Sustainable Novel Membranes Based on Carboxymethyl Cellulose Modified with ZIF-8 for Isopropanol/Water Pervaporation Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Dense Membranes
2.2.2. Porous Membranes (Substrates)
2.2.3. Supported Membranes
2.3. Characterization Techniques
2.3.1. Study of Structure
2.3.2. Study of Morphology
2.3.3. Study of Transport Properties
2.4. Theoretical Consideration
3. Results and Discussion
- Section 3.1 describes the development of dense CMC and CMC+ZIF-8 membranes, examining their transport properties in pervaporation (Section 3.1.1) and their structural and physicochemical characteristics (Section 3.1.2).
- Section 3.2 details the computational investigation, focusing on the creation and analysis of hypothetical associates (Section 3.2.1) and the study of non-covalent interactions (Section 3.2.2).
- Section 3.3 presents the development and investigation of supported CMC and CMC+ZIF-8 membranes. This includes the development and characterization of porous substrates (Section 3.3.1), followed by the analysis of untreated supported membranes (Section 3.3.2) and cross-linked supported membranes (Section 3.3.3).
3.1. The Development and Investigation of Dense CMC and CMC+ZIF-8 Membranes
3.1.1. Pervaporation Performance of Dense Membranes
3.1.2. Structure and Physicochemical Properties of Dense CMC and CMC+ZIF-8 Membranes
3.2. Computational Investigation
3.2.1. The Creation and Investigation of Hypothetical Associates
3.2.2. Investigation of Non-Covalent Interactions
3.3. The Development and Investigation of Supported CMC and CMC+ZIF-8 Membranes
3.3.1. The Development and Investigation of Porous Substrates
3.3.2. The Development and Investigation of Untreated Supported CMC and CMC+ZIF-8 Membranes
3.3.3. The Development and Investigation of Cross-Linked Supported CMC and CMC+ZIF-8 Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atlaskin, A.A.; Trubyanov, M.M.; Yanbikov, N.R.; Bukovsky, M.V.; Drozdov, P.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Total Reflux Operating Mode of Apparatuses of a Membrane Column Type during High Purification of Gases to Remove a Highly Permeable Impurity. Pet. Chem. 2018, 58, 508–517. [Google Scholar] [CrossRef]
- Davletbaeva, I.; Zaripov, I.; Mazilnikov, A.; Davletbaev, R.; Sharifullin, R.; Atlaskin, A.; Sazanova, T.; Vorotyntsev, I. Synthesis and Study of Gas Transport Properties of Polymers Based on Macroinitiators and 2,4-Toluene Diisocyanate. Membranes 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Besha, A.T.; Tsehaye, M.T.; Tiruye, G.A.; Gebreyohannes, A.Y.; Awoke, A.; Tufa, R.A. Deployable Membrane-Based Energy Technologies: The Ethiopian Prospect. Sustainability 2020, 12, 8792. [Google Scholar] [CrossRef]
- Gui, S.; Mai, Z.; Fu, J.; Wei, Y.; Wan, J. Transport Models of Ammonium Nitrogen in Wastewater from Rare Earth Smelteries by Reverse Osmosis Membranes. Sustainability 2020, 12, 6230. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, C.; Ding, S.; Du, H.; Tan, Z.; Li, M.; Wang, B.; Wang, T. The strategies to improve the interfacial compatibility in mixed-matrix membranes: A review. Mater. Today Energy 2025, 49, 101811. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Laptenkova, A.V.; Selyutin, A.A.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, F.; Wu, Y. Fluorinated Metal–Organic Framework–Polymer Mixed Matrix Membrane with Tunable Hydrophobic Channel for Efficient Pervaporation of Butanol/Water. Small Struct. 2024, 5, 2300333. [Google Scholar] [CrossRef]
- Liu, R.; Sui, Y.; Wang, X. Metal–organic framework-based ultrafiltration membrane separation with capacitive-type for enhanced phosphate removal. Chem. Eng. J. 2019, 371, 903–913. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Smith, S.J.D.; Jiang, S.; Wang, H.; Zhang, K.; Ladewig, B.P. Long-term stable metal organic framework (MOF) based mixed matrix membranes for ultrafiltration. J. Membr. Sci. 2021, 635, 119339. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wong, D.A.; Yang, J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers 2021, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. Metal-organic frameworks in mixed-matrix membranes for gas separation. Dalton. Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef] [PubMed]
- Daglar, H.; Aydin, S.; Keskin, S. MOF-based MMMs breaking the upper bounds of polymers for a large variety of gas separations. Sep. Purif. Technol. 2022, 281, 119811. [Google Scholar] [CrossRef]
- Yao, A.; Hua, D.; Zhao, F.; Zheng, D.; Pan, J.; Hong, Y.; Liu, Y.; Rao, X.; Zhou, S.; Zhan, G. Integration of P84 and porphyrin–based 2D MOFs (M−TCPP, M = Zn, Cu, Co, Ni) for mixed matrix membranes towards enhanced performance in organic solvent nanofiltration. Sep. Purif. Technol. 2022, 282, 120022. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef]
- Lalawmpuia, R.; Lalhruaitluangi, M.; Lalhmunsiama; Tiwari, D. Metal organic framework (MOF): Synthesis and fabrication for the application of electrochemical sensing. Environ. Eng. Res. 2024, 29, 230636. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, J.; Gao, R.; Lin, D.-Y.; Wang, F.; Zhang, J. Design and synthesis of zeolitic tetrazolate-imidazolate frameworks. Mater. Today Adv. 2021, 10, 100145. [Google Scholar] [CrossRef]
- Nazir, M.A.; Ullah, S.; Shahid, M.U.; Hossain, I.; Najam, T.; Ismail, M.A.; Rehman, A.U.; Karim, M.R.; Shah, S.S.A. Zeolitic imidazolate frameworks (ZIF-8 & ZIF-67): Synthesis and application for wastewater treatment. Sep. Purif. Technol. 2025, 356, 129828. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Cai, P.; Li, J.; Song, D.; Zhang, N.; Wang, N.; An, Q.-F. Enhancing permeability and stability of ZIF-8/PEBA pervaporation membrane through interface-induced directional nanoparticle distribution. J. Membr. Sci. 2024, 695, 122489. [Google Scholar] [CrossRef]
- Fang, L.J.; Chen, J.H.; Yang, Q.; Lin, W.W.; Lin, Q.J.; He, Y.S.; Zhuo, Y.Z. S-ZIF-8/PEBA/ZIF-8 pervaporation membrane with in situ growing of ZIF-8 active layer on the surface owing outstanding phenol enrichment performance. J. Taiwan Inst. Chem. Eng. 2022, 134, 104356. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Carn, F.; Tavares, S.R.; Menguy, N.; Giménez-Marqués, M.; Bellido, E.; Horcajada, P.; Berthelot, T.; Kuzminova, A.I.; et al. Covalent and Selective Grafting of Polyethylene Glycol Brushes at the Surface of ZIF-8 for the Processing of Membranes for Pervaporation. ACS Sustain. Chem. Eng. 2019, 7, 6629–6639. [Google Scholar] [CrossRef]
- Fazlifard, S.; Allahgholi, N.; Mohammadi, T.; Bakhtiari, O. P84/ZIF-8 mixed matrix membranes for pervaporation dehydration of isopropanol. Desalination Water Treat. 2017, 77, 282–290. [Google Scholar] [CrossRef]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Yin, H.; Lau, C.Y.; Rozowski, M.; Howard, C.; Xu, Y.; Lai, T.; Dose, M.E.; Lively, R.P.; Lind, M.L. Free-standing ZIF-71/PDMS nanocomposite membranes for the recovery of ethanol and 1-butanol from water through pervaporation. J. Membr. Sci. 2017, 529, 286–292. [Google Scholar] [CrossRef]
- Zhang, F.; Dou, J.; Zhang, H. Mixed Membranes Comprising Carboxymethyl Cellulose (as Capping Agent and Gas Barrier Matrix) and Nanoporous ZIF-L Nanosheets for Gas Separation Applications. Polymers 2018, 10, 1340. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Araújo, T.; Parnell, A.J.; Bernardo, G.; Mendes, A. Cellulose-based carbon membranes for gas separations—Unraveling structural parameters and surface chemistry for superior separation performance. Carbon 2023, 204, 398–410. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Sajjan, A.M.; Yunus Khan, T.M.; Rajhi, A.A.; Achappa, S.; Banapurmath, N.R.; Ashwini, M.; Duhduh, A.A. Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Jo, S.; Chaudhari, S.; Shin, H.; Fitriasari, E.I.; Shon, M.; Nam, S.; Park, Y. Strategies to overcome the limitations of cross-linked hydrophilic PVA membranes; carboxy methyl cellulose blending for epichlorohydrin-isopropanol-water pervaporation dehydration. J. Water Process Eng. 2023, 55, 104101. [Google Scholar] [CrossRef]
- Gasemloo, S.; Khosravi, M.; Sohrabi, M.R.; Dastmalchi, S.; Gharbani, P. Response surface methodology (RSM) modeling to improve removal of Cr (VI) ions from tannery wastewater using sulfated carboxymethyl cellulose nanofilter. J. Clean. Prod. 2019, 208, 736–742. [Google Scholar] [CrossRef]
- Kuzminova, A.I.; Dmitrenko, M.E.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; Emeline, A.V.; Mikhailovskii, V.Y.; Solovyev, N.D.; Ermakov, S.S.; Penkova, A.V. Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol. J. Membr. Sci. 2021, 626, 119194. [Google Scholar] [CrossRef]
- Sales, P.d.T.F.; de Souza, K.M.; Bezerra, A.G.; Ojala, S.A.; de Oliveira, S.B.; Bara, M.T.F. Cálculos químicos quânticos e seus usos. Res. Soc. Dev. 2021, 10, e45910817567. [Google Scholar] [CrossRef]
- Keskin, S.; Liu, J.; Rankin, R.B.; Johnson, J.K.; Sholl, D.S. Progress, Opportunities, and Challenges for Applying Atomically Detailed Modeling to Molecular Adsorption and Transport in Metal−Organic Framework Materials. Ind. Eng. Chem. Res. 2009, 48, 2355–2371. [Google Scholar] [CrossRef]
- Odoh, S.O.; Cramer, C.J.; Truhlar, D.G.; Gagliardi, L. Quantum-Chemical Characterization of the Properties and Reactivities of Metal–Organic Frameworks. Chem. Rev. 2015, 115, 6051–6111. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Lü, Z.; Yu, S. Modification of polysulfone ultrafiltration membrane by sequential deposition of cross-linked poly(vinyl alcohol) (PVA) and sodium carboxymethyl cellulose (CMCNa) for nanofiltration. Desalination Water Treat. 2016, 57, 17658–17669. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 16, Revision A.03; Gaussian, Inc., Wallingford CT. 2016. Available online: https://gaussian.com/citation_a03/ (accessed on 18 April 2025).
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Batonneau-Gener, I.; Sachse, A. Determination of the Exact Microporous Volume and BET Surface Area in Hierarchical ZSM-5. J. Phys. Chem. C 2019, 123, 4235–4242. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Mazur, A.; Ermakov, S.; Penkova, A. Novel Pervaporation Membranes Based on Biopolymer Sodium Alginate Modified by FeBTC for Isopropanol Dehydration. Sustainability 2021, 13, 6092. [Google Scholar] [CrossRef]
- Hidayat, S.; Ardiaksa, P.; Riveli, N.; Rahayu, I. Synthesis and characterization of carboxymethyl cellulose (CMC) from salak-fruit seeds as anode binder for lithium-ion battery. In Proceedings of the 3rd Padjadjaran International Physics Symposium, Bandung, Indonesia, 14–15 November 2017; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 1080, p. 012017. [Google Scholar] [CrossRef]
- Hernández, M.; Leyva, G.; Magaña, J.J.; Guzmán-Vargas, A.; Felipe, C.; Lara, V.; Lima, E. New copolymers as hosts of ribosomal RNA. BMC Chem. 2019, 13, 33. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Z.; Grande, C.; Zhang, W.; Dou, Y.; Li, X.; Fu, J.; Shezad, N.; Akhtar, F.; Kaiser, A. A phase conversion method to anchor ZIF-8 onto a PAN nanofiber surface for CO2 capture. RSC Adv. 2022, 12, 664–670. [Google Scholar] [CrossRef]
- Yáñez-S, M.; Matsuhiro, B.; Maldonado, S.; González, R.; Luengo, J.; Uyarte, O.; Serafine, D.; Moya, S.; Romero, J.; Torres, R.; et al. Carboxymethylcellulose from bleached organosolv fibers of Eucalyptus nitens: Synthesis and physicochemical characterization. Cellulose 2018, 25, 2901–2914. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Dubovenko, R.; Mikulan, A.; Puzikova, M.; Selyutin, A.; Mazur, A.; Ermakov, S.; Su, R.; Penkova, A. Carboxymethyl cellulose/Zn-based metal organic frameworks membranes for pervaporation-assisted esterification reactor. Sep. Purif. Technol. 2024, 332, 125720. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Mikhailovskaya, O.; Dubovenko, R.; Kuzminova, A.; Myznikov, D.; Mazur, A.; Semenov, K.; Rusalev, Y.; Soldatov, A.; Ermakov, S.; et al. Pervaporation Membranes Based on Polyelectrolyte Complex of Sodium Alginate/Polyethyleneimine Modified with Graphene Oxide for Ethanol Dehydration. Polymers 2024, 16, 1206. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Trindle, C. Bond index description of delocalization. J. Am. Chem. Soc. 1969, 91, 219–220. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for ‘fuzzy’ atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Georgiou, D.C.; Butler, P.; Browne, E.C.; Wilson, D.J.D.; Dutton, J.L. On the Bonding in Bis-pyridine Iodonium Cations. Aust. J. Chem. 2013, 66, 1179. [Google Scholar] [CrossRef]
- Naik, P.V.; Bernstein, R.; Vankelecom, I.F.J. Influence of support layer and PDMS coating conditions on composite membrane performance for ethanol/water separation by pervaporation. J. Appl. Polym. Sci. 2016, 133, 43670. [Google Scholar] [CrossRef]
- Kattula, M.; Ponnuru, K.; Zhu, L.; Jia, W.; Lin, H.; Furlani, E.P. Designing ultrathin film composite membranes: The impact of a gutter layer. Sci. Rep. 2015, 5, 15016. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Hao, P. Influence of the porous support on diffusion in composite membranes. J. Membr. Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar] [CrossRef]
- Kachhadiya, D.D.; Murthy, Z.V.P. Preparation and characterization of ZIF-8 and ZIF-67 incorporated poly(vinylidene fluoride) membranes for pervaporative separation of methanol/water mixtures. Mater. Today Chem. 2021, 22, 100591. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Li, M.; Hou, L. Influence of the 2-methylimidazole/zinc nitrate hexahydrate molar ratio on the synthesis of zeolitic imidazolate framework-8 crystals at room temperature. Sci. Rep. 2018, 8, 9597. [Google Scholar] [CrossRef]
- Cravillon, J.; Münzer, S.; Lohmeier, S.-J.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Innovative layer by layer and continuous growth methods for synthesis of ZIF-8 membrane on porous polymeric support using poly(ether- block -amide) as structure directing agent for gas separation. Microporous Mesoporous Mater. 2016, 234, 43–54. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]



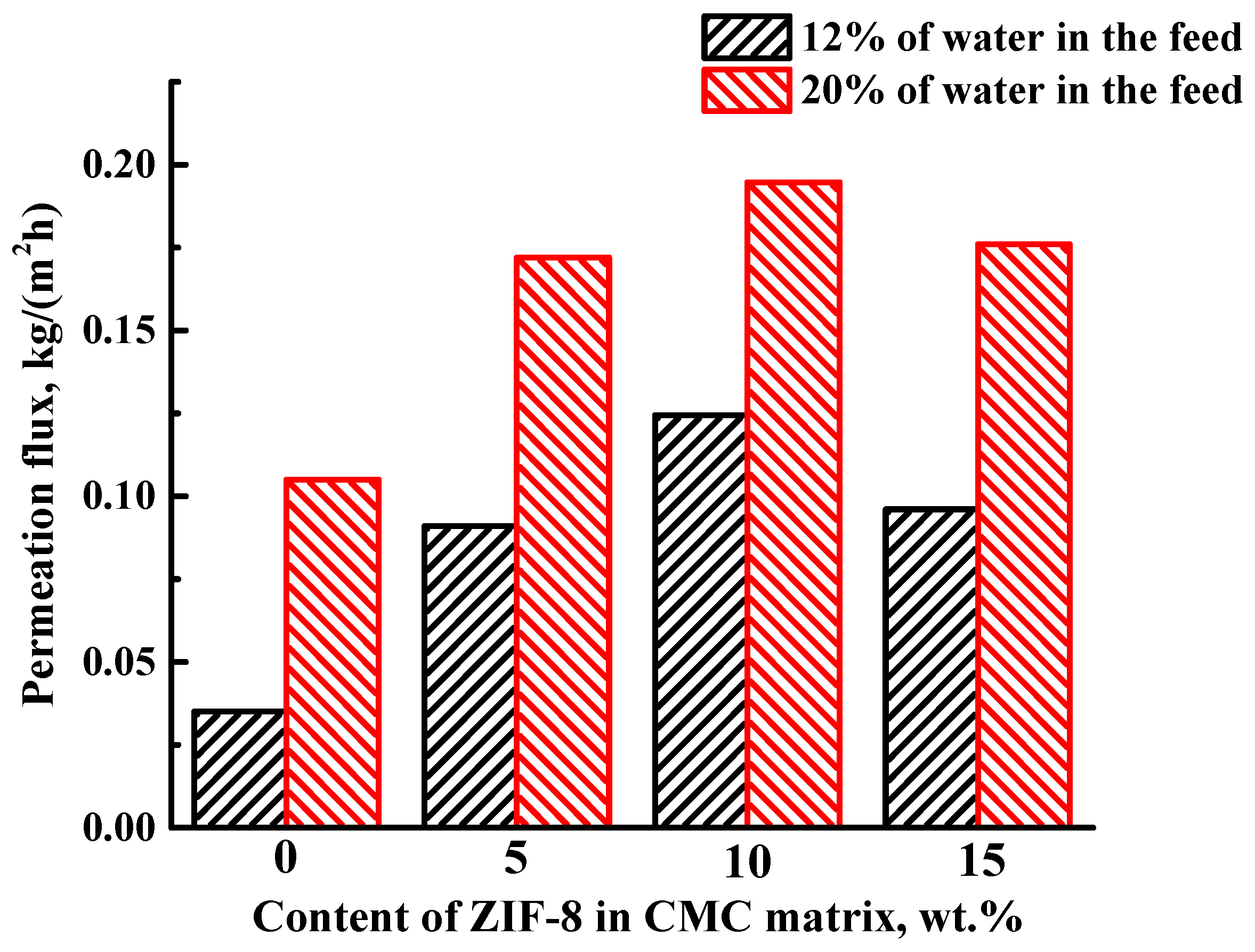

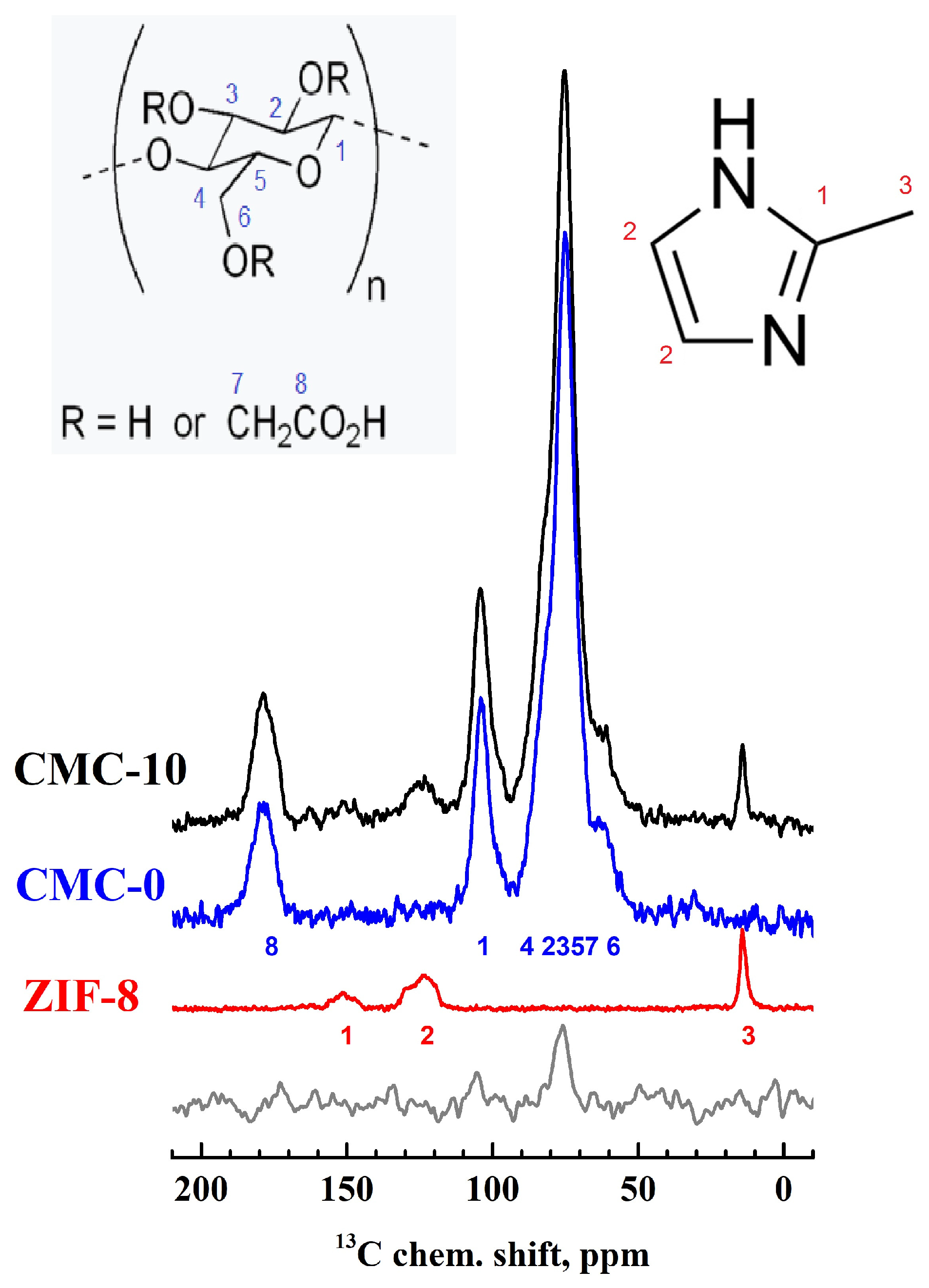
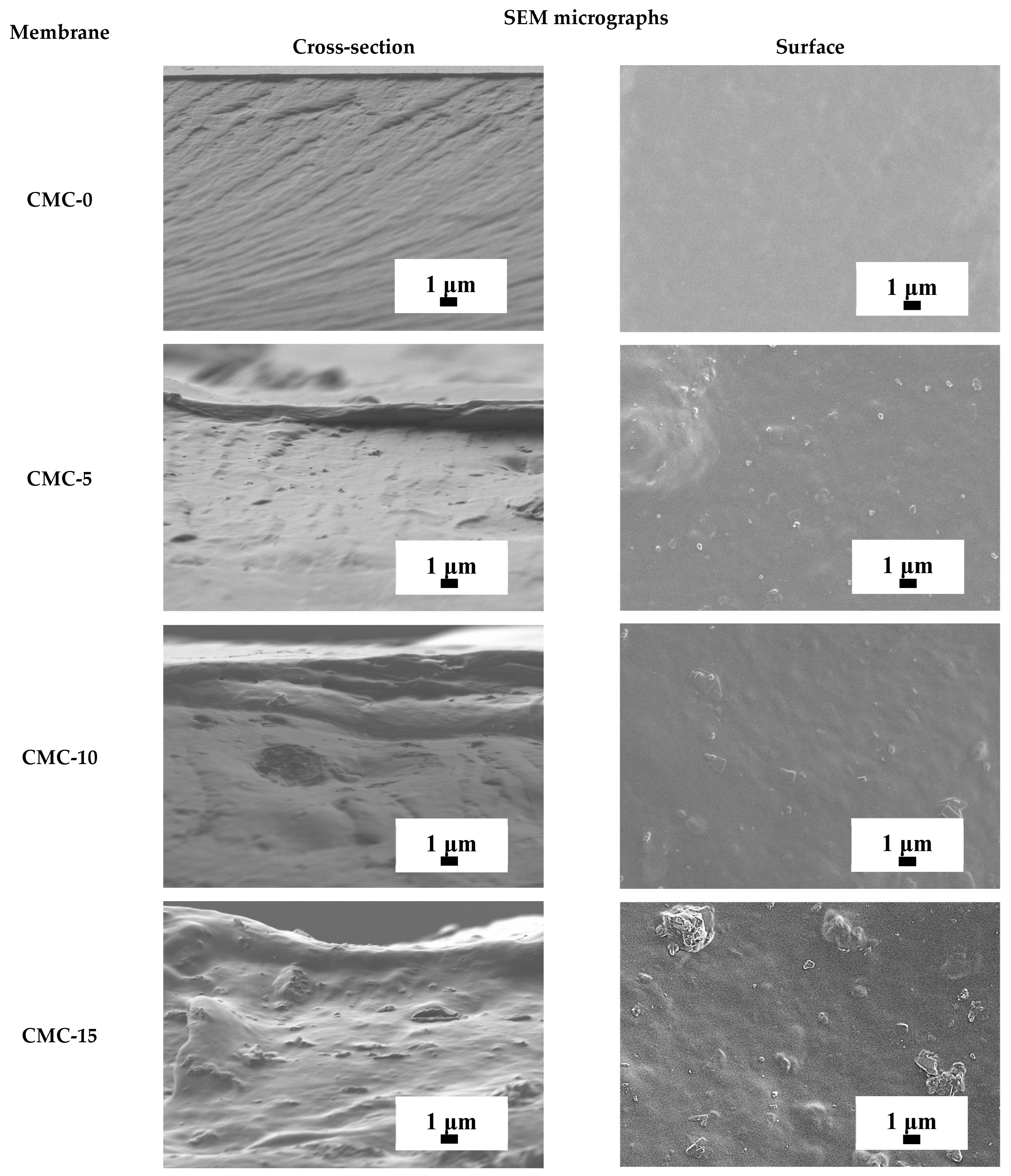


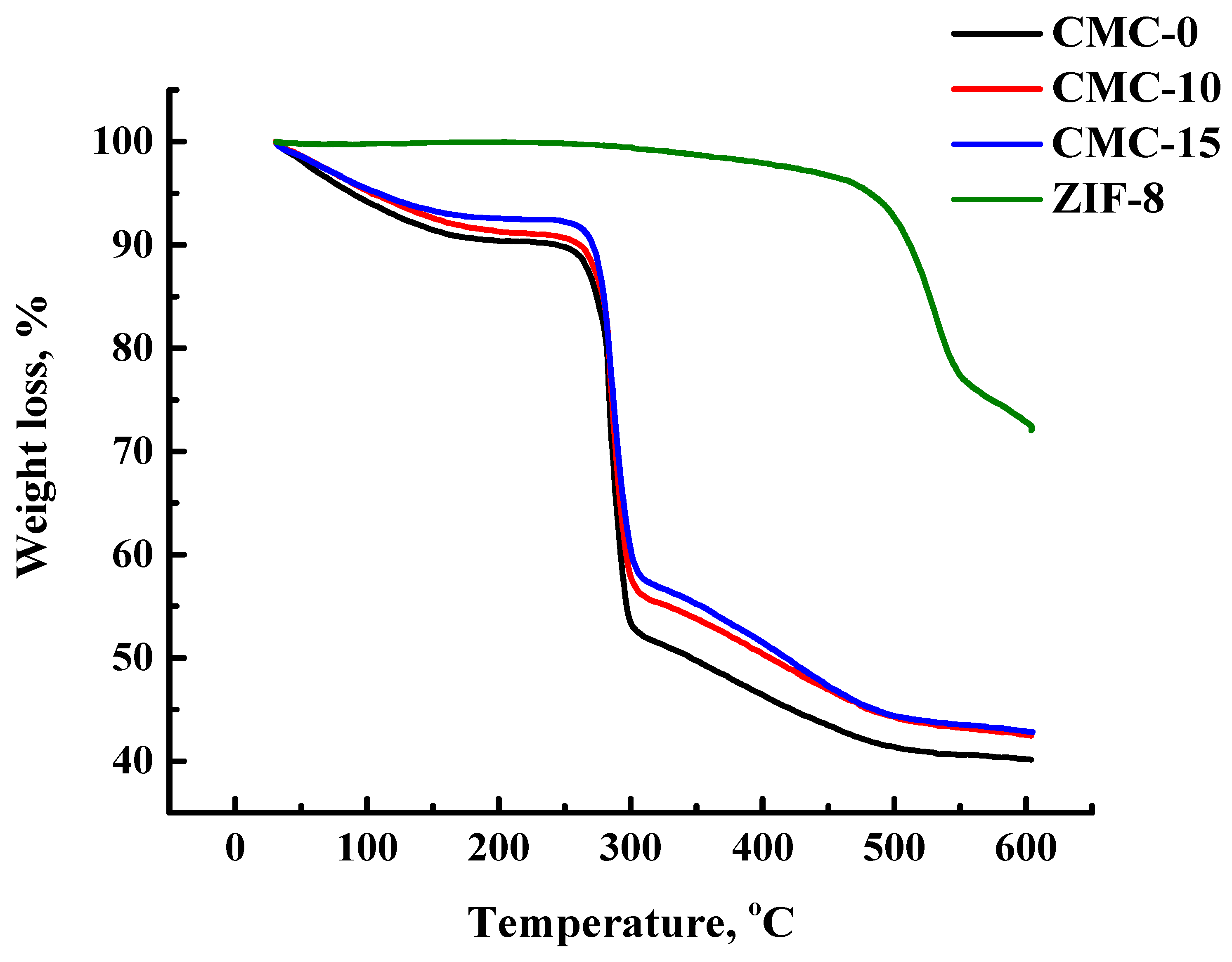
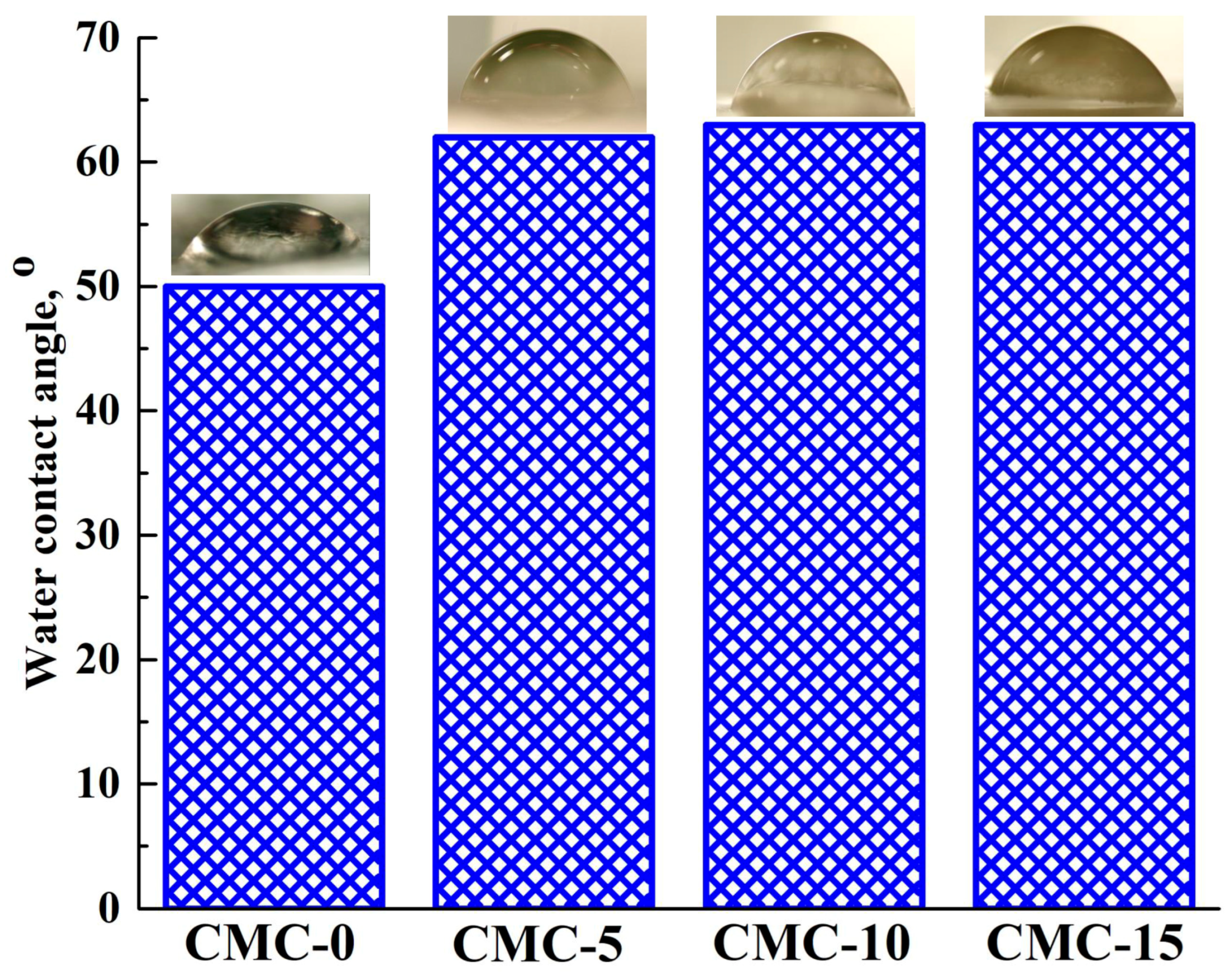

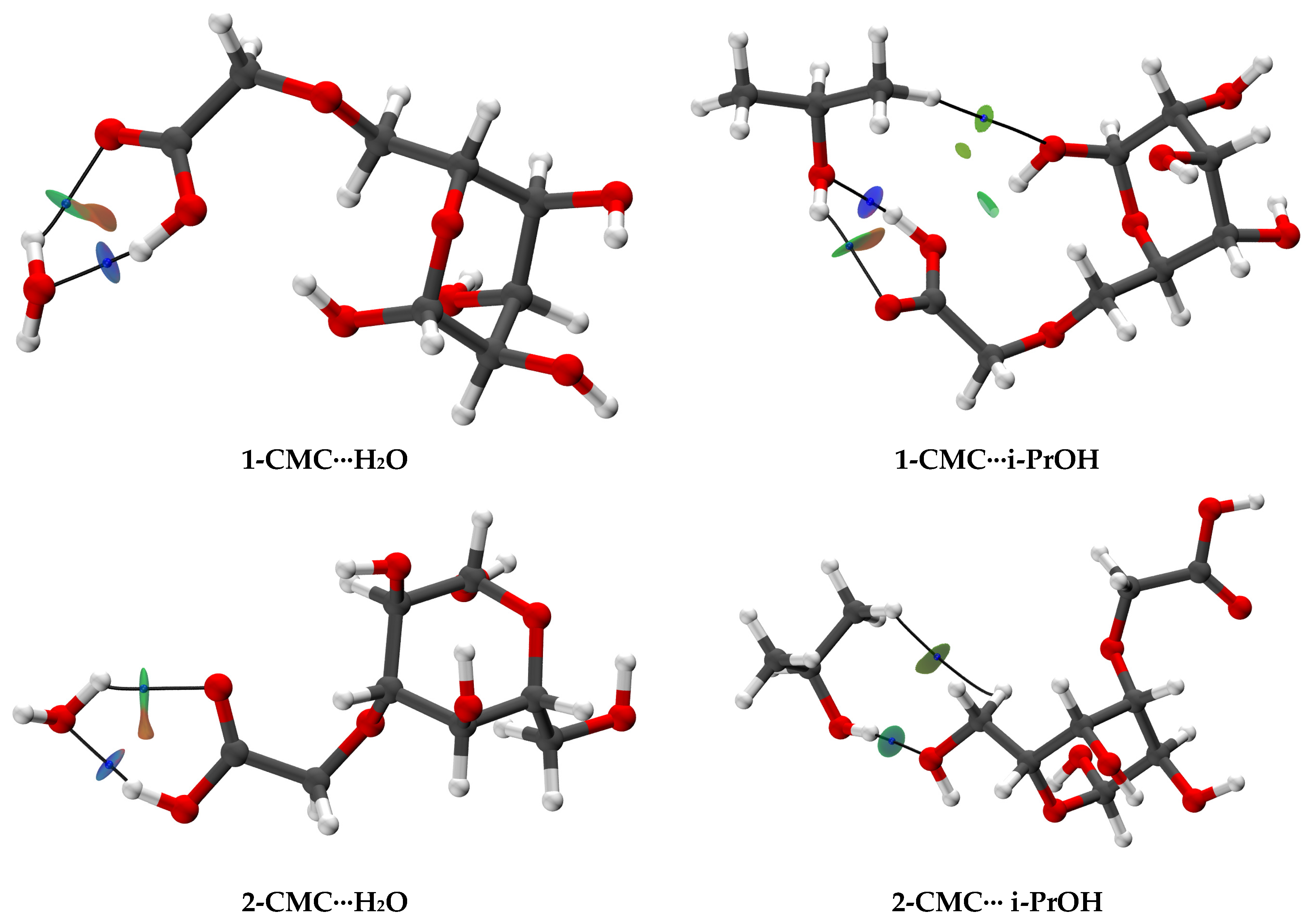
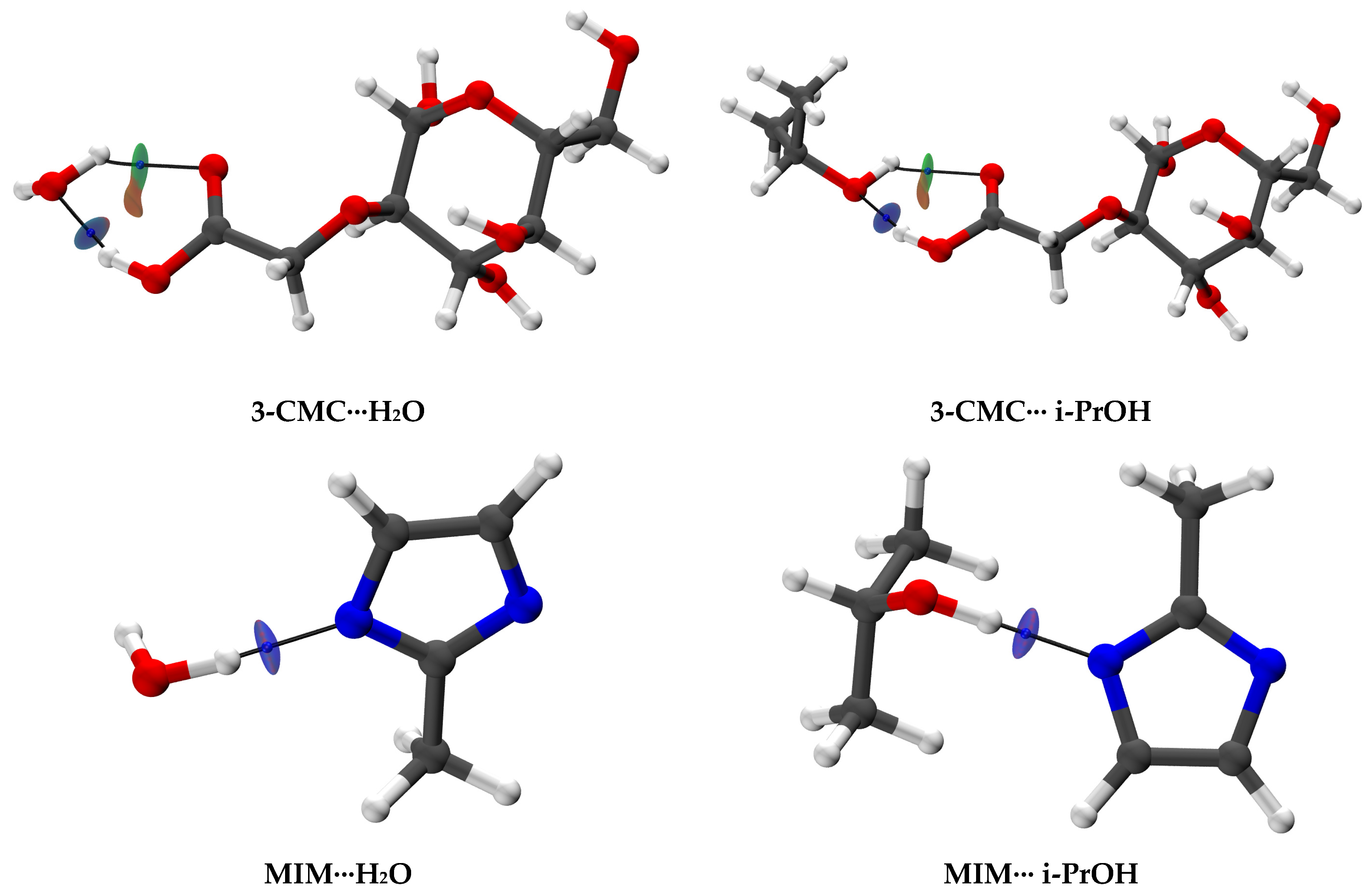
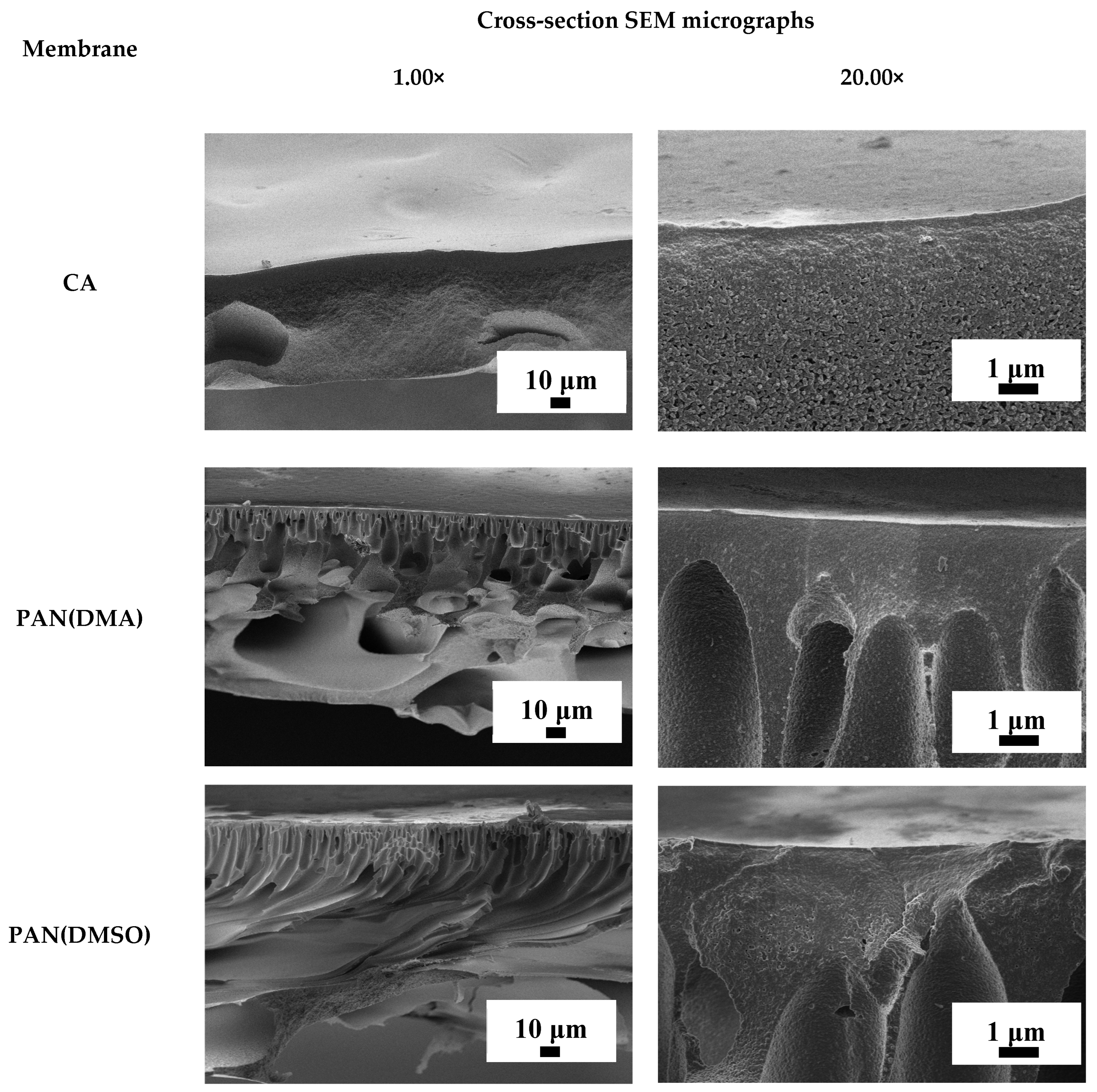
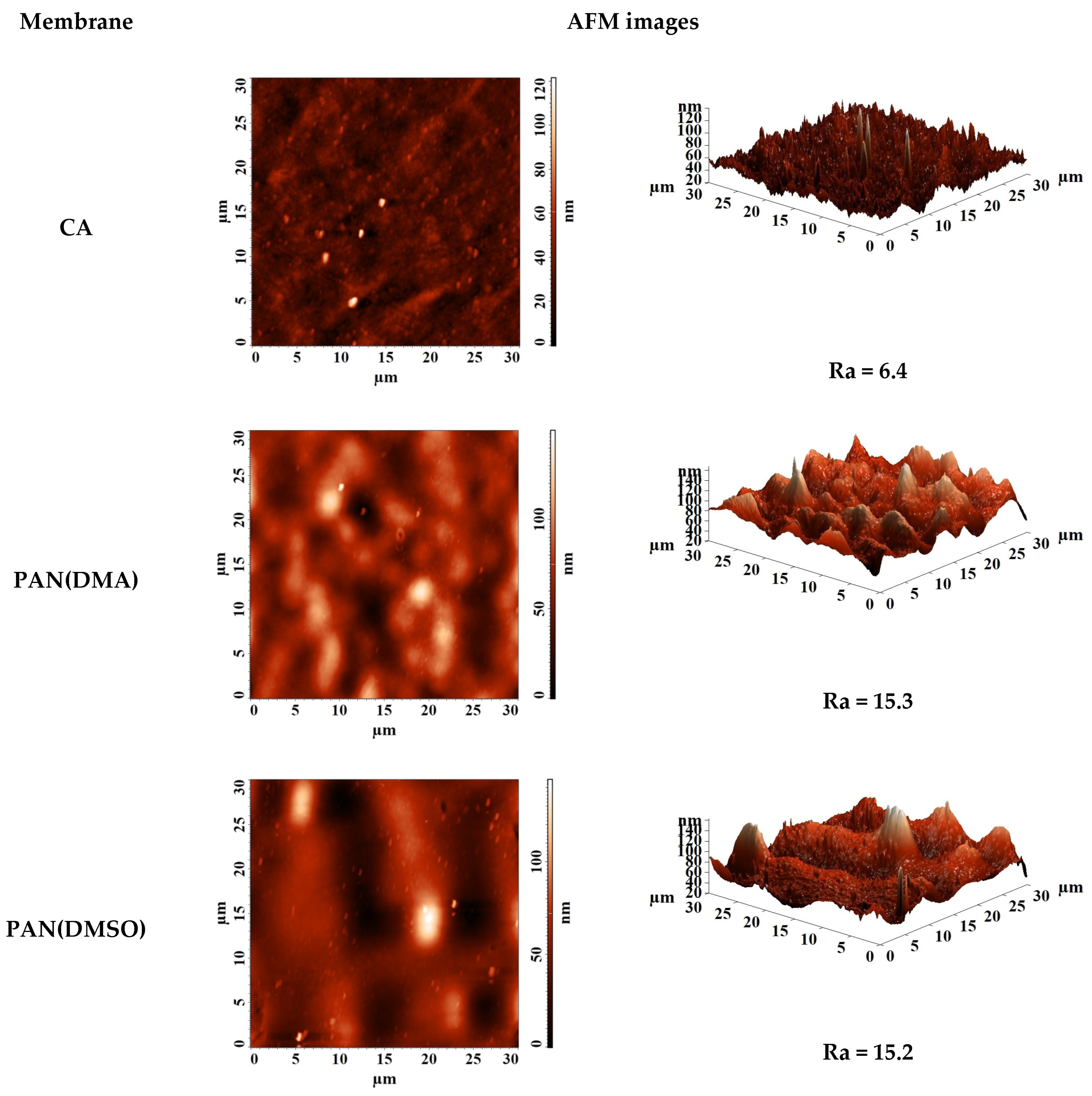
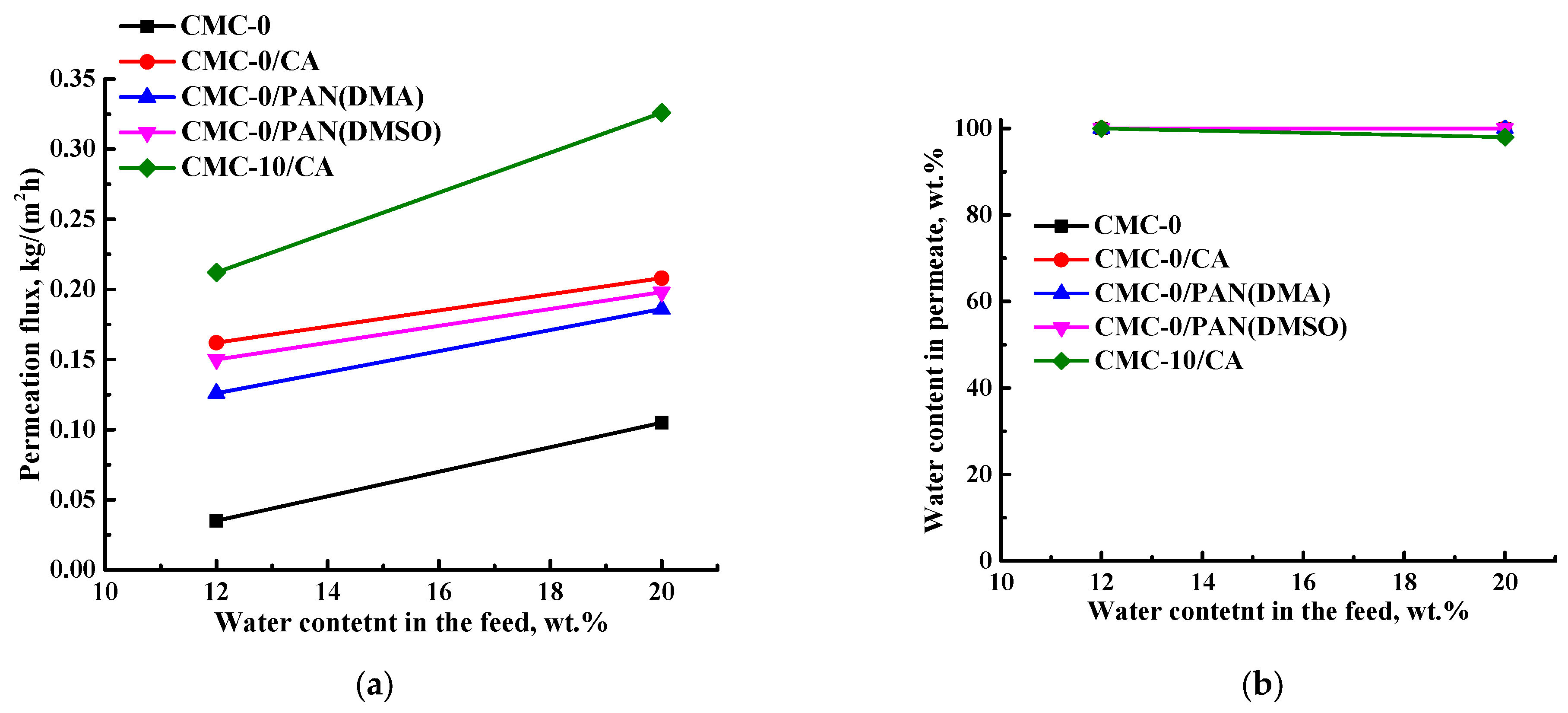
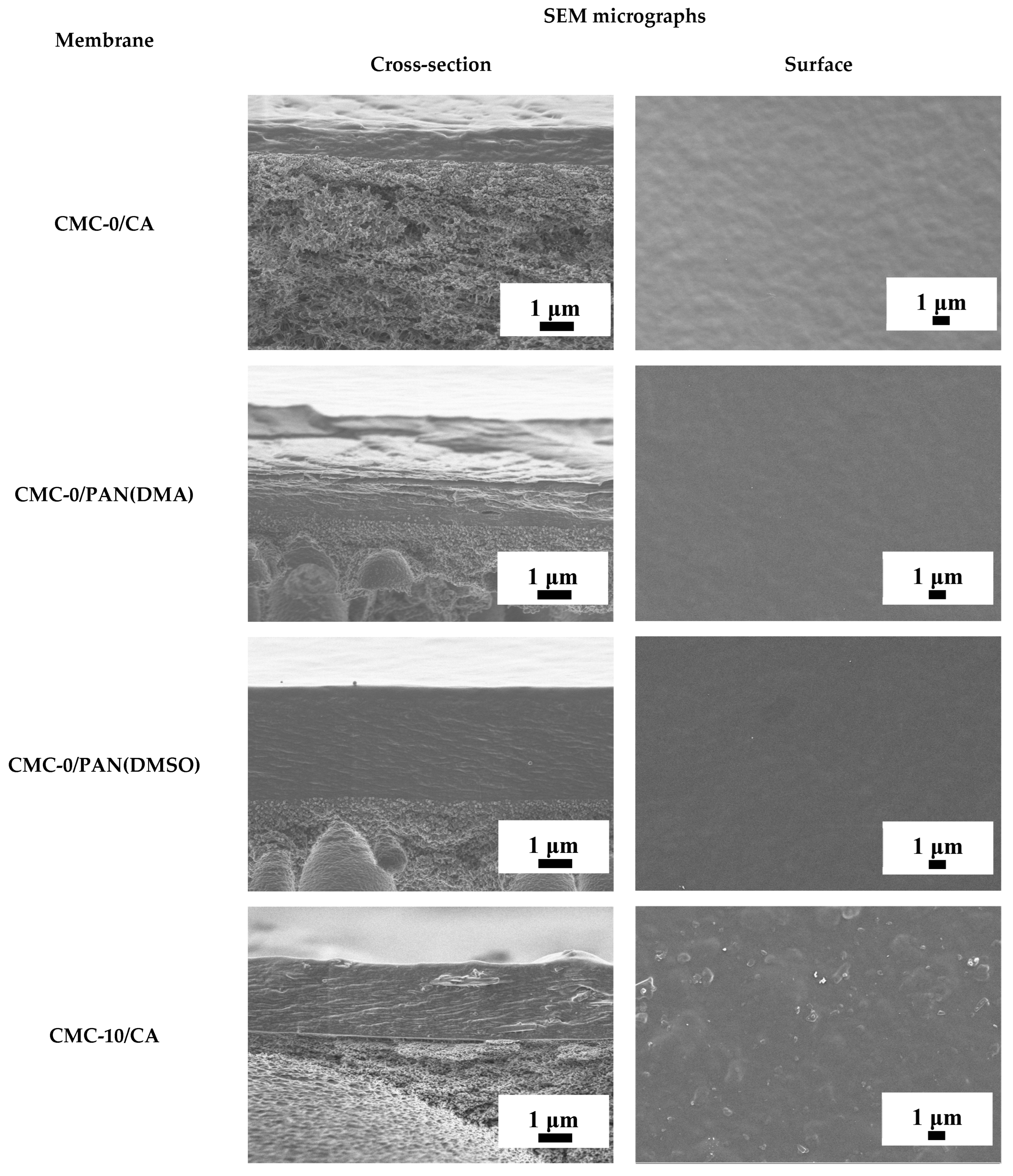


| Membrane | Type | Content of ZIF-8, wt% | Support | Cross-Linking |
|---|---|---|---|---|
| CMC-0 | dense | 0 | - | - |
| CMC-5 | dense | 5 | - | - |
| CMC-10 | dense | 10 | - | - |
| CMC-15 | dense | 15 | - | - |
| CMC-0/CA | supported | 0 | CA | - |
| CMC-0/PAN(DMA) | supported | 0 | PAN(DMA) | - |
| CMC-0/PAN(DMSO) | supported | 0 | PAN(DMSO) | - |
| CMC-10/CA | supported | 10 | CA | - |
| CMC-0/CACL | supported | 0 | CA | + |
| CMC-10/CACL | supported | 10 | CA | + |
| Membrane | Swelling Degree in Water/Isopropanol (12/88 wt%), % |
|---|---|
| CMC-0 | 5.8 |
| CMC-5 | 8.1 |
| CMC-10 | 9.9 |
| CMC-15 | 10.9 |
| ΔG0, (kJ/mol) | |||
|---|---|---|---|
| B3LYP/6-311++G** | MIM | i-PrOH | H2O |
| 1-CMC | −146.5 | −0.7 | −1.0 |
| 2-CMC | −148.9 | 4.1 | 1.8 |
| 3-CMC | −155.0 | 0.4 | 1.4 |
| MIM | ~ | −32.4 | −32.3 |
| i-PrOH | ~ | 10.7 | |
| B3LYP/6-311++G** | ||||||
|---|---|---|---|---|---|---|
| Associate | Interaction | WBI | FBO | d, Å | R, % | |
| 1-CMC | MIM | O(CO*OH)///H(CH3) | 0.042 | 0.049 | 2.37669 | 87.4% |
| H(COOH)//N | 0.856 | 0.691 | 1.03888 | 37.8% | ||
| O(COO*H)///H(COOH) | 0.089 | 0.094 | 1.78273 | 65.5% | ||
| i-PrOH | O(CO*OH)///H(OH) | 0.048 | 0.039 | 2.21791 | 81.5% | |
| H(COOH)///O | 0.185 | 0.122 | 1.68038 | 61.8% | ||
| O(OH)///H(CH3) | 0.005 | 0.009 | 3.25592 | 119.7% | ||
| H2O | O(CO*OH)///H | 0.072 | 0.054 | 2.06025 | 75.7% | |
| H(COOH)///O | 0.154 | 0.116 | 1.74939 | 64.3% | ||
| 2-CMC | MIM | O(CO*OH)///H(CH3) | 0.030 | 0.036 | 2.58091 | 94.9% |
| H(COOH)//N | 0.842 | 0.670 | 1.04734 | 38.1% | ||
| O(COO*H)///H(COOH) | 0.189 | 0.130 | 1.68682 | 62.0% | ||
| i-PrOH | H(CH2*OH)///H(CH3) | 0.002 | 0.002 | 3.15786 | 116.1% | |
| O(CH2OH)///H(OH) | 0.090 | 0.074 | 1.94578 | 71.5% | ||
| H2O | O(CO*OH)///H | 0.068 | 0.052 | 2.07838 | 76.4% | |
| H(COOH)///O | 0.143 | 0.109 | 1.76818 | 65.0% | ||
| 3-CMC | MIM | O(COO*H)///H(COOH) | 0.185 | 0.127 | 1.70157 | 62.6% |
| H(COOH)//N | 0.841 | 0.670 | 1.04724 | 38.1% | ||
| i-PrOH | O(CO*OH)///H(OH) | 0.051 | 0.041 | 2.18732 | 80.4% | |
| H(COOH)///O | 0.165 | 0.112 | 1.71006 | 62.9% | ||
| H2O | O(CO*OH)///H | 0.068 | 0.052 | 2.07584 | 76.3% | |
| H(COOH)///O | 0.144 | 0.110 | 1.76643 | 64.9% | ||
| MIM | i-PrOH | N///H(OH) | 0.214 | 0.147 | 1.73743 | 63.2% |
| H2O | N///H | 0.184 | 0.146 | 1.74683 | 63.5% | |
| IPA | H2O | O///H | 0.108 | 0.081 | 1.8857 | 69.3% |
| Porosity Characteristics | Porous Support | ||
|---|---|---|---|
| CA | PAN(DMA) | PAN(DMSO) | |
| Porosity over weight (cm3/g) | 2.0 | 2.5 | 4.9 |
| Porosity over volume (cm3/cm3) | 0.8 | 0.9 | 0.9 |
| Meso- and macro-pore surface over weight (m2/g) | 30.1 | 61.2 | 84.0 |
| Meso- and macro-pore surface over volume (m2/cm3) | 12.2 | 20.9 | 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzminova, A.; Dmitrenko, M.; Dubovenko, R.; Mikulan, A.; Stepanova, A.; Puzikova, M.; Rakovskaya, N.; Mazur, A.; Shurukhina, A.; Rudakova, A.; et al. Sustainable Novel Membranes Based on Carboxymethyl Cellulose Modified with ZIF-8 for Isopropanol/Water Pervaporation Separation. Sustainability 2025, 17, 3801. https://doi.org/10.3390/su17093801
Kuzminova A, Dmitrenko M, Dubovenko R, Mikulan A, Stepanova A, Puzikova M, Rakovskaya N, Mazur A, Shurukhina A, Rudakova A, et al. Sustainable Novel Membranes Based on Carboxymethyl Cellulose Modified with ZIF-8 for Isopropanol/Water Pervaporation Separation. Sustainability. 2025; 17(9):3801. https://doi.org/10.3390/su17093801
Chicago/Turabian StyleKuzminova, Anna, Mariia Dmitrenko, Roman Dubovenko, Anna Mikulan, Anastasia Stepanova, Margarita Puzikova, Nadezhda Rakovskaya, Anton Mazur, Anna Shurukhina, Aida Rudakova, and et al. 2025. "Sustainable Novel Membranes Based on Carboxymethyl Cellulose Modified with ZIF-8 for Isopropanol/Water Pervaporation Separation" Sustainability 17, no. 9: 3801. https://doi.org/10.3390/su17093801
APA StyleKuzminova, A., Dmitrenko, M., Dubovenko, R., Mikulan, A., Stepanova, A., Puzikova, M., Rakovskaya, N., Mazur, A., Shurukhina, A., Rudakova, A., Emeline, A., Su, R., & Penkova, A. (2025). Sustainable Novel Membranes Based on Carboxymethyl Cellulose Modified with ZIF-8 for Isopropanol/Water Pervaporation Separation. Sustainability, 17(9), 3801. https://doi.org/10.3390/su17093801










