Eco-Friendly Utilization of Phosphogypsum via Mechanical Activation for Sustainable Heavy Metal Removal from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mechanical Activation
2.2.2. Characterization
2.2.3. Adsorption Experiments
3. Results and Discussion
3.1. Physico-Chemical Characterizations
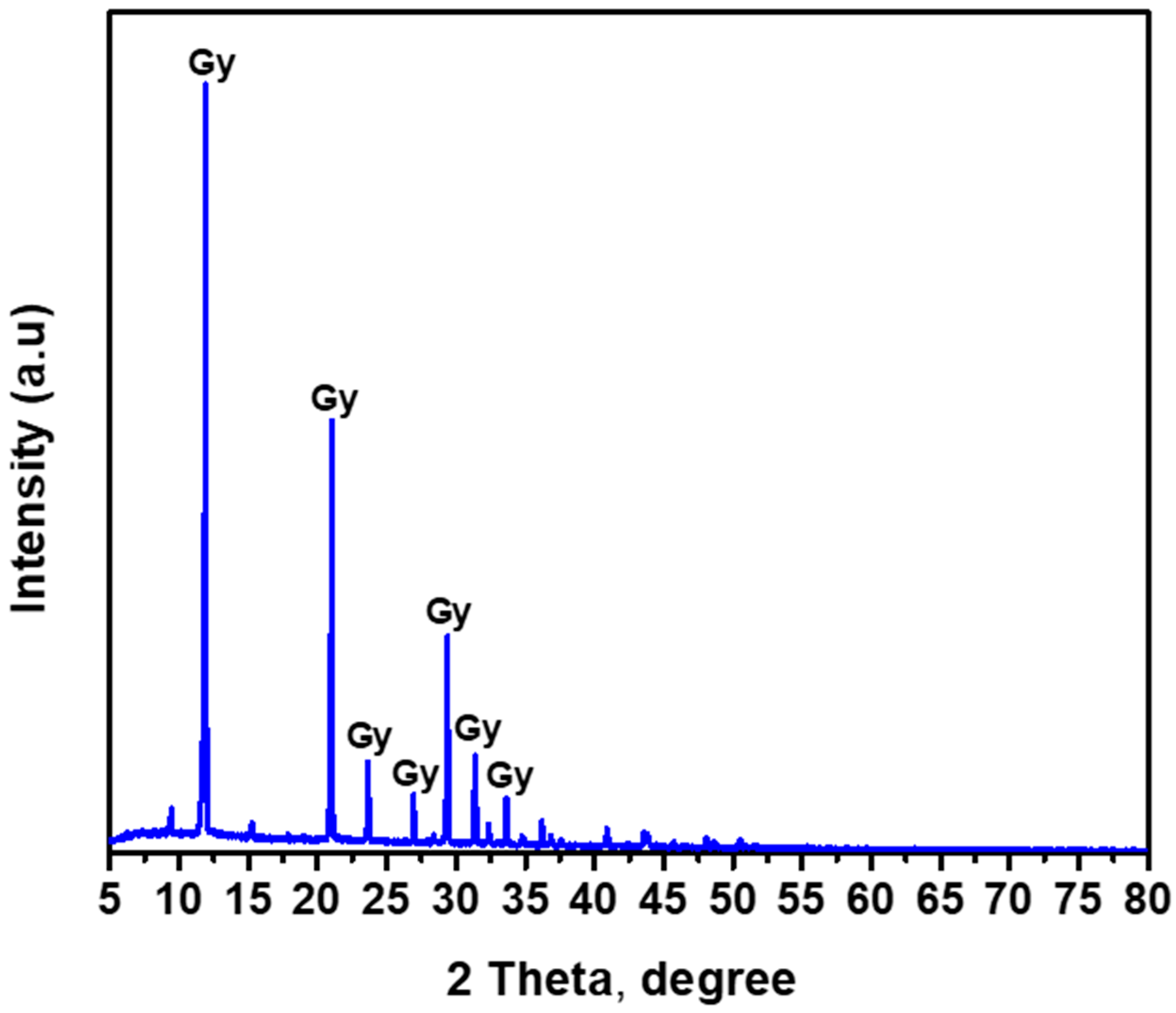
3.1.1. XRD Analysis
3.1.2. XPS Analysis
3.1.3. Microstructure Analysis
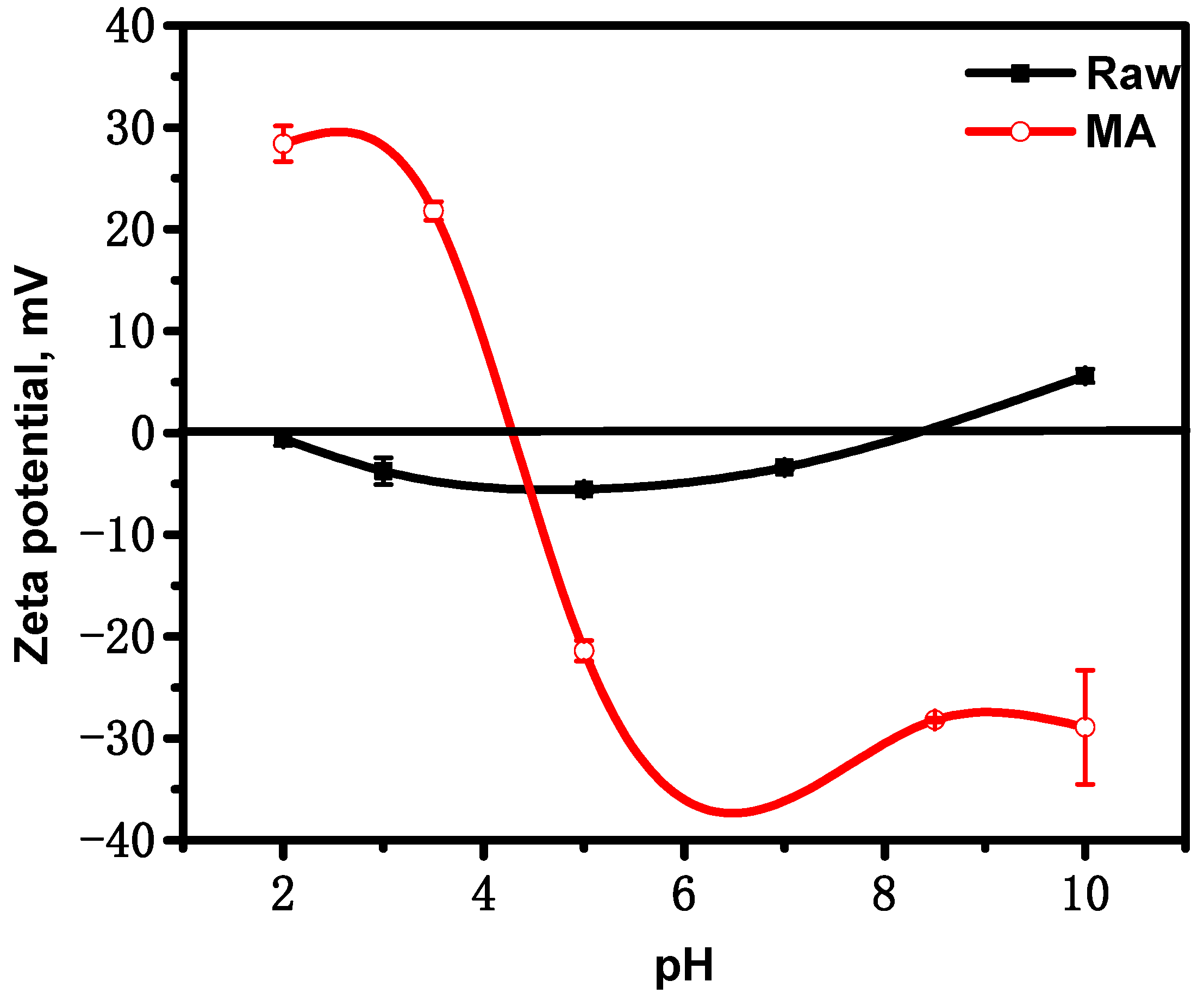
3.1.4. Zeta Potential
3.1.5. Surface Area
3.2. Adsorption Study
3.2.1. Effect of pH
3.2.2. Effect of Initial Concentration
3.2.3. Isotherm Models Study
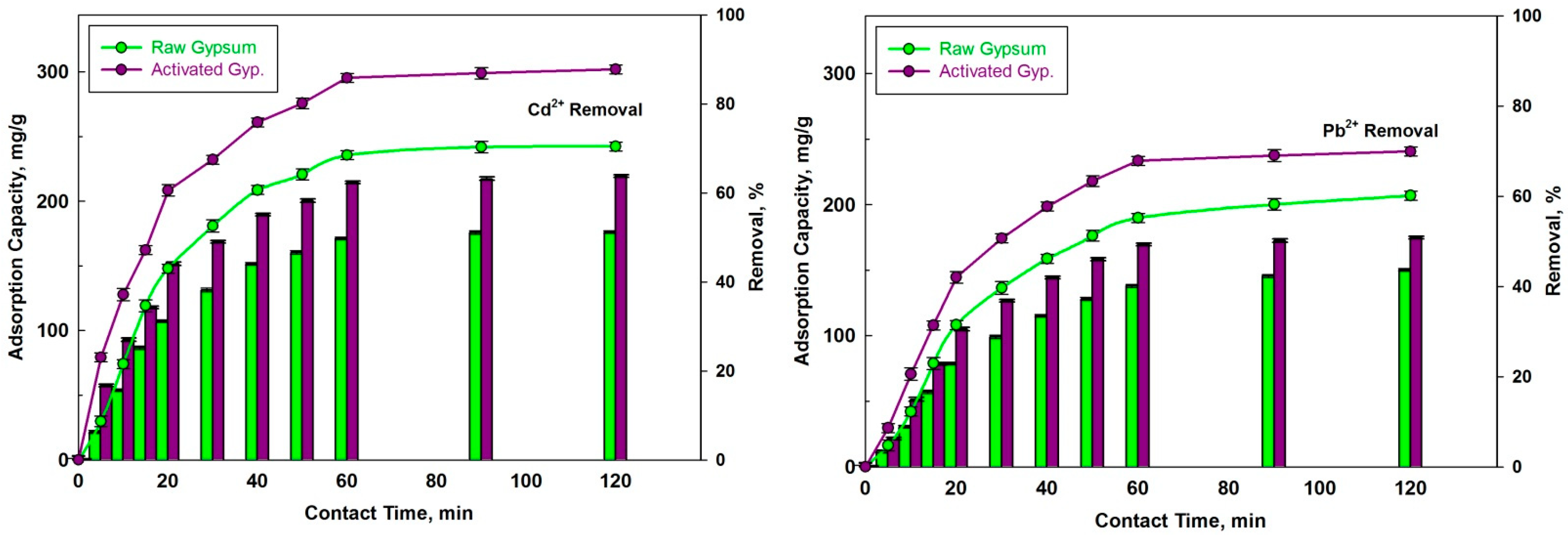
3.2.4. Effect of Contact Time
3.2.5. The Adsorption Kinetics
3.2.6. Effect of Temperature
3.2.7. Thermodynamic Study
3.2.8. Regeneration and Reusability of Adsorbent
3.2.9. Comparison Studies
3.2.10. Application in Real Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- AlJaberi, F.Y.; Hawaas, Z.A. Electrocoagulation removal of Pb, Cd, and Cu ions from wastewater using a new configuration of electrodes. MethodsX 2023, 10, 101951. [Google Scholar] [CrossRef]
- Liang, Y.; Jun, M.; Liu, W. Enhanced Removal of Lead(II) and Cadmium(II) from Water in Alum Coagulation by Ferrate(VI) Pretreatment. Water Environ. Res. 2007, 79, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Kavak, D. Removal of lead from aqueous solutions by precipitation: Statistical analysis and modeling. Desalination Water Treat. 2013, 51, 1720–1726. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Thriveni, T.; Mulatu, D.; Lee, M.h.; Jung, S.h.; Ahn, J.W. Removal of Cd(II) and Pb(II) from Wastewater via Carbonation of Aqueous Ca(OH)2 Derived from Eggshell. Process Saf. Environ. Prot. 2020, 141, 278–287. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto MM, S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Yu, Z.G. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder MA, J.; Al-Ahmed, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wen, N.; Wei, D.; Zhang, Y. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica. Sep. Purif. Technol. 2021, 265, 118341. [Google Scholar] [CrossRef]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A calcined clay fixed bed adsorption studies for the removal of heavy metals from aqueous solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Pfeifer, A.; Škerget, M.; Čolnik, M. Removal of iron, copper, and lead from aqueous solutions with zeolite, bentonite, and steel slag. Sep. Sci. Technol. 2021, 56, 2989–3000. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, Y.; Xing, B.; Qin, X.; Zhang, C.; Xia, H. Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust. J. Clean. Prod. 2021, 314, 128074. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy Metals Removal Using Activated Carbon, Silica and Silica Activated Carbon Composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Huang, Z.; Liu, Y.-H.; Hu, L.-X.; He, L.-Y.; Liu, Y.-S.; Zhao, J.-L.; Ying, G.-G. Occurrence and risks of 23 tire additives and their transformation products in an urban water system. Environ. Int. 2023, 171, 107715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Y.; Chen, A.J.Y.; Cheng, Z.; Bi, Z.; Zhang, R.; Lou, Z. Environmental impacts and nutrient distribution routes for food waste separated disposal on large-scale anaerobic digestion/composting plants. J. Environ. Manag. 2022, 318, 115624. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Amin, Y.; Ibrahim, S.; Al-Bich, F. Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 2004, 19, 747–753. [Google Scholar] [CrossRef]
- Rosales, J.; Gázquez, M.; Cabrera, M.; Bolivar, J.P.; Agrela, F. Application of phosphogypsum for the improvement of eco-efficient cements. In Waste and Byproducts in Cement-Based Materials; Woodhead Publishing: Sawston, UK, 2021; pp. 153–189. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.L. Exploring the potential reuse of phosphogypsum: A waste or a resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef]
- Outbakat, M.B.; Choukr-Allah, R.; Bouray, M.; El Gharous, M.; El Mejahed, K. Phosphogypsum: Properties and potential use in agriculture. In Biosaline Agriculture as a Climate Change Adaptation for Food Security; Springer International Publishing: Cham, Switzerland, 2023; pp. 229–255. [Google Scholar] [CrossRef]
- Pliaka, M.; Gaidajis, G. Potential uses of phosphogypsum: A review. Journal of Environmental Science and Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2022, 57, 746–763. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Es-said, A.; Nafai, H.; Lamzougui, G.; Bouhaouss, A.; Bchitou, R. Comparative adsorption studies of cadmium ions on phosphogypsum and natural clay. Sci. Afr. 2021, 13, e00960. [Google Scholar] [CrossRef]
- Es-said, A.; Nafai, H.; Zerki, N.; Bchitou, R. Chemometrics approach for multi-response optimisation of heavy metals Zn(II), Cu(II) and Cd(II) removal by phosphogypsum: Ternary aqueous solution. Int. J. Environ. Anal. Chem. 2021, 103, 3044–3058. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.; Román-Ross, G.; Cuello, G.J.; Turrillas, X.; Charlet, L.; Johnson, M.R.; Bardelli, F. Arsenic uptake by gypsum and calcite: Modelling and probing by neutron and X-ray scattering. Phys. B Condens. Matter 2006, 385–386, 935–937. [Google Scholar] [CrossRef]
- Cesur, H.; Balkaya, N. Zinc removal from aqueous solution using an industrial by-product phosphogypsum. Chem. Eng. J. 2007, 131, 203–208. [Google Scholar] [CrossRef]
- Balkaya, N.; Cesur, H. Adsorption of cadmium from aqueous solution by phosphogypsum. Chem. Eng. J. 2008, 140, 247–254. [Google Scholar] [CrossRef]
- Dai, Q.; Xie, L.; Ma, L.; Yang, J.; Yang, X.; Ren, N.; Tian, G.; Guo, Z.; Ning, P. Effects of flocculant-modified phosphogypsum on sludge treatment: Investigation of the operating parameters, variations of the chemical groups, and heavy metals in the sludge. Environ. Sci. Water Res. Technol. 2021, 7, 184–196. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, Y.; Li, P.; Liu, D.; Ren, Y.; Li, D.; Liu, Z.; Chen, Y.; Ye, Y. Reuse of phosphogypsum and phosphorus ore flotation tailings as adsorbent: The adsorption performance and mechanism of phosphate. J. Phys. Chem. Solids 2023, 178, 111313. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Mou, R. Phosphogypsum-Modified Vinasse Shell Biochar as a Novel Low-Cost Material for High-Efficiency Fluoride Removal. Molecules 2023, 28, 7617. [Google Scholar] [CrossRef]
- Shang, C.; Geng, Z.; Sun, Y.; Che, D.; Zhao, Q.; Chen, T.; Tang, M.; Huo, L. Lanthanum-Modified Phosphogypsum Red Mud Composite for the Co-Adsorption of Cadmium and Arsenic: Mechanism Study and Soil Remediation. Agriculture 2024, 14, 464. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Li, X.; Ye, J.; Chen, J. Adsorption of Cu(II) by phosphogypsum modified with sodium dodecyl benzene sulfonate. J. Hazard. Mater. 2020, 387, 121808. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Chen, M.; Hu, H.; Lei, Z.; Zhang, Q.; Yuan, W. Mechanochemical activation of serpentine for recovering Cu (II) from wastewater. Appl. Clay Sci. 2017, 149, 1–7. [Google Scholar] [CrossRef]
- Pechishcheva, N.V.; Burdina, L.G.; Kel’, P.V.; Estemirova, S.K.; Konysheva, E.Y.; Sushnikova, A.A. Mechanical activation of anatase as a way to improve hexavalent chromium removal from solutions by the photoreduction and adsorption under near visible LED irradiation. Mater. Chem. Phys. 2024, 314, 128872. [Google Scholar] [CrossRef]
- Xiyili, H.; Çetintaş, S.; Bingöl, D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 109, 288–300. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Xu, Q.; Hu, K.; Chen, H.; Liu, Y.; Wan, Y.; Zhang, J.; Li, X. Effective adsorption and recovery of rare earth elements from wastewater by activated talc. Appl. Clay Sci. 2024, 251, 107312. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Guan, B.; Ye, Q.; Zhang, J.; Lou, W.; Wu, Z. Interaction between α-calcium sulfate hemihydrate and superplasticizer from the point of adsorption characteristics, hydration and hardening process. Cem. Concr. Res. 2010, 40, 253–259. [Google Scholar] [CrossRef]
- Jia, X.J.; Wang, J.; Wu, J.; Teng, W.; Zhao, B.; Li, H.; Du, Y. Facile synthesis of MoO2/CaSO4 composites as highly efficient adsorbents for congo red and rhodamine B. RSC Adv. 2018, 8, 1621. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Y.; Podkolzin, S.G.; Lee, W. Band gap of reduced graphene oxide tuned by controlling functional groups. J. Mater. Chem. C 2020, 8, 4885–4894. [Google Scholar] [CrossRef]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and application of zeta-potential. Rud.-Geol.-Naft. Zb. 1992, 4, 147–151. [Google Scholar]
- Mahmoud, G.A.; Abdel Khalek, M.A.; Shoukry, E.M.; Amin, M.; Abdulghany, A.H. Removal of Phosphate Ions from Wastewater by Treated Hydrogel Based on Chitosan. Egypt. J. Chem. 2019, 62, 1537–1549. [Google Scholar] [CrossRef]
- Hałas, P.; Kołodyńska, D.; Płaza, A.; Gęca, M.; Hubicki, Z. Modified fly ash and zeolites as an effective adsorbent for metal ions from aqueous solution. Adsorpt. Sci. Technol. 2017, 35, 519–533. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Cheng, F.; Song, H.; Zheng, N.; Wang, X. Application of Modified Coal Fly Ash as an Absorbent for Ammonia-Nitrogen Wastewater Treatment. Adv. Mater. Res. 2012, 518–523, 2380–2384. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chen, H.R. Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manure-derived biochar. Int. J. Environ. Sci. Technol. 2013, 10, 1349–1356. [Google Scholar] [CrossRef]
- Goldberg, S. Equations and models describing adsorption processes in soils. Chem. Process. Soils 2018, 8, 489–517. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Masoumi, M. Efficient removal of Pb(II) and Cd(II) from water by cross-linked poly (N-vinylpyrrolidone-co-maleic anhydride)@eggshell/Fe3O4 environmentally friendly nanocomposite. Desalination Water Treat. 2018, 106, 209–219. [Google Scholar] [CrossRef]
- Chung, N.T. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Vietnam J. Sci. Technol. 2018, 54, 314. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mansour Lakouraj, M.; Ramezani, A. Efficient sorption of Pb(II) from an aqueous solution using a poly(aniline-co-3-aminobenzoic acid)-based magnetic core–shell nanocomposite. New J. Chem. 2016, 40, 2521–2529. [Google Scholar] [CrossRef]
- Lopes EC, N.; dos Anjos FS, C.; Vieira EF, S.; Cestari, A.R. An alternative Avrami equation to evaluate kinetic parameters of the interaction of Hg(II) with thin chitosan membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, H.; Sallem, F.; Lafaille, J.; Grassl, B.; Charrier–El Bouhtoury, F. Biosorption of Heavy Metals from Water onto Phenolic Foams Based on Tannins and Lignin Alkaline Liquor. Int. J. Environ. Res. 2021, 15, 369–381. [Google Scholar] [CrossRef]
- Plaza, L.; Castellote, M.; Nevshupa, R.; Jimenez-Relinque, E. High-capacity adsorbents from stainless steel slag for the control of dye pollutants in water. Environ. Sci. Pollut. Res. 2021, 28, 23896–23910. [Google Scholar] [CrossRef] [PubMed]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent Progress on Biosorption of Heavy Metals from Liquids Using Low Cost Biosorbents: Characterization, Biosorption Parameters and Mechanism Studies. CLEAN Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Fakari, S.; Nezamzadeh-Ejhieh, A. Synergistic effects of ion exchange and complexation processes in cysteine-modified clinoptilolite nanoparticles for removal of Cu (II) from aqueous solutions in batch and continuous flow systems. New J. Chem. 2017, 41, 3811–3820. [Google Scholar] [CrossRef]
- Rukayat, O.O.; Usman, M.F.; Elizabeth, O.M.; Abosede, O.O.; Faith, I.U. Kinetic Adsorption of Heavy Metal (Copper) On Rubber (Hevea brasiliensis) Leaf Powder. S. Afr. J. Chem. Eng. 2021, 37, 74–80. [Google Scholar] [CrossRef]
- Murray, J.W.; Dillard, J.G. The oxidation of cobalt (II) adsorbed on manganese dioxide. Geochim. Cosmochim. Acta 1979, 43, 781–787. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Çelikçapa, S.; Doğan, M. Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 2004, 116, 135–145. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Farahmandkia, Z.; Taghibeigloo, B.; Taromi, A. Adsorption of Lead and Cadmium from Aqueous Solution by Using Almond Shells. Water. Air Soil Pollut. 2009, 199, 343–351. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and Cadmium Remediation Using Magnetized and Nonmagnetized Biochar from Douglas Fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Cui, L.; Chen, T.; Yin, C.; Yan, J.; Ippolito, J.A.; Hussain, Q. Mechanism of Adsorption of Cadmium and Lead Ions by Iron-Activated Biochar. BioResources 2019, 14, 842–857. [Google Scholar] [CrossRef]
- Abdulrahman Oyekanmi, A.; Abd Latiff, A.A.; Daud, Z.; Saphira Radin Mohamed, R.M.; Ismail, N.; Ab Aziz, A.; Rafatullah, M.; Hossain, K.; Ahmad, A.; Kamoldeen Abiodun, A. Adsorption of Cadmium and Lead from Palm Oil Mill Effluent Using Bone-Composite: Optimisation and Isotherm Studies. Int. J. Environ. Anal. Chem. 2019, 99, 707–725. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, J.Q.; Huang, L.; Yuan, Z.H.; Li, Z.J.; Liu, M.C. Removal of Cd and Pb with Biochar Made from Dairy Manure at Low Temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef]
- Kavand, M.; Eslami, P.; Razeh, L. The Adsorption of Cadmium and Lead Ions from the Synthesis Wastewater with the Activated Carbon: Optimization of the Single and Binary Systems. J. Water Process Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, Z.; Asma, M.; Ahmad, M.; Rehan, K.; Munir, M.; Bazmi, A.A.; Ali, H.M.; Mazroua, Y.; Salem, M.A.; et al. Effective Adsorption of Cadmium and Lead Using SO3H-Functionalized Zr-MOFs in Aqueous Medium. Chemosphere 2022, 307, 135633. [Google Scholar] [CrossRef]
- Vishwakarma, M.C.; Joshi, H.K.; Tiwari, P.; Bhandari, N.S.; Joshi, S.K. Thermodynamic, Kinetic, and Equilibrium Studies of Cu(II), Cd(II), Ni(II), and Pb(II) Ion Biosorption onto Treated Ageratum Conyzoid Biomass. Int. J. Biol. Macromol. 2024, 274, 133001. [Google Scholar] [CrossRef]








| Item | CaO | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| % | 36.5 | 0.21 | 0.12 | 0.63 | 3.3 | 4.2 | 34.85 | 16.5 |
| Isotherm | Parameter | Cadmium | Lead | ||
|---|---|---|---|---|---|
| Raw | MA | Raw | MA | ||
| Langmuir | R2 | 0.8545 | 0.8955 | 0.8479 | 0.9043 |
| qmax (Cal.) | 36 | 5 | 100 | 44 | |
| qmax (Exp.) | 171 | 214 | 138 | 169 | |
| b | 6.5 × 10−3 | 4.5 × 10−3 | |||
| Freundlich | R2 | 0.9966 | 0.9940 | 0.9986 | 0.9965 |
| n | 1.35 | 1.34 | 1.32 | 1.27 | |
| KF | 6.91 | 15.77 | 3.91 | 5.78 | |
| Item | Cadmium Ions | Lead Ions | ||
|---|---|---|---|---|
| Raw | MA | Raw | MA | |
| The pseudo-first-order model | ||||
| R2 | 0.9770 | 0.9760 | 0.9746 | 0.9798 |
| K1 | −5.12 × 10−2 | −5.36 × 10−2 | −4.02 × 10−2 | −5.18 × 10−2 |
| Calculated qe | 218 | 286 | 216 | 239 |
| Experimental qe | 176 | 219 | 150 | 174 |
| The pseudo-second-order model | ||||
| R2 | 0.9929 | 0.9959 | 0.9948 | 0.9944 |
| K2 | 2.98 × 10−4 | 2.69 × 10−4 | 2.33 × 10−4 | 3.18 × 10−4 |
| Calculated qe | 188 | 250 | 172 | 191 |
| Experimental qe | 176 | 219 | 150 | 174 |
| Adsorbent | Ads. Capacity (mg/g) | pH | Reference | |
|---|---|---|---|---|
| Pb2+ | Cd2+ | |||
| Alkali-modified almond shells | 9 | 7 | 5-6 | [61] |
| Magnetic biochar | 40 | 16 | 5.0 | [62] |
| Modified reed biochar | 17 | 3 | 7.0 | [63] |
| Bone composite | 29 | 42 | 4.0 | [64] |
| Modified dairy manure biochar | 175 | 68 | 7.0 | [65] |
| Activated carbon | 9.3 | 9.2 | 6.3 | [66] |
| Zirconium organic frameworks | 176 | 195 | 6.0 | [67] |
| Ageratum conyzoid biomass | 30 | 36 | 6.0 | [68] |
| Phosphogypsum | 230 | 234 | 6.0 | This work |
| Activated phosphogypsum | 235 | 243 | 6.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.M.; Aljabbab, A.A.; Alajmi, M.S.; Qadrouh, A.N.; Farahat, M.; Abdel Khalek, M.A.; Baioumy, H.; Alhumimidi, M.S.; Almutairi, R.S.; Alkhammali, S.A. Eco-Friendly Utilization of Phosphogypsum via Mechanical Activation for Sustainable Heavy Metal Removal from Wastewater. Sustainability 2025, 17, 2817. https://doi.org/10.3390/su17072817
Alotaibi AM, Aljabbab AA, Alajmi MS, Qadrouh AN, Farahat M, Abdel Khalek MA, Baioumy H, Alhumimidi MS, Almutairi RS, Alkhammali SA. Eco-Friendly Utilization of Phosphogypsum via Mechanical Activation for Sustainable Heavy Metal Removal from Wastewater. Sustainability. 2025; 17(7):2817. https://doi.org/10.3390/su17072817
Chicago/Turabian StyleAlotaibi, Abdulrahman M., Abdulrahman A. Aljabbab, Mamdoh S. Alajmi, Ayman N. Qadrouh, Mohsen Farahat, Mohamed Abdeldayem Abdel Khalek, Hassan Baioumy, Mansour S. Alhumimidi, Ramzi S. Almutairi, and Sultan A. Alkhammali. 2025. "Eco-Friendly Utilization of Phosphogypsum via Mechanical Activation for Sustainable Heavy Metal Removal from Wastewater" Sustainability 17, no. 7: 2817. https://doi.org/10.3390/su17072817
APA StyleAlotaibi, A. M., Aljabbab, A. A., Alajmi, M. S., Qadrouh, A. N., Farahat, M., Abdel Khalek, M. A., Baioumy, H., Alhumimidi, M. S., Almutairi, R. S., & Alkhammali, S. A. (2025). Eco-Friendly Utilization of Phosphogypsum via Mechanical Activation for Sustainable Heavy Metal Removal from Wastewater. Sustainability, 17(7), 2817. https://doi.org/10.3390/su17072817







