Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation
Abstract
1. Introduction
2. Technologies for Effective Contaminated Site Cleaning
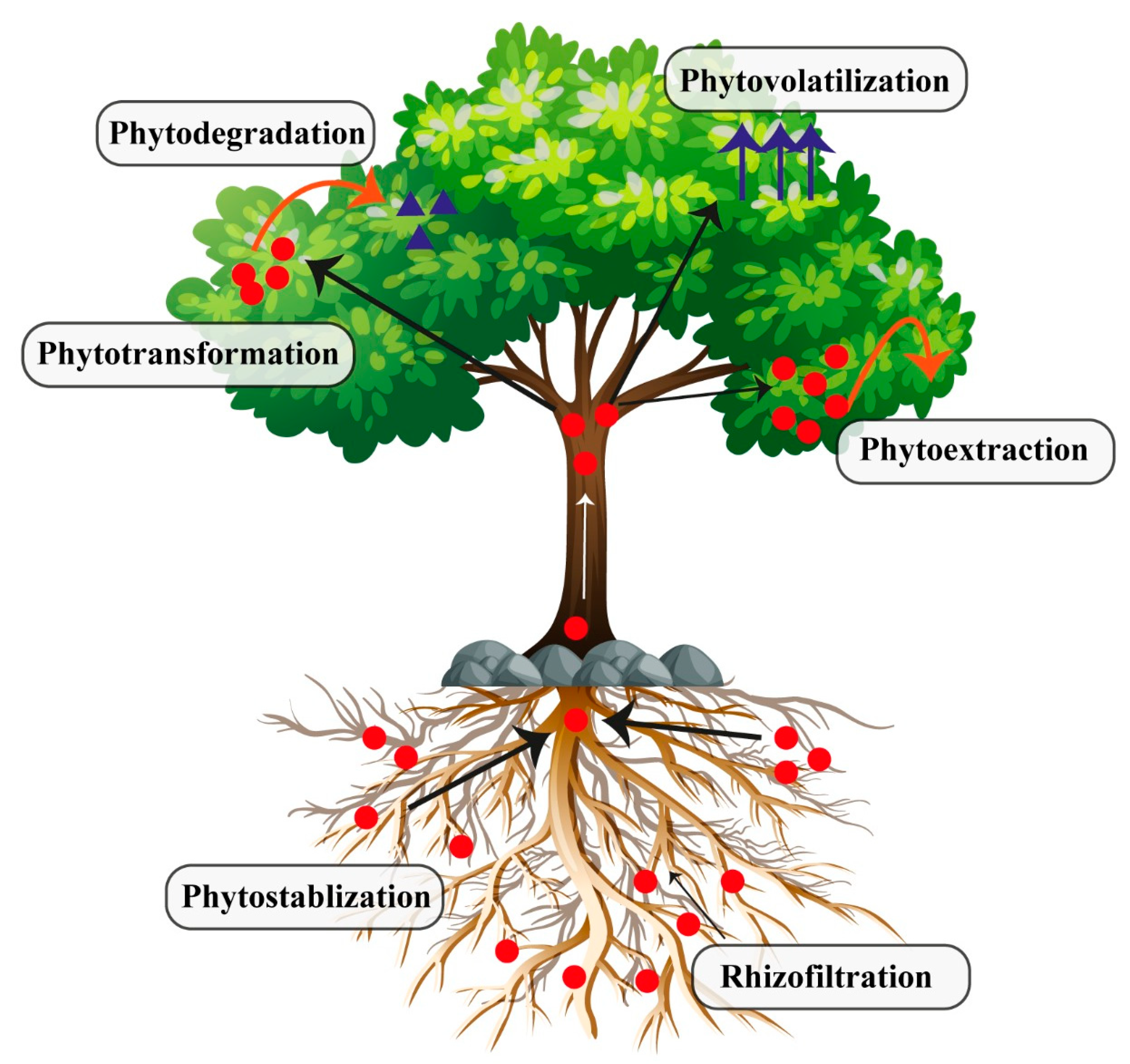
3. Phytoremediation as a Promising Technology for Site Remediation
4. Recent Advances in the Biofuel Industry
5. Potential of Biofuel-Producing Plants in Phytoremediation
6. Challenges of Coupling Phytoremediation with Bioenergy Production
6.1. Residual Contaminants in Biomass
6.2. Low Yield with Contaminated Biomass
6.3. Level of Site Cleaning
6.4. Phytoremediation Plant Selection
7. Research Gaps in Coupling Phytoremediation with Bioenergy Production
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Wang, C. Natural and Human Factors Affect the Distribution of Soil Heavy Metal Pollution: A Review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Hoang, H.G.; Chiang, C.F.; Lin, C.; Wu, C.Y.; Lee, C.W.; Cheruiyot, N.K.; Tran, H.T.; Bui, X.T. Human health risk simulation and assessment of heavy metal contamination in a river affected by industrial activities. Environ. Pollut. 2021, 285, 117414. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Mkhonza, P.; Tekere, M. Sources of Heavy Metals Pollution. In Remediation of Heavy Metals; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Chen, L.C.; Maciejczyk, P.; Thurston, G.D. Metals and air pollution. In Handbook on the Toxicology of Metals; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume I. [Google Scholar] [CrossRef]
- Sager, M. Urban soils and road dust—Civilization effects and metal pollution—A review. Environments 2020, 7, 98. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Zhang, Y.; Labianca, C.; Chen, L.; De Gisi, S.; Notarnicola, M.; Guo, B.; Sun, J.; Ding, S.; Wang, L. Sustainable ex-situ remediation of contaminated sediment: A review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Castiglione, S.; Guarino, F.; Pastore, M.C.; Labra, M. Beyond Cleansing: Ecosystem Services Related to Phytoremediation. Plants 2023, 12, 1031. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef]
- Ionata, E.; Caputo, E.; Mandrich, L.; Marcolongo, L. Moving towards Biofuels and High-Value Products through Phytoremediation and Biocatalytic Processes. Catalysts 2024, 14, 118. [Google Scholar] [CrossRef]
- Puthur, J. Soil and Sediment Contamination: An International Heavy Metal Phytoremediation by Bioenergy Plants and Associated Tolerance Mechanisms. Soil Sediment Contam. Int. J. 2020, 30, 253–274. [Google Scholar] [CrossRef]
- Department for International Development. Agenda 2030: The UK Government’s Approach to Delivering the Global Goals for Sustainable Development—At Home and Around the World. No. March, p. 47. 2017. Available online: https://www.gov.uk/government/publications/agenda-2030-delivering-the-global-goals (accessed on 5 December 2024).
- Zheng, X.; Lin, H.; Du, D.; Li, G.; Alam, O.; Cheng, Z.; Liu, X.; Jiang, S.; Li, J. Remediation of heavy metals polluted soil environment: A critical review on biological approaches. Ecotoxicol. Environ. Saf. 2024, 284, 116883. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Gerhard, J.I.; Grant, G.P.; Torero, J.L. Star: A uniquely sustainable in situ and ex situ remediation process. In Sustainable Remediation of Contaminated Soil and Groundwater; Butterworth-Heinemann: Oxford, UK, 2019; pp. 221–246. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, B. Recent Progress on Ex Situ Remediation Technology and Resource Utilization for Heavy Metal Contaminated Sediment. Toxics 2023, 11, 207. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Yang, J.; Chen, T. Three-year field experiment on the risk reduction, environmental merit, and cost assessment of four in situ remediation technologies for metal(loid)-contaminated agricultural soil. Environ. Pollut. 2020, 266, 115193. [Google Scholar] [CrossRef]
- Wadgaonkar, S.L.; Ferraro, A.; Nancharaiah, Y.V.; Dhillon, K.S.; Fabbricino, M.; Esposito, G.; Lens, P.N.L. In situ and ex situ bioremediation of seleniferous soils from northwestern India. J. Soils Sediments 2019, 19, 762–773. [Google Scholar] [CrossRef]
- Cioica, N.; Tudora, C.; Iuga, D.; Deak, G.; Matei, M.; Nagy, E.M.; Gyorgy, Z. A review on phytoremediation as an ecological method for in situ clean up of heavy metals contaminated soils. E3S Web Conf. 2019, 112, 03024. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Athar, T.; Mustafa, U.; Iqbal, R. Economic feasibility of phytoremediation. In Phytoremediation; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Usman, K.; Ahmed, B.; Rizwan, M.; Saleem, M.H.; Al Jabri, H. Understanding the Phytoremediation Mechanisms of Potentially Toxic Elements: A Proteomic Overview of Recent Advances. Front. Plant Sci. 2022, 13, 881242. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead Responses and Tolerance Mechanisms of Koelreuteria paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef]
- Mileti, Z.; Pavlovi, D.; Mati, M.; Pavlovi, P. Heliyon Phytoremediation potential of invasive plant species for potentially toxic elements along the Sava River upstream. Heliyon 2024, 10, e33798. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xiong, Y.; Wang, Y.; Li, Q. Combination effects of heavy metal and inter-specific competition on the invasiveness of Alternanthera philoxeroides. Environ. Exp. Bot. 2021, 189, 104532. [Google Scholar] [CrossRef]
- Sołtysiak, J. Heavy Metals Tolerance in an Invasive Weed (Fallopia japonica) under Different Levels of Soils Contamination. J. Ecol. Eng. 2020, 21, 81–91. [Google Scholar] [CrossRef]
- Gruntman, M.; Segev, U.; Tielbörger, K. Shade-induced plasticity in invasive Impatiens glandulifera populations. Weed Res. 2020, 60, 16–25. [Google Scholar] [CrossRef]
- Khan, I.U.; Zhang, Y.F.; Shi, X.N.; Qi, S.S.; Zhang, H.Y.; Du, D.L.; Gul, F.; Wang, J.H.; Naz, M.; Shah, S.W.A.; et al. Dose dependent effect of nitrogen on the phyto extractability of Cd in metal contaminated soil using Wedelia trilobata. Ecotoxicol. Environ. Saf. 2023, 264, 115419. [Google Scholar] [CrossRef]
- Khan, I.U.; Qi, S.S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.N.; Zhang, H.; Dai, Z.C.; Du, D.L. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rutherford, S.; Saif Ullah, M.; Ullah, I.; Javed, Q.; Rasool, G.; Ajmal, M.; Azeem, A.; Nazir, M.J.; Du, D. Plant-soil feedback during biological invasions: Effect of litter decomposition from an invasive plant (Sphagneticola trilobata) on its native congener (S. calendulacea). J. Plant Ecol. 2022, 15, 610–624. [Google Scholar] [CrossRef]
- Mousavi Kouhi, S.M.; Moudi, M. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline–sodic soil. Environ. Sci. Pollut. Res. 2020, 27, 10027–10038. [Google Scholar] [CrossRef]
- Li, J.; Leng, Z.; Wu, Y.; Du, Y.; Dai, Z.; Biswas, A.; Zheng, X.; Li, G.; Mahmoud, E.K.; Jia, H.; et al. Interactions between invasive plants and heavy metal stresses: A review. J. Plant Ecol. 2022, 15, 429–436. [Google Scholar] [CrossRef]
- Khan, S.; Masoodi, T.H.; Pala, N.A.; Murtaza, S.; Mugloo, J.A.; Sofi, P.A.; Zaman, M.U.; Kumar, R.; Kumar, A. Phytoremediation Prospects for Restoration of Contamination in the Natural Ecosystems. Water 2023, 15, 1498. [Google Scholar] [CrossRef]
- Mohamed El-Mahrouk, E.S.; El-Hakim Eisa, E.A.; Ali, H.M.; Abd El-naby Hegazy, M.; Abd El-Gayed, M.E.S. Populus nigra as a phytoremediator for Cd, Cu, and Pb in contaminated soil. BioResources 2020, 15, 869–893. [Google Scholar] [CrossRef]
- Anerao, P.; Kaware, R.; Khedikar, A.K.; Kumar, M.; Singh, L. Phytoremediation of persistent organic pollutants: Concept challenges and perspectives. In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Elsevier B.V.: Amsterdam, The Netherlands, 2022; pp. 375–404. [Google Scholar] [CrossRef]
- Matzen, S.L.; Lobo, G.P.; Fakra, S.C.; Kakouridis, A.; Nico, P.S.; Pallud, C.E. Arsenic hyperaccumulator Pteris vittata shows reduced biomass in soils with high arsenic and low nutrient availability, leading to increased arsenic leaching from soil. Sci. Total Environ. 2022, 818, 151803. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.M.; ZakeriNia, M.; Farasati, M. The effects of phytoremediation of treated urban wastewater on the discharge of surface and subsurface drippers (Case study: Gorgan wastewater treatment plant in northern Iran). Clean. Eng. Technol. 2021, 4, 100210. [Google Scholar] [CrossRef]
- Maurya, A.; Sharma, D.; Partap, M.; Kumar, R.; Bhargava, B. Microbially-assisted phytoremediation toward air pollutants: Current trends and future directions. Environ. Technol. Innov. 2023, 31, 103140. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
- Ahmad, I.; Alserae, H.; Zhu, B.; Zahoor, A.; Farooqi, Z.U.R.; Mihoub, A.; Ain, Q.U.; Radicetti, E. Phytoremediation of Cadmium: A Review. In Cadmium Toxicity in Water; Springer Water; Springer: Berlin/Heidelberg, Germany, 2024; Volume Part F2532, pp. 75–99. [Google Scholar] [CrossRef]
- Sivanantha, N.; Wijesinghe, M.R.; Wijesekara, R.D. Distribution of five toxic heavy metals in biotic and abiotic constituents of the Negombo Lagoon, Sri Lanka. Sri Lankan J. Biol. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Syam, N.; Wardiyati, T.; Maghfoer, M.D.; Handayanto, E.; Ibrahim, B.; Muchdar, A. Effect of Accumulator Plants on Growth and Nickel Accumulation of Soybean on Metal-contaminated Soil. Agric. Agric. Sci. Procedia 2016, 9, 13–19. [Google Scholar] [CrossRef]
- Sahay, S.; Inam, A.; Iqbal, S. Risk analysis by bioaccumulation of Cr, Cu, Ni, Pb and Cd from wastewater-irrigated soil to Brassica species. Int. J. Environ. Sci. Technol. 2020, 17, 2889–2906. [Google Scholar] [CrossRef]
- Liu, S.; Ali, S.; Yang, R.; Tao, J.; Ren, B. A newly discovered Cd-hyperaccumulator Lantana camara L. J. Hazard. Mater. 2019, 371, 233–242. [Google Scholar] [CrossRef]
- Hafshajani, E.J.; Hoodaji, M.; Ghanati, F.; Hosseini, Y.; Alipour, V. The Effect of Magnetized Water on the Absorption of Cadmium Using Synthetic Effluents by Lantana camara Species. J. Health Sci. Surveill. Syst. 2022, 10, 408–419. [Google Scholar] [CrossRef]
- Negrin, V.L.; Botté, S.E.; La Colla, N.S.; Marcovecchio, J.E. Uptake and accumulation of metals in Spartina alterniflora salt marshes from a South American estuary. Sci. Total Environ. 2019, 649, 808–820. [Google Scholar] [CrossRef]
- Pennington, P.; Reed, L.A. Evaluating the Potential for Transfer of Heavy Metals Through Trophic Interactions in Spartina alterniflora and Littorina irrorata. Master’s Thesis, College of Charleston, Charleston, SC, USA, 2023. [Google Scholar]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Manabe, S. Role of greenhouse gas in climate change. Tellus Ser. A Dyn. Meteorol. Oceanogr. 2019, 71, 1–13. [Google Scholar] [CrossRef]
- Kalair, A.R.; Seyedmahmoudian, M.; Stojcevski, A.; Abas, N.; Khan, N. Waste to energy conversion for a sustainable future. Heliyon 2021, 7, e08155. [Google Scholar] [CrossRef]
- Siddik, M.; Islam, M.; Zaman, A.K.; Hasan, M. Current status and correlation of fossil fuels consumption and greenhouse gas emissions. Int. J. Energy Environ. Econ. 2021, 28, 103–119. [Google Scholar]
- Sebestyén, V. Renewable and Sustainable Energy Reviews: Environmental impact networks of renewable energy power plants. Renew. Sustain. Energy Rev. 2021, 151, 111626. [Google Scholar] [CrossRef]
- EIA. Annual Energy Outlook 2021. 2020; pp. 1–81. Available online: www.eia.gov/aeo (accessed on 5 December 2024).
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2022, 28, 127–139. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Bru, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Attard, T.; Clark, J.H.; Mcelroy, C.R. Recent developments in key bio-refinery areas. Curr. Opin. Green Sustain. Chem. 2020, 21, 64–74. [Google Scholar] [CrossRef]
- Syahirah, N.; Aron, M.; Shiong, K.; Kit, K.; Chew, W. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Vera, I.; Hoefnagels, R.; Junginger, M. Supply potential of lignocellulosic energy crops grown on marginal land and greenhouse gas footprint of advanced A spatially explicit assessment under the sustainability criteria of the Renewable Energy Directive Recast. GCB Bioenergy 2021, 13, 1425–1447. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I. Sustainable Valorization of Biomass: From Assisted Phytoremediation to Green Energy Production Bioenergy: The Role of Biomass. In Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 29–51. [Google Scholar]
- Nadari, O.; Hemda, A.; Huw, G.; Diane, J. Combining phytoremediation with bioenergy production: Developing a multi-criteria decision matrix for plant species selection. Environ. Sci. Pollut. Res. 2023, 30, 40698–40711. [Google Scholar] [CrossRef]
- Werle, S.; Tran, K.-Q.; Magdziarz, A.; Sobek, S.; Pogrzeba, M.; Løvås, T. Energy crops for sustainable phytoremediation—Fuel characterization. Energy Procedia 2019, 158, 867–872. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation potential of fast-growing energy plants: Challenges and perspectives—A review. Polish J. Environ. Stud. 2020, 29, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Kwakye, J.M.; Ekechukwu, D.E.; Ogundipe, O.B. Climate Change Adaptation Strategies for Bioenergy Crops: A Global Synthesis. Int. J. Eng. Res. Dev. 2024, 20, 434–443. [Google Scholar]
- Nikolić, M.; Tomasević, V.; Ugrinov, D. Energy plants as biofuel source and as accumulators of heavy metals. Hem. Ind. 2022, 76, 209–225. [Google Scholar] [CrossRef]
- Takase, M.; Kipkoech, R.; Essandoh, P.K. A comprehensive review of energy scenario and sustainable energy in Kenya. Fuel Commun. 2021, 7, 100015. [Google Scholar] [CrossRef]
- Arbaoui, S.; Evlard, A.; Mhamdi, M.E.W.; Campanella, B.; Paul, R.; Bettaieb, T. Potential of kenaf (Hibiscus cannabinus L.) and corn (Zea mays L.) for phytoremediation of dredging sludge contaminated by trace metals. Biodegradation 2013, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mathur, J. Phytoremediation efficiency of Helianthus annuus L. for reclamation of heavy metals-contaminated industrial soil. Environ. Sci. Pollut. Res. 2020, 27, 29954–29966. [Google Scholar] [CrossRef]
- Zubairu, A.; Gimba, A.S.B.; Abdullahi, A.M. Production and characterization of biodiesel from hybrid feedstock of Jatropha curcas and Thevetia peruviana seeds oil. Arid Zone J. Eng. Technol. Environ. 2021, 17, 469–480. [Google Scholar]
- Singh, P.; Verma, Y.; Avtar, R.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2022, 48, 1178–1184. [Google Scholar] [CrossRef]
- Maliha, A.; Abu, B. A Review on the Current Status and Post-Pandemic Prospects of Third-Generation Biofuels; Springer: Berlin/Heidelberg, Germany, 2023; Volume 14. [Google Scholar] [CrossRef]
- Juli, P.; Ileana, V. Coupling Plant Biomass Derived from Phytoremediation of Potential Toxic-Metal-Polluted Soils to Bioenergy Production and High-Value by-Products—A Review. Appl. Sci. 2021, 11, 2982. [Google Scholar]
- Kick, C.; Kidikas, Ž.; Kasiulienė, A.; Maletić, S.; Zeremski, T.; Rubežius, M.; Eschen, M.; Ortner, M. Feasibility of using phytoremediation biomass for sustainable biofuel production via thermochemical conversion. Biofuels Bioprod. Biorefining 2024, 18, 1010–1026. [Google Scholar] [CrossRef]
- Prabha, J.; Kumar, M.; Tripathi, R. Opportunities and Challenges of Utilizing Energy Crops in Phytoremediation of Environmental Pollutants: A Review; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Naveed, S.; Oladoye, P.O.; Alli, Y.A. Toxic heavy metals: A bibliographic review of risk assessment, toxicity, and phytoremediation technology. Sustain. Chem. Environ. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Mohamed, A.A.A.; Dardiry, M.H.O.; Samad, A.; Abdelrady, E. Exposure to Lead (Pb) Induced Changes in the Metabolite Content, Antioxidant Activity and Growth of Jatropha curcas (L.). Trop. Plant Biol. 2020, 13, 150–161. [Google Scholar] [CrossRef]
- Pathak, G.; Dudhagi, S.S. Bioenergy Crops as an Alternate Energy Resource. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Wiley: Hoboken, NJ, USA, 2021; pp. 357–376. [Google Scholar] [CrossRef]
- Ahmed, Y.; Mukhtar, M.D.; Yahaya, S.; Faggo, A.A. Potency of Some Savanna Herbaceous Weeds in the Production of Bioethanol Using Zymomonas mobilis and Saccharomyces cerevisiae. J. Biochem. Microbiol. Biotechnol. 2023, 11, 51–56. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Zhu, J.Y.; Headlee, W.L.; Gleisner, R.; Pilipović, A.; Acker, J.V.; Bauer, E.O.; Birr, B.A.; Wiese, A.H. Ecosystem Services, Physiology, and Biofuels Recalcitrance of Poplars Grown for Landfill Phytoremediation. Plants 2020, 9, 1357. [Google Scholar] [CrossRef]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef]
- Learning, X.; Premkumar, G. Bioenergy Crops A Sustainable Means of Phytoremediation; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The Impact and Invasive Mechanisms of Pueraria montana var. lobata, One of the World’s Worst Alien Species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef]
- Herod, M. Environmental Factors Influencing the Spread and Invasion Potential of Arundo donax in Central Texas. Master’s Thesis, Texas State University, San Marcos, TX, USA, 2022. [Google Scholar]
- Assaf, J.C.; Mortada, Z.; Rezzoug, S.; Maache-rezzoug, Z.; Louka, N. Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn. Processes 2024, 12, 1001. [Google Scholar] [CrossRef]
- Ibrahimpašić, J.; Jogić, V.; Džaferović, A.; Makić, H.; Toromanović, M.; Dedić, S. The potential of corn. Technol. Acta 2021, 14, 31–38. [Google Scholar] [CrossRef]
- De Bari, I.; Liuzzi, F.; Ambrico, A.; Trupo, M. Arundo donax refining to second generation bioethanol and furfural. Processes 2020, 8, 1591. [Google Scholar] [CrossRef]
- Azizi, A.; Krika, A.; Krika, F. Heavy metal bioaccumulation and distribution in typha latifolia and arundo donax: Implication for phytoremediation. Casp. J. Environ. Sci. 2020, 18, 21–29. [Google Scholar] [CrossRef]
- Accardi, D.S.; Russo, P.; Lauri, R.; Pietrangeli, B.; Di Palma, L. From soil remediation to biofuel: Process simulation of bioethanol production from Arundo donax. Chem. Eng. Trans. 2015, 43, 2167–2172. [Google Scholar] [CrossRef]
- Corno, L. Arundo donax L. (Giant Cane) as a Feedstock for Bioenergy and Green Chemistry. Ph.D. Thesis, Università Degli Studi Di Milano, Milano, Italy, 2015; pp. 1–217. [Google Scholar]
- Rahman, S.U.; Yasin, G.; Nawaz, M.F.; Cheng, H.; Azhar, M.F.; Riaz, L.; Javed, A.; Li, Y. Evaluation of heavy metal phytoremediation potential of six tree species of Faisalabad city of Pakistan during summer and winter seasons. J. Environ. Manag. 2022, 320, 115801. [Google Scholar] [CrossRef]
- Oni, B.A.; Oluwatosin, D. Emission characteristics and performance of neem seed (Azadirachta indica) and Camelina (Camelina sativa) based biodiesel in diesel engine. Renew. Energy 2020, 149, 725–734. [Google Scholar] [CrossRef]
- Jumare, F.I.; Aliyu, M.; Dabai, I.A.; Bello, H.; Bala, A. Production of Bioethanol Using Neem Tree Leaves. Caliphate J. Sci. Technol. (CaJoST) 2021, 1, 5–9. [Google Scholar]
- BABA, A.; Mohammed, A.I.; Inuwa, L.B.; Chellube, Z.M. Determination of the sources of heavy metals pollution using neem tree (azadirachtaindica) stem barks in Maiduguri, Borno State, Nigeria. Proc. Niger. Soc. Phys. Sci. 2024, 1, 119. [Google Scholar] [CrossRef]
- Awotedu, O.L.; Ogunbamowo, P.O. Comparative Heavy Metal Uptake and Phytoremediation Potential of Three Jatropha Species. Environ. Ecosyst. Sci. 2019, 3, 26–30. [Google Scholar] [CrossRef]
- Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.Q.; Qin, R.; Adhikari, P. Growing jatropha (Jatropha curcas L.) as a potential second-generation biodiesel feedstock. Inventions 2021, 6, 60. [Google Scholar] [CrossRef]
- Madian, H.R.; Abdelhamid, A.E.; Hassan, H.M.; Labena, A. Potential applicability of Jatropha curcas leaves in bioethanol production and their composites with polymer in wastewater treatment. Biomass Convers. Biorefinery 2024, 14, 20991–21005. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Okoh Ezennia Valentine Charles Bioethanol production from Jatropha seed cake via dilute acid hydrolysis and fermentation by Saccharomyces cerevisiae. GSC Biol. Pharm. Sci. 2021, 15, 049–054. [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- BİLGİN, F.D. Phytoremediation by Guinea grass (Panicum maximum): A Focused Review. Turkish J. Range Forage Sci. 2023, 4, 85–92. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.; Cui, J.; Wang, Q. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytoremediation 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Ali, S.; Serba, D.D.; Walker, D.; Jenkins, J.; Schmutz, J.; Bhamidimarri, S.; Saha, M.C. Genome-wide quantitative trait loci detection for biofuel traits in switchgrass (Panicum virgatum L.). GCB Bioenergy 2020, 12, 923–940. [Google Scholar] [CrossRef]
- Taranenko, A.; Kulyk, M.; Galytska, M.; Taranenko, S. Effect of cultivation technology on switchgrass (Panicum virgatum L.) productivity in marginal lands in Ukraine. Acta Agrobot. 2019, 72, 1–11. [Google Scholar] [CrossRef]
- Manmai, N.; Unpaprom, Y.; Mariano, A.P.B.; Ramaraj, R. Bioethanol Production from Sunflower Stalk: Comparison between the Impact of Optimal Chemical and Biological Pretreatments Bioethanol Production from Sunflower Stalk: Comparison between the Impact of Optimal Chemical and Biological Pretreatments. In Proceedings of the 1st Thailand Biorefinery Conference, Nakhon Ratchasima, Thailand, 25–26 July 2019; pp. 24–31. [Google Scholar]
- Thirumarimurugan, M.; Sivakumar, V.M.; Xavier, A.M.; Prabhakaran, D.; Kannadasan, T. Preparation of Biodiesel from Sunflower Oil by Transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441–444. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Nguyen, T.T.; Le, H.T.; Nguyen, T.T.; Bach, L.G.; Nguyen, T.D.; Vo, D.V.; Van Tran, T. The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture: A review. Environ. Chem. Lett. 2021, 19, 3701–3726. [Google Scholar] [CrossRef]
- Anita; Kulsoom, M.; Yadav, A.K.; Kumar, M.; Raw, K.P.; Prasad, S.; Kumar, N. Accumulation and Translocation of Heavy Metals in Hibiscus cannabinus Grown in Tannery Sludge Amended Soil. Nat. Environ. Pollut. Technol. 2024, 23, 1133–1139. [Google Scholar] [CrossRef]
- Meryemoǧlu, B.; Hasanoǧlu, A.; Irmak, S.; Erbatur, O. Biofuel production by liquefaction of kenaf (Hibiscus cannabinus L.) biomass. Bioresour. Technol. 2014, 151, 278–283. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Hakeem, K.R.; Paridah, M.T.; Khalina, A.; Alothman, O.Y. Potential of bioenergy production from industrial kenaf (Hibiscus cannabinus L.) based on Malaysian perspective. Renew. Sustain. Energy Rev. 2015, 42, 446–459. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, P.; Purchase, D.; Chandra, R. Distillery wastewater detoxification and management through phytoremediation employing Ricinus communis L. Bioresour. Technol. 2021, 333, 125192. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.R.; Prasad, M.N.V. Ricinus communis L. (Castor bean), a potential multi-purpose environmental crop for improved and integrated phytoremediation. EuroBiotech J. 2017, 1, 101–116. [Google Scholar] [CrossRef]
- Abel, S.; Jule, L.T.; Gudata, L.; Nagaraj, N.; Shanmugam, R.; Dwarampudi, L.P.; Stalin, B.; Ramaswamy, K. Preparation and characterization analysis of biofuel derived through seed extracts of Ricinus communis (castor oil plant). Sci. Rep. 2022, 12, 11021. [Google Scholar] [CrossRef]
- Abada, E.; Al-Fifi, Z.; Osman, M. Bioethanol production with carboxymethylcellulase of Pseudomonas poae using castor bean (Ricinus communis L.) cake. Saudi J. Biol. Sci. 2019, 26, 866–871. [Google Scholar] [CrossRef] [PubMed]
- waoo, A.A.; Khare, S.; Ganguly, S. Comparative in-vitro Studies on Native Plant Species at Heavy Metal Polluted Soil Having Phytoremediation Potential. Int. J. Sci. Res. Environ. Sci. 2014, 2, 40–55. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A. Bioethanol production from Lantana camara (red sage): Pretreatment, saccharification and fermentation. Bioresour. Technol. 2010, 101, 8348–8354. [Google Scholar] [CrossRef]
- Kandari, V.; Bajpayee, I.; Kamal, B.; Jadon, V.S.; Gupta, S. Production of Bioethanol from Enzymatic and Dilute Acid Hydrolysate of Lantana camara in Batch Fermentation. J. Adv. Microbiol. 2014, 1, 170–183. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazyńska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Wani, K.A.; Sofi, Z.M.; Malik, J.A. Bioremediation Biotechnol; Springer Nature: Berlin, Germany, 2020; Volume 2. [Google Scholar] [CrossRef]
- Rosso, L.; Facciotto, G.; Bergante, S.; Vietto, L.; Nervo, G. Selection and testing of Populus alba and Salix spp. as bioenergy feedstock: Preliminary results. Appl. Energy 2013, 102, 87–92. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jin, Y.S.; Cha, Y.L.; Seo, J.H. Bioethanol production from cellulosic hydrolysates by engineered industrial Saccharomyces cerevisiae. Bioresour. Technol. 2017, 228, 355–361. [Google Scholar] [CrossRef]
- Lee, W.C.; Kuan, W.C. Miscanthus as cellulosic biomass for bioethanol production. Biotechnol. J. 2015, 10, 840–854. [Google Scholar] [CrossRef]
- Dubis, B.; Management, A.P.; Production, S.; Lodzki, P. Biomass production and energy balance of Miscanthus over a period of 11 years: A case study in a large-scale farm in Poland. GCB Bioenergy 2019, 11, 1187–1201. [Google Scholar] [CrossRef]
| Common Name | Scientific Name | Type of Contaminant | References |
|---|---|---|---|
| Tumu merah | Bruguiera gymnorhiza | Pb Cr Hg | [46] |
| Sunflower | Helianthus annus | Pd Cd | [47] |
| Indian rhododendron | M. melastoma | Ni | [48] |
| Knob weed | Hyptis capitata | Ni | [49] |
| Gandapana | Lantana camara L. | Zn Pb Cd | [50,51] |
| Smooth cord grass | Spartina altiniflora | Zn Pb Ni Cu | [52,53] |
| Plant Type | Contaminant Type | Plant Part | Possibility of Use in Biofuel Production | References |
|---|---|---|---|---|
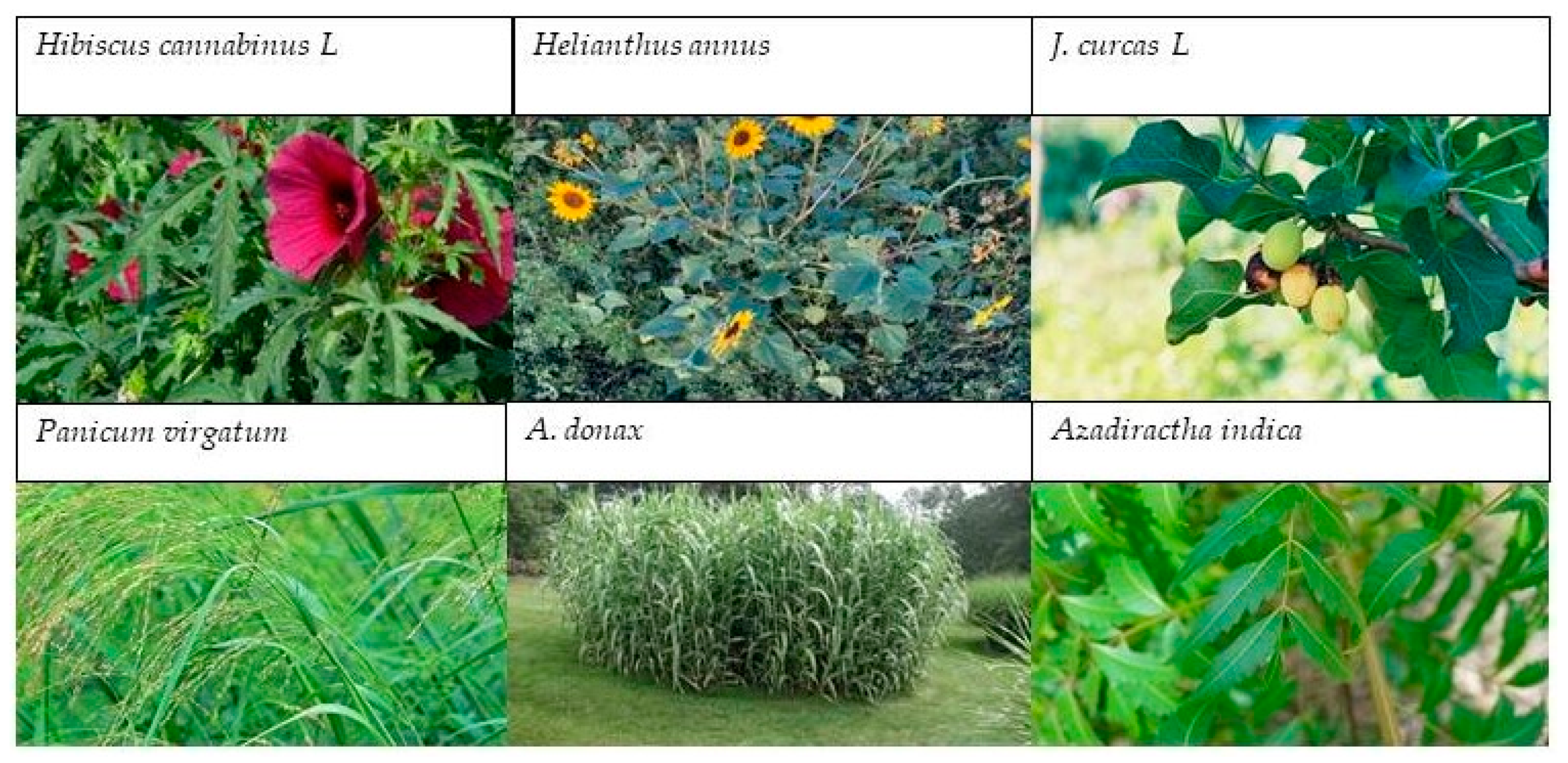
| Hibiscus cannabinus L. | Cd, Zn, Pb, Cr, Ni | Shoots and roots | Bioethanol Biodiesel | [73,74] |
| Helianthus annus | Pd, Cd, Cu, As, Zn | Biomass and seeds | Bioethanol Biodiesel | [75] |
| J. curcas L. | Zn, Cd, Al, Cr, Pd, Mn, Cu | Shoots and roots | Bioethanol Biodiesel | [59,60,61,76] |
| Panicum virgatum | Cd, Pb, Cr, Ni, Ba, Cu | Biomass | Bioethanol | [62,64,77] |
| A. donax | Zn, Hg, Pb, Ni, As, Cd, Cu | Roots | Bioethanol Biogas | [63,78] |
| Azadiractha indica | Zn, Pb, Cd, Cu | Leaves and Stems | Biodiesel Bioethanol | [65,66,79] |
| Plant | Contaminant Type | Climatic Growing Condition | Harvesting Condition | Biofuel Type | References |
|---|---|---|---|---|---|
| Gian reed (A. donax) | Zn, Cr, Pb, Ni, As, Cd, Cu | Subtropical, and semi-arid climates (Hot areas) | Multiple harvests per year for about 10–15 years | Bioethanol Biogas | [87,93,94,95,96] |
| Neem (A. indica) | Zn, Pb, Cd, Cr, Ni, Co | Sub-arid and sub-humid areas with tropical and subtropical climates | Once in year, harvesting can be carried out for about 10–11 years | Biodiesel Bioethanol | [97,98,99,100] |
| Jatropha (J. curcas) | Fe, Cd, Al, Cr, Pb, Mn | Tropical and subtropical regions | Harvest seeds around 90 days after flowering regions | Bioethanol Biodiesel | [101,102,103,104,105,106] |
| Switch grass (P. virgatum) | Cd, Pb, Cr, Ni, As, Fe | Temperate and subtropical zones and tropical climates | Once a year (later in winter or even early spring) for over 10 years | Bioethanol | [107,108,109,110] |
| Sunflower (H. annus) | Pd, Cd, Ni, Cr, As | Temperate and tropical climates | Harvest seeds 70 to 100 days after planting | Bioethanol Biodiesel | [47,111,112,113] |
| Java jute (H. cannabinus) | Cd, Cr, Cu, Ni | Tropics and subtropics where temperatures are greater than 20 °C | Harvesting at 180 days after planting | Bioethanol Biodiesel | [114,115,116] |
| Castor (R. communis) | Cr, Ni, Pb, Zn, Cu, Mn | Tropical and subtropical regions with hot and dry climates | Harvest seeds 140–180 days after planting | Biodiesel Bioethanol | [117,118,119,120] |
| Gandapana (L. camera) | Ni, Cr, Cd | Temperate climate and drought conditions | Harvest seeds once a year | Bioethanol | [121,122,123] |
| White willow (Salix alba) | Cd, Cu, Hg, Pb, Zn | Temperate climates, tolerate both high and low temperatures | Start harvesting 3 years after establishing | Bioethanol | [124,125,126] |
| Silver grass (Miscanthus sinensis) | Cd, Cr, Cu, Ni, Pb, Zn | Temperate and tropical climates | Harvest in the flowering stage | Bioethanol | [67,127,128,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijekoon, W.; Priyashantha, H.; Gajanayake, P.; Manage, P.; Liyanage, C.; Jayarathna, S.; Kumarasinghe, U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability 2025, 17, 822. https://doi.org/10.3390/su17030822
Wijekoon W, Priyashantha H, Gajanayake P, Manage P, Liyanage C, Jayarathna S, Kumarasinghe U. Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability. 2025; 17(3):822. https://doi.org/10.3390/su17030822
Chicago/Turabian StyleWijekoon, Wimukthika, Hasitha Priyashantha, Pradeep Gajanayake, Pathmalal Manage, Champika Liyanage, Shishanthi Jayarathna, and Udayagee Kumarasinghe. 2025. "Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation" Sustainability 17, no. 3: 822. https://doi.org/10.3390/su17030822
APA StyleWijekoon, W., Priyashantha, H., Gajanayake, P., Manage, P., Liyanage, C., Jayarathna, S., & Kumarasinghe, U. (2025). Review and Prospects of Phytoremediation: Harnessing Biofuel-Producing Plants for Environmental Remediation. Sustainability, 17(3), 822. https://doi.org/10.3390/su17030822







