jia, M.; Jing, H.; Wang, T.; Zhen, R.Q.; Huang, M.T.; Tian, J.; Guo, J.; Li, L.; et al. Iron and steel industry emissions and contribution to the air quality in China. Atmos. Environ. 2020, 237, 117668. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.; Fadlallah, S.; Al-Rashdi, M. Evaluation of the impact of ground-level concentrations of SO2, NOx, CO, and PM10 emitted from a steel melting plant on Muscat, Oman. Sustain. Cities Soc. 2018, 38, 675–683. [Google Scholar] [CrossRef]
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.S.; Kara, S.; Hauschild, M.; Chen, W.Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef]
- Li, X.; Lu, L.; Mu, X.; Qin, C. Emission Reduction Potential of Pollutants Emissions from Iron and Steel Industry over Beijing-Tianjin-Hebei Region based on LEAP. Res. Environ. Sci. 2019, 32, 365–371. [Google Scholar]
- Li, Z.L.; Hanaoka, T. Plant-level mitigation strategies could enable carbon neutrality by 2060 and reduce non-CO2 emissions in China’s iron and steel sector. One Earth 2022, 5, 932–943. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, B.Y.; An, R.Y.; Sun, F.H.; Xu, S. An integrated analysis of China’s iron and steel industry towards carbon neutrality. Appl. Energy 2022, 322, 119453. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Lu, C. Research on the driving factors and carbon emission reduction pathways of China’s iron and steel industry under the vision of carbon neutrality (vol 357, 131990, 2022). J. Clean. Prod. 2022, 360, 132238. [Google Scholar] [CrossRef]
- Yue, Q.; Chai, X.C.; Zhang, Y.J.; Wang, Q.; Wang, H.M.; Zhao, F.; Ji, W.; Lu, Y.Q. Analysis of iron and steel production paths on the energy demand and carbon emission in China’s iron and steel industry. Environ. Dev. Sustain. 2023, 25, 4065–4085. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Lu, C.; Guan, Z.; Cai, B.; Lei, Y.; Yan, G. China’s Iron and Steel Industry Carbon Emissions Peak Pathways. Res. Environ. Sci. 2022, 35, 339–346. [Google Scholar]
- Duan, H.Y.; Hou, C.H.; Yang, W.; Song, J.N. Towards lower CO2 emissions in iron and steel production: Life cycle energy demand-LEAP based multi-stage and multi-technique simulation. Sustain. Prod. Consump. 2022, 32, 270–281. [Google Scholar] [CrossRef]
- Li, X.; Li, B. Low carbon transition path of China’s iron and steel industry under global temperature-control target. Iron Steel 2019, 54, 224–231. [Google Scholar]
- Zhang, Q.; Wang, Y.J.; Zhang, W.; Xu, J. Energy and resource conservation and air pollution abatement in China’s iron and steel industry. Resour. Conserv. Recycl. 2019, 147, 67–84. [Google Scholar] [CrossRef]
- Bo, X.; Jia, M.; Xue, X.D.; Tang, L.; Mi, Z.F.; Wang, S.Y.; Cui, W.G.; Chang, X.Y.; Ruan, J.H.; Dong, G.X.; et al. Effect of strengthened standards on Chinese ironmaking and steelmaking emissions. Nat. Sustain. 2021, 4, 811–820. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Chen, J.; Jiang, F. Pollution control efficiency of China’s iron and steel industry: Evidence from different manufacturing processes. J. Clean. Prod. 2019, 240, 118184. [Google Scholar] [CrossRef]




| Absorbents | CO2 Absorption Capacity Mol CO2/mol | Reaction Rate k1/(106 mol·m−2sPa) | Absorption of Reaction Heat kJ/mol | Regeneration Energy Consumption GJ/t CO2 | Ref. |
|---|---|---|---|---|---|
| AMP | 1–1.5 | 0.7 | 73 | 2.0–2.5 | [22] |
| MDEA | 1–1.68 | 0.26 | 54.6 | 2.5–3.5 | [27] |
| PZ | 0.8–0.9 | 2.5 | 70 | 3.15 | [22] |
| MEA | 0.5–0.7 | 1.6 | 147 | 3.3–4.4 | [24] |
| DEA | 0.25–0.5 | 1.1 | 79 | 2.1–2.6 | [5] |
| Mineralization Type | Minerals and Chemicals | Advantages | Disadvantages |
|---|---|---|---|
| Direct mineralization | Gas-solid dry direct mineralization technology | The process is simple and does not require the use of solvents. The reaction is spontaneous and consumes less energy. | Reaction rates are slow and require high temperature and pressure conditions to improve efficiency. Carbonation reaction rates and conversions are difficult to meet the demands of industrial applications. |
| Wet direct mineralization technology | Faster reaction rate than dry process. Suitable for industrial solid waste with high reactivity. | Costly, requires ore pre-treatment (crushing, milling). High temperature and pressure requirements increase energy consumption. | |
| Indirect mineralization | Mineralization by hydrochloric acid leaching | Capable of producing high purity, high value carbonate products. Can effectively remove impurities such as silica and iron. | Requires energy-intensive ore pretreatment steps. Recovery and recycling of acid leachate is difficult. |
| Mineralization by leaching of strong acid and weak base salts | The regeneration of leaching agent consumes less energy. Recycling of leaching agent can be realized and chemical consumption can be reduced. | Fine control of the reaction conditions is required to maintain the stability and selectivity of the leaching agent. |
| CO V/V% | H2 V/V% | CH4 V/V% | N2 V/V% | CO2 V/V% | O2 V/V% | Sulfide | Densities kg/m3 | Calorific Value kJ/m3 |
|---|---|---|---|---|---|---|---|---|
| 25.0~30.0 | 1.5~3.0 | 0.2~0.5 | 55.0~60.0 | 9.0~12.0 | 0.2~0.4 | <0.1% | 1.29~1.30 | 3300~4200 |
| Comparison of Energy Consumption Between Conventional MEA Capture Technology and Integrated Absorption Mineralization (in kJ/kgCO2) | |||
|---|---|---|---|
| Pre-treatment and absorption units | |||
| Equipment type | Energy consumption | / | |
| Bellow | 106.0 | ||
| Pump | 18.0 | ||
| Subtotal | 124.0 | ||
| Desorption and compression units | |||
| Equipment type | Traditional MEA workers | Integration technology | Energy conservation |
| Reboiling of vapor stripping Tower | 760.0 | - | 760.0 |
| Compactor | 397.0 | - | 397.0 |
| Pump | 9.9 | 9.9 | 0.0 |
| Filter press | - | 51.7 | −51.7 |
| Subtotal | 1166.9 | 61.6 | 1105.3 |
| Total system energy consumption | |||
| Traditional MEA workers | 1290.9 | ||
| Integration technology | 185.6 | 1105.3 | |
| Percentage of energy savings | - | 85.6% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Cheng, S.; Tong, Y.; Li, G.; Yue, T. An Approach to CO2 Emission Reduction in the Iron and Steel Industry: Research Status and Development Trends of Integrated Absorption-Mineralization Technologies. Sustainability 2025, 17, 702. https://doi.org/10.3390/su17020702
Zhang C, Cheng S, Tong Y, Li G, Yue T. An Approach to CO2 Emission Reduction in the Iron and Steel Industry: Research Status and Development Trends of Integrated Absorption-Mineralization Technologies. Sustainability. 2025; 17(2):702. https://doi.org/10.3390/su17020702
Chicago/Turabian StyleZhang, Chuanbo, Sihong Cheng, Yali Tong, Guoliang Li, and Tao Yue. 2025. "An Approach to CO2 Emission Reduction in the Iron and Steel Industry: Research Status and Development Trends of Integrated Absorption-Mineralization Technologies" Sustainability 17, no. 2: 702. https://doi.org/10.3390/su17020702
APA StyleZhang, C., Cheng, S., Tong, Y., Li, G., & Yue, T. (2025). An Approach to CO2 Emission Reduction in the Iron and Steel Industry: Research Status and Development Trends of Integrated Absorption-Mineralization Technologies. Sustainability, 17(2), 702. https://doi.org/10.3390/su17020702










