Restoring Soil Health with Legume-Based Integrated Farming Systems
Abstract
1. Introduction
2. Materials and Methods
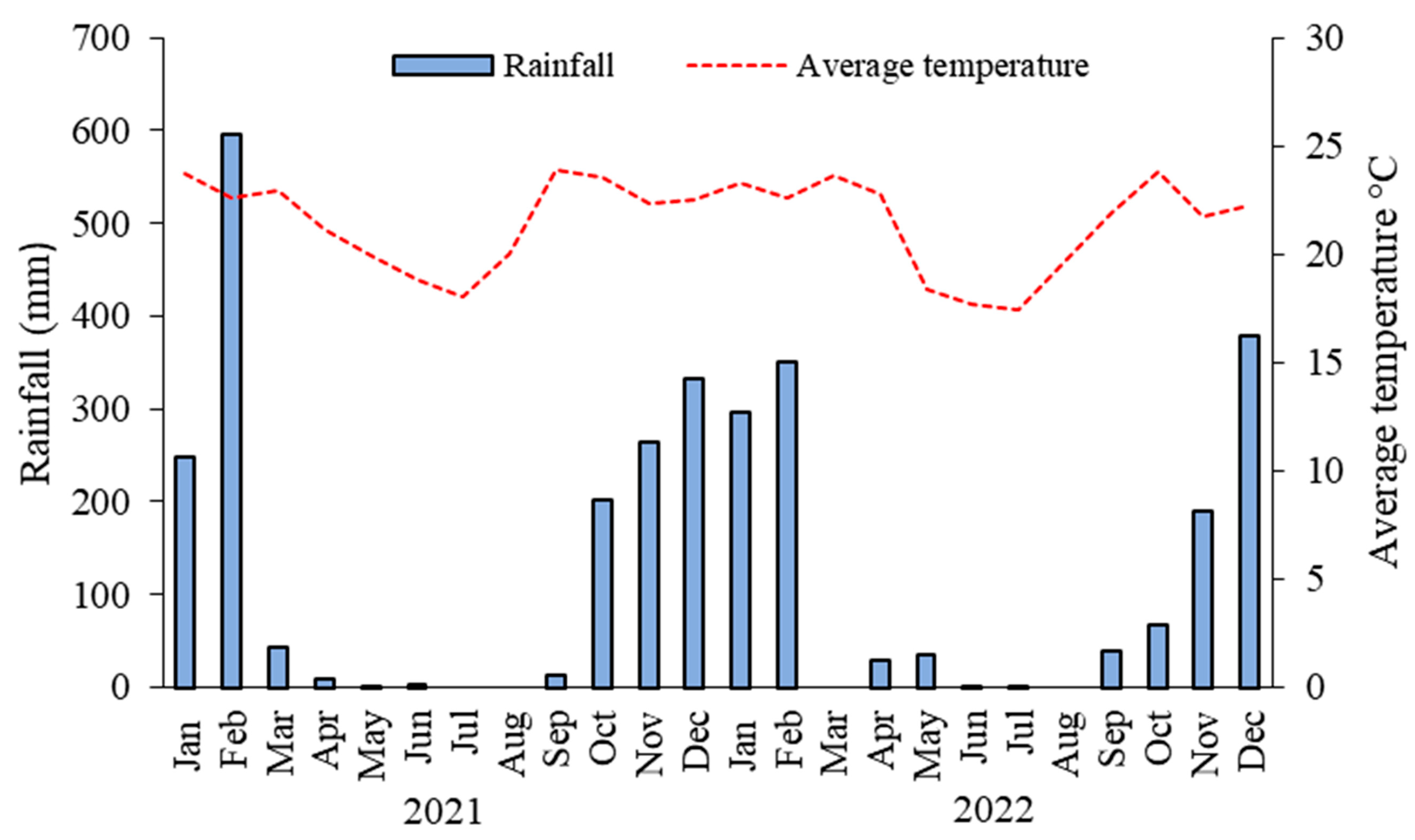
2.1. Location and Characterization of the Study Areas
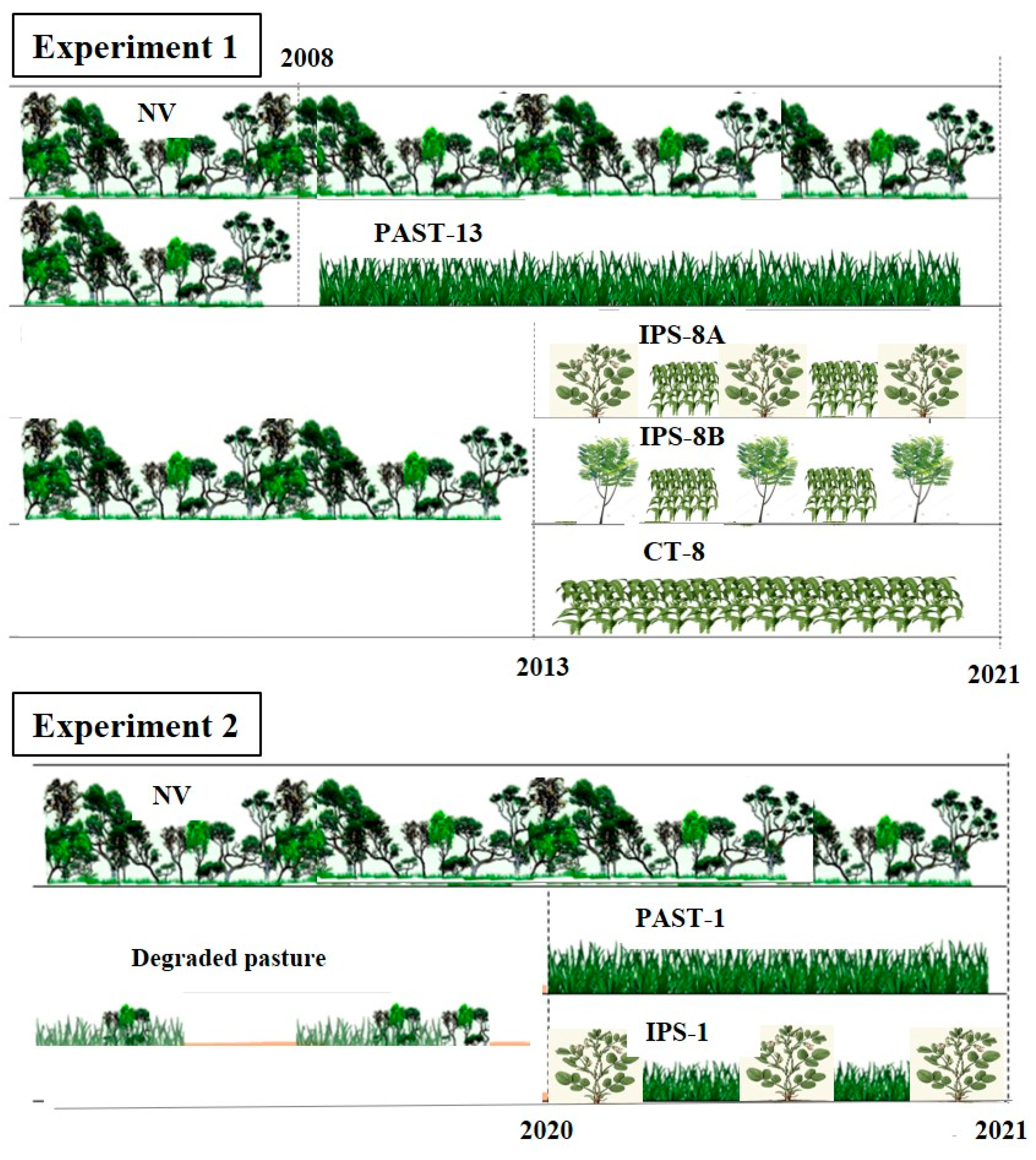
2.2. Experiment 1: Integrated Farming Systems Using Legumes and Annual Crop
2.3. Experiment 2: Integrated Farming Systems Using Legumes and Pasture
2.4. Soil Sampling and Laboratory Analysis
2.5. Data Analysis
3. Results
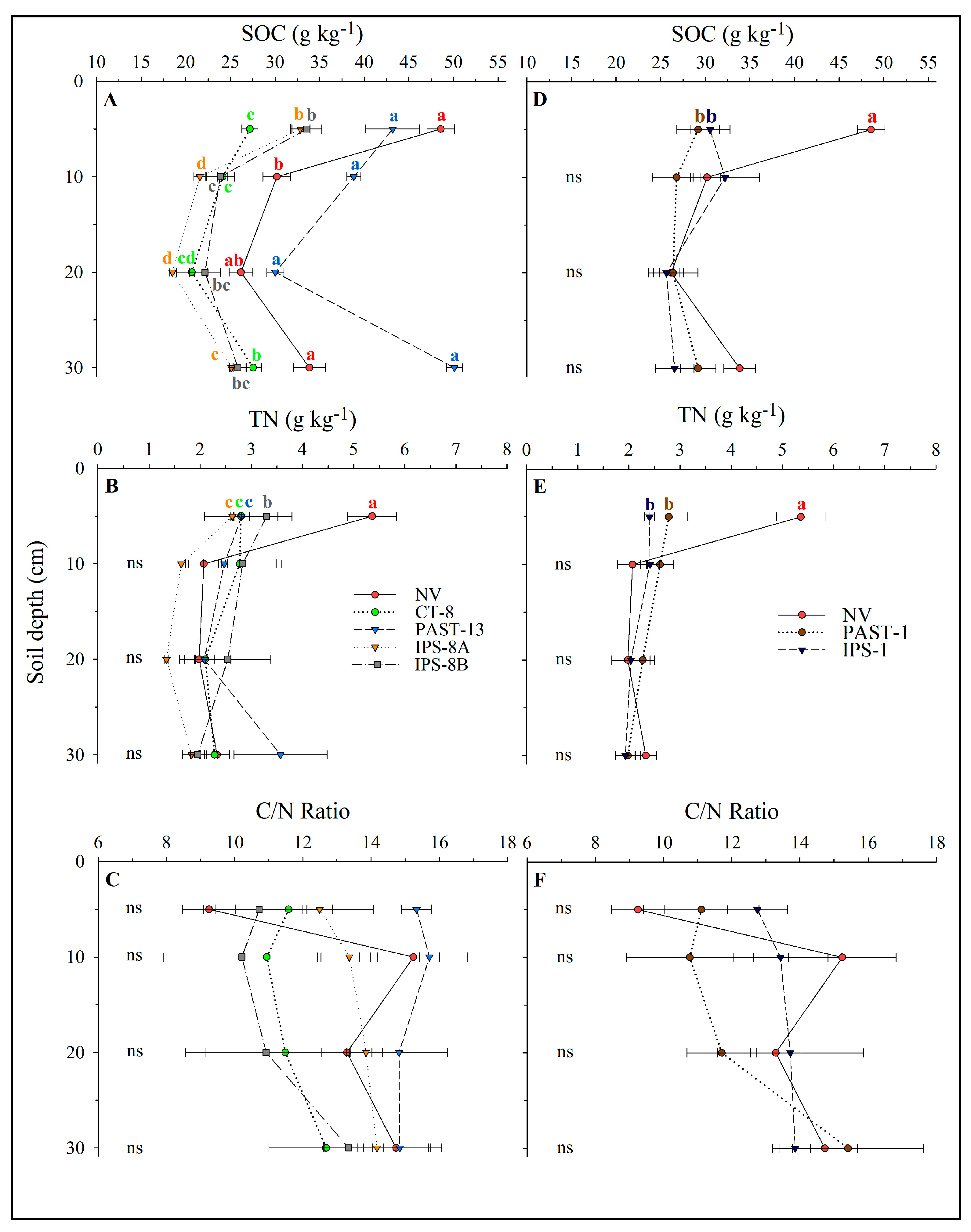
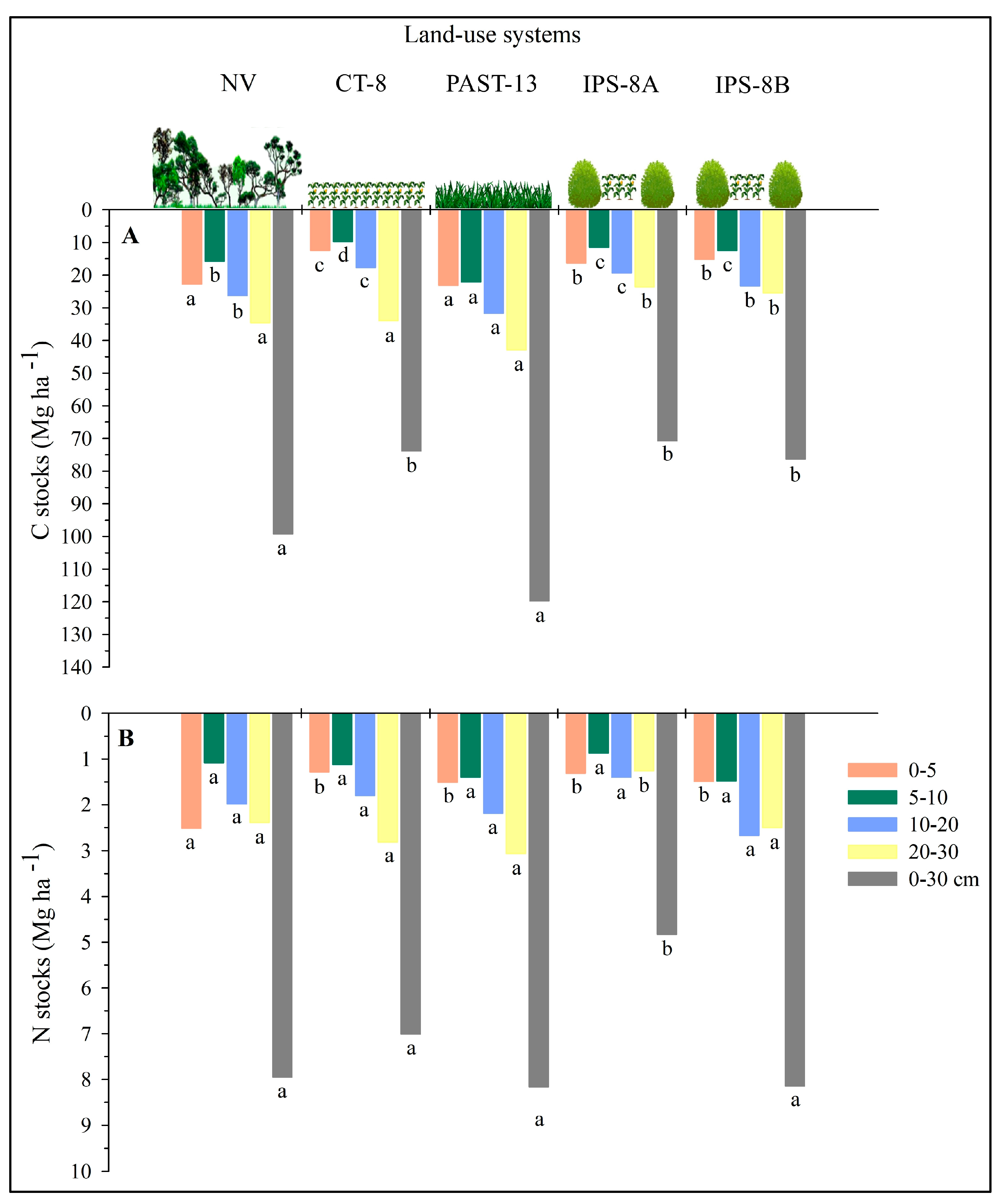
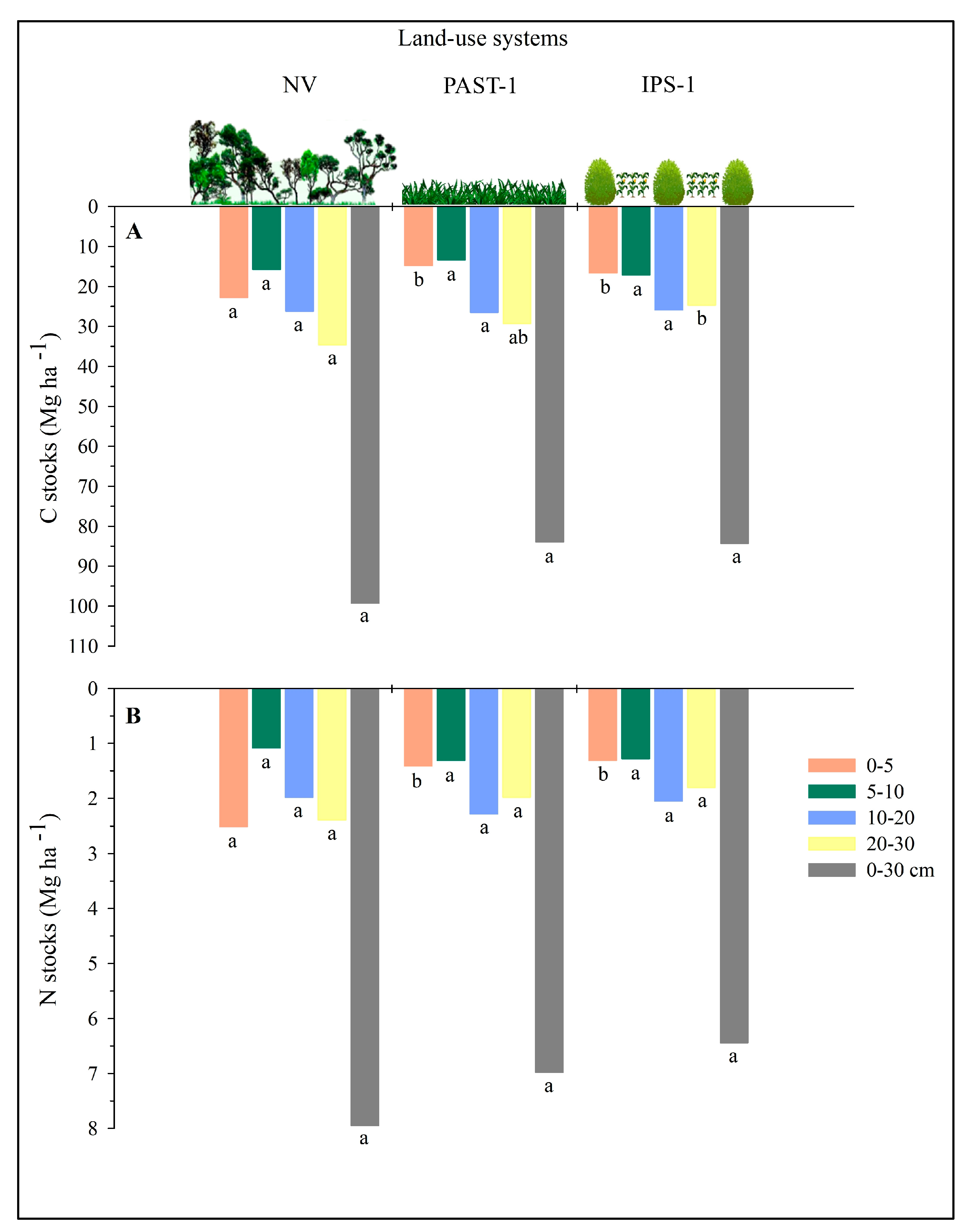
3.1. Soil Content and Stocks of C and N and Bulk Density
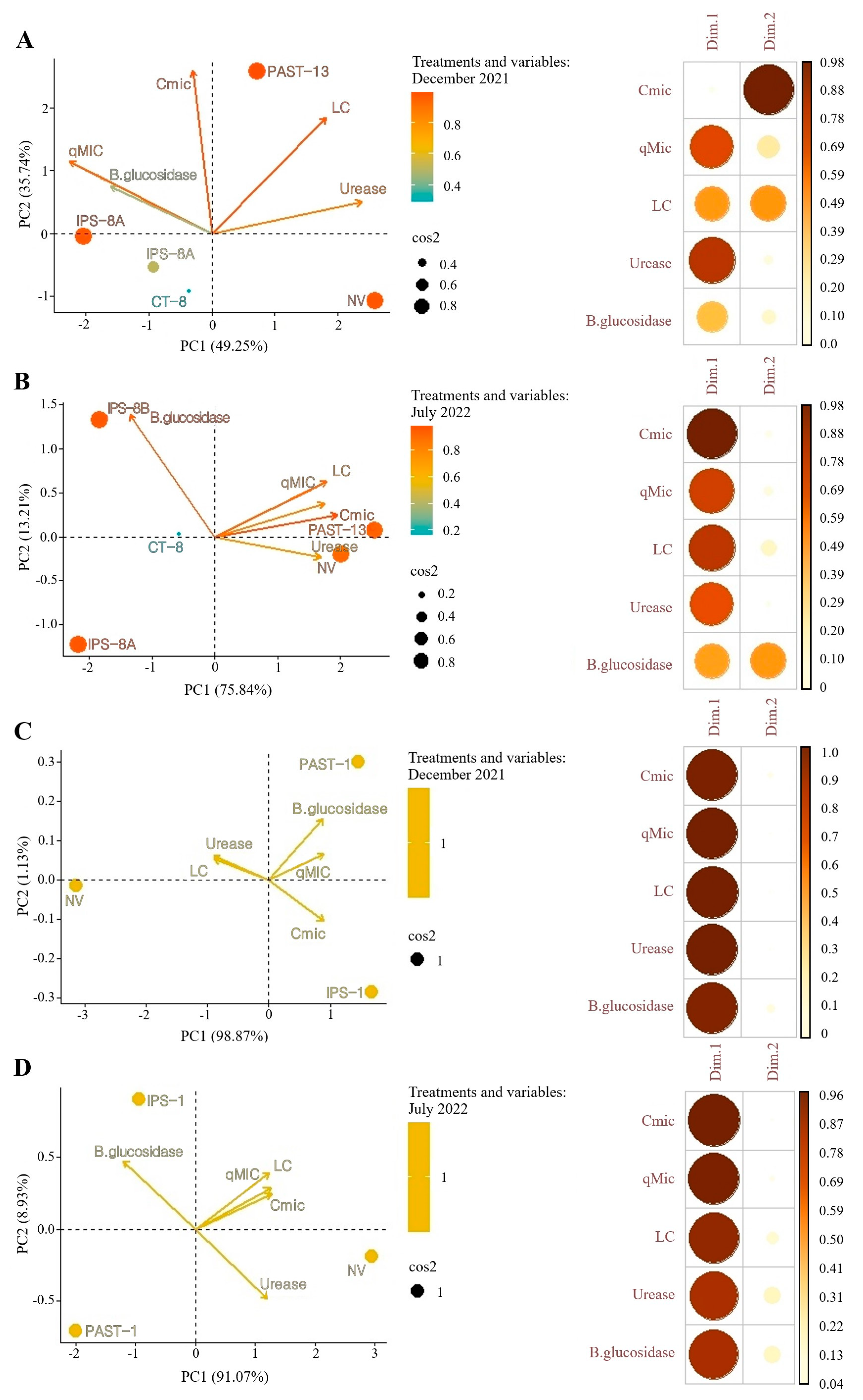
3.2. Soil Microbial Attributes
4. Discussion
4.1. Soil C and N Stocks in the Land-Use Systems
4.2. Soil Microbial Attributes in the Land-Use Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brazil. Ministry of Agriculture, Livestock and Food Supply. Plan for Adaptation and Low Carbon Emission in Agriculture: Strategic Vision for a New Cycle. 2021. Available online: https://www.gov.br/agricultura/pt-br/assuntos/sustentabilidade/planoabc-abcmais/publicacoes/abc-english.pdf (accessed on 27 April 2024).
- Kennedy, C.M.; Hawthorne, P.L.; Miteva, D.A.; Baumgarten, L.; Sochi, K.; Matsumoto, M.; Evans, J.S.; Polasky, S.; Hamel, P.; Vieira, E.M.; et al. Optimizing land use decision-making to sustain Brazilian agricultural profits, biodiversity and ecosystem services. Biol. Conserv. 2016, 204, 221–230. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Sixth Assessment Report. Working Group 1: The Physical Science Basis. Summary for Policymakers. 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/chapter/summary-for-policymakers/ (accessed on 31 May 2024).
- Intergovernmental Panel on Climate Change (IPCC). AR6 Synthesis Report: Climate Change 2023. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III. 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 23 April 2024).
- Bieluczyk, W.; de Cássia Piccolo, M.; Pereira, M.G.; de Moraes, M.T.; Soltangheisi, A.; de Campos Bernardi, A.C.; Pezzopane, J.R.; Oliveira, P.P.; Moreira, M.Z.; de Camargo, P.B.; et al. Integrated Farming Systems Influence Soil Organic Matter Dynamics in Southeastern Brazil. Geoderma 2020, 371, 114368. [Google Scholar] [CrossRef]
- Balbino, L.C.; Kichel, N.A.; Bungenstab, D.J.; Almeida, R.G. Sistemas de integração: Conceitos, considerações, contribuições e desafios. In ILPF: Inovação com Integração de Lavoura, Pecuária e Floresta; Bungenstab, J.D., Almeida, R.G., Laura, V.A., Balbino, L.C., Ferreira, A.D., Eds.; Embrapa: Brasília, Brazil, 2019; pp. 31–48. [Google Scholar]
- Bonanomi, J.; Tortato, F.R.; Gomes, R.d.S.R.; Penha, J.M.; Bueno, A.S.; Peres, C.A. Protecting Forests at the Expense of Native Grasslands: Land-Use Policy Encourages Open-Habitat Loss in the Brazilian Cerrado Biome. Perspect. Ecol. Conserv. 2019, 17, 26–31. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Krishnamurthy, P.; Rajagopal, I.; Solares, A.P. Can agroforestry systems thrive in the drylands? Characteristics of successful agroforestry systems in the arid and semi-arid regions of Latin America. Agrofor. Syst. 2019, 93, 503–513. [Google Scholar] [CrossRef]
- Calazans, G.M.; de Oliveira, C.A.; Cruz, J.C.; Matrangolo, W.J.R.; Marriel, I.E. Seleção de Rizóbios Eficientes Na Simbiose Com Cratylia Argentea No Bioma Cerrado. Cienc. Rural. 2016, 46, 1594–1600. [Google Scholar] [CrossRef][Green Version]
- de Figueiredo, C.C.; Moreira, T.N.; Coser, T.R.; da Silva, L.P.; Leite, G.G.; de Carvalho, A.M.; Malaquias, J.V.; Marchão, R.L.; Urquiaga, S. Nitrogen Use Efficiency in an Agrisilviculture System with Gliricidia Sepium in the Cerrado Region. Plants 2023, 12, 1647. [Google Scholar] [CrossRef]
- Matrangolo, W.J.; Brasileiro, B.P.; da Silva, C.J.; Netto, D.A.; Mattar, E.P.; Frade Júnior, E.F.; da Silva, I.H.; da Silva, I.S.; Crivelaro, J.C.; Ribeiro, J.P.; et al. Aspectos de Cratylia argentea na região central de Minas Gerais e potencialidades em sistemas agrobiodiversos. In Comunicado Técnico 233; Embrapa: Sete Lagoas, Brazil, 2018; pp. 1–48. [Google Scholar]
- Valani, G.P.; Martíni, A.F.; da Silva, L.F.S.; Bovi, R.C.; Cooper, M. Soil Quality Assessments in Integrated Crop–Livestock–Forest Systems: A Review. Soil. Use Manag. 2021, 37, 22–36. [Google Scholar] [CrossRef]
- Frazão, L.A.; Cardoso, P.H.S.; Almeida Neta, M.N.; Mota, M.F.C.; Almeida, L.L.D.S.; Ribeiro, J.M.; Bicalho, T.F.; Feigl, B.J. Carbon and Nitrogen Stocks and Organic Matter Fractions in the Topsoil of Traditional and Agrisilvicultural Systems in the Southeast of Brazil. Soil. Res. 2021, 59, 794–805. [Google Scholar] [CrossRef]
- Freitas, I.C.d.; Ribeiro, J.M.; Araújo, N.C.A.; Santos, M.V.; Sampaio, R.A.; Fernandes, L.A.; Azevedo, A.M.; Feigl, B.J.; Cerri, C.E.P.; Frazão, L.A. Agrosilvopastoral Systems and Well-Managed Pastures Increase Soil Carbon Stocks in the Brazilian Cerrado. Rangel. Ecol. Manag. 2020, 73, 776–785. [Google Scholar] [CrossRef]
- Dhaliwal, J.K.; Laxmisagra, S.K.; Chellappa, J.; Sekaran, U.; Kumar, S. Labile soil carbon and nitrogen fractions under short and long-term integrated crop-livestock agroecosystems. Soil. Res. 2021, 60, 511–519. [Google Scholar] [CrossRef]
- de Souza Almeida, L.L.; Frazão, L.A.; Lessa, T.A.; Fernandes, L.A.; de Carvalho Veloso, Á.L.; Lana, A.M.; de Souza, I.A.; Pegoraro, R.F.; Ferreira, E.A. Soil Carbon and Nitrogen Stocks and the Quality of Soil Organic Matter under Silvopastoral Systems in the Brazilian Cerrado. Soil. Tillage Res. 2021, 205, 104785. [Google Scholar] [CrossRef]
- Pires, G.C.; Denardin, L.G.O.; Silva, L.S.; Freitas, C.M.; Gonçalves, E.C.; Camargo, T.A.; Bremm, C.; Carvalho, P.C.F.; Souza, E.D. System Fertilization Increases Soybean Yield Through Soil Quality Improvements in Integrated Crop-Livestock System in Tropical Soils. J. Soil. Sci. Plant Nutr. 2022, 22, 4487–4495. [Google Scholar] [CrossRef]
- Stieven, A.C.; Mendes, W.M.; Wruck, F.; Couto, E.G.; da Campos, D.T.S. Atributos Do Solo Em Sistemas Diferenciados de Uso e Manejo Do Solo Em Mato Grosso, MT, Brasil. Colloq. Agrar. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Zago, L.M.S.; Ramalho, W.P.; Silva-Neto, C.M.; Caramori, S.S. Biochemical indicators drive soil quality in integrated crop–livestock–forestry systems. Agrofor. Syst. 2020, 94, 2249–2260. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–376. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of a organic matter and nutrients stored in soil under contrasting management regimes. Can. J. Soil. Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Moraes, J.F.L.; Volkoff, B.; Cerri, C.C.; Bernoux, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma 1996, 70, 63–81. [Google Scholar] [CrossRef]
- Shang, C.; Tiessen, H. Organic matter lability in a tropical oxisol: Evidence from shifting cultivation, chemical oxidation, particle size, density and magnetic fractionations. Soil. Sci. 1997, 11, 795–807. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil. Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Silva, E.E.; Azevedo, P.H.S.; De-Polli, H. Determinação do carbono da biomassa microbiana do solo (BMS-C). In Comunicado Técnico 98; Embrapa: Seropédica, Brazil, 2007; pp. 1–6. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Scott, A., Bottomeley, P.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 778–835. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org// (accessed on 25 September 2024).
- Schaffrath, V.R.; Tormena, C.A.; Gonçalves, A.C.A.; Fidalski, J. Variabilidade e correlação espacial de propriedades físicas de solo sob plantio direto e preparo convencional. Rev. Bras. Ciência Do Solo 2008, 32, 1369–1377. [Google Scholar] [CrossRef]
- Ketema, H.; Yimer, F. Soil Property Variation under Agroforestry Based Conservation Tillage and Maize Based Conventional Tillage in Southern Ethiopia. Soil. Tillage Res. 2014, 141, 25–31. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Jiang, X.; Wu, J. Effects of Rubber-Based Agroforestry Systems on Soil Aggregation and Associated Soil Organic Carbon: Implications for Land Use. Geoderma 2017, 299, 13–24. [Google Scholar] [CrossRef]
- Silva, J.F.d.; Gontijo Neto, M.M.; Silva, G.F.d.; Borghi, E.; Calonego, J.C. Soil Organic Matter and Aggregate Stability in Soybean, Maize and Urochloa Production Systems in a Very Clayey Soil of the Brazilian Savanna. Agronomy 2022, 12, 1652. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Borges, W.L.B.; Sarto, J.R.W.; Rice, C.W.; Rosolem, C.A. Deep Soil Carbon Stock, Origin, and Root Interaction in a Tropical Integrated Crop–Livestock System. Agrofor. Syst. 2020, 94, 1865–1877. [Google Scholar] [CrossRef]
- Hazra, K.K.; Nath, C.P.; Singh, U.; Praharaj, C.S.; Kumar, N.; Singh, S.S.; Singh, N.P. Diversification of Maize-Wheat Cropping System with Legumes and Integrated Nutrient Management Increases Soil Aggregation and Carbon Sequestration. Geoderma 2019, 353, 308–319. [Google Scholar] [CrossRef]
- Barros, V.D.C.; Lira Junior, M.A.; Fracetto, F.J.C.; Fracetto, G.G.M.; da Ferreira, J.S.; de Barros, D.J.; da Silva Júnior, A.F. Effects of Different Legume Green Manures on Tropical Soil Microbiology after Corn Harvest. Bragantia 2020, 79, 505–515. [Google Scholar] [CrossRef]
- López-Santiago, J.G.; Casanova-Lugo, F.; Villanueva-López, G.; Díaz-Echeverría, V.F.; Solorio-Sánchez, F.J.; Martínez-Zurimendi, P.; Aryal, D.R.; Chay-Canul, A.J. Carbon Storage in a Silvopastoral System Compared to That in a Deciduous Dry Forest in Michoacán, Mexico. Agrofor. Syst. 2019, 93, 199–211. [Google Scholar] [CrossRef]
- Aryal, D.R.; De Jong, B.H.J.; Ochoa-Gaona, S.; Mendoza-Vega, J.; Esparza-Olguin, L. Successional and seasonal variation of litterfall and associated nutrient transfer in semi-evergreen tropical secondary forests of SE Mexico. Nutr. Cycl. Agroecosyst 2015, 103, 45–60. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Frazão, L.A.; Cardoso, P.H.S.; Oliveira, A.L.G.; Sampaio, R.A.; Fernandes, L.A. Soil Fertility and Carbon and Nitrogen Stocks under Agroforestry Systems in the Cerrado of Minas Gerais State. Cienc. Florest. 2019, 29, 920–930. [Google Scholar] [CrossRef]
- Coser, T.R.; de Figueiredo, C.C.; Jovanovic, B.; Moreira, T.N.; Leite, G.G.; Cabral Filho, S.L.S.; Kato, E.; Malaquias, J.V.; Marchão, R.L. Short-Term Buildup of Carbon from a Low-Productivity Pastureland to an Agrisilviculture System in the Brazilian Savannah. Agric. Syst. 2018, 166, 184–195. [Google Scholar] [CrossRef]
- Alves, T.d.S.; Campos, L.L.; Neto, N.E.; Matsuoka, M.; Loureiro, M.F. Biomassa e Atividade Microbiana de Solo Sob Vegetação Nativa e Diferentes Sistemas de Manejos. Acta Sci. Agron. 2011, 33, 341–347. [Google Scholar] [CrossRef]
- Silva, A.S.; Colozzi Filho, A.; Nakatani, A.S.; Alves, S.J.; de Andrade, D.S.; de Guimarães, M.F. Atributos Microbiológicos do Solo em Sistema de Integração. Rev. Bras. Cienc. Solo 2015, 39, 40–48. [Google Scholar] [CrossRef]
- Lira Junior, M.A.; Fracetto, F.J.C.; da Ferreira, J.S.; Silva, M.B.; Fracetto, G.G.M. Legume-Based Silvopastoral Systems Drive C and N Soil Stocks in a Subhumid Tropical Environment. Catena 2020, 189, 104508. [Google Scholar] [CrossRef]
- Brito, G.S.; Bautista, S.; López-Poma, R.; Pivello, V.R. Labile Soil Organic Carbon Loss in Response to Land Conversion in the Brazilian Woodland Savanna (Cerradão). Biogeochemistry 2019, 144, 31–46. [Google Scholar] [CrossRef]
- Loss, A.; Coutinho, F.S.; Pereira, M.G.; Silva, R.A.C.; Torres, J.L.R.; Ravelli Neto, A. Fertilidade e Carbono Total e Oxidável de Latossolo de Cerrado sob Pastagem Irrigada e de Sequeiro. Cienc. Rural. 2013, 433, 426–432. [Google Scholar] [CrossRef]
- Ansari, M.A.; Choudhury, B.U.; Layek, J.; Das, A.; Lal, R.; Mishra, V.K. Green Manuring and Crop Residue Management: Effect on Soil Organic Carbon Stock, Aggregation, and System Productivity in the Foothills of Eastern Himalaya (India). Soil. Tillage Res. 2022, 218, 105318. [Google Scholar] [CrossRef]
- Vargas, R.S.; Bataiolli, R.; da Costa, P.B.; Lisboa, B.; Passaglia, L.M.P.; Beneduzi, A.; Vargas, L.K. Microbial Quality of Soil from the Pampa Biome in Response to Different Grazing Pressures. Genet. Mol. Biol. 2015, 38, 205–212. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil. Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Kuscu, I.S.K. Changing of Soil Properties and Urease–Catalase Enzyme Activity Depending on Plant Type and Shading. Environ. Monit. Assess. 2019, 191, 178. [Google Scholar] [CrossRef]
- Longo, R.M.; Melo, W.J. Hidrólise da Uréia em Latossolos: Efeito da Concentração de Uréia, Temperatura, pH, Armazenamento e Tempo de Incubação. Rev. Bras. Cienc. Solo 2005, 29, 651–657. [Google Scholar] [CrossRef]
- Yang, C.; Feng, M.; Song, L.; Jing, B.; Xie, Y.; Wang, C.; Qin, M.; Yang, W.; Xiao, L.; Sun, J.; et al. Hyperspectral Monitoring of Soil Urease Activity under Different Water Regulation. Plant Soil. 2022, 477, 779–792. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Soon, Y.K. Soil Microbial Properties during Decomposition of Pulse Crop and Legume Green Manure Residues in Three Consecutive Subsequent Crops. Can. J. Soil. Sci. 2016, 96, 413–426. [Google Scholar] [CrossRef]
- Miguel, D.L.; da Silva, E.M.R.; da Silva, C.F.; Pereira, M.G.; Leite, L.F.C. Soil Microbiological Properties and Enzyme Activity in Agroforestry Systems Compared with Monoculture, Natural Regeneration, and Native Caatinga. Biosci. J. 2020, 36, 1–16. [Google Scholar] [CrossRef]
- Turner, B.L.; Hopkins, D.W.; Haygarth, P.M.; Ostle, N. β-Glucosidase Activity in Pasture Soils. Appl. Soil. Ecol. 2022, 20, 157–162. [Google Scholar] [CrossRef]
- Knight, T.R.; Dick, R.P. Differentiating Microbial and Stabilized ?-glucosidase Activity Relative to Soil Quality. Soil. Biol. Biochem. 2004, 36, 2089–2096. [Google Scholar] [CrossRef]
- Yan, J.; Pan, G.; Li, L.; Quan, G.; Ding, C.; Luo, A. Adsorption, Immobilization, and Activity of β-Glucosidase on Different Soil Colloids. J. Colloid. Interface Sci. 2010, 348, 565–570. [Google Scholar] [CrossRef]
- Yadav, R.S.; Yadav, B.L.; Chhipa, B.R.; Dhyani, S.K.; Ram, M. Soil biological properties under different tree based traditional agroforestry systems in a semi-arid region of Rajasthan, India. Agrofor Syst. 2011, 81, 195–202. [Google Scholar] [CrossRef]

| a Land-Use | Soil Depth (cm) | |||
|---|---|---|---|---|
| 0–5 | 5–10 | 10–20 | 20–30 | |
| Experiment 1 | ||||
| NV | b 0.94 ± 0.13 * A | 1.04 ± 0.04 A | 1.00 ± 0.07 A | 1.02 ± 0.01 B |
| CT-8 | 0.92 ± 0.08 A | 0.81 ± 0.07 B | 0.85 ± 0.06 A | 0.73 ± 0.08 D |
| PAST-13 | 1.07 ± 0.01 A | 1.13 ± 0.06 A | 1.05 ± 0.06 A | 1.07 ± 0.01 A |
| IPS-8A | 1.03 ± 0.02 A | 1.04 ± 0.02 A | 1.03 ± 0.03 A | 1.00 ± 0.01 C |
| IPS-8B | 0.96 ± 0.06 A | 1.05 ± 0.05 A | 1.03 ± 0.03 A | 0.98 ± 0.04 C |
| Experiment 2 | ||||
| NV | b 0.93 ± 0.13 A | 1.04 ± 0.04 A | 1.00 ± 0.07 A | 1.02 ± 0.01 A |
| PAST-1 | 1.01 ± 0.04 A | 0.99 ± 0.07 A | 1.00 ± 0.14 A | 0.98 ± 0.13 A |
| IPS-1 | 1.08 ± 0.02 A | 1.04 ± 0.03 A | 1.00 ± 0.02 A | 0.97 ± 0.01 A |
| a Land Use | Soil Depth (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5 | 5–10 | 10–20 | |||||||
| Dec b | Jul | Mean c | Dec | Jul | Mean | Dec | Jul | Mean | |
| Cmic (mg kg −1) | |||||||||
| NV | 398 Ba | 269 Aa | 333 A | 257 Aa | 182 Aa | 219 B | 199 Aa | 113 Ab | 156 B |
| CT-8 | 424 Ba | 204 Ab | 314 A | 340 Aa | 115 Ab | 227 B | 227 Aa | 72 Ab | 149 B |
| PAST-13 | 708 Aa | 218 Ab | 463 A | 479 Aa | 246 Ab | 362 A | 424 Aa | 183 Ab | 303 A |
| IPS-8A | 481 Ba | 110 Ab | 296 A | 367 Aa | 63 Ab | 215 B | 297 Aa | 50 Ab | 173 B |
| IPS-8B | 368 Ba | 154 Ab | 261 A | 339 Aa | 90 Ab | 215 B | 297 Aa | 66 Ab | 182 B |
| Mean d | 476 a | 191 b | 356 a | 139 b | 289 a | 97 b | |||
| qMIC (%) | |||||||||
| NV | 0.82 Aa | 0.55 Aa | 0.68 A | 0.87 Ba | 0.62 Aa | 0.75 A | 0.74 Aa | 0.44 Aa | 0.59 A |
| CT-8 | 1.58 Aa | 0.75 Ab | 1.17 A | 1.42 Aa | 0.48 Ab | 0.95 A | 1.15 Aa | 0.35 Ab | 0.75 A |
| PAST-13 | 1.64 Aa | 0.51 Ab | 1.07 A | 1.24 Aa | 0.63 Ab | 0.93 A | 1.42 Aa | 0.61 Ab | 1.01 A |
| IPS-8A | 1.49 Aa | 0.34 Ab | 0.91 A | 1.69 Aa | 0.29 Ab | 0.99 A | 1.62 Aa | 0.27 Ab | 0.95 A |
| IPS-8B | 1.10 Aa | 0.46 Ab | 0.78 A | 1.42 Aa | 0.37 Ab | 0.89 A | 1.37 Aa | 0.31 Ab | 0.84 A |
| Mean | 1.33 a | 0.52 b | 1.33 a | 0.48 b | 1.26 a | 0.39 b | |||
| Labile C (g kg −1) | |||||||||
| NV | 1.43 Ab | 3.85 Aa | 2.64 A | 0.98 Ab | 1.84 Aa | 1.41 B | 0.79 Ab | 1.75 Aa | 1.27 A |
| CT-8 | 1.21 Ab | 2.67 Ba | 1.94 A | 0.97 Ab | 2.08 Aa | 1.53 B | 0.67 Ab | 1.58 Aa | 1.13 A |
| PAST-13 | 1.60 Ab | 3.06 Ba | 2.33 A | 1.26 Ab | 2.62 Aa | 1.94 A | 0.83 Ab | 1.52 Aa | 1.17 A |
| IPS-8A | 1.40 Ab | 2.80 Ba | 2.10 A | 0.75 Ab | 1.70 Aa | 1.22 B | 0.46 Ab | 1.26 Aa | 0.86 A |
| IPS-8B | 1.31 Ab | 3.06 Ba | 2.18 A | 0.88 Ab | 1.90 Aa | 1.39 B | 0.52 Ab | 1.55 Aa | 1.04 A |
| Mean | 1.39 | 3.09 | 0.97 b | 2.03 a | 0.66 b | 1.53 a | |||
| Urease (µg NH4+ g−1 2 h−1) | |||||||||
| NV | 126 Ab | 270 Aa | 198 A | 101 Ab | 172 Ba | 136 A | 71 Ab | 159 Ba | 115 A |
| CT-8 | 101 Ab | 113 Ba | 107 A | 32 Aa | 78 Ca | 55 B | 18 Ba | 38 Ca | 28 B |
| PAST-13 | 94 Ab | 261 2Aa | 178 A | 59 Ab | 242 Aa | 150 A | 97 Ab | 302 Aa | 199 A |
| IPS-8A | 99 Ab | 271 Aa | 185 A | 36 Aa | 77 Ca | 56 B | 19 Ba | 27 Ca | 23 B |
| IPS-8B | 125 Ab | 224 Aa | 175 A | 47 Aa | 67 Ca | 57 B | 20 Ba | 21 Ca | 21 B |
| Mean | 109 b | 228 a | 55 b | 127 a | 45 b | 109 a | |||
| β-glucosidase (µg pNP g−1 h−1) | |||||||||
| NV | 134 Aa | 136 Ca | 135 A | 75 Aa | 47 Aa | 61 B | 47 Aa | 36 Aa | 41 A |
| CT-8 | 140 Aa | 198 Ba | 169 A | 94 Aa | 91 Ab | 75 B | 38 Aa | 34 Aa | 36 A |
| PAST-13 | 176 Aa | 153 Ca | 164 A | 75 Aa | 86 Aa | 81 B | 48 Aa | 25 Aa | 37 A |
| IPS-8A | 189 Aa | 221 Ba | 205 A | 88 Aa | 62 Aa | 75 B | 59 Aa | 26 Aa | 42 A |
| IPS-8B | 178 Ab | 350 Aa | 264 A | 104 Ba | 124 Aa | 114 A | 61 Aa | 61 Aa | 53 A |
| Mean | 163 a | 212 a | 80 a | 82 a | 51 a | 33 a | |||
| a Land-Use | Soil Depth (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5 | 5–10 | 10–20 | |||||||
| Dec b | Jul | Mean c | Dec | Jul | Mean | Dec | Jul | Mean | |
| Cmic (mg kg −1) | |||||||||
| NV | 398 Aa b | 269 Ab | 333 A | 257 Ba | 182 Aa | 219 A | 199 Aa | 113 Aa | 156 A |
| PAST-1 | 596 Aa | 144 Ab | 370 A | 396 Aa | 132 Ab | 264 A | 339 Aa | 93 Aa | 216 A |
| IPS-1 | 566 Aa | 182 Ab | 374 A | 495 Aa | 154 Ab | 324 A | 368 Aa | 109 Aa | 238 A |
| average d | 520 a | 198 b | 383 a | 156 b | 302 Aa | 105 Ba | |||
| qMIC (%) | |||||||||
| NV | 0.82 Ba | 0.55 Aa | 0.68 A | 0.87Aa | 0.62 Aa | 0.75 A | 0.74 Aa | 0.44 Aa | 0.59 A |
| PAST-1 | 2.09 Aa | 0.52 Ab | 1.30 A | 1.56Aa | 0.51 Aa | 1.03 A | 1.41 Aa | 0.37 Aa | 0.89 A |
| IPS-1 | 1.84 Aa | 0.59 Ab | 1.22 A | 1.65Aa | 0.48 Aa | 1.06 A | 1.47 Aa | 0.42 Aa | 0.94 A |
| Mean | 1.58Aa | 0.55Ab | 1.36 Aa | 0.54 Ab | 1.21 Aa | 0.41 Ab | |||
| LC (g kg −1) | |||||||||
| NV | 1.43 Ab | 3.85 Aa | 2.64 A | 0.98 Ab | 1.84 Aa | 1.41 A | 0.79 Aa | 1.75 Aa | 1.27 A |
| PAST-1 | 1.05 Ab | 2.15 Ba | 1.60 A | 0.81 Ab | 1.80 Aa | 1.30 A | 0.75 Aa | 1.81 Aa | 1.28 A |
| IPS-1 | 0.90 Ab | 2.25 Ba | 1.57 A | 0.88 Ab | 2.29 Aa | 1.58 A | 0.76 Aa | 2.05 Aa | 1.41 A |
| Mean | 1.13 a | 2.75 a | 0.89 b | 1.98 a | 0.77 b | 1.87 a | |||
| Urease (µg NH4+ g−1 2 h−1) | |||||||||
| NV | 126 Ab | 270 Aa | 198 A | 101 Aa | 172 Aa | 136 A | 71 Ab | 159 Aa | 115 A |
| PAST-1 | 73 Ab | 149 Ba | 111 A | 56 Aa | 107 Aa | 81 B | 43 Aa | 89 Aa | 66 B |
| IPS-1 | 70 Ab | 127 Ba | 98 A | 47 Aa | 90 Aa | 69 B | 38 Aa | 69 Aa | 53 B |
| Mean | 90 b | 182 a | 68 b | 123 a | 51 b | 106 a | |||
| β-glucosidase (µg pNP g−1 h−1) | |||||||||
| NV | 134 Aa | 136 Aa | 135 A | 75 Aa | 47 Ba | 61 A | 47 Aa | 36 Aa | 41 B |
| PAST-1 | 111 Aa | 169 Aa | 140 A | 113 Aa | 98 Aa | 105 A | 68 Aa | 82 Aa | 75 A |
| IPS-1 | 124 Aa | 173 Aa | 149 A | 84 Ab | 121 Aa | 103 A | 79 Aa | 82 Aa | 80 A |
| Mean | 123 b | 159 a | 91 a | 89 a | 65 a | 67 a | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, A.C.S.; Oliveira, J.d.C.d.; Oliveira, W.R.d.; Freitas, I.C.d.; Cardoso, Á.d.S.; Couto, A.J.S.; Matrangolo, W.J.R.; Silva, K.T.d.; Pegoraro, R.F.; Frazão, L.A. Restoring Soil Health with Legume-Based Integrated Farming Systems. Sustainability 2025, 17, 3340. https://doi.org/10.3390/su17083340
Duarte ACS, Oliveira JdCd, Oliveira WRd, Freitas ICd, Cardoso ÁdS, Couto AJS, Matrangolo WJR, Silva KTd, Pegoraro RF, Frazão LA. Restoring Soil Health with Legume-Based Integrated Farming Systems. Sustainability. 2025; 17(8):3340. https://doi.org/10.3390/su17083340
Chicago/Turabian StyleDuarte, Ana Clara Santos, Jaqueline de Cássia de Oliveira, Warley Rodrigues de Oliveira, Igor Costa de Freitas, Álissam de Sá Cardoso, Alex José Silva Couto, Walter José Rodrigues Matrangolo, Karina Toledo da Silva, Rodinei Facco Pegoraro, and Leidivan Almeida Frazão. 2025. "Restoring Soil Health with Legume-Based Integrated Farming Systems" Sustainability 17, no. 8: 3340. https://doi.org/10.3390/su17083340
APA StyleDuarte, A. C. S., Oliveira, J. d. C. d., Oliveira, W. R. d., Freitas, I. C. d., Cardoso, Á. d. S., Couto, A. J. S., Matrangolo, W. J. R., Silva, K. T. d., Pegoraro, R. F., & Frazão, L. A. (2025). Restoring Soil Health with Legume-Based Integrated Farming Systems. Sustainability, 17(8), 3340. https://doi.org/10.3390/su17083340







