Promoting Sustainability in the Recycling of End-of-Life Photovoltaic Panels and Li-Ion Batteries Through LIBS-Assisted Waste Sorting
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Microscopic Examination and LIBS Analysis
2.3. Contact Angle Measurements
2.4. X-Ray Diffraction Studies
3. Results
3.1. Analysis of PV Panels’ Back Sheets
3.2. Analysis of Components of Li-Ion Batteries
3.2.1. Analysis of Li-Ion Anodes
3.2.2. Analysis of Separator
3.2.3. Analysis of Cathode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BESS | battery energy storage system |
| CA | contact angle |
| CMC | carboxymethyl cellulose |
| DMC | dimethyl carbonate |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| LCO | lithium cobalt oxide |
| LIBS | laser-induced breakdown spectroscopy |
| LNMO | lithium manganese nickel oxide |
| MCMB | mesocarbon microbeads |
| Nd:YAG | neodymium-doped yttrium aluminum garnet laser |
| NMC | lithium nickel manganese cobalt oxides |
| PE | polyethylene |
| PP | polypropylene |
| PTFE | polytetrafluoroethylene |
| PV | photovoltaic panel |
| PVDF | polyvinylidene fluoride |
| RSD | relative standard deviation |
| SBR | styrene-butadiene rubber |
| SEM-EDS | scanning electron microscopy–energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
References
- Solar Power Generation. Available online: https://ourworldindata.org/ (accessed on 29 October 2024).
- Jordan, D.; Kurtz, S. Photovoltaic Degradation Rates-an Analytical Review. Prog. Photovolt. 2013, 21, 12–29. [Google Scholar] [CrossRef]
- Jordan, D.; Kurtz, S.; VanSant, K.; Newmiller, J. Compendium of photovoltaic degradation rates. Prog. Photovolt. 2016, 24, 978–989. [Google Scholar] [CrossRef]
- Theristis, M.; Livera, A.; Jones, C.; Makrides, G.; Georghiou, G.; Stein, J. Nonlinear Photovoltaic Degradation Rates: Modeling and Comparison Against Conventional Methods. IEEE J. Photovolt. 2020, 10, 1112–1118. [Google Scholar] [CrossRef]
- Energy Storage Worldwide (Report). Available online: https://www.statista.com/study/55020/energy-storage-worldwide (accessed on 29 October 2024).
- Huan, Z.; Sun, C.; Ge, M. Progress in Profitable Fe-Based Flow Batteries for Broad-Scale Energy Storage. Wiley Interdiscip. Rev.-Energy Environ. 2024, 13, e541. [Google Scholar] [CrossRef]
- Choi, J.; Jo, H.; Han, S. BESS life span evaluation in terms of battery wear through operation examples of BESS for frequency Regulation. In Proceedings of the 2017 IEEE Innovative Smart Grid Technologies—Asia (Isgt-Asia), Auckland, New Zealand, 4–7 December 2017; pp. 682–686. [Google Scholar]
- Statista. Estimated Number of Electric Vehicles in Use Worldwide Between 2016 and 2022, by Type. 2024. Available online: https://www.statista.com/statistics/1101415/number-of-electric-vehicles-by-type/ (accessed on 29 October 2024).
- Keil, P.; Schuster, S.F.; von Lüders, C.; Hesse, H.; Arunachala, R.; Jossen, A. Lifetime Analyses of Lithium-Ion EV Batteries. In Proceedings of the 3rd Electromobility Challenging Issues conference (ECI), Singapore, 1–4 December 2015. [Google Scholar]
- Xu, J.; Sun, C.; Ni, Y.; Lyu, C.; Wu, C.; Zhang, H.; Yang, Q.; Feng, F. Fast Identification of Micro-Health Parameters for Retired Batteries Based on a Simplified P2D Model by Using Pade Approximation. Batteries 2023, 9, 64. [Google Scholar] [CrossRef]
- Spotnitz, R. Simulation of capacity fade in lithium-ion batteries. J. Power Sources 2003, 113, 72–80. [Google Scholar] [CrossRef]
- Niu, B.; Shanshan, E.; Song, Q.; Xu, Z.; Han, B.; Qin, Y. Physicochemical reactions in e-waste recycling. Nat. Rev. Chem. 2024, 8, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, H.-J.; Taylor, P.; Aleksandrov, K.; Bergdolt, P.; Bologa, A.; Blye, D.; Dalal, P.; Gunasekar, P.; Herremanns, S.; Kapoor, D.; et al. Mineralization of fluoropolymers from combustion in a pilot plant under representative european municipal and hazardous waste combustor conditions. Chemosphere 2024, 365, 143403. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Zhang, B.; Tran, P.; Zhang, J.; Balasubramanian, R. Recycling of spent lithium-ion batteries for a sustainable future: Recent advancements. Chem. Soc. Rev. 2024, 53, 5552–5592. [Google Scholar] [CrossRef]
- Królicka, A.; Maj, A.; Łój, G. Application of laser-induced breakdown spectroscopy for depth profiling of multilayer and graded materials. Materials 2023, 16, 6641. [Google Scholar] [CrossRef]
- Legnaioli, S.; Campanella, B.; Poggialini, F.; Pagnotta, S.; Harith, M.; Abdel-Salam, Z.; Palleschi, V. Industrial applications of laser-induced breakdown spectroscopy: A review. Anal. Methods 2020, 12, 1014–1029. [Google Scholar] [CrossRef]
- Cui, M.; Xiong, S.; Yang, N.; Wang, Y.; Wang, Z.; Luo, M.; Yao, C.; Deguchi, Y. Applications of laser-induced breakdown spectroscopy in industrial measurement and monitoring: Multi-technology combination. Appl. Spectrosc. Rev. 2024. [Google Scholar] [CrossRef]
- Costa, V.; de Mello, M.; Babos, D.; Castro, J.; Pereira-Filho, E. Calibration strategies for determination of Pb content in recycled polypropylene from car batteries using laser-induced breakdown spectroscopy (LIBS). Microchem. J. 2020, 159, 105558. [Google Scholar] [CrossRef]
- Peng, L.; Sun, D.; Su, M.; Han, J.; Dong, C. Rapid analysis on the heavy metal content of spent zinc-manganese batteries by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2012, 44, 2469–2475. [Google Scholar] [CrossRef]
- Paradis, M.; Doucet, F.; Rousselot, S.; Hernández-García, A.; Rifai, K.; Touag, O.; Özcan, L.; Azami, N.; Dollé, M. Deep Learning Classification of Li-Ion Battery Materials Targeting Accurate Composition Classification from Laser-Induced Breakdown Spectroscopy High-Speed Analyses. Batteries 2022, 8, 231. [Google Scholar] [CrossRef]
- Królicka, A.; Maj, A.; Łój, G.; Murzyn, P.; Mochalski, P. Atypical methods for characterization of used photovoltaic panels during their pre- and Post-Thermal treatment assessment. Waste Manag. 2024, 175, 315–327. [Google Scholar] [CrossRef]
- Rouhi, H.; Serna-Guerrero, R.; Santasalo-Aarnio, A. Electrochemical discharge of Li-ion batteries—A methodology to evaluate the potential of discharge electrolytes without corrosion. J. Energy Storage 2022, 55, 105734. [Google Scholar] [CrossRef]
- Torabian, M.; Jafari, M.; Bazargan, A. Discharge of lithium-ion batteries in salt solutions for safer storage, transport, and resource recovery. Waste Manag. Res. 2022, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Pan, Y.; Wang, J.; Liu, H.; Dou, S. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Ahn, K. Role of carboxymethyl cellulose binder and its effect on the preparation process of anode slurries for Li-ion batteries. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 664, 131130. [Google Scholar] [CrossRef]
- Park, J.; Willenbacher, N.; Ahn, K. How the interaction between styrene-butadiene-rubber (SBR) binder and a secondary fluid affects the rheology, microstructure and adhesive properties of capillary-suspension-type graphite slurries used for Li-ion battery anodes. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 579, 123692. [Google Scholar] [CrossRef]
- Gallego, N.; Contescu, C.; Meyer, H.; Howe, J.; Meisner, R.; Payzant, E.; Lance, M.; Yoon, S.; Denlinger, M.; Wood, D. Advanced surface and microstructural characterization of natural graphite anodes for lithium ion batteries. Carbon 2014, 72, 393–401. [Google Scholar] [CrossRef]
- Nam, K.; Cho, J.; Yeo, H. Thermomechanical Behavior of Polymer Composites Based on Edge-Selectively Functionalized Graphene Nanosheets. Polymers 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Imashuku, S.; Taguchi, H.; Fujieda, S.; Suzuki, S.; Wagatsuma, K. Three-dimensional lithium mapping of graphite anode using laser-induced breakdown spectroscopy. Electrochim. Acta 2019, 293, 78–83. [Google Scholar] [CrossRef]
- Zheng, Y.; Pfäffl, L.; Seifert, H.; Pfleging, W. Lithium Distribution in Structured Graphite Anodes Investigated by Laser-Induced Breakdown Spectroscopy. Appl. Sci. 2019, 9, 4218. [Google Scholar] [CrossRef]
- Petz, D.; Baran, V.; Park, J.; Schökel, A.; Kriele, A.; Kornmeier, J.; Paulmann, C.; Koch, M.; Nilges, T.; Müller-Buschbaum, P.; et al. Heterogeneity of Lithium Distribution in the Graphite Anode of 21700-Type Cylindrical Li-Ion Cells during Degradation. Batteries 2024, 10, 68. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.; Wang, W.; Cordeiro, I.; Wang, C.; Chen, T.; Zhang, J.; Chan, Q.; Yeoh, G. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.; Lee, T. The wettability of fluoropolymer surfaces: Influence of surface dipoles. Langmuir 2008, 24, 4817–4826. [Google Scholar] [CrossRef] [PubMed]
- Zdziennicka, A.; Krawczyk, J.; Szymczyk, K.; Janczuk, B. Components and parameters of liquids and some polymers surface tension at different temperature. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 529, 864–875. [Google Scholar] [CrossRef]
- Hawley, W.; Parejiya, A.; Bai, Y.; Meyer, H.; Wood, D.; Li, J. Lithium and transition metal dissolution due to aqueous processing in lithium-ion battery cathode active materials. J. Power Sources 2020, 466, 228315. [Google Scholar] [CrossRef]
- Cui, X.; Feng, H.; Xue, Y.; Geng, S.; Li, S. Convenient synthesis and electrochemical performance investigation of nano-sized LiMn2O4. J. Mater. Sci.-Mater. Electron. 2017, 28, 8529–8536. [Google Scholar] [CrossRef]
- Tian, L.; Yuan, A. Electrochemical performance of nanostructured spinel LiMn2O4 in different aqueous electrolytes. J. Power Sources 2009, 192, 693–697. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Li, S.; Geng, S.; Zhao, J. Effects of different precipitants on LiNi0.5Mn1.5O4 for lithium ion batteries prepared by modified co-precipitation method. Ionics 2017, 23, 2993–2999. [Google Scholar] [CrossRef]
- Lobo, L.; Kumar, A. Synthesis, structural and electrical properties of spinel LiNi0.5Mn1.5O4 synthesized by sol-gel method. J. Sol-Gel Sci. Technol. 2016, 80, 821–826. [Google Scholar] [CrossRef]
- Chen, R.; Mei, J.; Xu, J.; Xu, W.; Wang, L.; Chen, Y.; Peng, D. Preparation of LiNi0.5Mn1.5O4 cathode materials by using different-sized Mn3O4 nanocrystals as precursors. J. Solid State Electrochem. 2022, 26, 1359–1368. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Wu, B.; Lin, W.; Li, J.; Zhao, J. A homogeneous intergrown material of LiMn2O4 and LiNi0.5Mn1.5O4 as a cathode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 2353–2360. [Google Scholar] [CrossRef]
- Okudur, F.; Mylavarapu, S.; Safari, M.; De Sloovere, D.; D’Haen, J.; Joos, B.; Kaliyappan, P.; Kelchtermans, A.; Samyn, P.; Van Bael, M.; et al. LiNi0.5Mn1.5O4−δ (LNMO) as Co-free cathode for lithium ion batteries via solution-gel synthesis: Particle size and morphology investigation. J. Alloys Compd. 2022, 892, 162175. [Google Scholar] [CrossRef]
- Jin, J.; Wei, J.; Zhou, Z.; Xie, Z. Application of 5V spinel material LiNi0.5Mn1.5O4 in Li-ion batteries: Single crystalline or polycrystalline? Rsc. Adv. 2023, 13, 12394–12401. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M. An investigation of the lattice parameter and micro-strain behaviour of LiMn2O4 coated with LiMn1.5Ni0.5O4 to attain high-rate capability and cycling stability. J. Energy Storage 2023, 72, 108602. [Google Scholar] [CrossRef]
- Potapenko, A.; Kirillov, S. Enhancing high-rate electrochemical properties of LiMn2O4 in a LiMn2O4LiNi0.5Mn1.5O4 core/shell composite. Electrochim. Acta 2017, 258, 9–16. [Google Scholar] [CrossRef]
- Künne, S.; Hesper, J.; Lein, T.; Voigt, K.; Mikhailova, D.; Michaelis, A.; Winter, M.; Placke, T.; Heubner, C. Hybrid High-Voltage LiNi0.5Mn1.5O4 Graphite Cathodes Enabling Rechargeable Batteries with Simultaneous Anion- and Cation Storage. Batter. Supercaps 2023, 6, e202300284. [Google Scholar] [CrossRef]
- Chen, K.; Barai, P.; Kahvecioglu, O.; Wu, L.; Pupek, K.; Ge, M.; Ma, L.; Ehrlich, S.; Zhong, H.; Zhu, Y.; et al. Cobalt-free composite-structured cathodes with lithium-stoichiometry control for sustainable lithium-ion batteries. Nat. Commun. 2024, 15, 430. [Google Scholar] [CrossRef] [PubMed]


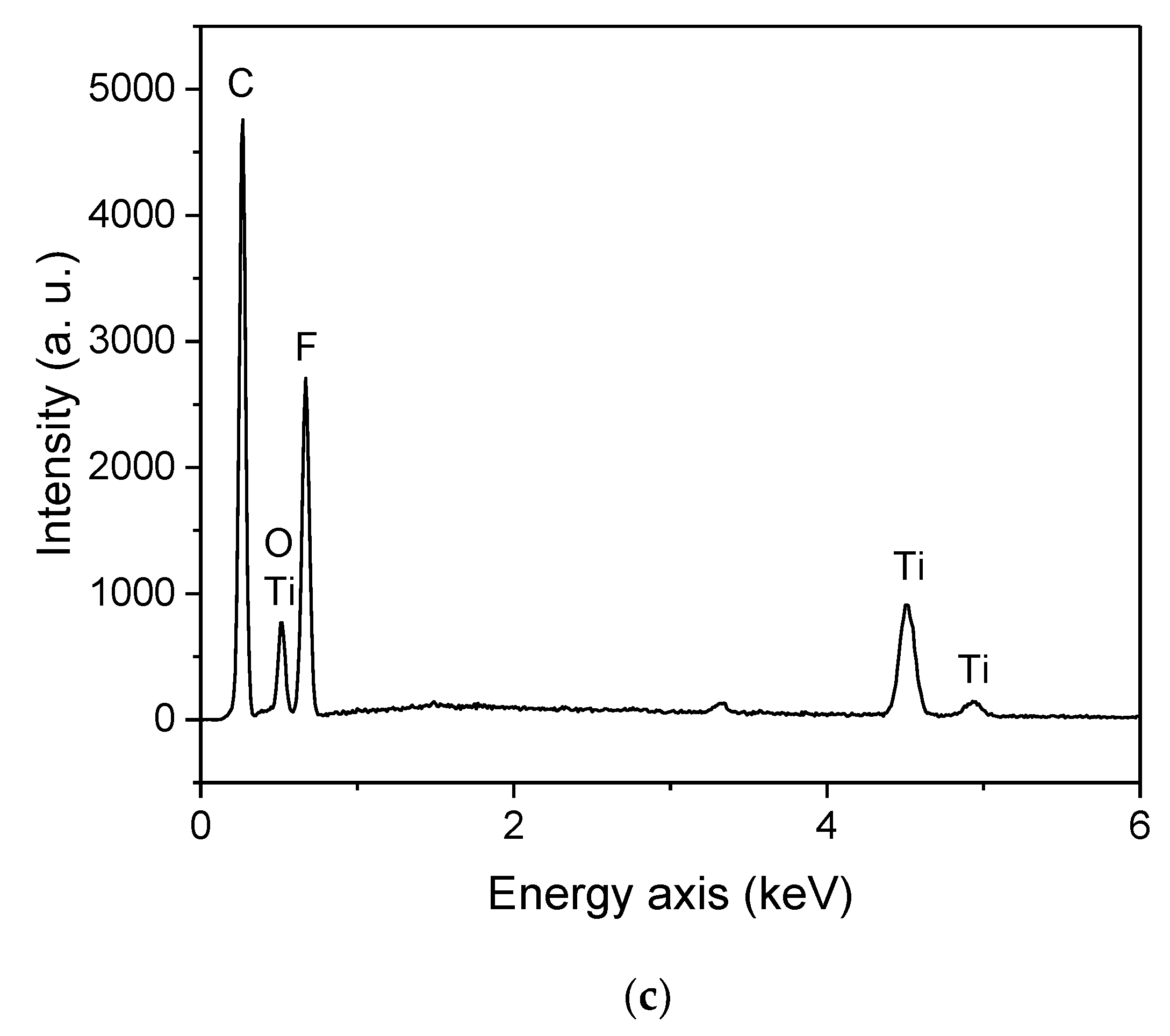
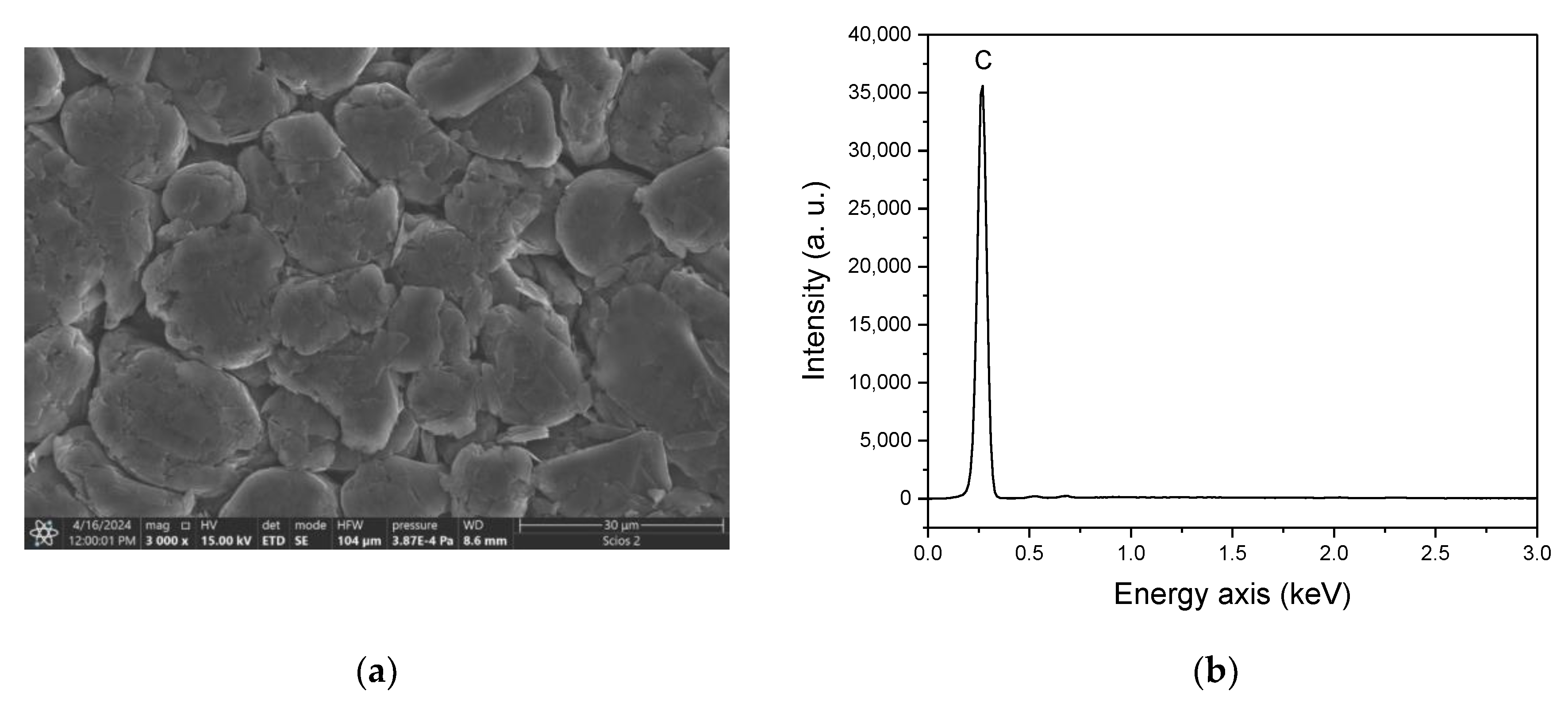
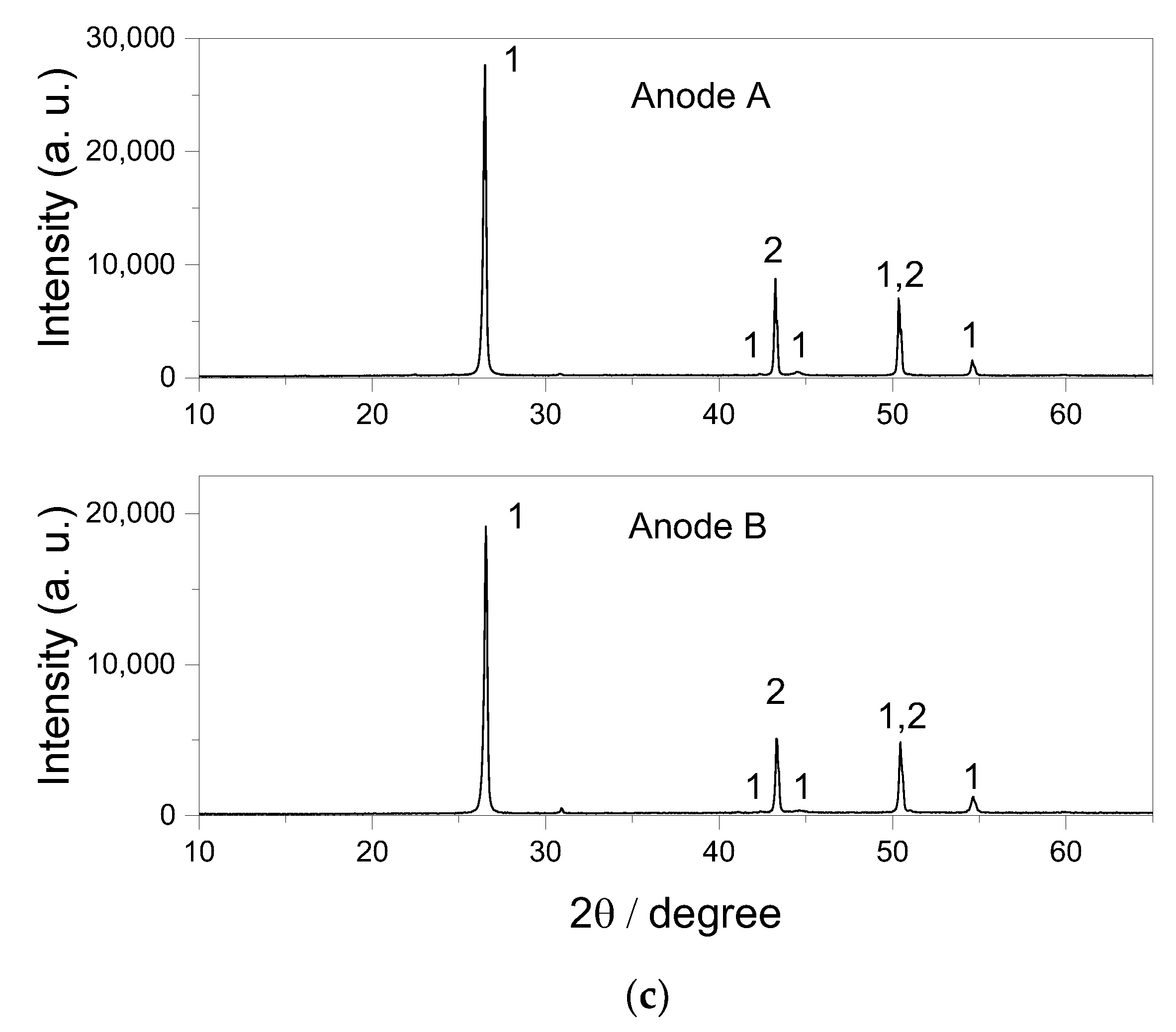
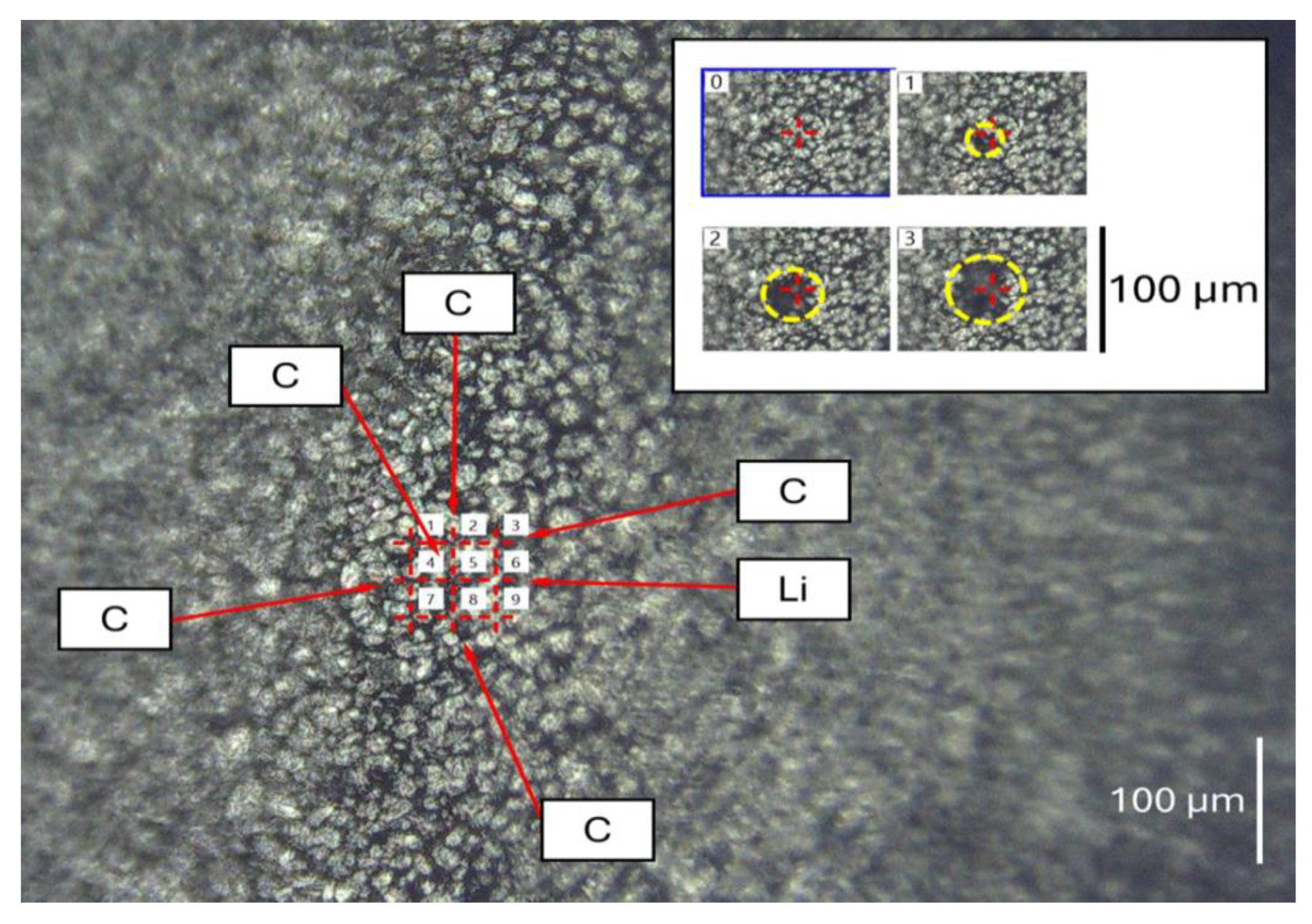


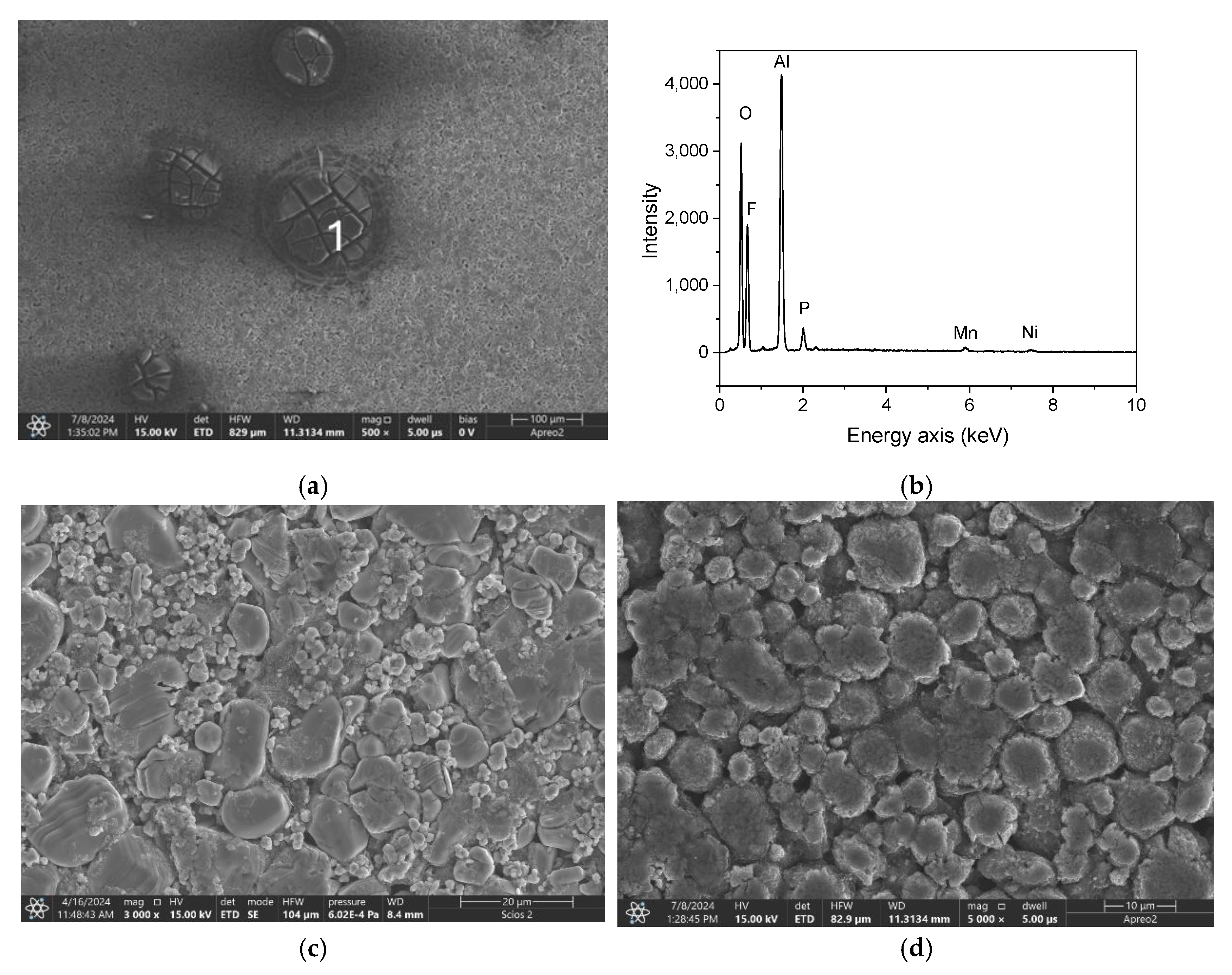



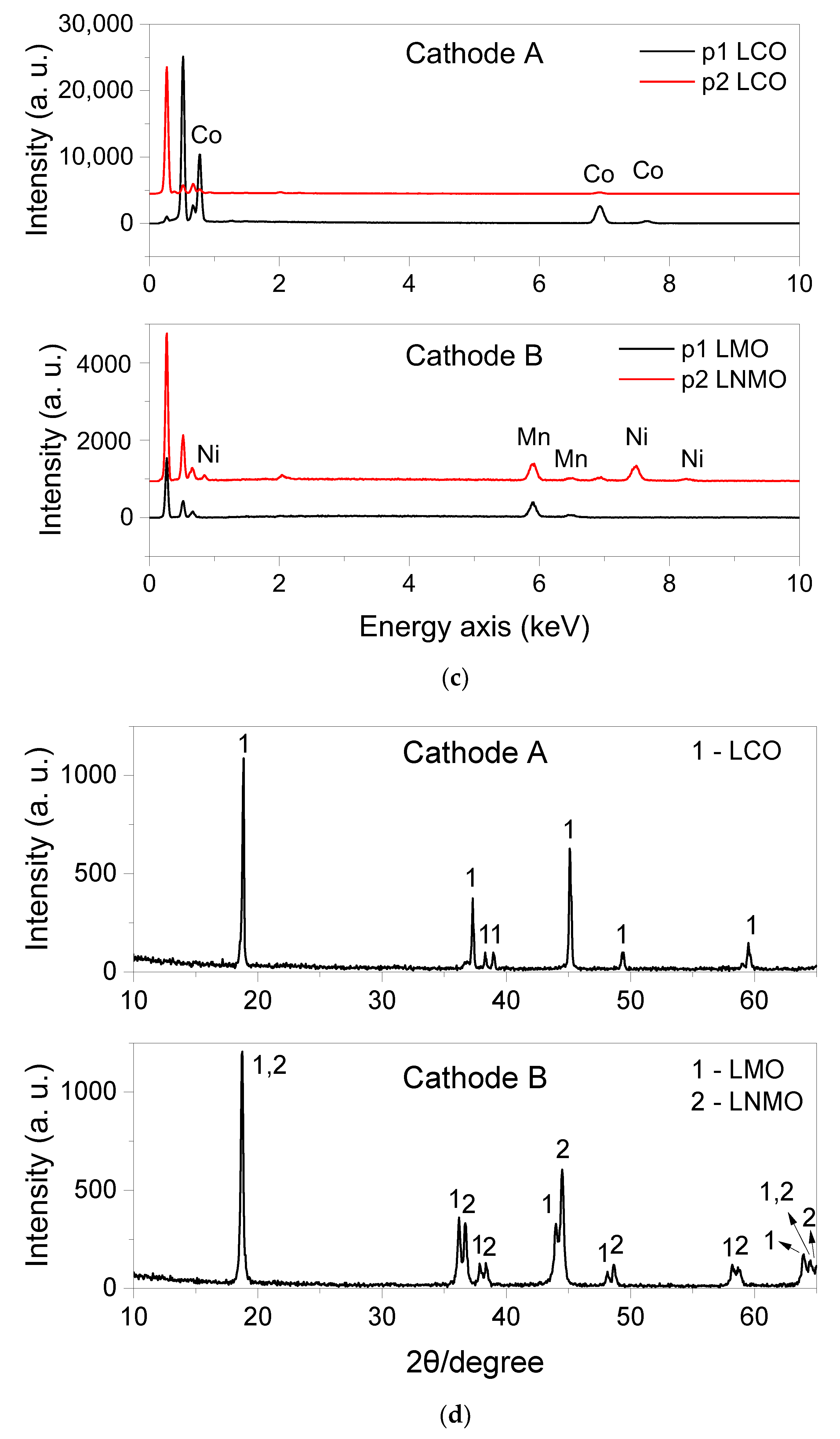
| Ablation | Laser Pulse Power | Crater Depth (mm) | Crater Diameter (mm) | Detected Elements |
|---|---|---|---|---|
| A | Weak | 1.0 ± 0.1 | 24 ± 2 | Ti, O |
| B | Normal | 1.9 ± 0.3 | 26 ± 2 | Ti, O, C, H |
| C | Strong | 6.7 ± 1.0 | 33 ± 1 | Ti, O, C, H, F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królicka, A.; Maj, A.; Łój, G. Promoting Sustainability in the Recycling of End-of-Life Photovoltaic Panels and Li-Ion Batteries Through LIBS-Assisted Waste Sorting. Sustainability 2025, 17, 838. https://doi.org/10.3390/su17030838
Królicka A, Maj A, Łój G. Promoting Sustainability in the Recycling of End-of-Life Photovoltaic Panels and Li-Ion Batteries Through LIBS-Assisted Waste Sorting. Sustainability. 2025; 17(3):838. https://doi.org/10.3390/su17030838
Chicago/Turabian StyleKrólicka, Agnieszka, Anna Maj, and Grzegorz Łój. 2025. "Promoting Sustainability in the Recycling of End-of-Life Photovoltaic Panels and Li-Ion Batteries Through LIBS-Assisted Waste Sorting" Sustainability 17, no. 3: 838. https://doi.org/10.3390/su17030838
APA StyleKrólicka, A., Maj, A., & Łój, G. (2025). Promoting Sustainability in the Recycling of End-of-Life Photovoltaic Panels and Li-Ion Batteries Through LIBS-Assisted Waste Sorting. Sustainability, 17(3), 838. https://doi.org/10.3390/su17030838






