Comparison of the Synthesis Method of Zeolite Catalysts Based on Pozzolan, Pumice, and Ignimbrite Applied to the Sustainable Pyrolysis of Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zeolite Synthesis
2.2.1. Methodology 1: Alkaline Fusion/Hydrothermal Reaction Method with NaOH
2.2.2. Methodology 2: Alkaline Fusion/Hydrothermal Reaction Method with Concentrated NaOH
2.2.3. Methodology 3: Hydrothermal Method for Directing ZSM-5-Type Zeolite Synthesis
2.3. Characterization of the Zeolites Obtained
2.4. Catalytic Pyrolysis
3. Results
3.1. XRD
3.2. BET Analysis
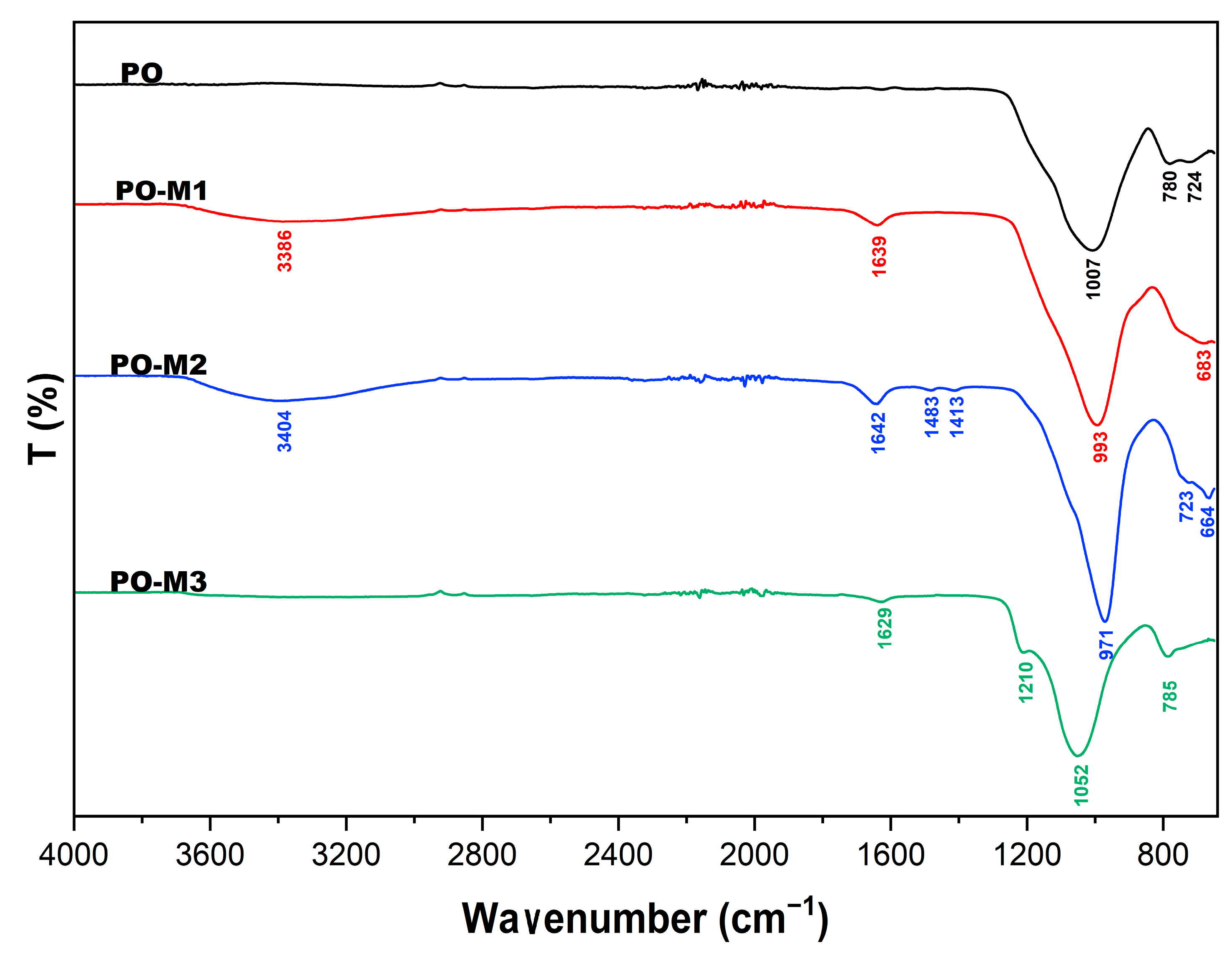
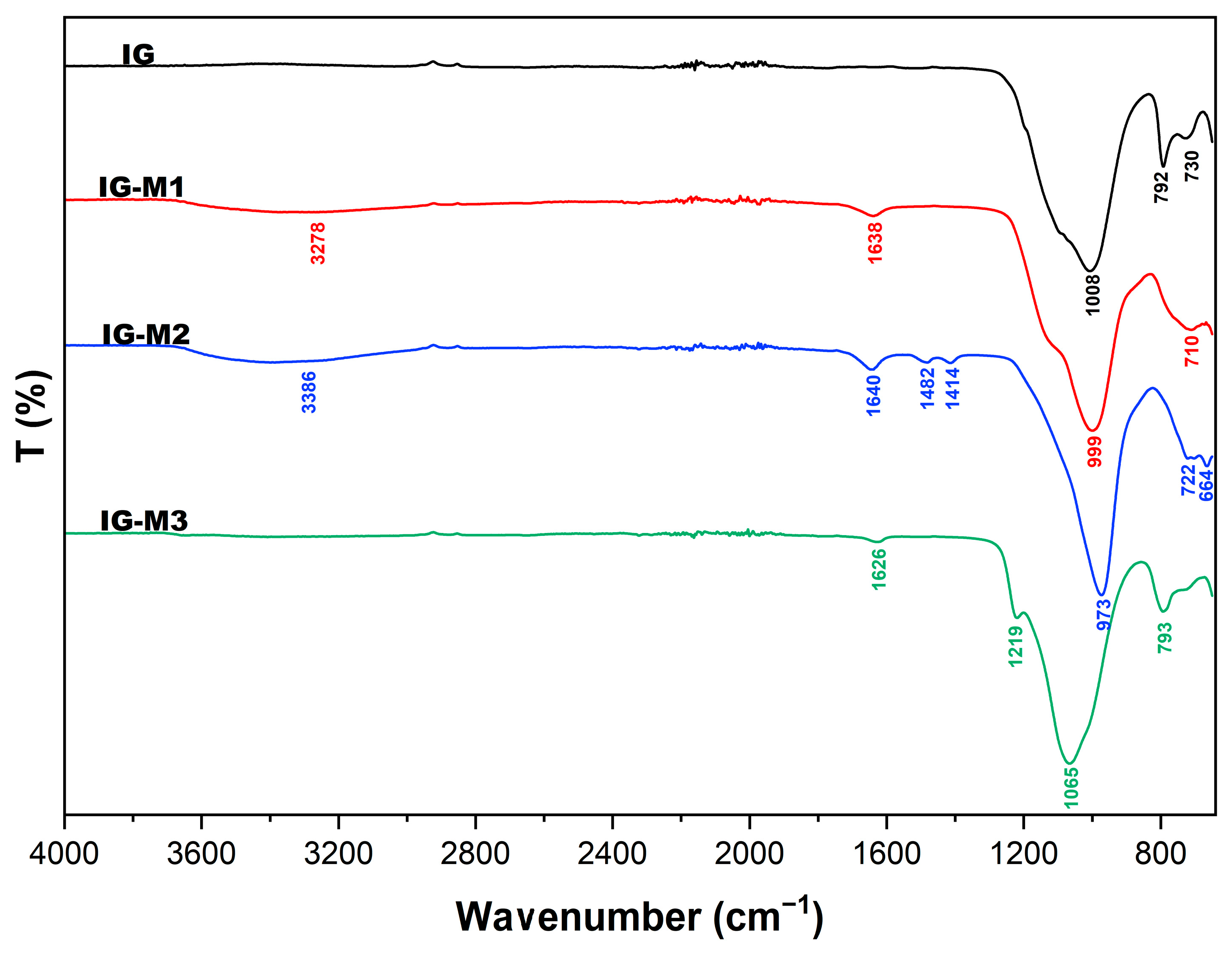
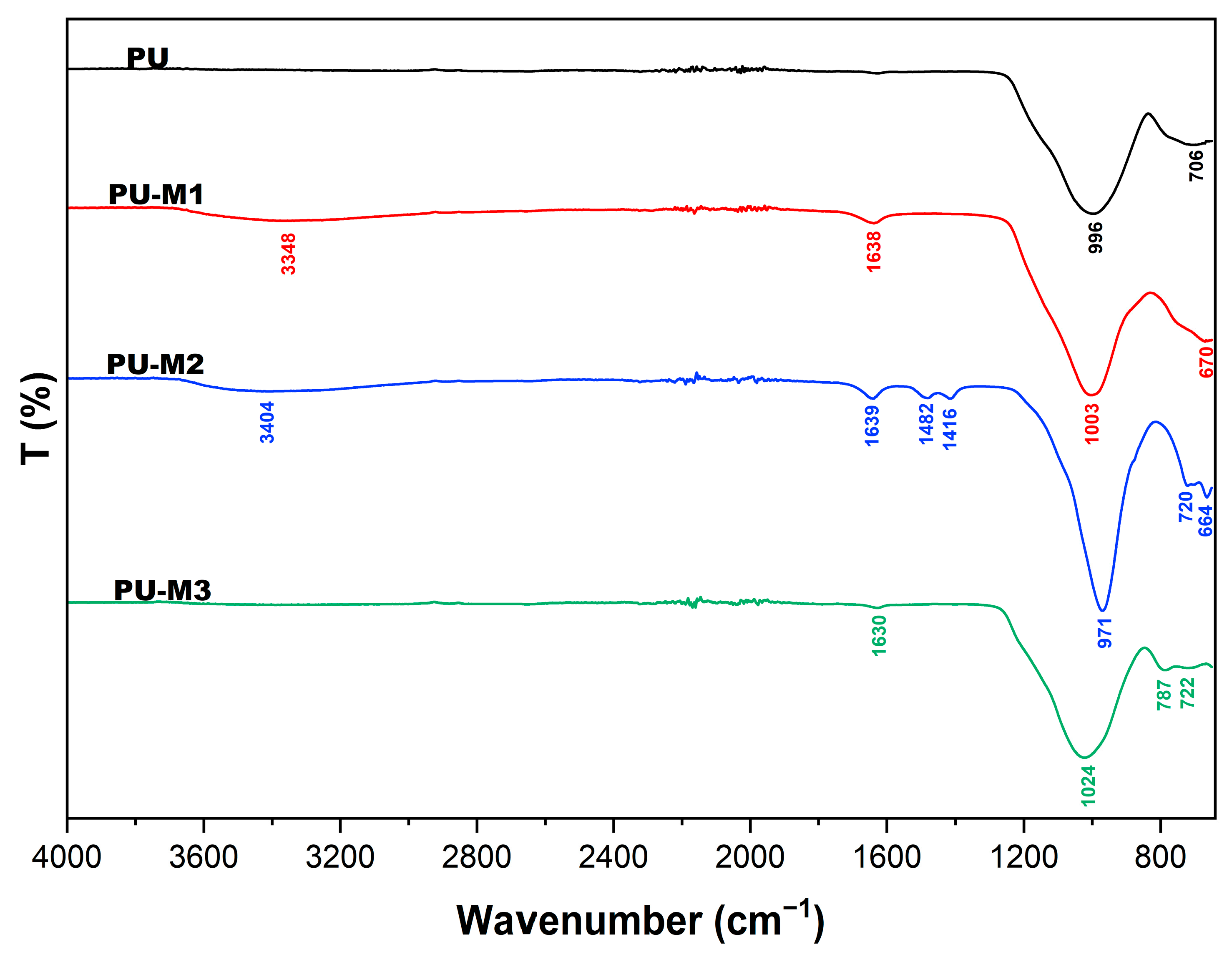
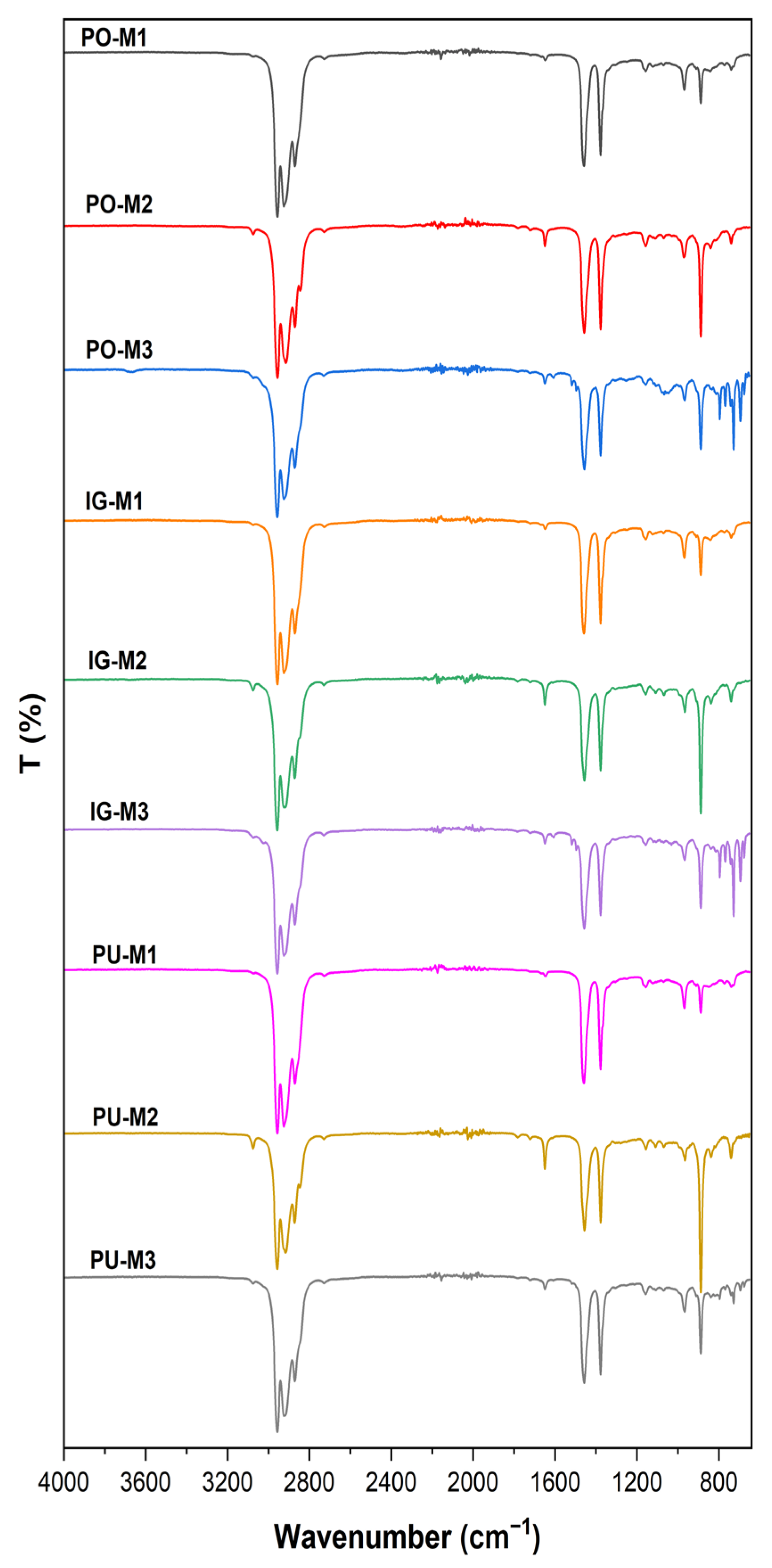
3.3. FTIR
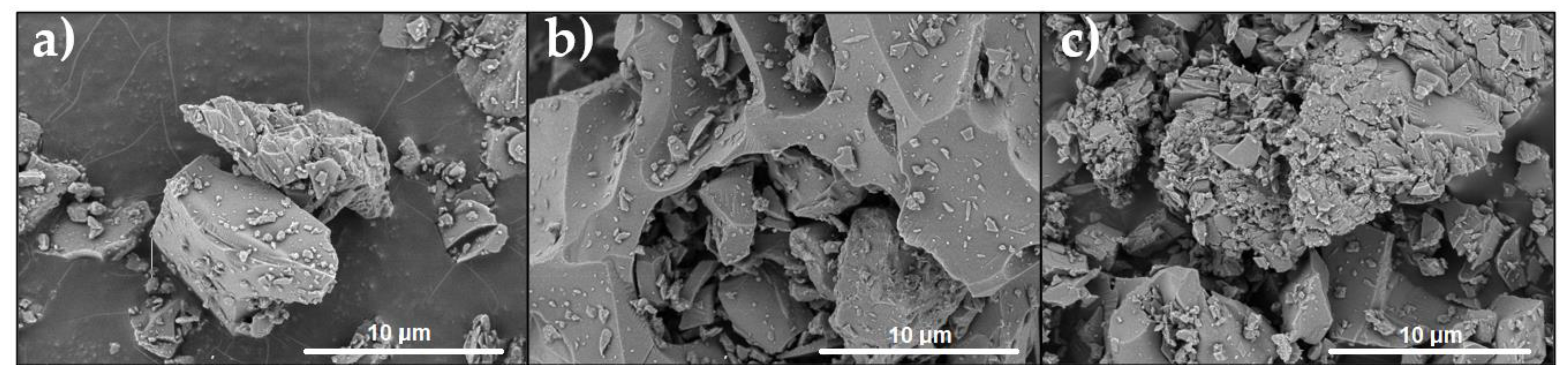
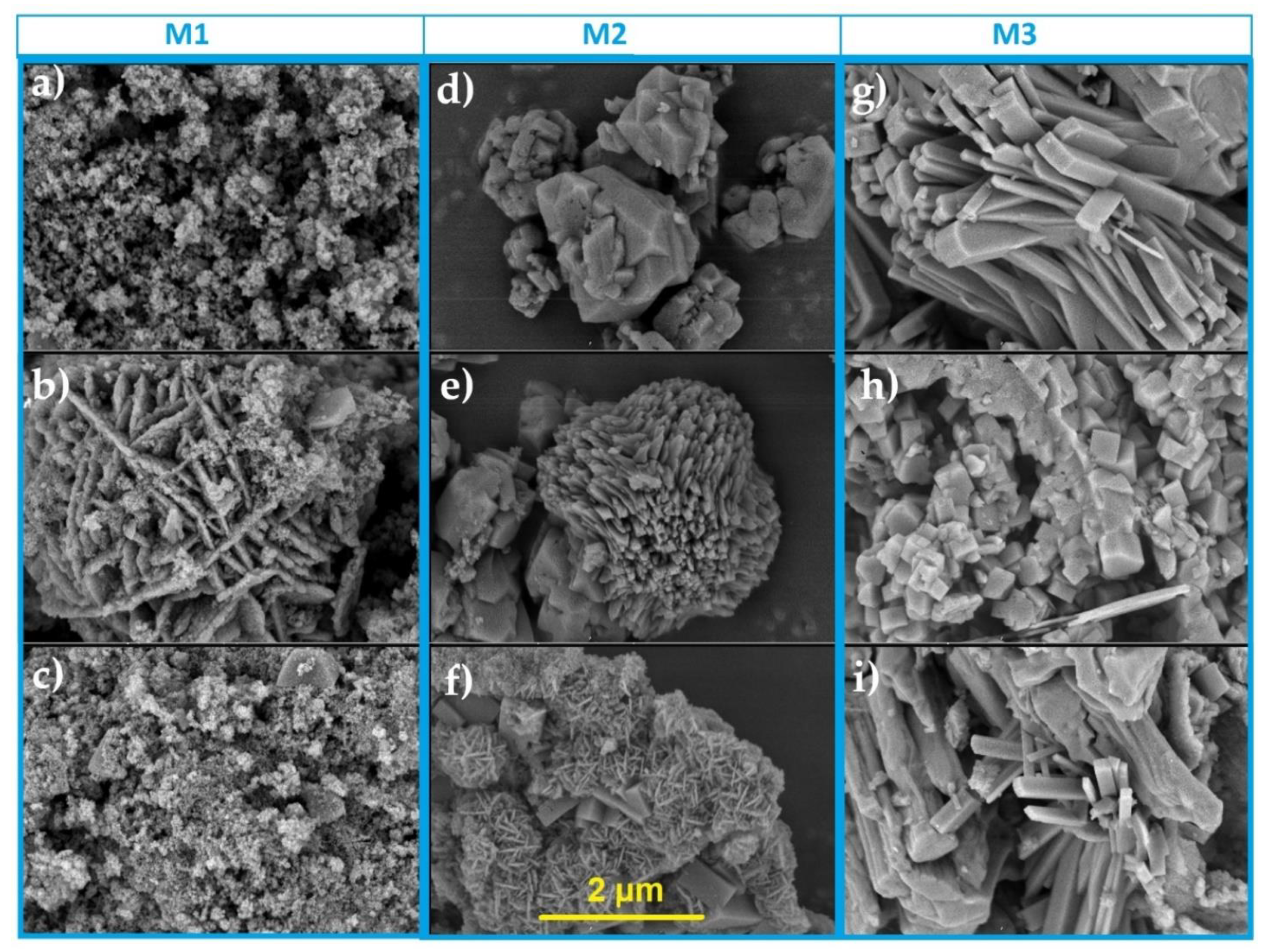
3.4. SEM Analysis
4. Pyrolysis Products
Analysis of Pyrolysis Liquid Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belviso, C.; Abdolrahimi, M.; Peddis, D.; Gagliano, E.; Sgroi, M.; Lettino, A.; Roccaro, P.; Vagliasindi, F.G.A.; Falciglia, P.P.; Di Bella, G.; et al. Synthesis of Zeolite from Volcanic Ash: Characterization and Application for Cesium Removal. Microporous Mesoporous Mater. 2021, 319, 111045. [Google Scholar] [CrossRef]
- Hussein, Z.A.; Shakor, Z.M.; Alzuhairi, M.; Al-Sheikh, F. Thermal and Catalytic Cracking of Plastic Waste: A Review. Int. J. Environ. Anal. Chem. 2023, 103, 5920–5937. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; Legorreta-Garcia, F.; Aguirre-Cruz, G.; Stanciu, L.; Aguirre-Alvarez, G. Synthesis of Hierarchical Silica Zeolites for Heterogenous Catalysis and Adsorption. Microporous Mesoporous Mater. 2022, 345, 112274. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Yin, C.; Shan, Z.; Xiao, F.S. Hierarchical Mesoporous Zeolites with Controllable Mesoporosity Templated from Cationic Polymers. Microporous Mesoporous Mater. 2010, 131, 58–67. [Google Scholar] [CrossRef]
- Song, C.; Chu, Y.; Wang, M.; Shi, H.; Zhao, L.; Guo, X.; Yang, W.; Shen, J.; Xue, N.; Peng, L.; et al. Cooperativity of Adjacent Brønsted Acid Sites in MFI Zeolite Channel Leads to Enhanced Polarization and Cracking of Alkanes. J. Catal. 2017, 349, 163–174. [Google Scholar] [CrossRef]
- Derouane, E.G.; Védrine, J.C.; Pinto, R.R.; Borges, P.M.; Costa, L.; Lemos, M.A.N.D.A.; Lemos, F.; Ribeiro, F.R. The Acidity of Zeolites: Concepts, Measurements and Relation to Catalysis: A Review on Experimental and Theoretical Methods for the Study of Zeolite Acidity. Catal. Rev. Sci. Eng. 2013, 55, 454–515. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, S.; Yu, J. Metal Sites in Zeolites: Synthesis, Characterization and Catalysis. Chem. Rev. 2023, 123, 6039–6106. [Google Scholar] [CrossRef]
- Yang, G.; Peng, P.; Guo, H.; Song, H.; Li, Z. The Catalytic Pyrolysis of Waste Polyolefins by Zeolite-Based Catalysts: A Critical Review on the Structure-Acidity Synergies of Catalysts. Polym. Degrad. Stab. 2024, 222, 110712. [Google Scholar] [CrossRef]
- Maghfirah, A.; Ilmi, M.M.; Fajar, A.T.N.; Kadja, G.T.M. A Review on the Green Synthesis of Hierarchically Porous Zeolite. Mater. Today Chem. 2020, 17, 100348. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite Synthesis from Low-Cost Materials and Environmental Applications: A Review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Jamil, T.S.; Ibrahim, H.S.; Abd El-Maksoud, I.H.; El-Wakeel, S.T. Application of Zeolite Prepared from Egyptian Kaolin for Removal of Heavy Metals: I. Optimum Conditions. Desalination 2010, 258, 34–40. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, X.; Zhang, H.Q.; Liu, J.D. Ammonium Removal from Aqueous Solutions by Zeolite Adsorption Together with Chemical Precipitation. Water Sci. Technol. 2010, 61, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Belviso, C.; Cavalcante, F.; Lettino, A.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Ammonium from Wastewater by Zeolite Synthetized from Volcanic Ash: Batch and Column Tests. J. Environ. Chem. Eng. 2022, 10, 107539. [Google Scholar] [CrossRef]
- Lee, M.G.; Park, J.W.; Kam, S.K.; Lee, C.H. Synthesis of Na-A Zeolite from Jeju Island Scoria Using Fusion/Hydrothermal Method. Chemosphere 2018, 207, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Garcia-Vallès, M.; Martínez-Manent, S. Synthesis of Na–X Zeolites from Tripolaceous Deposits (Crotone, Italy) and Volcanic Zeolitised Rocks (Vico Volcano, Italy). Microporous Mesoporous Mater. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Otieno, S.O.; Kengara, F.O.; Kowenje, C.O.; Mokaya, R. Hydrothermal Synthesis of Zeolites Using Silica Extracted from Tropical Volcanic Ash. Mater. Adv. 2023, 4, 2292–2300. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Singh, E.; Kumar, S. Recent Research Advancements in Catalytic Pyrolysis of Plastic Waste. ACS Sustain. Chem. Eng. 2023, 11, 2033–2049. [Google Scholar] [CrossRef]
- Li, K.; Lee, S.W.; Yuan, G.; Lei, J.; Lin, S.; Weerachanchai, P.; Yang, Y.; Wang, J.Y. Investigation into the Catalytic Activity of Microporous and Mesoporous Catalysts in the Pyrolysis of Waste Polyethylene and Polypropylene Mixture. Energies 2016, 9, 431. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: A Review. Process. Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Tan, T.; Wang, W.; Zhang, K.; Zhan, Z.; Deng, W.; Zhang, Q.; Wang, Y. Upcycling Plastic Wastes into Value-Added Products by Heterogeneous Catalysis. ChemSusChem 2022, 15, e202200522. [Google Scholar] [CrossRef] [PubMed]
- Buekens, A. Introduction to Feedstock Recycling of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; Scheirs, J., Kaminsky, W., Eds.; J. Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–41. ISBN 0470021527. [Google Scholar]
- Christopher, F.J.; Senthil Kumar, P.; Jayaraman, L.; Rangasamy, G. Assessment of Product Distribution of Plastic Waste from Catalytic Pyrolysis Process. Fuel 2023, 332, 126168. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; De Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Catalytic Stepwise Pyrolysis of Packaging Plastic Waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of Real Waste (MSW and MPW) Pyrolysis in Batch Reactor over Different Catalysts. Part I: Product Yields, Gas and Pyrolysis Oil Properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of Catalyst Contact Mode and Gas Atmosphere during Catalytic Pyrolysis of Waste Plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, Y.; Hu, Z.; Wang, S.; Jiao, X.; Zhu, J.; Sun, Y.; Xie, Y. Progress and Perspective for Conversion of Plastic Wastes into Valuable Chemicals. Chem. Soc. Rev. 2023, 52, 8–29. [Google Scholar] [CrossRef]
- Cocchi, M.; De Angelis, D.; Mazzeo, L.; Nardozi, P.; Piemonte, V.; Tuffi, R.; Ciprioti, S.V. Catalytic Pyrolysis of a Residual Plastic Waste Using Zeolites Produced by Coal Fly Ash. Catalysts 2020, 10, 1113. [Google Scholar] [CrossRef]
- Obali, Z.; Sezgi, N.A.; Doǧu, T. Performance of Acidic MCM-Like Aluminosilicate Catalysts in Pyrolysis of Polypropylene. Proc. Chem. Eng. Commun. 2009, 196, 116–130. [Google Scholar] [CrossRef]
- Luo, W.; Hu, Q.; Fan, Z.-Y.; Wan, J.; He, Q.; Huang, S.-X.; Zhou, N.; Song, M.; Zhang, J.-C.; Zhou, Z. The Effect of Different Particle Sizes and HCl-Modified Kaolin on Catalytic Pyrolysis Characteristics of Reworked Polypropylene Plastics. Energy 2020, 213, 119080. [Google Scholar] [CrossRef]
- ASTM E11-22; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM: West Conshohocken, PA, USA, 2022.
- Wu, T.-L.; Chen, Y.-H.; Hsu, W.-D. Phase Transition Pathway of Hydrothermal Zeolite Synthesis. Phys. Chem. Min. 2021, 48, 1. [Google Scholar] [CrossRef]
- Soongprasit, K.; Vichaphund, S.; Sricharoenchaikul, V.; Atong, D. Activity of Fly Ash-Derived ZSM-5 and Zeolite X on Fast Pyrolysis of Millettia (Pongamia) Pinnata Waste. Waste Biomass Valorization 2020, 11, 715–724. [Google Scholar] [CrossRef]
- Ramadhani, A.N.; Abdullah, I.; Krisnandi, Y.K. Effect of Physicochemical Properties of Co and Mo Modified Natural Sourced Hierarchical ZSM-5 Zeolite Catalysts on Vanillin and Phenol Production from Diphenyl Ether. Bull. Chem. React. Eng. Catal. 2022, 17, 225–239. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Characteristic of Fly Ash Derived-Zeolite and Its Catalytic Performance for Fast Pyrolysis of Jatropha Waste. Environ. Technol. 2014, 35, 2254–2261. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.D.; de Marques, M.F.V.; Henriques, C.A. Degradation of Polypropylene and Polyethylene Wastes Over HZSM-5 and USY Zeolites. Catal. Lett. 2019, 149, 798–812. [Google Scholar] [CrossRef]
- Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Duquesne, S.; Roudet, F.; Silva-Vela, A. Influence of the Process of Synthesis of Zeolites from Volcanic Ash in Its Synergistic Action as a Flame-Retardant for Polypropylene Composites. Buildings 2022, 12, 24. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- Murukutti, M.K.; Jena, H. Synthesis of Nano-Crystalline Zeolite-A and Zeolite-X from Indian Coal Fly Ash, Its Characterization and Performance Evaluation for the Removal of Cs+ and Sr2+ from Simulated Nuclear Waste. J. Hazard. Mater. 2022, 423, 127085. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yu, G.; Liu, J.; Du, Y.; Li, Y.; Li, H. Controlled Synthesis of Zeolite Adsorbent from Low-Grade Diatomite: A Case Study of Self-Assembled Sodalite Microspheres. J. Environ. Sci. 2020, 91, 92–104. [Google Scholar] [CrossRef]
- Krisnandi, Y.; Yanti, F.M.; Murti, S.D.S. Synthesis of ZSM-5 Zeolite from Coal Fly Ash and Rice Husk: Characterization and Application for Partial Oxidation of methane to Methanol. J. Phys. Conf. Ser. 2017, 755, 011001. [Google Scholar] [CrossRef]
- Verrecchia, G.; Cafiero, L.; de Caprariis, B.; Dell’Era, A.; Pettiti, I.; Tuffi, R.; Scarsella, M. Study of the Parameters of Zeolites Synthesis from Coal Fly Ash in Order to Optimize Their CO2 Adsorption. Fuel 2020, 276, 118041. [Google Scholar] [CrossRef]
- Salem, K.; Kasera, N.; Rahman, A.; Jameel, H.; Habibi, Y.; Eichhorn, S.; French, A.; Pal, L.; Lucia, L. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of Pure Na–X and Na–A Zeolite from Bagasse Fly Ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Mintova, S.; Hölzl, M.; Valtchev, V.; Mihailova, B.; Bouizi, Y.; Bein, T. Closely Packed Zeolite Nanocrystals Obtained via Transformation of Porous Amorphous Silica. Chem. Mater. 2004, 16, 5452–5459. [Google Scholar] [CrossRef]
- Kordatos, K.; Gavela, S.; Ntziouni, A.; Pistiolas, K.N.; Kyritsi, A.; Kasselouri-Rigopoulou, V. Synthesis of Highly Siliceous ZSM-5 Zeolite Using Silica from Rice Husk Ash. Microporous Mesoporous Mater. 2008, 115, 189–196. [Google Scholar] [CrossRef]
- Valencia-Huaman, A.G.; Fuentes-Mamani, S.H.; Mamani-De La Cruz, L.F.; Velasco, F.; Churata, R.; Silva-Vela, A.; Mamani-Quispe, J.; Almirón, J. Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers. Sustainability 2024, 16, 5910. [Google Scholar] [CrossRef]
- Liang, G.; Li, Y.; Yang, C.; Hu, X.; Li, Q.; Zhao, W. Synthesis of ZSM-5 Zeolites from Biomass Power Plant Ash for Removal of Ionic Dyes from Aqueous Solution: Equilibrium Isotherm, Kinetic and Thermodynamic Analysis. RSC Adv. 2021, 11, 22365–22375. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Munagapati, V.S.; Wen, J.C.; Shu, C.M. Synthesis of Novel ZSM-22 Zeolite from Taiwanese Coal Fly Ash for the Selective Separation of Rhodamine 6G. J. Mater. Res. Technol. 2020, 9, 15381–15393. [Google Scholar] [CrossRef]
- Szostak, R. Molecular Sieves; Springer Netherlands: Dordrecht, The Netherlands, 1989; ISBN 978-94-010-9531-0. [Google Scholar]
- Frantz, T.S.; Ruiz, W.A.; Da Rosa, C.A.; Mortola, V.B. Synthesis of ZSM-5 with High Sodium Content for CO2 Adsorption. Microporous Mesoporous Mater. 2016, 222, 209–217. [Google Scholar] [CrossRef]
- Sumari, S.; Santoso, A.; Cahyanti, R.; Yahmin, Y.; Wijaya, A.R. Synthesis of Zeolite Na/H-X Using Silica Based of Coastal Sand by Hydrothermal Method. AIP Conf. Proc. 2020, 2251, 040026. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.; Liu, H.; Yang, L.; Liu, D.; Tang, J.; Luo, Y. Synthesis of Na-X Zeolite from Low Aluminum Coal Fly Ash: Characterization and High Efficient As(V) Removal. Adv. Powder Technol. 2019, 30, 199–206. [Google Scholar] [CrossRef]
- Xu, J.; Wen, Y.; Li, D.; Zhang, S.; Han, Z.; Hu, H.; Jin, L. Catalytic Upgrading of Biomass Pyrolysis Volatiles over Y Zeolites Modified with Different Metal Oxides. Fuel 2024, 371, 131936. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure–Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Le, T.T.; Chawla, A.; Rimer, J.D. Impact of Acid Site Speciation and Spatial Gradients on Zeolite Catalysis. J. Catal. 2020, 391, 56–68. [Google Scholar] [CrossRef]
- Peral, A.; Escola, J.M.; Serrano, D.P.; Přech, J.; Ochoa-Hernández, C.; Čejka, J. Bidimensional ZSM-5 Zeolites Probed as Catalysts for Polyethylene Cracking. Catal. Sci. Technol. 2016, 6, 2754–2765. [Google Scholar] [CrossRef]
- Novembre, D.; di Sabatino, B.; Gimeno, D.; Pace, C. Synthesis and Characterization of Na-X, Na-A and Na-P Zeolites and Hydroxysodalite from Metakaolinite. Clay Miner. 2011, 46, 339–354. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Microwave-Assisted Hydrothermal Synthesis of Hydroxy-Sodalite Zeolite Membrane. Microporous Mesoporous Mater. 2004, 75, 173–181. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the Fly Ash Fraction after Grinding Process on the Hydrothermal Synthesis Efficiency of Na-A, Na-P1, Na-X and Sodalite Zeolite Types. Int. J. Coal Sci. Technol. 2021, 8, 291–311. [Google Scholar] [CrossRef]
- Kuroki, S.; Hashishin, T.; Morikawa, T.; Yamashita, K.; Matsuda, M. Selective Synthesis of Zeolites A and X from Two Industrial Wastes: Crushed Stone Powder and Aluminum Ash. J. Environ. Manag. 2019, 231, 749–756. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal Synthesis of Mixtures of NaA Zeolite and Sodalite from Ti-Bearing Electric Arc Furnace Slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Zou, J.; Guo, C.; Wei, C.; Li, F.; Jiang, Y. Synthesis of Pure Na-X and Na-P Zeolite from Acid-Extracting Residues of CFB Fly Ash by a Single-Step Hydrothermal Method. Mater. Trans. 2016, 57, 726–731. [Google Scholar] [CrossRef]
- Sousa, L.V.; Silva, A.O.S.; Silva, B.J.B.; Teixeira, C.M.; Arcanjo, A.P.; Frety, R.; Pacheco, J.G.A. Fast Synthesis of ZSM-22 Zeolite by the Seed-Assisted Method of Crystallization with Methanol. Microporous Mesoporous Mater. 2017, 254, 192–200. [Google Scholar] [CrossRef]
- Munusamy, K.; Das, R.K.; Ghosh, S.; Kishore Kumar, S.A.; Pai, S.; Newalkar, B.L. Synthesis, Characterization and Hydroisomerization Activity of ZSM-22/23 Intergrowth Zeolite. Microporous Mesoporous Mater. 2018, 266, 141–148. [Google Scholar] [CrossRef]
- Chareonpanich, M.; Namto, T.; Kongkachuichay, P.; Limtrakul, J. Synthesis of ZSM-5 Zeolite from Lignite Fly Ash and Rice Husk Ash. Fuel Process. Technol. 2004, 85, 1623–1634. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Effect of Regeneration Temperature and Time on the Activity of HUSY and HZSM5 Zeolites during the Catalytic Pyrolysis of Polyethylene. J. Anal. Appl. Pyrolysis 2005, 74, 361–369. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical Routes for the Valorization of Waste Polyolefinic Plastics to Produce Fuels and Chemicals. A Review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Tekin, K.; Akalın, M.K.; Kadı, Ç.; Karagöz, S. Catalytic Degradation of Waste Polypropylene by Pyrolysis. J. Energy Inst. 2012, 85, 150–155. [Google Scholar] [CrossRef]
- Shi, H.; Cui, Y.; Zhang, Y.; Zhao, W.; Liu, W.; Ruan, R. Gases production from microwave-assisted pyrolysis of polypropylene plastic. J. Environ. Chem. Eng. 2023, 11, 110851. [Google Scholar] [CrossRef]
- Khair, H.; Listiany, B.; Utami, R. Pyrolysis of polypropylene plastic waste: An analysis of oil quantity, density, viscosity, and calorific value. Earth Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Dhahak, A.; Hild, G.; Rouaud, M.; Mauviel, G.; Burkle-Vitzthum, V. Slow pyrolysis of polyethylene terephthalate: Online monitoring of gas production and quantitative analysis of waxy products. J. Anal. Appl. Pyrolysis 2019, 142, 104664. [Google Scholar] [CrossRef]


| Oxide | PO (%) | PU (%) | IG (%) |
|---|---|---|---|
| SiO2 | 77.90 | 78.60 | 67.60 |
| Al2O3 | 15.10 | 14.80 | 20.30 |
| K2O | 3.65 | 3.49 | 2.29 |
| Fe2O3 | 1.33 | 1.35 | 4.43 |
| CaO | 1.30 | 1.14 | 4.22 |
| TiO2 | 0.26 | 0.23 | 0.60 |
| Si/Al | 9.10 | 9.40 | 5.90 |
| Other | 0.46 | 0.389 | 0.56 |
| Method | Zeolite Precursor | Sample Code | Merger Conditions | Hydrothermal Treatment Conditions | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | NaOH/Precursor Ratio by Weight | Temperature (°C) | Time (h) | Liquid/Solid Ratio by Weight | NaOH (mol/L) | |||
| 1 | Pozzolan | PO-M1 | ||||||
| Ignimbrite | IG-M1 | 500 | 1.2 | 90 | 7 | 10 | - | |
| Pumice | PU-M1 | |||||||
| 2 | Pozzolan | PO-M2 | ||||||
| Ignimbrite | IG-M2 | 550 | 1.2 | 90 | 12 | 5 | - | |
| Pumice | PU-M2 | |||||||
| 3 | Pozzolan | PO-M3 | ||||||
| Ignimbrite | IG-M3 | - | - | 160 | 72 | 8.3 | 3 | |
| Pumice | PU-M3 | |||||||
| Zeolite | Surface Specific Area | Micropore Area | Micropore Volume | Pore Diameter |
|---|---|---|---|---|
| m2·g−1 | m2·g−1 | cm3·g−1 | nm | |
| PO-M1 | 56.8 | 3.4 | 0.0014 | 9.6 |
| IG-M1 | 37.1 | 1.3 | 0.0004 | 8.5 |
| PU-M1 | 103.0 | 0.9 | 0.0000 | 8.3 |
| PO-M2 | 451.3 | 408.2 | 0.212 | 6.0 |
| IG-M2 | 365 | 328.5 | 0.170 | 5.6 |
| PU-M2 | 289.6 | 196.6 | 0.102 | 5.6 |
| PO-M3 | 229.4 | 114.4 | 0.076 | 3.8 |
| IG-M3 | 205.4 | 146.9 | 0.076 | 4.8 |
| PU-M3 | 156.5 | 101.3 | 0.053 | 5.8 |
| Material/Zeolite | Stretching/Bending H-O-H | Asymmetric Stretching Si-O-T | Symmetric Stretching Si-O-T | ||
|---|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | cm−1 | cm−1 | |
| PO | - | - | 1007 | 780 | 724 |
| PO-M1 | 3386 | 1639 | 993 | - | 683 |
| PO-M2 | 3404 | 1642 | 971 | - | 664 |
| PO-M3 | - | 1629 | 1052 | 785 | - |
| IG | - | - | 1008 | 792 | 730 |
| IG-M1 | 3278 | 1638 | 999 | - | 710 |
| IG-M2 | 3386 | 1640 | 973 | - | 664 |
| IG-M3 | - | 1626 | 1065 | 793 | - |
| PU | - | - | 996 | - | 706 |
| PU-M1 | 3348 | 1638 | 1003 | - | 670 |
| PU-M2 | 3404 | 1639 | 971 | - | 664 |
| PU-M3 | - | 1630 | 1024 | 787 | 722 |
| Peaks cm−1 | Bond | Functional Group |
|---|---|---|
| 3072 | Stretching =C-H | Alkenes |
| 2960–2955 | Asymmetric C-H stretching of CH3 | Methyl alkanes |
| 2925–2915 | Asymmetric C-H stretching of CH2 | Methylene alkanes |
| 2875, 2870 | Symmetric C-H stretching of CH3 | Methyl alkanes |
| 1654–1648 | C=C stretching | Alkenes or aromatics |
| 1460–1455 | Asymmetric C-H stretching of CH3 | Methyl and methylene alkanes |
| 1379–1376 | C-H plane bending (scissoring) of CH2 | Methyl alkanes |
| 970–964 | C-H symmetrical bending of CH3 | Alkenes |
| 888 | C-H bending | Aromatics |
| 795 | C-H out-of-plane bending | Aromatics |
| 740–728 | C-H out-of-plane bending | Methylene Alkanes |
| 694 | C-H plane bending (Swing) of CH2 | Aromatics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamani-De La Cruz, L.F.; Churata, R.; Valencia-Huaman, A.G.; Fuentes-Mamani, S.H.; Almirón, J. Comparison of the Synthesis Method of Zeolite Catalysts Based on Pozzolan, Pumice, and Ignimbrite Applied to the Sustainable Pyrolysis of Polymers. Sustainability 2025, 17, 2986. https://doi.org/10.3390/su17072986
Mamani-De La Cruz LF, Churata R, Valencia-Huaman AG, Fuentes-Mamani SH, Almirón J. Comparison of the Synthesis Method of Zeolite Catalysts Based on Pozzolan, Pumice, and Ignimbrite Applied to the Sustainable Pyrolysis of Polymers. Sustainability. 2025; 17(7):2986. https://doi.org/10.3390/su17072986
Chicago/Turabian StyleMamani-De La Cruz, Luis Fernando, Rossibel Churata, Angel Gabriel Valencia-Huaman, Sandro Henry Fuentes-Mamani, and Jonathan Almirón. 2025. "Comparison of the Synthesis Method of Zeolite Catalysts Based on Pozzolan, Pumice, and Ignimbrite Applied to the Sustainable Pyrolysis of Polymers" Sustainability 17, no. 7: 2986. https://doi.org/10.3390/su17072986
APA StyleMamani-De La Cruz, L. F., Churata, R., Valencia-Huaman, A. G., Fuentes-Mamani, S. H., & Almirón, J. (2025). Comparison of the Synthesis Method of Zeolite Catalysts Based on Pozzolan, Pumice, and Ignimbrite Applied to the Sustainable Pyrolysis of Polymers. Sustainability, 17(7), 2986. https://doi.org/10.3390/su17072986






