Potential of Lipids from Polymer-Based Dewatered Sewage Sludge as Feedstock for Biodiesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagent
2.2. Preparation of Sludge Sample
2.3. Experiment of Lipid Extraction
2.4. Separation of Lipid
2.5. Lipid Yield
2.6. Characterisation of Lipid
2.7. Conversion of Lipid to Fatty Acid Methyl Esters (FAME)
2.8. Characterisation of FAME
3. Results and Discussion
3.1. Influence of Extraction Parameters on Lipid Yield
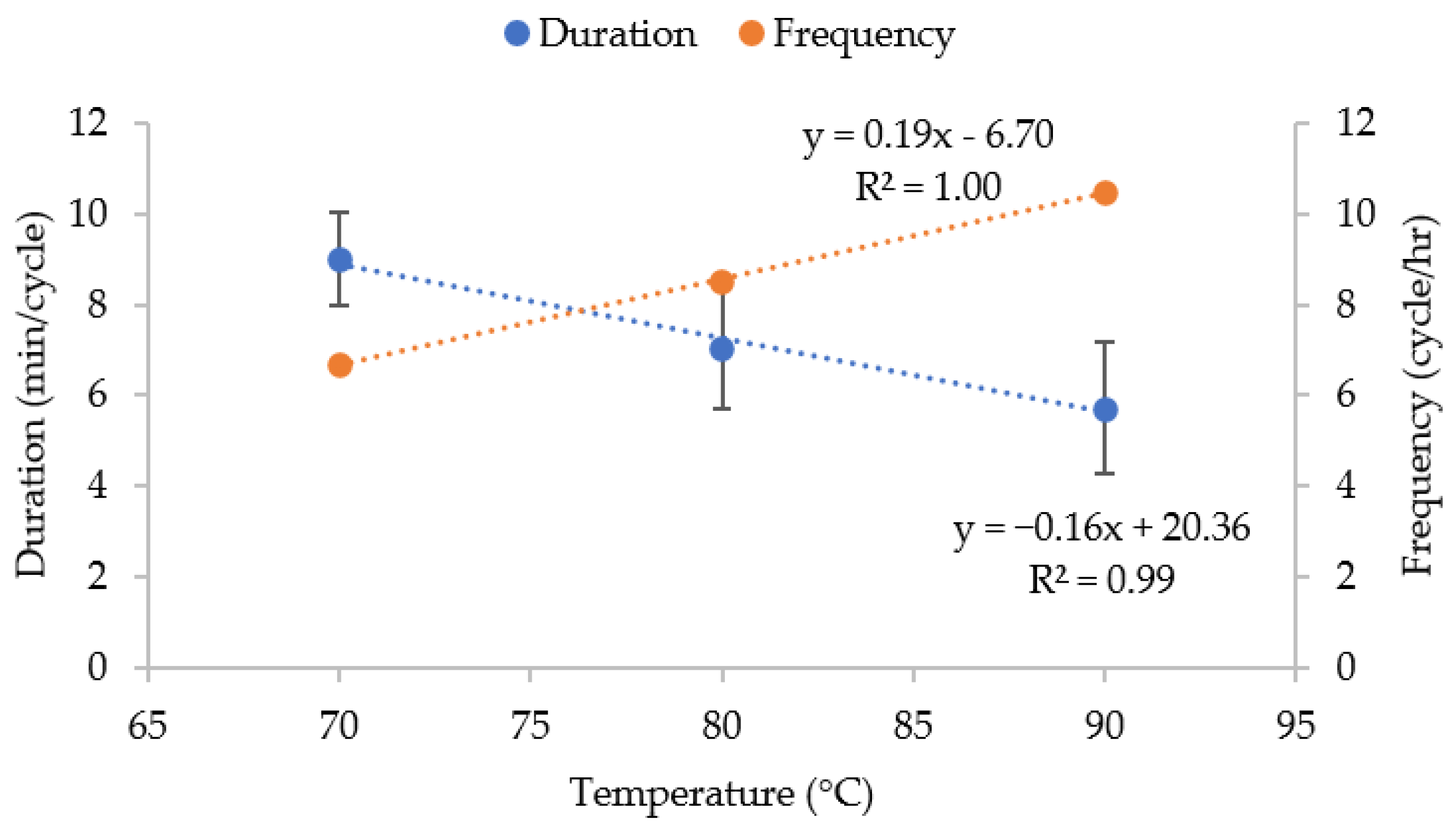
3.1.1. Extraction Temperature
3.1.2. Extraction Time
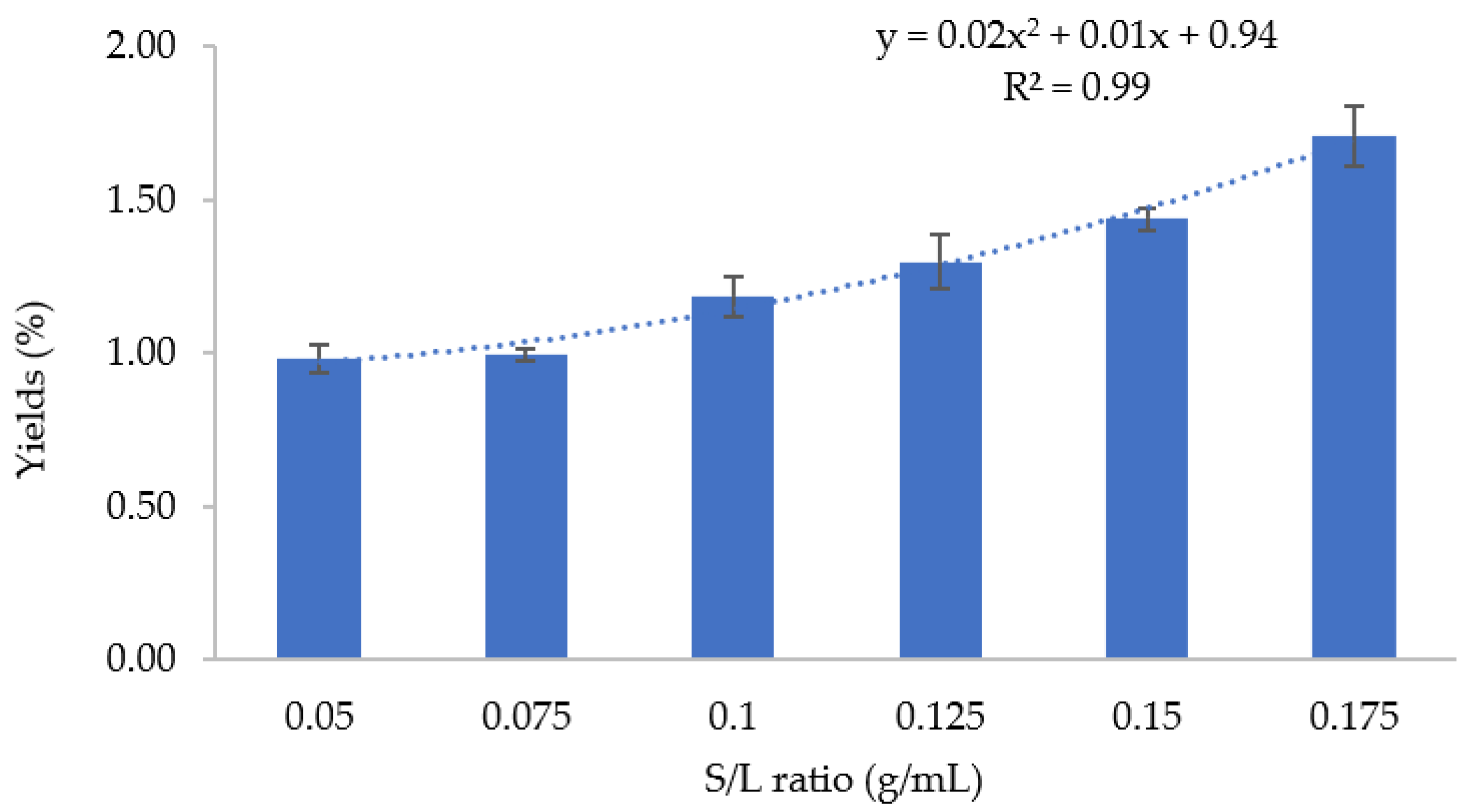
3.1.3. Sludge-to-Solvent (S/L) Ratio
3.2. Optimum Lipid Yield
3.3. Lipid Yield from Various Types of Sludge
3.4. Characteristics of DS Lipid and DS FAME
3.4.1. FTIR Analysis of DS Lipid
3.4.2. TGA Analysis of DS Lipid
3.5. Characteristics of DS FAME
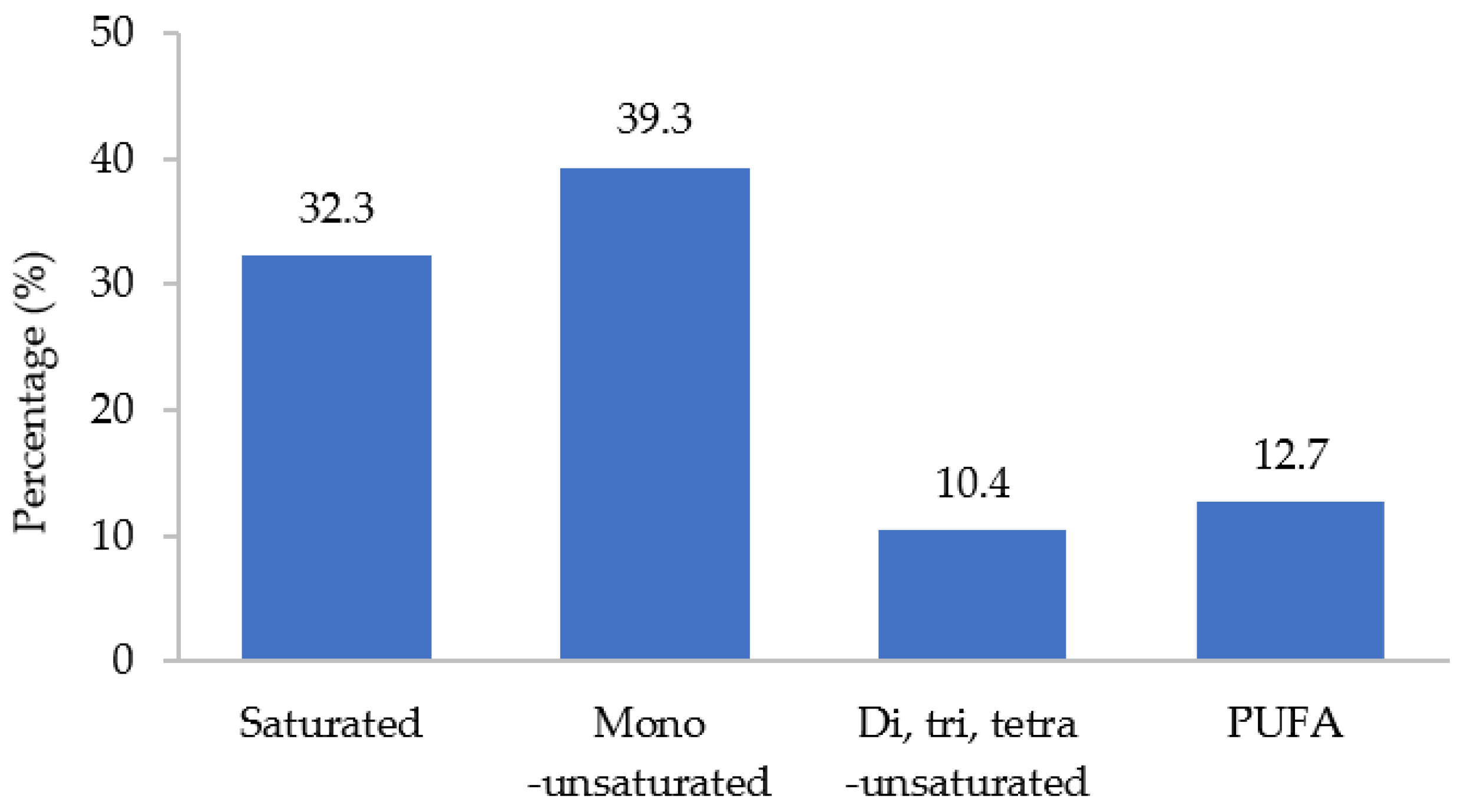
3.5.1. Fatty Acid Profile of DS FAME
3.5.2. Potential of DS FAME as Biodiesel
3.6. Production of Biodiesel from Sewage Sludge and Its Economic and Environmental Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasanya, O.O.; Osigbesan, A.A.; Avbenake, O.P. Biodiesel Production From Non-Edible and Waste Lipid Sources. In Biodiesel Technology and Applications; Inamuddin, Ahamed, M.I., Boddula, R., Rezakazemi, M., Eds.; Scrivener Publishing: Beverly, MA, USA, 2021; pp. 389–428. ISBN 9781119724643. [Google Scholar]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Soratana, K.; Khanna, V.; Landis, A.E. Re-Envisioning the Renewable Fuel Standard to Minimize Unintended Consequences: A Comparison of Microalgal Diesel with Other Biodiesels. Appl. Energy 2013, 112, 194–204. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Adnan, A.; Mukhtar, H.; Rashid, U.; Danish, M. Biodiesel Production Through Chemical and Biochemical Transesterification. In Clean Energy for Sustainable Development; Rasul, M.G., Azad, A.k., Sharma, S.C., Eds.; Academic Press (Elsevier): New York, NY, USA, 2017; pp. 465–485. ISBN 9780128054239. [Google Scholar]
- Rehan, M.; Gardy, J.; Demirbas, A.; Rashid, U.; Budzianowski, W.M.; Pant, D.; Nizami, A.S. Waste to Biodiesel: A Preliminary Assessment for Saudi Arabia. Bioresour. Technol. 2018, 250, 17–25. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Feedstocks for Biodiesel Production. In Biodiesel Science and Technology; Bart, J.C.J., Palmeri, N., Cavallaro, S., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 130–225. ISBN 9781845695910. [Google Scholar]
- Zhu, F.; Zhao, L.; Jiang, H.; Zhang, Z.; Xiong, Y.; Qi, J.; Wang, J. Comparison of the Lipid Content and Biodiesel Production from Municipal Sludge Using Three Extraction Methods. Energy Fuels 2014, 28, 5277–5283. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage Sludge to Bio-Fuel: A Review on the Sustainable Approach of Transforming Sewage Waste to Alternative Fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Alsaedi, A.A.; Hossain, S.; Balakrishnan, V.; Naim, A.; Yahaya, A.; Ismail, N.; Naushad, M.; Bathula, C.; Ahmad, M.I. Extraction of Municipal Sewage Sludge Lipids Using Supercritical CO2 for Biodiesel Production: Mathematical and Kinetics Modeling. J. Chem. 2022, 2022, 11. [Google Scholar]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical Processing of Sewage Sludge to Energy and Fuel: Fundamentals, Challenges and Considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, X.; Zhao, L.; Liu, X.; Qi, J.; Wang, X.; Wang, J. Lipid Profiling in Sewage Sludge. Water Res. 2017, 116, 149–158. [Google Scholar] [CrossRef]
- Mtshali, J.S.; Tiruneh, A.T.; Fadiran, A.O. Sewage Sludge, Nutrient Value, Organic Fertilizer, Soil Amendment, Sludge Reuse, Nitrogen, Phosphorus; Sewage Sludge, Nutrient Value, Organic Fertilizer, Soil Amendment, Sludge Reuse, Nitrogen, Phosphorus. Resour. Environ. 2014, 4, 190–199. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; ten Hoeve, M.; Christensen, T.H.; Bruun, S.; Jensen, L.S.; Scheutz, C. Life Cycle Assessment of Sewage Sludge Management Options Including Long-Term Impacts after Land Application. J. Clean. Prod. 2018, 174, 538–547. [Google Scholar] [CrossRef]
- Minnesota Pollution Control Agency. Wastewater Treatment Technology; Minnesota Pollution Control Agency: Saint Paul, MN, USA, 1997. [Google Scholar]
- Volume 6: Sludge Treatment and Disposal; Andreoli, C.V., von Sperling, M., Fernendes, F., Eds.; IWA Publishing: London, UK, 2007; ISBN 9781843391661. [Google Scholar]
- De Oliveira Silva, J.; Filho, G.R.; Da Silva Meireles, C.; Ribeiro, S.D.; Vieira, J.G.; Da Silva, C.V.; Cerqueira, D.A. Thermal Analysis and FTIR Studies of Sewage Sludge Produced in Treatment Plants. The Case of Sludge in the City of Uberlândia-MG, Brazil. Thermochim. Acta 2012, 528, 72–75. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Rohani, S. Lipid Extraction and Biodiesel Production from Municipal Sewage Sludges: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1067–1072. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, X.; Wu, Y.; Wang, D.; Xu, Q.; Yang, J. The Fate and Impact of Coagulants/Flocculants in Sludge Treatment Systems. Environ. Sci. Water Res. Technol. 2021, 7, 1387–1401. [Google Scholar] [CrossRef]
- Luo, F.; Dong, B.; Dai, L.; He, Q.; Dai, X. Change of Thermal Drying Characteristics for Dewatered Sewage Sludge Based on Anaerobic Digestion. J. Therm. Anal. Calorim. 2013, 114, 307–312. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global Wastewater and Sludge Production, Treatment and Use. In Wastewater: Economic Asset in an Urbanizing World; Drechsel, P., Qadir, M., Wichelns, D., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 15–36. ISBN 978-94-017-9544-9. [Google Scholar]
- Gorazda, K.; Tarko, B.; Wzorek, Z.; Kominko, H.; Nowak, A.K.; Kulczycka, J.; Henclik, A.; Smol, M. Fertilisers Production from Ashes after Sewage Sludge Combustion—A Strategy towards Sustainable Development. Environ. Res. 2017, 154, 171–180. [Google Scholar] [CrossRef]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The Role of Wastewater Treatment in Achieving Sustainable Development Goals (SDGs) and Sustainability Guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Nazari, M.T.; Mazutti, J.; Basso, L.G.; Colla, L.M.; Brandli, L. Biofuels and Their Connections with the Sustainable Development Goals: A Bibliometric and Systematic Review. Environ. Dev. Sustain. 2021, 23, 11139–11156. [Google Scholar] [CrossRef]
- Wang, K.; Nakakubo, T. Design of a Sewage Sludge Energy Conversion Technology Introduction Scenario for Large City Sewage Treatment Plants in Japan: Focusing on Zero Fuel Consumption. J. Clean. Prod. 2022, 379, 134794. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, J.; Li, B.; Zhao, S.; Zhang, L. Residual and Ecological Risk Assessment of Heavy Metals in Fly Ash from Co-Combustion of Excess Sludge and Coal. Sci. Rep. 2021, 11, 2499. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Rohani, S. Experimental Analysis of Lipid Extraction and Biodiesel Production from Wastewater Sludge. Fuel Process. Technol. 2011, 92, 2241–2251. [Google Scholar] [CrossRef]
- Aiman, N.; Mustapha, H.; Sing, W.; Rahman, R.A. Optimisation of Lipid Extraction from Primary Sludge by Soxhlet Extraction. Chem. Eng. Trans. 2017, 56, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Energy Balance and Greenhouse Gas Emissions of Biodiesel Production from Oil Derived from Wastewater and Wastewater Sludge. Renew. Energy 2013, 55, 392–403. [Google Scholar] [CrossRef]
- Chan, W.P.; Wang, J.-Y. Comprehensive Characterisation of Sewage Sludge for Thermochemical Conversion Processes—Based on Singapore Survey. Waste Manag. 2016, 54, 131–142. [Google Scholar] [CrossRef]
- Khalil, N.A.; Lajulliadi, A.F.; Abedin, F.N.J.; Fizal, A.N.S.; Safie, S.I.; Zulkifli, M.; Taweepreda, W.; Hossain, M.S.; Ahmad Yahaya, A.N. Multifaceted Impact of Lipid Extraction on the Characteristics of Polymer-Based Sewage Sludge towards Sustainable Sludge Management. Polymers 2024, 16, 2646. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage Sludge Disposal Strategies for Sustainable Development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons Inc.: Danvers, MA, USA, 2013; ISBN 9781118146927. [Google Scholar]
- Sakthi Vignesh, N.; Vimali, E.; Sangeetha, R.; Arumugam, M.; Ashokkumar, B.; Ganeshmoorthy, I.; Varalakshmi, P. Sustainable Biofuel from Microalgae: Application of Lignocellulosic Wastes and Bio-Iron Nanoparticle for Biodiesel Production. Fuel 2020, 278, 118326. [Google Scholar] [CrossRef]
- Khalil, N.A.; Hamid, H.A.; Fizal, A.N.S.; Zulkifli, M.; Hossain, M.S.; Yahaya, A.N.A. Utilization of Supercritical Carbon Dioxide (SC-CO2) in Lipids Extraction from Sewage Sludge Cake: A Preliminary Study. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012054. [Google Scholar] [CrossRef]
- Hag Ibrahim, S.N. Statistical Optimization of Lipid Extraction from Wastewater Scum Sludge and Saponifiable Lipids Composition Analysis. Sci. J. Energy Eng. 2017, 5, 48. [Google Scholar] [CrossRef][Green Version]
- Baur, F.J.; Ensminger, L.G. The Association of Official Analytical Chemists (AOAC). J. Am. Oil Chem. Soc. 1977, 54, 171–172. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Method 996.06 Fat (Total, Saturated and Unsaturated) in Foods—Hyrolytic Extraction Gas Chromatographic Method; AOAC International: Rockville, MD, USA, 2001. [Google Scholar]
- Luque de Castro, M.D.; García Ayuso, L.E. ENVIRONMENTAL APPLICATIONS|Soxhlet Extraction. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 2701–2709. [Google Scholar]
- Thermo Scientific. Methods Optimization in Accelerated Solvent Extraction; Thermo Scientific: Boston, MA, USA, 2013. [Google Scholar]
- Gomaa, M.A.; Gombocz, N.; Schild, D.; Mjalli, F.S.; Al-Harrasi, A.; Abed, R.M.M. Effect of organic solvents and acidic catalysts on biodiesel yields from primary sewage sludge, and characterization of fuel properties. Biofuels 2021, 12, 405–413. [Google Scholar] [CrossRef]
- Abdulhussein Alsaedi, A.; Sohrab Hossain, M.; Balakrishnan, V.; Abdul Hakim Shaah, M.; Mohd Zaini Makhtar, M.; Ismail, N.; Naushad, M.; Bathula, C. Extraction and Separation of Lipids from Municipal Sewage Sludge for Biodiesel Production: Kinetics and Thermodynamics Modeling. Fuel 2022, 325, 124946. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An Evaluation of the Efficacy of Using Selected Solvents for the Extraction of Lipids from Algal Biomass by the Soxhlet Extraction Method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Melero, J.A.; Sánchez-Vázquez, R.; Vasiliadou, I.A.; Martínez Castillejo, F.; Bautista, L.F.; Iglesias, J.; Morales, G.; Molina, R. Municipal Sewage Sludge to Biodiesel by Simultaneous Extraction and Conversion of Lipids. Energy Convers. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Russo-Profili, A.; Eveleigh, A.; Aliev, A.; Kay, A.; Mills-Lamptey, B. Influence of Solvent Selection and Extraction Temperature on Yield and Composition of Lipids Extracted from Spent Coffee Grounds. Ind. Crops Prod. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid–Liquid Extraction. In Food Engineering Handbook; Varzakas, T., Tzia, C., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 253–286. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 162, ISBN 2135415065. [Google Scholar]
- Olkiewicz, M.; Fortuny, A.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Effects of Pre-Treatments on the Lipid Extraction and Biodiesel Production from Municipal WWTP Sludge. Fuel 2015, 141, 250–257. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Fortuny, A.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Evaluation of Different Sludges from WWTP as a Potential Source for Biodiesel Production. Procedia Eng. 2012, 42, 634–643. [Google Scholar] [CrossRef]
- Liu, S.; Luo, T.; Liu, G.; Xu, X.; Shao, Y.; Qi, L.; Wang, H. Characterization and Reutilization Potential of Lipids in Sludges from Wastewater Treatment Processes. Sci. Rep. 2020, 10, 12997. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of Extraction Parameters and Their Optimization on the Nutraceuticals and Antioxidant Properties of Aqueous Extract Mulberry Leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Safder, M.; Temelli, F.; Ullah, A. Extraction, Optimization, and Characterization of Lipids from Spent Hens: An Unexploited Sustainable Bioresource. J. Clean. Prod. 2019, 206, 622–630. [Google Scholar] [CrossRef]
- Syimir Fizal, A.N.; Hossain, M.S.; Zulkifli, M.; Khalil, N.A.; Abd Hamid, H.; Ahmad Yahaya, A.N. Implementation of the Supercritical CO2 Technology for the Extraction of Candlenut Oil as a Promising Feedstock for Biodiesel Production: Potential and Limitations. Int. J. Green Energy 2022, 19, 72–83. [Google Scholar] [CrossRef]
- Stillwell, W. Bioactive Lipids. In An Introduction to Biological Membranes; Stillwell, W., Ed.; Elsevier: Armsterdam, The Netherlands, 2016; pp. 453–478. ISBN 9781616684648. [Google Scholar]
- Xia, A.; Sun, C.; Fu, Q.; Liao, Q.; Huang, Y.; Zhu, X.; Li, Q. Biofuel Production from Wet Microalgae Biomass: Comparison of Physicochemical Properties and Extraction Performance. Energy 2020, 212, 118581. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Plechkova, N.V.; Fabregat, A.; Stüber, F.; Fortuny, A.; Font, J.; Bengoa, C. Efficient Extraction of Lipids from Primary Sewage Sludge Using Ionic Liquids for Biodiesel Production; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 153, ISBN 3497755966. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 10815–10837. ISBN 0471976709. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; Volume 21, ISBN 0471393622. [Google Scholar]
- Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. FTIR Spectroscopy for Evaluation and Monitoring of Lipid Extraction Efficiency for Oleaginous Fungi. PLoS ONE 2017, 12, e0170611. [Google Scholar] [CrossRef]
- Smith, B.C. The C=O Bond, Part VIII: Review. Spectroscopy 2018, 33, 24–29. [Google Scholar]
- Smith, B.C. The C=O Bond, Part VI: Esters and the Rule of Three. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Marina, A.M.; Wan Rosli, W.I.; Noorhidayah, M. Rapid Quantification of Free Fatty Acids in Virgin Coconut Oil by Ftir Spectroscopy. Malays. Appl. Biol. 2015, 44, 45–49. [Google Scholar]
- Siatis, N.G.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Polissiou, M.G. Improvement of Biodiesel Production Based on the Application of Ultrasound: Monitoring of the Procedure by FTIR Spectroscopy. J. Am. Oil Chem. Soc. 2006, 83, 53–57. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Salih, N. Hydrolysis Optimization and Characterization Study of Preparing Fatty Acids from Jatropha Curcas Seed Oil. Chem. Cent. J. 2011, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Kowalska, K.; Wiszniowski, J.; Turek-Szytow, J. Qualitative Analysis of Activated Sludge Using FT-IR Technique. Chem. Pap. 2018, 72, 2699–2706. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Wolfrum, E.J. Feasibility of Spectroscopic Characterization of Algal Lipids: Chemometric Correlation of NIR and FTIR Spectra with Exogenous Lipids in Algal Biomass. Bioenergy Res. 2011, 4, 22–35. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chu, Y.-S.; Liu, J.-L.; Chang, J.-S. Thermal Degradation of Carbohydrates, Proteins and Lipids in Microalgae Analyzed by Evolutionary Computation. Energy Convers. Manag. 2018, 160, 209–219. [Google Scholar] [CrossRef]
- Mettler Toledo Thermal Analysis Application No. UC 131: Interpreting TGA Curves. Available online: https://www.mt.com/us/en/home/supportive_content/matchar_apps/MatChar_UC131.html (accessed on 25 July 2023).
- Alves, C.T.; Peters, M.A.; Onwudili, J.A. Application of Thermogravimetric Analysis Method for the Characterisation of Products from Triglycerides during Biodiesel Production. J. Anal. Appl. Pyrolysis 2022, 168, 105766. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A Review on Latest Trends in Cleaner Biodiesel Production: Role of Feedstock, Production Methods, and Catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Carrero, A.; Pérez, Á. Advances in Biodiesel Quality Control, Characterisation and Standards Development. In Advances in Biodiesel Production; Luque, R., Melero, J.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 91–130. ISBN 978-0-85709-117-8. [Google Scholar]
- EN 14104:2003; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- Jariah, N.F.; Hassan, M.A.; Taufiq-Yap, Y.H.; Roslan, A.M. Technological Advancement for Efficiency Enhancement of Biodiesel and Residual Glycerol Refining: A Mini Review. Processes 2021, 9, 1198. [Google Scholar] [CrossRef]
- Gopinath, A.; Puhan, S.; Nagarajan, G. Relating the Cetane Number of Biodiesel Fuels to Their Fatty Acid Composition: A Critical Study. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2009, 223, 565–683. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Transesterification Processes for Biodiesel Production from Oils and Fats. In Biodiesel Science and Technology; Bart, J.C.J., Palmeri, N., Cavallaro, S., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 285–321. [Google Scholar]
- Knothe, G.; Dunn, R.O.; Moser, B.R.; Peterson, C.L.; Möller, G.; Bringe, N.A.; Calabotta, B.J.; Morgenstern, D.A. 6—Fuel Properties. In The Biodiesel Handbook: Second Edition; Knothe, G., Krahl, J., Van Gerpen, J., Eds.; AOCS Press: Champaign, IL, USA, 2010; Volume 2, pp. 137–251. ISBN 9780983507260. [Google Scholar]
- Dufreche, S.; Hernandez, R.; French, T.; Sparks, D.; Zappi, M.; Alley, E. Extraction of Lipids from Municipal Wastewater Plant Microorganisms for Production of Biodiesel. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 181–187. [Google Scholar] [CrossRef]
- Quéméneur, M.; Marty, Y. Fatty Acids and Sterols in Domestic Wastewaters. Water Res. 1994, 28, 1217–1226. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Emerging New Energy Crops for Biodiesel Production. In Biodiesel Science and Technology; Bart, J.C.J., Palmeri, N., Cavallaro, S., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 226–284. [Google Scholar]
- Kech, C.; Galloy, A.; Frippiat, C.; Piel, A.; Garot, D. Optimization of Direct Liquid-Liquid Extraction of Lipids from Wet Urban Sewage Sludge for Biodiesel Production. Fuel 2018, 212, 132–139. [Google Scholar] [CrossRef]
- EN 15779; Gas Chromatographic Analysis of Polyunsaturated FAME in Biodiesel Made from Algae and Marine Oils. Agilent Technologies: Santa Clara, CA, USA, 2011.
- Chandra, R.; Vishal, G.; Sánchez, C.E.G.; Uribe, J.A.G. Bioreactor for Algae Cultivation and Biodiesel Production. In Bioreactors; Singh, L., Yousuf, A., Mahapatra, D.M., Eds.; Elsevier: Armsterdam, The Netherlands, 2020; pp. 289–307. ISBN 9780128212646. [Google Scholar]
- EN14103:2003; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic Acid Methyl Ester Contents. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- European Committee for Standardization (CEN). EN 14214:2008; Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Biodiesel Engines—Requirements and Test Methods. CEN: Brussels, Belgium, 2008.
- Knothe, G. Analyzing Biodiesel: Standards and Other Methods. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Seng Liew, C.; Ren Mong, G.; Wei Lim, J.; Raksasat, R.; Rawindran, H.; Hong Leong, W.; Devendran Manogaran, M.; Ho Chai, Y.; Chia Ho, Y.; Ur Rahmah, A.; et al. Life Cycle Assessment: Sustainability of Biodiesel Production from Black Soldier Fly Larvae Feeding on Thermally Pre-Treated Sewage Sludge under a Tropical Country Setting. Waste Manag. 2023, 164, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Mondala, A.; Liang, K.; Toghiani, H.; Hernandez, R.; French, T. Biodiesel Production by in Situ Transesterification of Municipal Primary and Secondary Sludges. Bioresour. Technol. 2009, 100, 1203–1210. [Google Scholar] [CrossRef]
- Pokoo-Aikins, G.; Heath, A.; Mentzer, R.A.; Sam Mannan, M.; Rogers, W.J.; El-Halwagi, M.M. A Multi-Criteria Approach to Screening Alternatives for Converting Sewage Sludge to Biodiesel. J. Loss Prev. Process Ind. 2010, 23, 412–420. [Google Scholar] [CrossRef]
- Pastore, C.; Lopez, A.; Lotito, V.; Mascolo, G. Biodiesel from Dewatered Wastewater Sludge: A Two-Step Process for a More Advantageous Production. Chemosphere 2013, 92, 667–673. [Google Scholar] [CrossRef]





| Type of Sludge | Higher Heating Value, HHV (MJ/kg) | Lower Heating Value, LHV (MJ/kg) | Residual Energy (MJ/kg) |
|---|---|---|---|
| Primary 1 | 17.53 | 16.34 | −1.014 |
| Primary 2 | 18.20 | 17.03 | −1.074 |
| Secondary 1 | 19.66 | 18.34 | −0.880 |
| Secondary 2 | 18.65 | 17.45 | −1.109 |
| Dewatered 1 | 17.41 | 16.15 | 0.244 |
| Dewatered 2 | 13.19 | 12.36 | 1.629 |
| Dewatered 3 | 15.66 | 14.44 | 0.445 |
| Dewatered 4 | 17.48 | 16.29 | 0.688 |
| Properties | Value (wt.%) |
|---|---|
| Moisture content, MC | 80.82 ± 0.94 |
| Total solid content, TS | 19.18 ± 0.94 |
| Volatile solid content, VS | a 46.75 ± 0.74 |
| Type of Sludge | Point of Collection | TS (wt.%) | Extraction Parameters | Lipid Yield, Y (%) | Ref. | |
|---|---|---|---|---|---|---|
| Yds | Yww | |||||
| Primary | Primary treatment, after partial gravity thickening | 4.2 ± 1.2 | Temperature = NA Time = 5.5 h S/L = 0.1 g/mL Particle size = NA | 26 | 1.09 | [50,51] |
| Secondary | Activated sludge process, after partial thickening by flotation | 3.1 ± 0.7 | 9 | 0.28 | ||
| Sludge cake | Sludge storage yard of CSTF | 33..32 | Temperature = 80 °C Time = 6 h S/L = 0.1 g/mL Particle size ≤ 100 mesh | 5.15 | 1.72 | [36] |
| DS | Sludge compartment of RSTP | 19.18 ± 0.94 | Temperature = 70 °C Time = 4 h S/L = 0.175 g/mL Particle size ≤ 4 mm | 1.71 ± 0.10 | 0.33 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, N.A.; Banjar, M.F.; Abedin, F.N.J.; Fizal, A.N.S.; Ahmad, N.; Zulkifli, M.; Taweepreda, W.; Hossain, M.S.; Ahmad Yahaya, A.N. Potential of Lipids from Polymer-Based Dewatered Sewage Sludge as Feedstock for Biodiesel Production. Sustainability 2025, 17, 2991. https://doi.org/10.3390/su17072991
Khalil NA, Banjar MF, Abedin FNJ, Fizal ANS, Ahmad N, Zulkifli M, Taweepreda W, Hossain MS, Ahmad Yahaya AN. Potential of Lipids from Polymer-Based Dewatered Sewage Sludge as Feedstock for Biodiesel Production. Sustainability. 2025; 17(7):2991. https://doi.org/10.3390/su17072991
Chicago/Turabian StyleKhalil, Nor Afifah, Mohd Faizar Banjar, Fatin Najwa Joynal Abedin, Ahmad Noor Syimir Fizal, Norkhairi Ahmad, Muzafar Zulkifli, Wirach Taweepreda, Md Sohrab Hossain, and Ahmad Naim Ahmad Yahaya. 2025. "Potential of Lipids from Polymer-Based Dewatered Sewage Sludge as Feedstock for Biodiesel Production" Sustainability 17, no. 7: 2991. https://doi.org/10.3390/su17072991
APA StyleKhalil, N. A., Banjar, M. F., Abedin, F. N. J., Fizal, A. N. S., Ahmad, N., Zulkifli, M., Taweepreda, W., Hossain, M. S., & Ahmad Yahaya, A. N. (2025). Potential of Lipids from Polymer-Based Dewatered Sewage Sludge as Feedstock for Biodiesel Production. Sustainability, 17(7), 2991. https://doi.org/10.3390/su17072991















