Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing
Abstract
1. Introduction
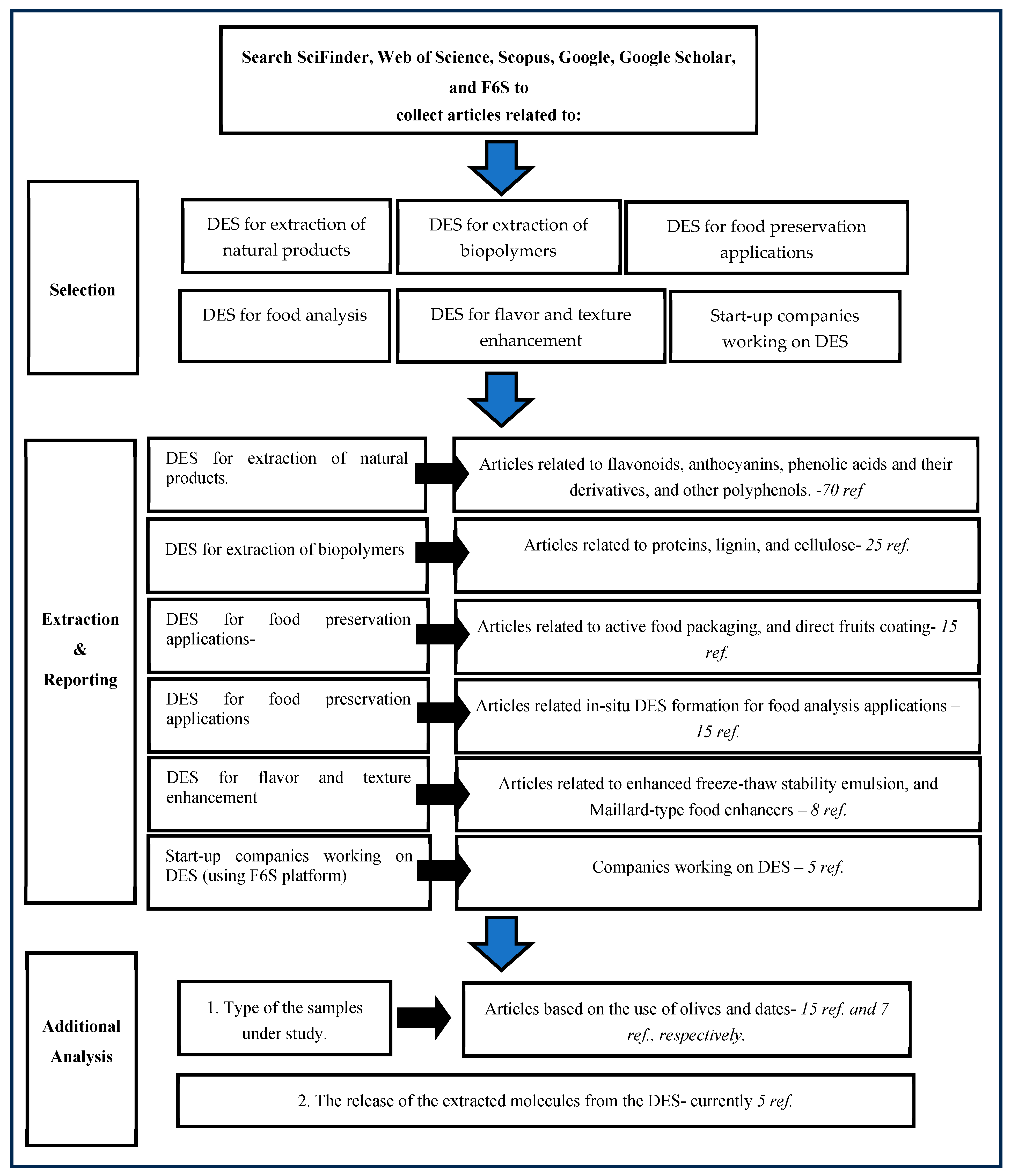
2. Methodology
3. Extraction of Biomolecules of Alimentary and Nutritional Value
3.1. Applications of Deep Eutectic Solvents in the Extraction of Phenols
3.1.1. Extraction of Flavonoids: Flavones, Flavanones, Flavanols, and Flavanonols
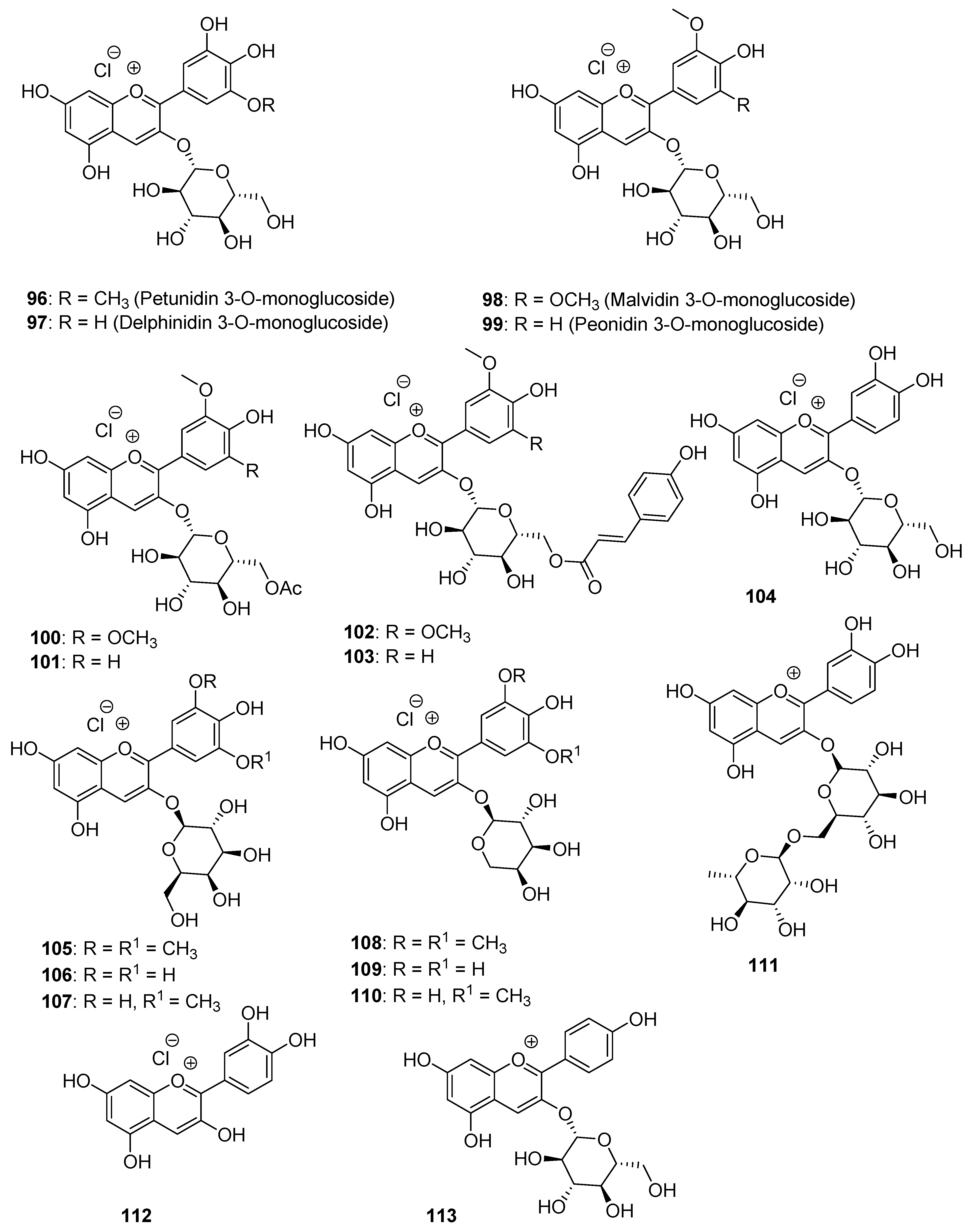
3.1.2. Extraction of Anthocyanins
3.1.3. Extraction of Phenolic Acids and Their Derivatives
3.1.4. Extraction of Polyphenols Other than Flavonoids and Phenolic Acids and Derivatives
3.1.5. DES-Mediated Extraction of Natural Compounds from Olives
3.1.6. DES-Mediated Extraction of Natural Compounds from Dates (Phoenix dactylifera L.)
3.1.7. Release of the Extracted Phenolic Compounds from DES
3.2. Applications of DESs for Biopolymer Extraction
3.2.1. Applications of DESs for Protein Extraction
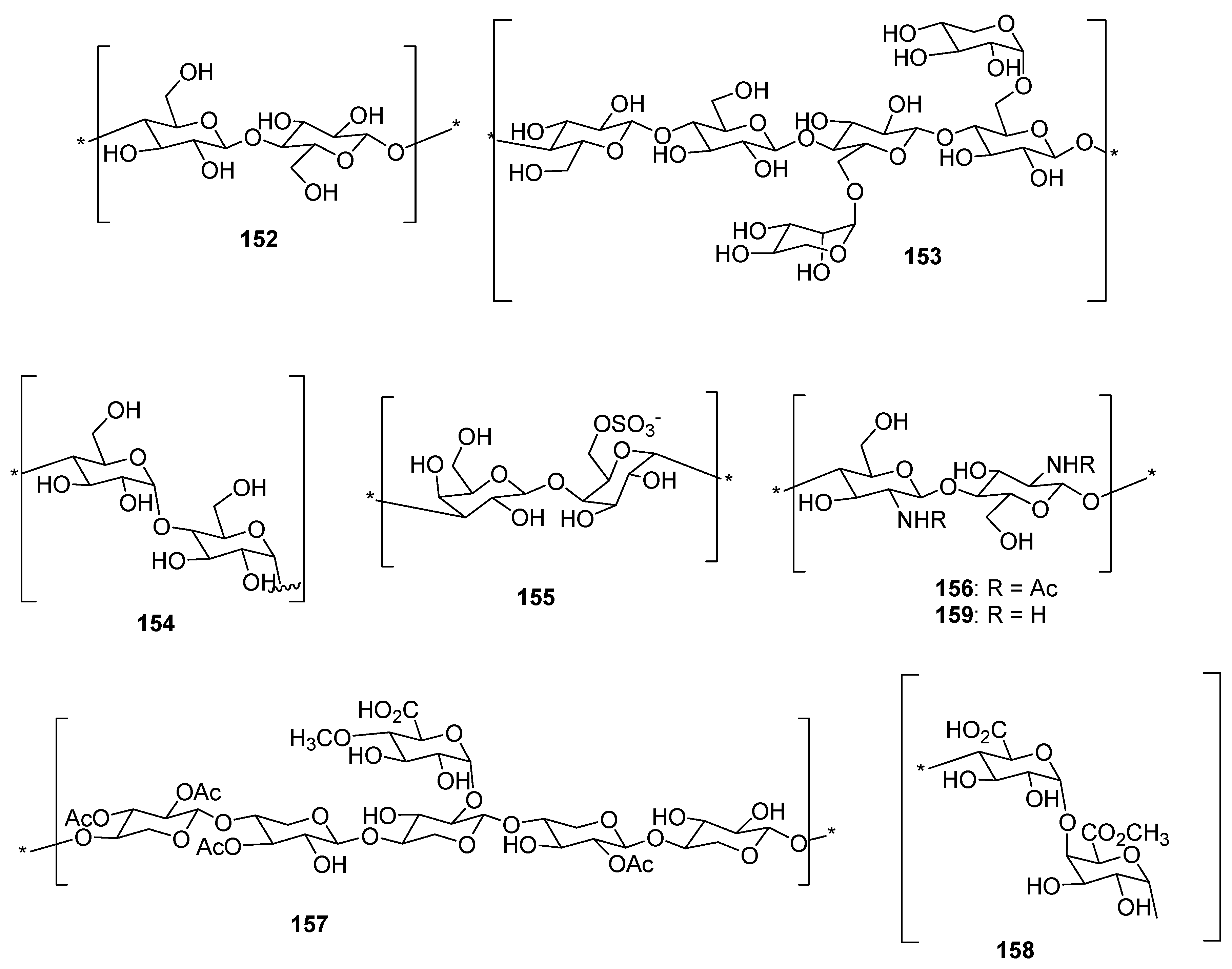
3.2.2. Biomass Utilization from Lignocellulosic Agricultural Waste Using DES for the Extraction of Keratin, Cellulose, Lignins, and Others
3.3. The Use of DES in Food Preservation
Deep Eutectic Solvents for Fruit Preservation Applications
3.4. In-Situ DES Formation for Food Analysis and the Purification of Food Additives
3.5. DESs for Flavor and Food Texture Enhancement
4. Considerations on the Commercialization of NADES
5. Shifting from Petroleum-Based Solvents to Green DES Solvents: Will Start-Up Companies Accelerate This Movement?
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAE | ascorbic acid equivalent(s) |
| ABTS | 2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid |
| AC | acetic acid |
| CE | cyanidin equivalent(s) |
| CGE | cyanidin-3-glucoside equivalent |
| ChCl | choline chloride |
| Cit | citric acid |
| CLSM | confocal laser scanning microscope |
| DES | deep eutectic solvent |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ECE | epicatechin equivalent |
| ESI-QAD-TOF | Electrospray ionization-quadrupole time-of-flight-mass spectrometry |
| GAE | gallic acid equivalent(s) |
| Gel | Gelatine |
| Gly | glycerine/glycerol |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HPLC-DAD-MS | high performance liquid chromatography–diode array detection–mass spectrometry |
| LA | lactic acid |
| LALP | lipid peroxidation inhibition assay |
| MA | malic acid |
| NADES | natural deep eutectic solvent |
| OAE | oleanolic acid equivalent(s) |
| Ox | oxalic acid |
| PCM | polarizable continuum model |
| PE | Polythene |
| PhMo | Phosphomolybdenum |
| Pro | Proline |
| QE | quercetin equivalent(s) |
| SML | specific migration limits |
| Sor | Sorbitol |
| TBAC | tetrabutylammonium chloride |
| TDES | ternary deep eutectic solvent |
| TE | Trolox equivalent(s) |
| TSS | total soluble solids |
| UHPLC | Ultra-high-performance liquid chromatography |
| Xyl | Xylitol |
| Xylo | Xylose |
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Xu, Z.; Zhu, Y.; Zhou, Y.; Wang, Z.; Liu, L.; Ren, W.; Xie, Y.; Li, C.; et al. The novel application of type II deep eutectic solvents (DES) for sludge dewatering. Sep. Purif. Technol. 2023, 306B, 122714. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Gontrani, L.; Donia, D.T.; Bauer, E.M.; Tagliatesta, P.; Carbone, M. Novel synthesis of zinc oxide nanoparticles from type IV deep eutectic solvents. Inorganica Chim. Acta 2023, 545, 121268. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Yong, W.F.; She, Q.; Esteban, J. Status and advances of deep eutectic solvents for metal separation and recovery. Green Chem. 2022, 24, 1859–1929. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, A.; Sharma, S.K.; Rawat, N.; Saini, D.; Sirohi, R.; Prakash, O.; Dubey, A.; Dutta, A.; Shahi, N.C. Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 2024, 10, e28784. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Z.-H.; Liu, L.-L.; Wang, H.; Yang, S.-H.; Zhang, J.-S.; Zhang, Y. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT 2021, 148, 111708. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharmacal Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.d.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, L.; Li, S.; Meng, T.; Wang, L.; Zhang, W. The effect of sonication-synergistic natural deep eutectic solvents on extraction yield, structural and physicochemical properties of pectins extracted from mango peels. Ultrason. Sonochemistry 2022, 86, 106045. [Google Scholar] [CrossRef]
- Chen, M.; Lahaye, M. Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocoll. 2021, 115, 106601. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Zhu, Z.; Yi, J.; Lan, Y.; Chen, B.; Rao, J. Structure and functionality of oat protein extracted by choline chloride–dihydric alcohol deep eutectic solvent and its water binary mixtures. Food Hydrocoll. 2021, 112, 106330. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.T.; Wei, Y.H.; Lan, J.C.W. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Hilt, P.; Streker, P.; Endreβ, H.-U.; Rentschler, C.; Carle, R. A new process of the combined recovery of pectin and phenolic compounds from apple waste. Innov. Food Sci. Emerg. Technol. 2003, 4, 99–107. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Food waste valorization. In Saving Food—Production, Supply Chain, Food Waste and Food Consumption; Academic Press: Cambridge, MA, USA, 2019; pp. 279–313. [Google Scholar] [CrossRef]
- Morgana, N.M.; Magdalena, E.; Fernandez, M.A.; Fernanda, S.M. NADES for food industry innovation: Novel bioadditives based on olive oil byproducts. Food Bioprod. Process. 2022, 134, 193–201. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Dragičević, T.L.; Radojčić Redovniković, I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.-S. Toxicity test profile for deep eutectic solvents: A detailed review and future prospects. Chemosphere 2024, 350, 141097. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Aktaş¸, H.; Kurek, M.A. Deep eutectic solvents for the extraction of polyphenols from food plants. Food Chem. 2024, 444, 138629. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from Agri-food waste biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Fernández, M.D.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Teo, Y.L.; Ho, H.K.; Chan, A. Metabolism-related pharmacokinetic drug-drug interactions with tyrosine kinase inhibitors: Current understanding, challenges and recommendations. Br. J. Clin. Pharmacol. 2015, 79, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sloane, B.F. Cathepsin B and cystatins: Evidence for a role in cancer progression. Semin. Cancer Biol. 1990, 1, 137–152. [Google Scholar] [PubMed]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmentally friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvent extraction of flavonoids of Scutellaria baicalensis as a replacement for conventional organic solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef]
- Vo, T.P.; Ho, M.T.; Nguyen, P.U.N.; Pham, N.D.; Truong, K.V.; Nguyen, T.H.Y.; Nguyen, D.Q.; Vo, T.T.H. Extracting phenolics, flavonoids, and terpenoids from Codonopsis pilosula using green solvents. Sustain. Chem. Pharm. 2024, 37, 101395. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Fang, J.; Song, Z.; Geng, J.; Zhao, J.; Fang, Y.; Wang, C.; Li, M. Green and efficient extraction of flavonoids from Perilla frutescens (L.) Britt. leaves based on natural deep eutectic solvents: Process optimization, component identification, and biological activity. Food Chem. 2024, 452, 139508. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Garg, M.; Kaur, S.; Sharma, A.; Kumari, A.; Tiwari, V.; Sharma, S.; Kapoor, P.; Sheoran, B.; Goyal, A.; Krishania, M. Rising demand for healthy foods-anthocyanin biofortified colored wheat is a new research trend. Frontiers in Nutrition, Sec. Nutr. Food Sci. Technol. 2022, 9, 878221. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources. Scientific opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar] [CrossRef]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs): Designer solvents for green extraction of anthocyanin. Sustain. Chem. Pharm. 2023, 4, 101168. [Google Scholar] [CrossRef]
- Guo, N.; Ping-Kou Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.T.; Pauletto, R.; Cavalheiro, S.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp.) using natural deep eutectic solvents. LWT 2022, 158, 113184. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Bochi, V.C.; Godoy, H.T.; Giusti, M.M. Anthocyanin and other phenolic compounds in Ceylon gooseberry (Dovyalis hebecarpa) fruits. Food Chem. 2015, 176, 234–243. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J. Degradation kinetics modeling of antioxidant compounds from the wastes of Mangifera pajang fruit in aqueous and choline chloride/ascorbic acid natural deep eutectic solvent. J. Food Eng. 2021, 294, 110401. [Google Scholar] [CrossRef]
- Heitmann, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2016, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Tang, B.; Row, K.H. Application of deep eutectic solvents as additives in ultrasonic extraction of two phenolic Acids from Herba Artemisiae Scopariae. Anal. Lett. 2014, 47, 1476–1484. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Huang, Q.; Fu, Y.; Chang, J. A quick selection of natural deep eutectic solvents for the extraction of chlorogenic acid from herba artemisiae scopariae. RSC Adv. 2020, 10, 22403–22409. [Google Scholar] [CrossRef]
- Yuniarti, E.; Saputri, F.C.; Mun’im, A. Natural deep eutectic solvent extraction and evaluation of caffeine and chlorogenic acid from green coffee beans of Coffea canephora. Indian J. Pharm. Sci. 2019, 81, 1062–1069. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Rachmawati Putri, F.A.; Puspita, F.; Prihadi, A.R. Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Fanali, C.; Posta, S.D.; Dugo, L.; Gentili, A.; Mondello, L.; de Gara, L. Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J. Pharm. Biomed. Anal. 2020, 189, 113421. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Ramos, G.S.M.; Carvalho, M.G.O.; Koblitz, M.G.B. Natural deep eutectic solvents characteristics determine their extracting and protective power on chlorogenic acids from sunflower meal. Sustain. Chem. Pharm. 2024, 37, 101430. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic acid intake guidance: Sources, health benefits, and safety. Asian Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef]
- Silva, C.N.d.; Silva, R.M.d.; Lemes, A.C.; Ribeiro, B.D. Recovery of phenolic compounds by deep eutectic solvents in orange by-products and spent coffee grounds. Sustainability 2024, 16, 7403. [Google Scholar] [CrossRef]
- Le, N.T.; Hoang, N.T.; Van, V.T.T.; Nguyen, T.P.D.; Chau, N.H.T.; Le, N.T.N.; Le, H.B.T.; Phung, H.T.; Nguyen, H.T.; Nguyen, H.M. Extraction of curcumin from turmeric residue (Curcuma longa L.) using deep eutectic solvents and surfactant solvents. Analytical Methods 2022, 14, 850–858. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crops Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Patil, S.S.; Pathak, A.; Rathod, V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochemistry 2021, 70, 105267. [Google Scholar] [CrossRef] [PubMed]
- Abouheif, S.A.; Sallam, S.M.; El Sohafy, S.M.; Kassem, F.F.; Shawky, E. A green extraction approach using natural deep eutectic solvents enhances the in-vivo bioavailability of curcuminoids from turmeric extracts. Ind. Crops Prod. 2022, 189, 115790. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural deep eutectic solvents as agents for improving solubility, stability and delivery of curcumin. Pharm. Res. 2019, 36, 119. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Tsolis, T.; Kyriakou, D.; Sifnaiou, E.; Thomos, D.; Glykos, D.; Tsiafoulis, C.G.; Garoufis, A. NMR Analysis of extra virgin olive oil of the Epirus region of Greece with emphasis on selected phenolic compounds. Molecules 2024, 29, 1111. [Google Scholar] [CrossRef]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef]
- Djaoudene, O.; Louaileche, H. Optimization of a green ultrasound-assisted extraction of phenolics and in vitro antioxidant potential of date fruit (Phoenix dactylifera L.). The Annals of the University Dunarea De Jos of Galati. Fascicle VI-Food Technol. 2018, 42, 109–122. [Google Scholar]
- Djaoudene, O.; Bachir-Bey, M.; Schisano, C.; Djebari, S.; Tenore, G.C.; Romano, A. A Sustainable extraction approach of phytochemicals from date (Phoenix dactylifera L.) fruit cultivars using ultrasound-assisted deep eutectic solvent: A comprehensive study on bioactivity and phenolic variability. Antioxidants 2024, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Samarawira, I. Date palm, potential source for refined sugar. Econ. Bot. 1983, 37, 181–186. [Google Scholar] [CrossRef]
- El Sharnouby, G.A.; Aleid, S.M.; Al-Otaibi, M.M. Liquid sugar extraction from date palm (Phoenix dactylifera L.) fruits. J. Food Process. Technol. 2014, 5, 1000402. [Google Scholar] [CrossRef]
- AlYammahi, J.; Darwish, A.S.; Almustafa, G.; Lemaoui, T.; AlNashef, I.M.; Hasan, S.W.; Taher, H.; Banat, F. Natural deep eutectic solvents for ultrasonic-assisted extraction of nutritious date sugar: Molecular screening, experimental, and prediction. Ultrason. Sonochemistry 2023, 98, 106514. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Mostafa, H.; Riaz, A.; Stathopoulos, C.; Maqsood, S. Natural deep eutectic solvents and microwave-assisted green extraction for efficient recovery of bioactive compounds from by-products of date fruit (Phoenix dactylifera L.). Processing: Modeling, optimization, and phenolic characterization. Food Bioprocess. Technol. 2023, 16, 824–843. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Mostafa, H.; Ranasinghe, M.; Maqsood, S. Influence of physicochemical properties of carboxylic acid-based natural deep eutectic solvents (CA-NADES) on extraction and stability of bioactive compounds from date (Phoenix dactylifera L.) seeds: An innovative and sustainable extraction technique. J. Mol. Liq. 2023, 388, 122767. [Google Scholar] [CrossRef]
- Morais, E.S.; Lopes, A.M.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharide dissolution and extractyion processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.S.; Schmatz, A.A.; de Freitas, C.; Masarin, F.; Brienzo, M. Fruit and restaurant waste polysaccharides recycling producing xylooligosaccharides. Recycling 2023, 8, 16. [Google Scholar] [CrossRef]
- Al Varqani, N.; Bastian, F. Review: Utilization of cellulose in food products. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012039. [Google Scholar] [CrossRef]
- Sabbaghi, H. Perspective Chapter: Cellulose in Food Production—Principles and Innovations; IntechOpen: London, UK, 2023; p. 109204. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Sethulekshmi, C. Chitin and its benefits. Int. J. Adv. Res. Biol. Sci. 2014, 1, 171–175. [Google Scholar]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.J.; Soares, N.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Z.; Hu, L.; Li, G.; Yao, Q.; Hu, Y. Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends Food Sci. Technol. 2021, 118A, 167–178. [Google Scholar] [CrossRef]
- Mika, L.; Cséfalvay, E.; Németh, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives—A review of recent advances in the production of furfural. Frontiers in Chemistry. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef]
- Dawsey, T.R.; McCormick, C.L. The lithium chloride/dimethylacetamide solvent for cellulose: A literature review. J. Macromol. Sci.—Rev. Macromol. Chem. Phys. 1990, 30, 405–440. [Google Scholar] [CrossRef]
- Radosta, S.; Haberer, M.; Vorwerg, W. Molecular characteristics of amylose and starch in dimethyl sulfoxide. Biomacromolecules 2001, 2, 970–978. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.A.; Laver, M.L. Molecular weight characterization of wood pulp cellulose: Dissolution and size exclusion chromatography analysis. Tappi J. 1997, 80, 173. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promosing technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- De Bhowmick, C.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Prasath, V.A.; Pandiselvam, R. Deep eutectic solvent: An emerging trend for extraction of plant proteins. J. Mol. Liq. 2023, 389, 122887. [Google Scholar] [CrossRef]
- Contreras, M.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verman, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Karimi, A.; Bhowmik, P.; Yang, T.C.; Samaranayaka, A.; Chen, L. Extraction of canola protein via natural deep eutectic solvents compared to alkaline treatments: Isolate characteristics and protein structural and functional properties. Food Hydrocoll. 2024, 152, 109922. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2013, 52, 676–684. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep eutectic solvents as new media for green extraction of food proteins: Opportunity and challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; House, J.D.; Aluko, R.E.; Bandara, N. Improved protein extraction technology using deep eutectic solvent system for producing high purity fava bean protein isolates at mild conditions. Food Hydrocoll. 2024, 147, 109283. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochemistry 2014, 21, 216–225. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, M.; Zheng, Y. Lignin biorefinery: Lignin source, isolation, characterization, and bioconversion. In Advances in Bioenergy; Li, Y., Zhou, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 6; Volume 7, pp. 211–270. [Google Scholar] [CrossRef]
- Pang, C.; Xie, T.; Lin, L.; Zhuang, J.; Liu, Y.; Shi, J.; Yang, Q. Changes of the surface structure of corn stalk in the cooking process with active oxygen and MgO-based solid alkali as a pretreatment of its biomass conversion. Bioresour. Technol. 2012, 103, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Moccia, F.; Gallucci, N.; Giovando, S.; Zuorro, A.; Lavecchia, R.; D’Errico, G.; Panzella, L.; Napolitano, A. A tunable deep eutectic solvent-based processing for valorization of chestnut wood fiber as a source of ellagic acid and lignin. J. Environ. Chem. Eng. 2022, 10, 107773. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Hou, Q.; Liu, H.; Lei, H.; Jian, B.; Li, X. Preparation of cellulose nanofibrils from okara by high pressure homogenization method using deep eutectic solvents. Cellulose 2020, 27, 2511–2520. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Samani, E.S.; Jooyandeh, H.; Behbahani, B.A. Shelf-life extension of buffalo meat using Farsi gum edible coating containing Shirazi thyme essential oil. Food Sci. Nutr. 2022, 11, 1599. [Google Scholar] [CrossRef]
- Khaledian, S.; Basiri, S.; Shekarforoush, S. Shelf-life extension of pacific white shrimp using tragacanth gum-based coatings containing Persian lime peel (Citrus latifolia) extract. LWT 2021, 141, 110937. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Shankar, S.; Salmieri, S.; Lacroix, M. Influence of cellulose nanocrystals gellan gum-based coating on color and respiration rate of Agaricus bisporus mushrooms. J. Food Sci. 2021, 86, 420–425. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Kua, Y.L.; Chew, Z.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Insights on the potential of natural deep eutectic solvents (NADES) to fine-tune durian seed gum for use as edible food coating. Food Hydrocoll. 2022, 132, 107861. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, S.; Yan, L. Deep eutectic solvents eutectogels: Progress and challenges. Green Chem. Eng. 2021, 2, 359–371. [Google Scholar] [CrossRef]
- Panzer, M.J. Holding it together: Noncovalent cross-linking strategies for ionogels and eutectogels. Mater. Adv. 2022, 3, 7709–7725. [Google Scholar] [CrossRef]
- Zeng, C.; Zhao, H.; Wan, Z.; Xiao, Q.; Xia, H.; Guo, S. Highly biodegradable, thermostable eutectogels prepared by gelation of natural deep eutectic solvents using xanthan gum: Preparation and characterization. RSC Adv. 2020, 10, 28376. [Google Scholar] [CrossRef]
- Wan, H.; Zhu, Z.; Sun, D.-W. Deep eutectic solvents (DESs) films based on gelatin as active packaging for moisture regulation in fruit preservation. Food Chem. 2024, 439, 138114. [Google Scholar] [CrossRef]
- Wan, H.; Zhu, Z.; Sun, D.-W. Buffering moisture variation during cherry tomatoes preservation through deep eutectic solvent films. Postharvest Biol. Technol. 2024, 218, 113171. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.; Barik, M.; Das, A.B. Effect of high amylose starch-natural deep eutectic solvent based edible coating on quality parameters of strawberry during storage. J. Agric. Food Res. 2023, 11, 100487. [Google Scholar] [CrossRef]
- Ortega-Zamora, C.; González-Sálamo, J.; Hernández-Borges, J. Deep eutectic solvents application in food analysis. Molecules 2021, 26, 6846. [Google Scholar] [CrossRef]
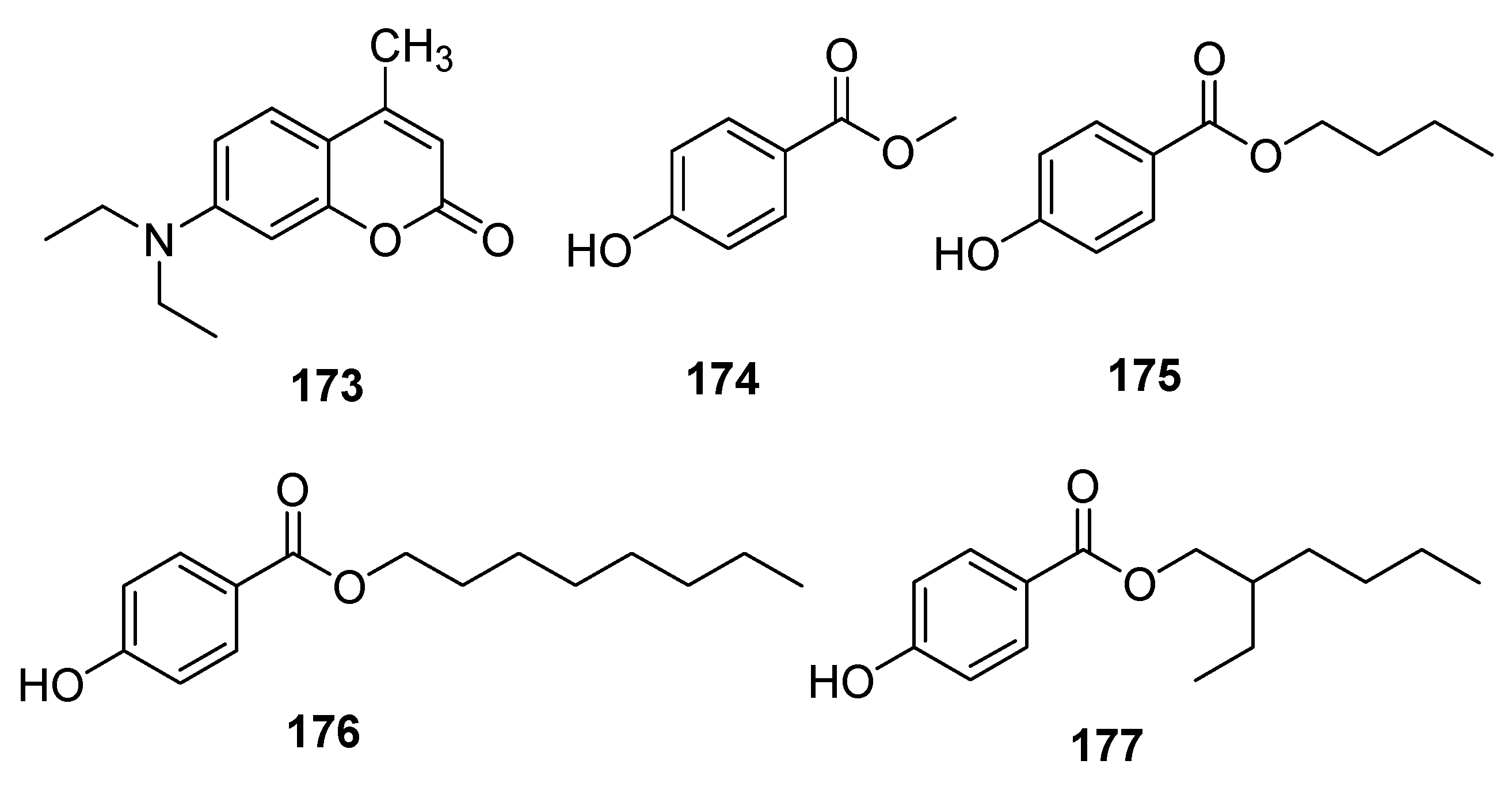
- Zhu, W.; Jin, P.; Yang, H.; Li, F.; Wang, C.; Li, T.; Fan, J. A green extraction strategy for the detection of antioxidants in food simulants and beverages migrated from plastic packaging materials. Food Chem. 2023, 406, 135060. [Google Scholar] [CrossRef]
- Xu, Y.; Jiao, Y.; Luo, J.; He, Z.; Zeng, M.; Shen, Q.W.; Chen, J.; Quan, W. The influence of deep eutectic solvents extract from ginger on the formation of heterocyclic amines and advanced glycation end products in roast beef patties. Foods 2022, 11, 3161. [Google Scholar] [CrossRef]
- Muncke, J. Tackling the toxics in plastics packaging. PLoS Biol. 2021, 19, e3000961. [Google Scholar] [CrossRef] [PubMed]
- GB 9685-2016; National Food Safety Standard: Standard for Uses of Additives in Food Contact Materials and Their Products. ChemSafetyPro.com: Shrewsbury, UK, 2016. Available online: https://chemsafetypro.com/Topics/Food_Contact/GB_9685_2016_National_Food_Safety_Standard_Standard_for_the_Use_of_Additives_in_Food_Contact_Materials_and_Articles.html (accessed on 20 October 2024).
- Shi, Y.; Li, X.; Shang, Y.; Li, T.; Zhang, K.; Fan, J. Effective extraction of fluorescent brightener 52 from foods by in situ formation of hydrophobic deep eutectic solvent. Food Chem. 2020, 311, 125870. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, K.; Yang, G.; Yu, J. A highly efficient microextraction technique based on deep eutectic solvent formed by choline chloride and p-cresol for simultaneous determination of lignans in sesame oils. Food Chem. 2019, 281, 140–146. [Google Scholar] [CrossRef]
- Shabani, E.; Zappi, D.; Berisha, L.; Dini, D.; Antonelli, M.L.; Sadun, C. Deep eutectic solvents (DES) as green extraction media for antioxidants electrochemical quantification in extra-virgin olive oils. Talanta 2020, 215, 120880. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ho Row, K.; Tang, W. Deep eutectic solvents based in situ isolation technique for extractive deterpenation of essential oils. Food Chem. 2024, 431, 137153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wu, M.; Cheng, H.; Chen, L.; Qi, Z. Deep deterpenation of citrus essential oils Intensified by in situ formation of a deep eutectic solvent in associative extraction. Ind. Eng. Chem. Res. 2020, 59, 9223–9232. [Google Scholar] [CrossRef]
- Liu, X.; Deng, Z.-Y.; Huang, Y.-Q.; Yanfg, X.-Q. Preparation of high freeze-thaw wheat gluten-based emulsions by incorporated deep eutectic solvents. Food Hydrocoll. 2020, 98, 105280. [Google Scholar] [CrossRef]
- Kranz, M.; Hofmann, T. Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES). Molecules 2018, 23, 261. [Google Scholar] [CrossRef]
- Troise, A.D.; Vitiello, D.; Tsang, C.; Fiore, A. Encapsulation of ascorbic acid promotes the reduction of Maillard reaction products in UHT milk. Food Funct. 2016, 7, 2591–2602. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Moccand, C.; Davidek, T.; Ghanbari, R.; Martiel, I.; Negrini, R.; Mezzenga, R.; Leser, M.E.; Blank, I.; Michel, M. Lipid self-assembled structures for reactivity control in food. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150136. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Choline Chloride Prices—Historical Graph. Available online: https://procurementtactics.com/choline-chloride-prices/ (accessed on 2 March 2025).
- Intratec. Ethylene Glycol Prices—Current and Forecast. 2025. Available online: https://www.intratec.us/chemical-markets/ethylene-glycol-price (accessed on 2 March 2025).
- International Sugar Organization. Daily Sugar Prices. 2025. Available online: https://www.isosugar.org/prices.php (accessed on 2 March 2025).
- Methanex. Pricing—Methanex. 2025. Available online: https://www.methanex.com/about-methanol/pricing/ (accessed on 2 March 2025).
- Statista. Wholesale Price of U.S. Omaha Ethanol From 1990 to 2023. 2025. Available online: https://www.statista.com/statistics/1440733/global-fuel-ethanol-benchmark-price/#:~:text=The%20world%20benchmark%20for%20fuel%20ethanol%20-%20U.S.,a%20high%20of%20%2470.75%20per%20hectoliter%20in%202022. (accessed on 2 March 2025).
- Procurement Resource. Dichloromethane Price Trend and Forecast. 2025. Available online: https://www.procurementresource.com/resource-center/dichloromethane-price-trends (accessed on 3 March 2025).
- IMARC Group. Hexane Prices Report Analysis. Available online: https://www.supermarketresearch.com/2025/03/03/hexane-prices-report/#:~:text=In%20December%202024%2C%20hexane%20prices%20in%20the%20United,of%20hexane%2C%20which%20helped%20avoid%20major%20price%20changes (accessed on 2 March 2025).
- Mišan, A.; Pojić, M. Applications of NADES in stabilizing food and protecting food compounds against oxidation. Adv. Bot. Res. 2021, 97, 333–356. [Google Scholar] [CrossRef]
- Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients (Recast) (Text with EEA Relevance)—Document 32009L0032. Available online: https://eur-lex.europa.eu/eli/dir/2009/32/oj/eng (accessed on 2 March 2025).
- Source Reduction Research Partnership Metropolitan, Water District of Southern California Environmental Defense Fund, Source Reduction of Chlorinated Solvents, Food Products Manufacture. 1991. Available online: https://p2infohouse.org/ref/19/18697.pdf (accessed on 2 March 2025).
- Trager, R. EPA Proposes ban on most uses of dichloromethane. ChemistryWorld. 2023, p. 4017332. Available online: https://www.chemistryworld.com/news/epa-proposes-ban-on-most-uses-of-dichloromethane/4017332.article (accessed on 2 March 2025).
- Hoffmann, C.; Sturm, C.; Rothman, A. The State of Change in Chemical Innovation; Boston Consulting Group: Boston, MA, USA, 2022; Available online: https://www.bcg.com/publications/2022/state-of-change-in-chemical-innovation (accessed on 28 February 2025).
- Cao, R.; Lueck, K.; Musso, C.; Sarathy, V. The Chemicals Industry of Tomorrow: Collaborate to Innovate; McKinsey & Company: New York, NY, USA, 2024; Available online: https://www.mckinsey.com/industries/chemicals/our-insights/the-chemicals-industry-of-tomorrow-collaborate-to-innovate#/ (accessed on 28 February 2025).
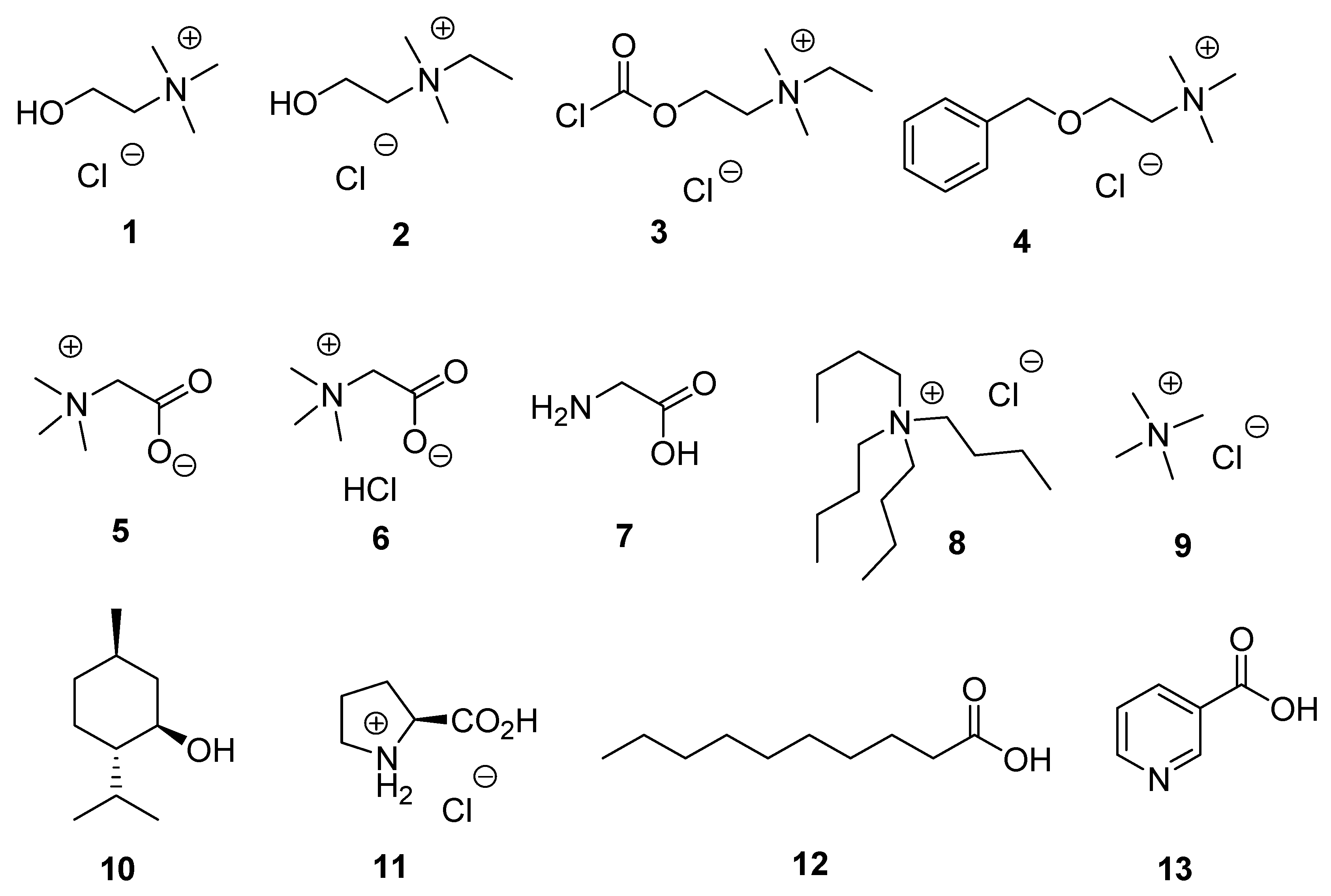

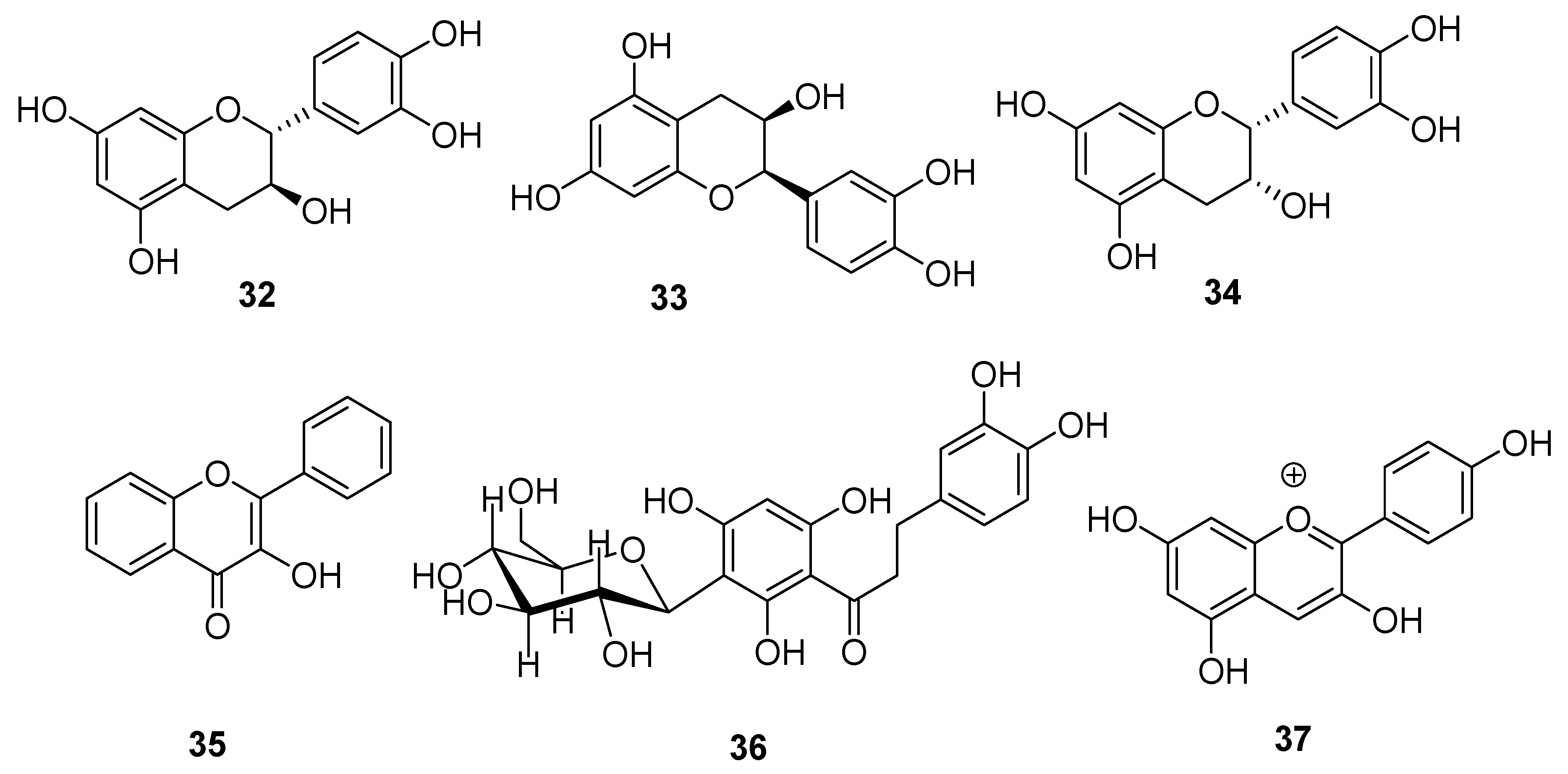
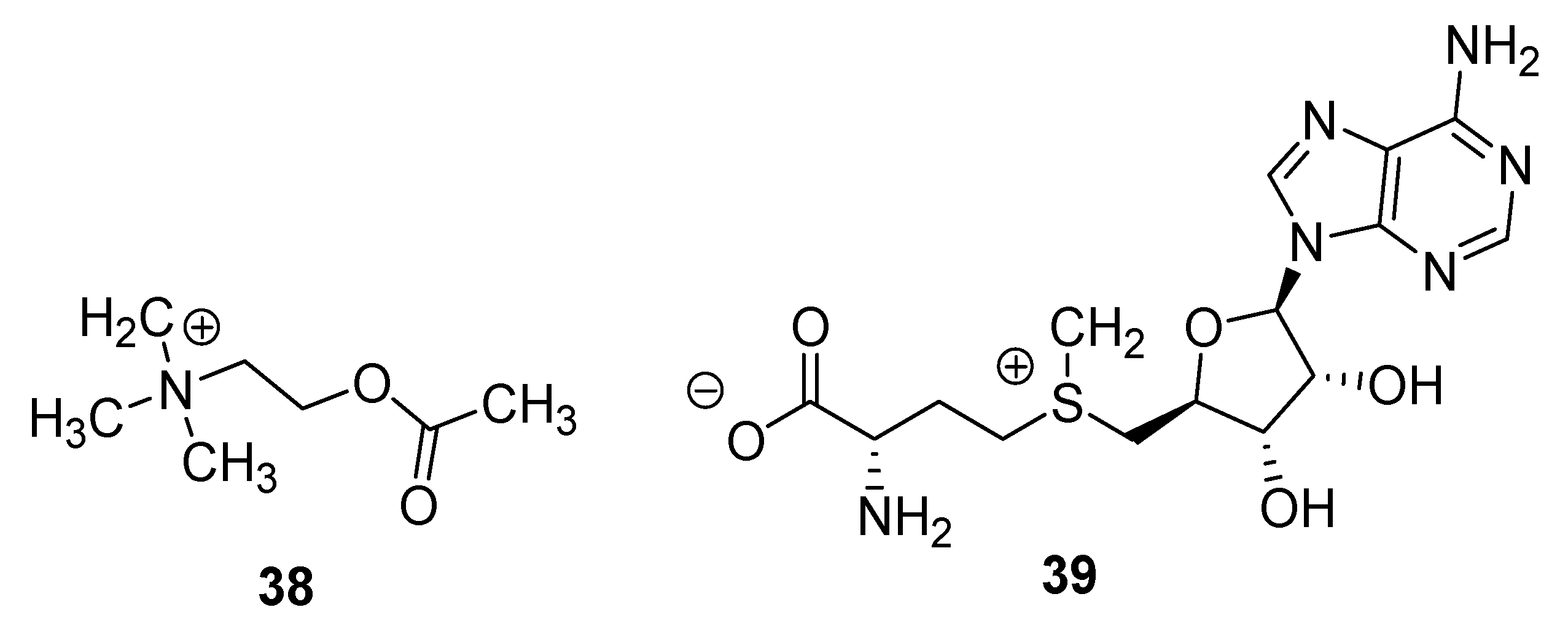


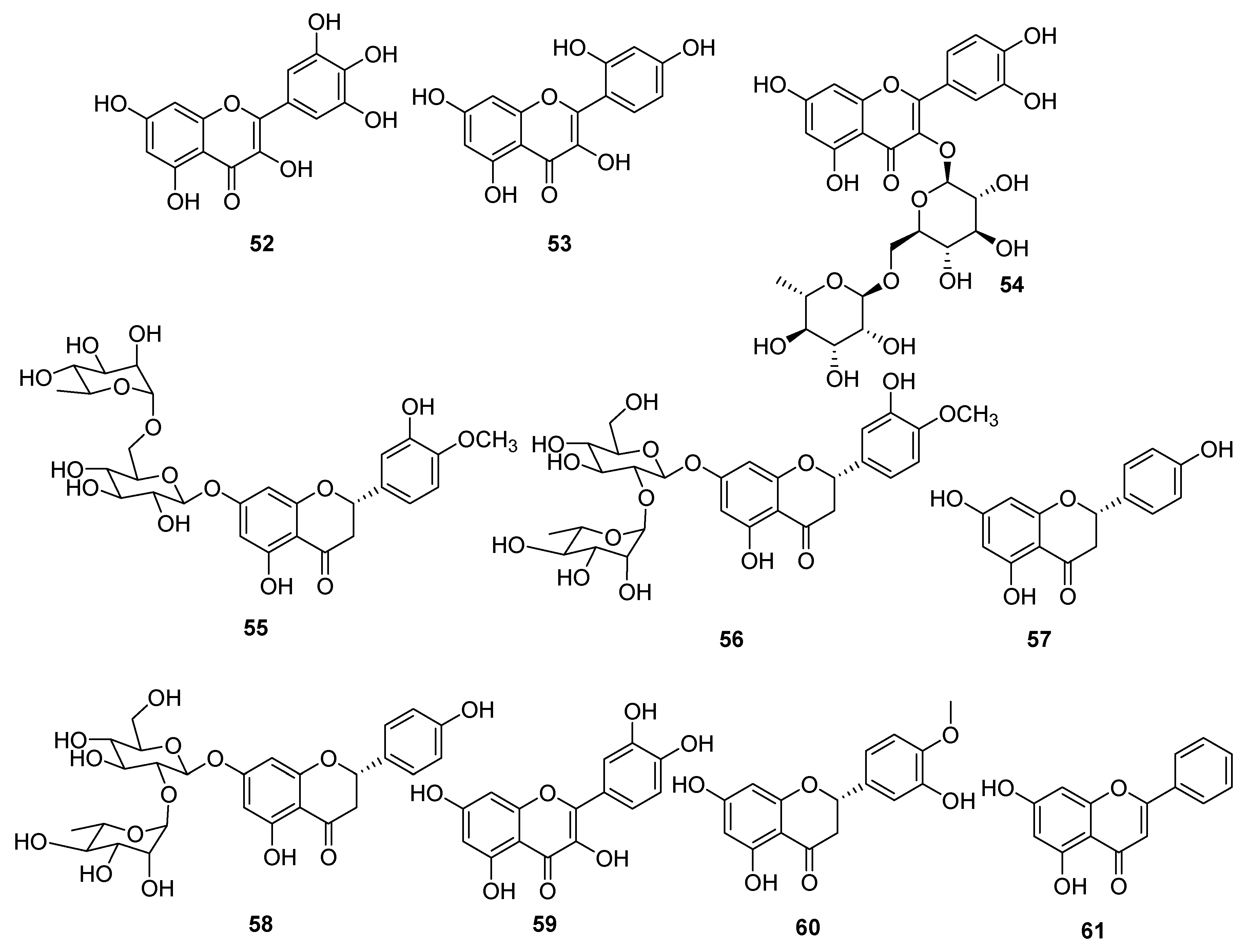




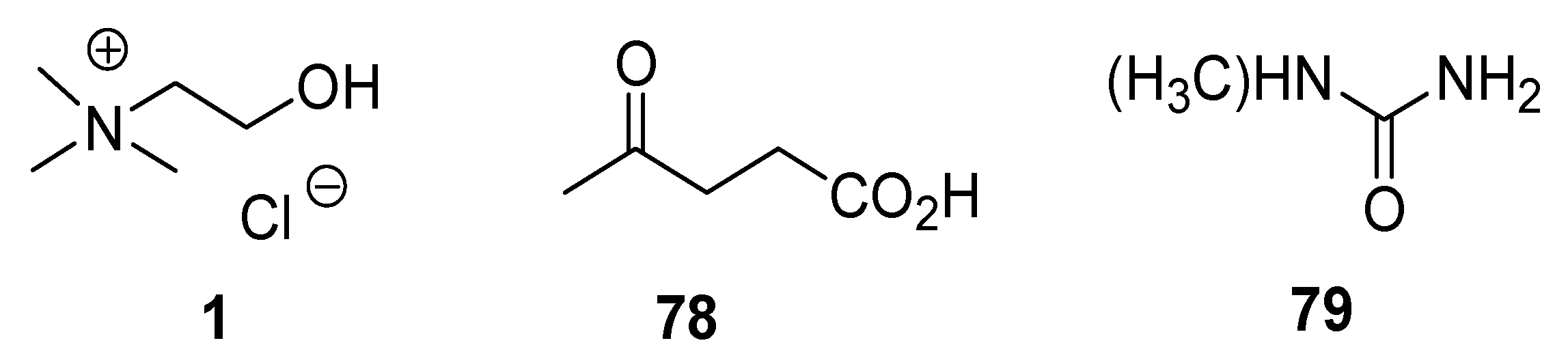
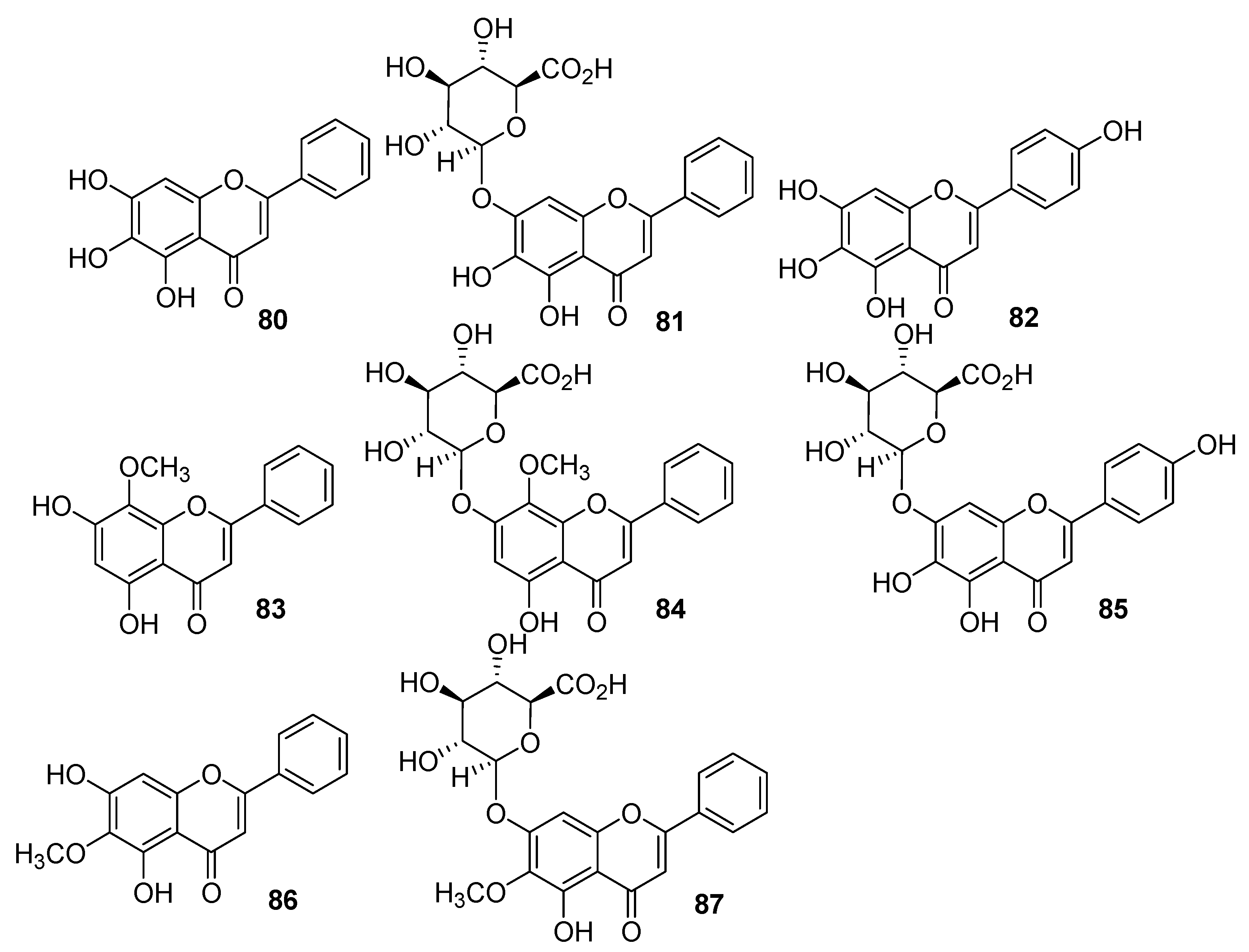
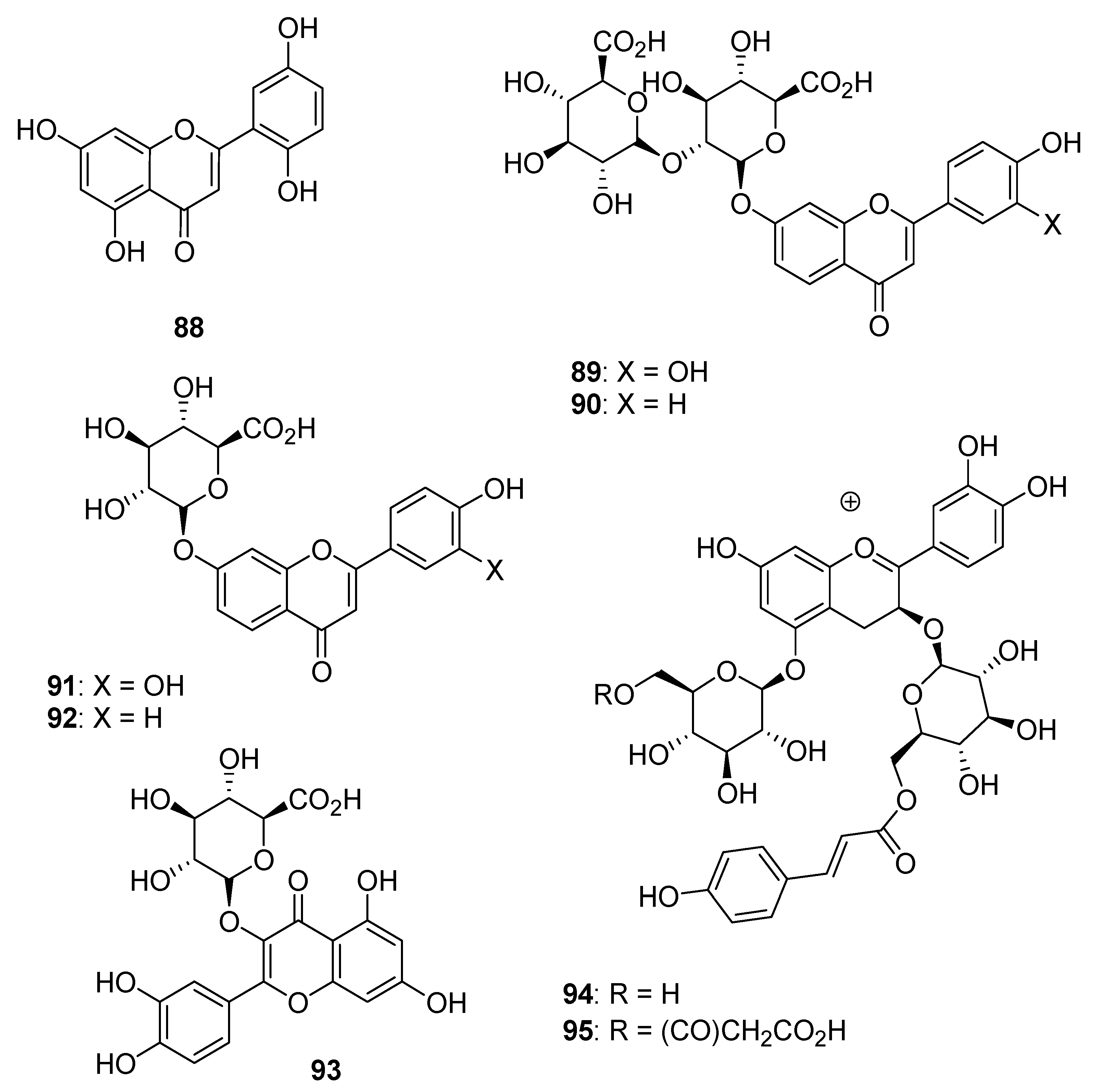










| Type I | Organic salt (mostly organic ammonium salt)/metal salt (mostly metal halide) |
| Type II | Organic salt (mostly organic ammonium salt)/metal salt hydrate (mostly metal halide hydrate) |
| Type III | Organic salt (mostly organic ammonium salt)/hydrogen bond donor |
| Type IV | Metal salt hydrate (mostly metal halide hydrate)/hydrogen bond donor |
| Type V (recently added) | Alkanols/phenols |
| Sample | NADES Mixture | Extraction Method | Reference |
|---|---|---|---|
| Mulberry (Fructus mori) | Choline chloride/citric acid/glucose (1:1:1) with 40% H2O | High-speed homogenization/cavitation-burst extraction | Guo et al. [65] |
| Grape pomace (Vitis vinifera cv) | Choline chloride/citric acid | Simultaneous ultrasonication/microwave irradiation | Panić et al. [38] |
| Grape pomace (Vitis vinifera cv) | Choline chloride/proline/malic acid | Simultaneous ultrasonication/microwave irradiation | Panić et al. [38] |
| Pomace of the Brazilian grape-tree (Myrciaria cauliflora) fruit | Choline chloride/propyleneglycol Choline chloride/citric acid | 50 °C | Benevutti et al. [23] |
| Blueberry (O’Neal and Florida cultivars) | Choline chloride/glycerol/25% H2O | Ultrasonication 40 kHz, at room temperature for 50 min. | Silva et al. [66] |
| Blueberry pomace | Choline chloride/oxalic acid | Pulsed ultrasonication | Fu et al. [68] |
| Blackberry (Rubus spp.) | Choline chloride/glycerol/20% water | Ultrasonication at 25 °C for 20 min. | Zannou and Koca [67] |
| Blackberry (Rubus spp.) | Choline chloride/butanediol/20% water | Ultrasonication at 25 °C for 20 min. | Zannou and Koca [67] |
| Blackberry (Rubus spp.) | Choline chloride/ethyleneglycol/20% H2O | Ultrasonication at 25 °C for 20 min. | Zannou and Koca [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaidi, R.; Thiemann, T. Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing. Sustainability 2025, 17, 2293. https://doi.org/10.3390/su17052293
Alsaidi R, Thiemann T. Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing. Sustainability. 2025; 17(5):2293. https://doi.org/10.3390/su17052293
Chicago/Turabian StyleAlsaidi, Rana, and Thies Thiemann. 2025. "Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing" Sustainability 17, no. 5: 2293. https://doi.org/10.3390/su17052293
APA StyleAlsaidi, R., & Thiemann, T. (2025). Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing. Sustainability, 17(5), 2293. https://doi.org/10.3390/su17052293






