Plant Growth Regulators and Short-Term Irrigation for Berry Maturation Homogeneity and Increased Coffea arabica Bean Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Conditions and Plant Material
2.2. Treatment with Plant Growth Regulators (PGRs)
2.3. Leaf Gas Exchange Measurements and Photosynthetic Capacity Modeling
2.4. Plant Coding, 3D Reconstructions and Plant Photosynthesis Computing
2.5. Berry Harvest
2.6. Coffee Bean Chemical Analysis
2.7. Statistical Analysis
3. Results
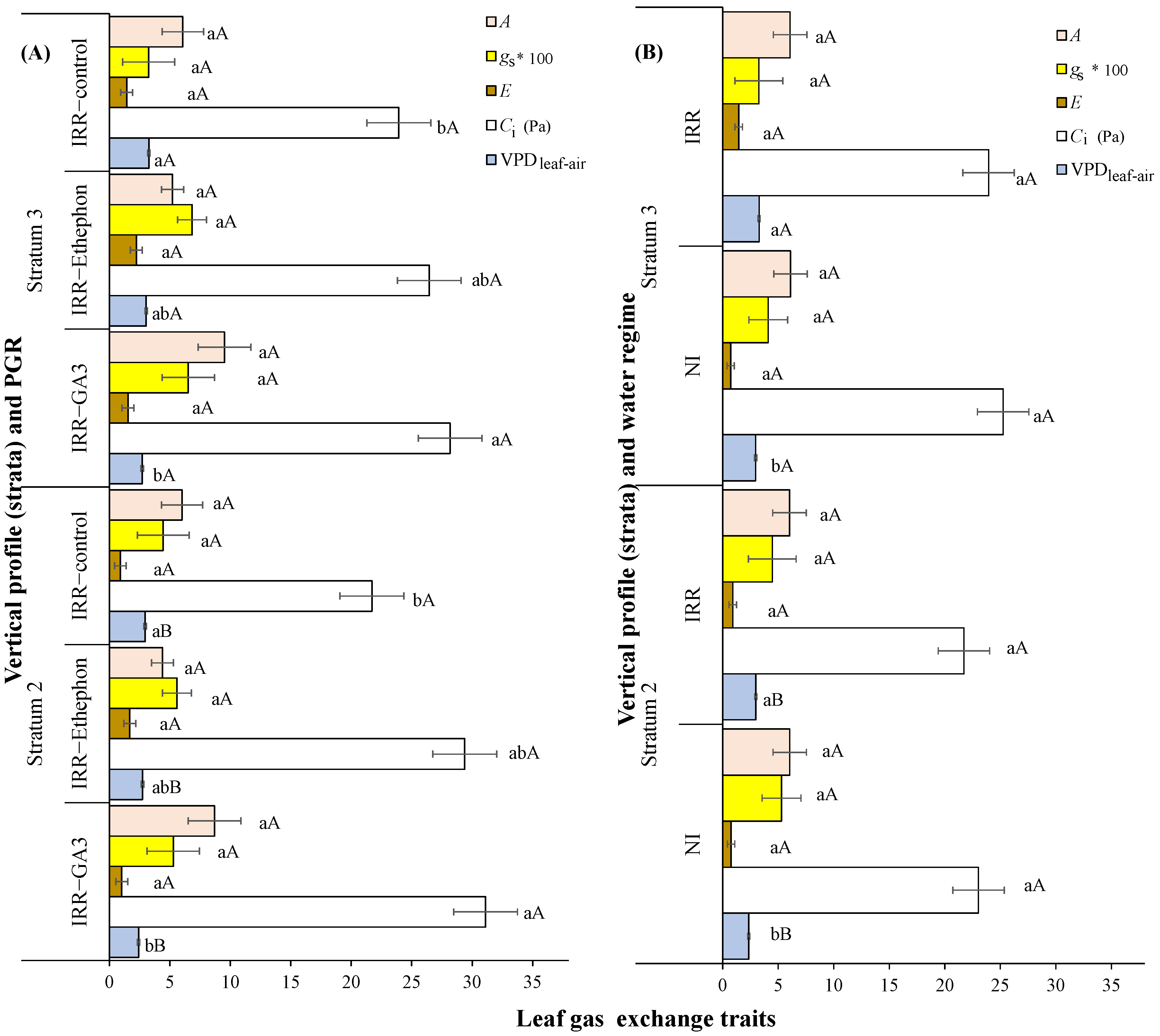
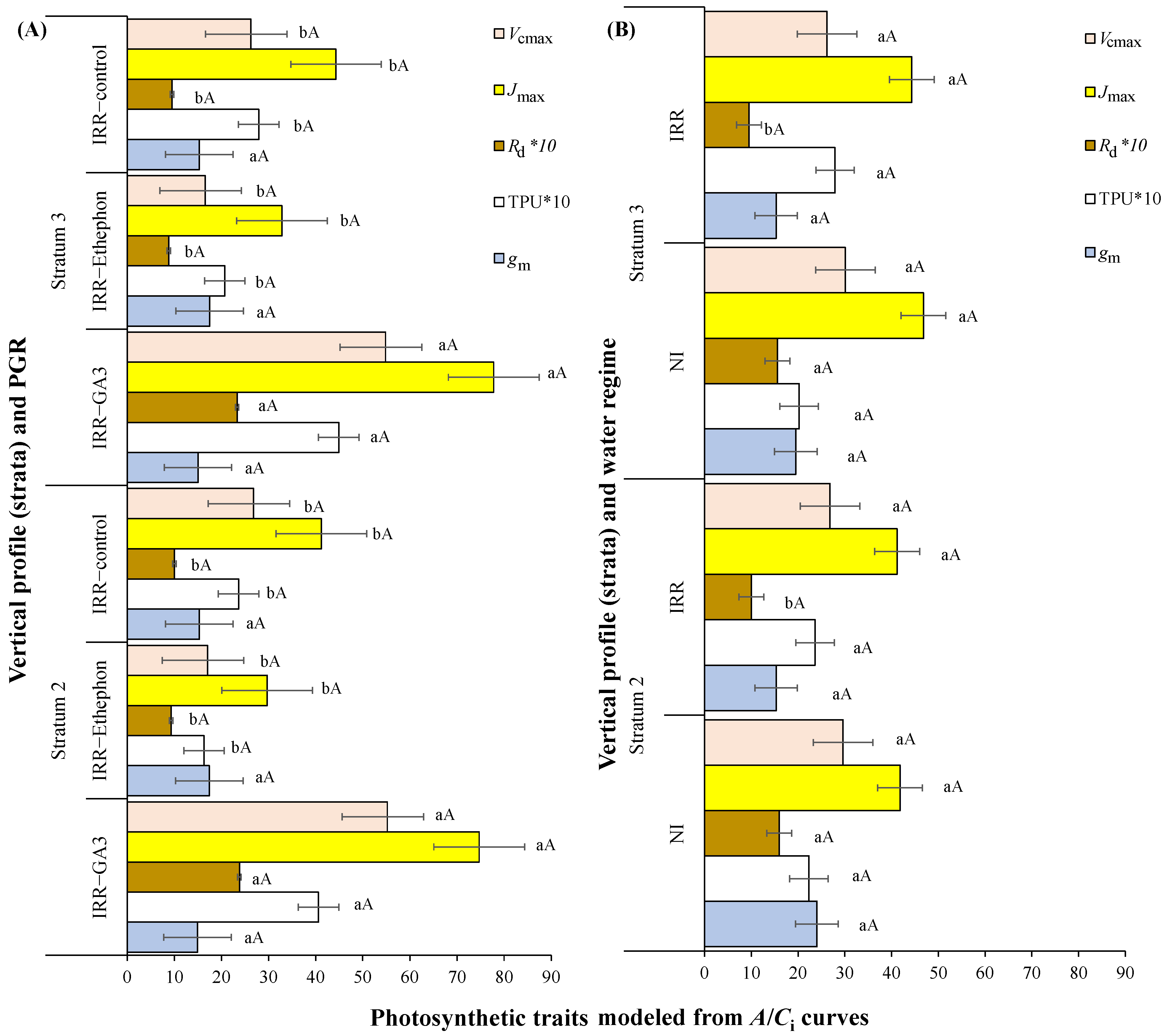
3.1. Leaf Gas Exchanges over the Plant Vertical Profile in Relation to PGRs or Water Regime
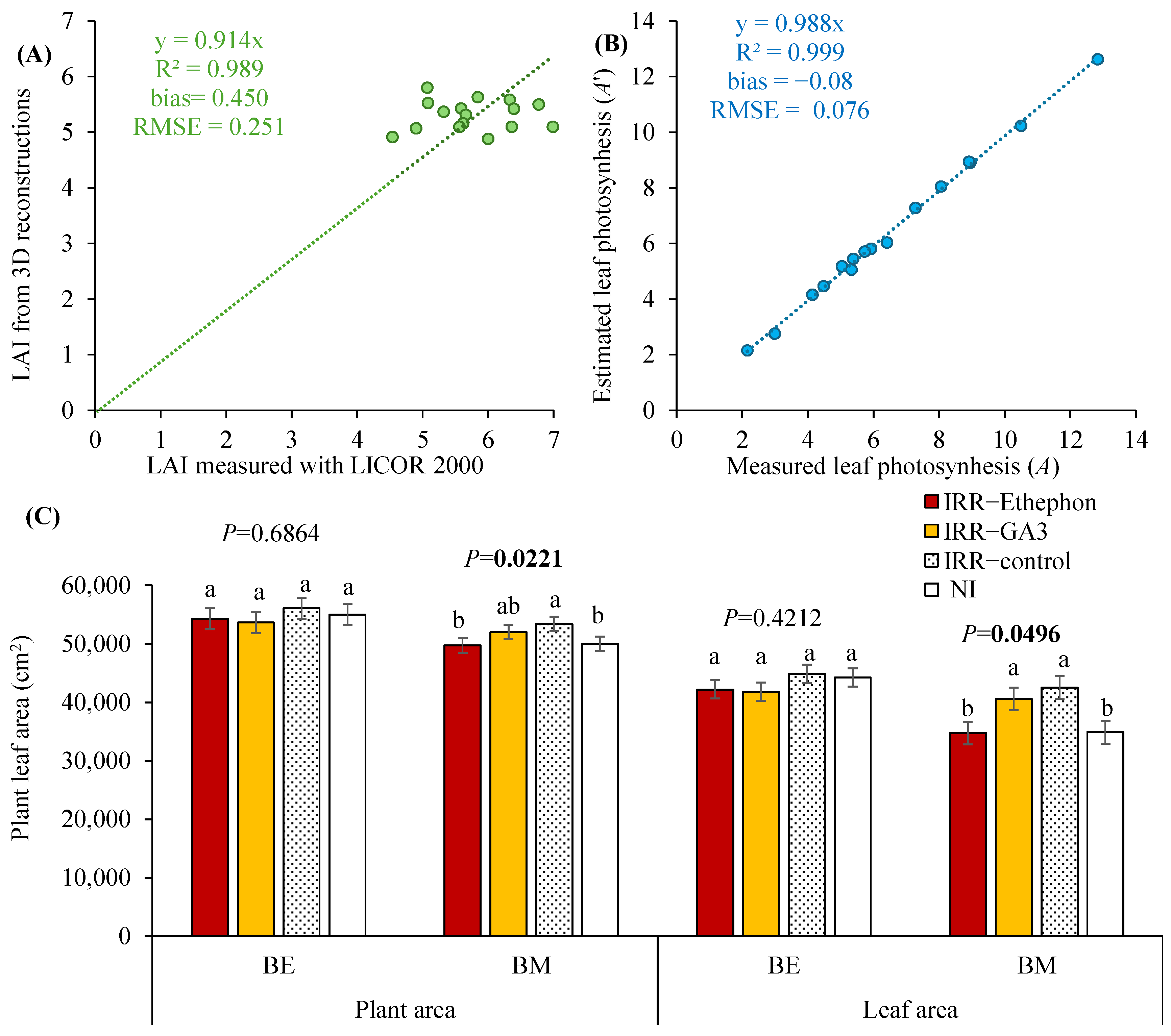
3.2. Validation of 3D Plant Reconstructions, Estimations of Leaf Area and Plant Scale Photosynthesis in Relation to PGRs or Water Regime
3.3. The Distribution of Red and Green Berries over Branching Orders as Dependent on PGRs or Water Regime
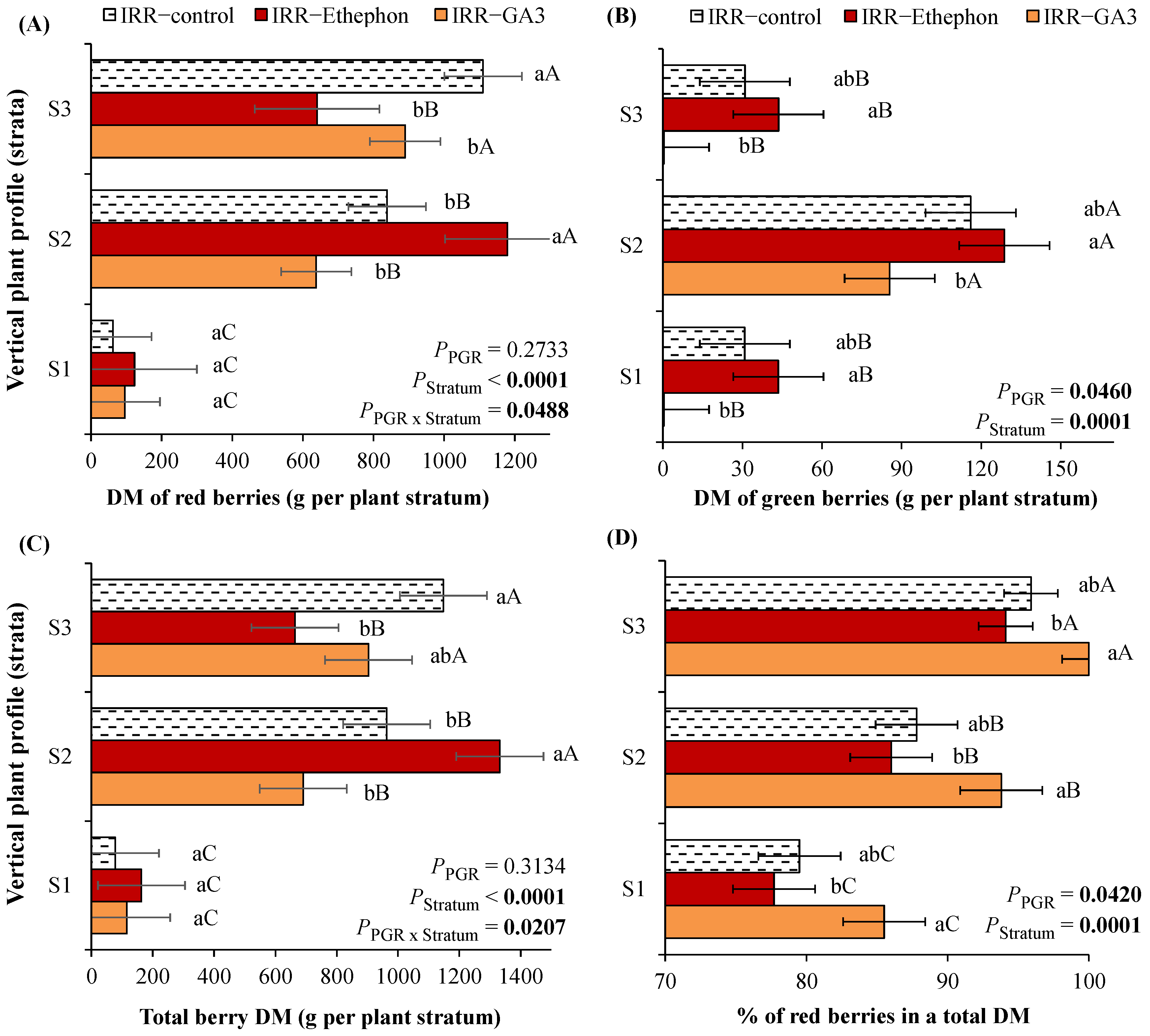
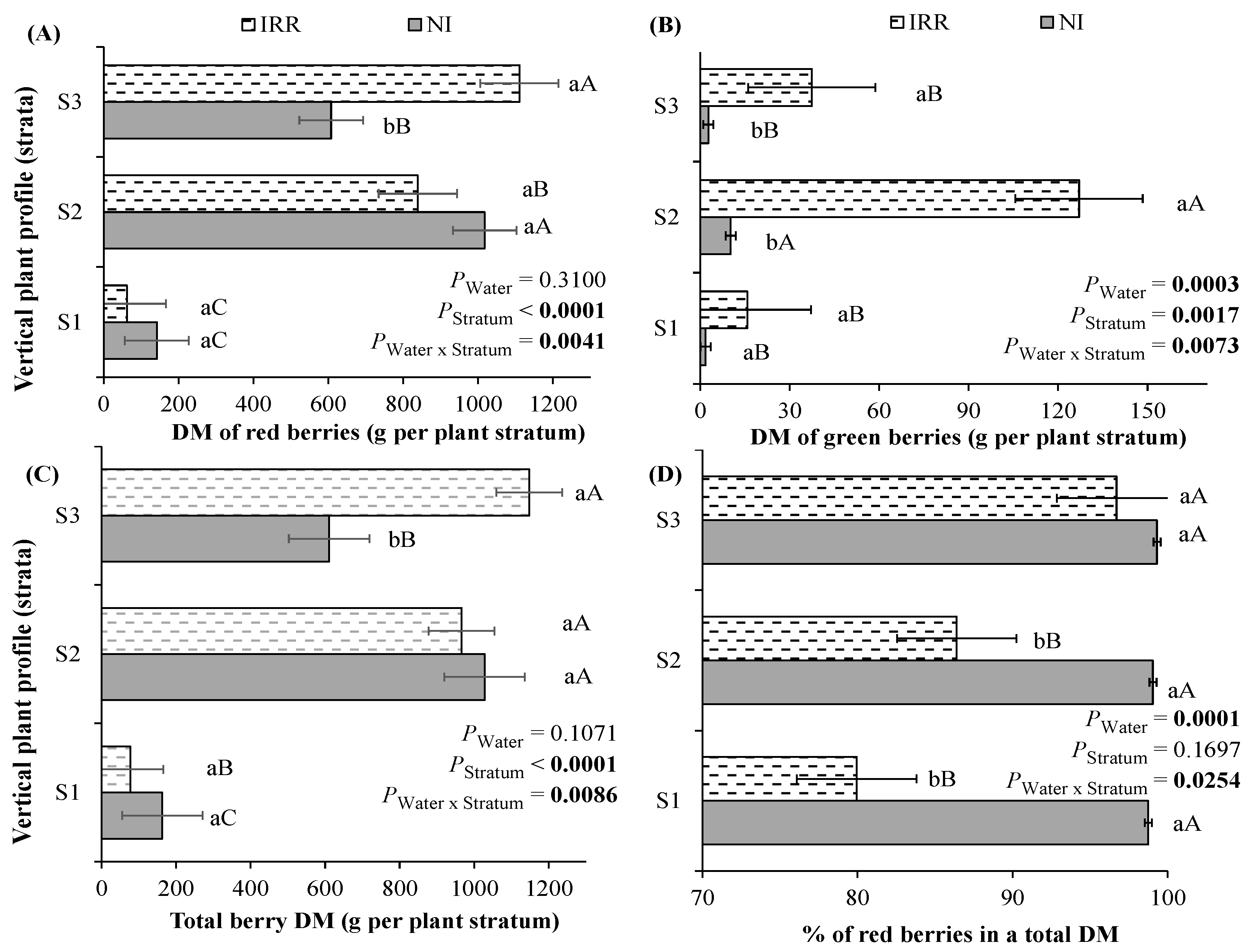
3.4. The Fresh and Dry Mass of Red and Green Berries over the Plant Vertical Profile as Dependent on PGRs or Water Regime
3.5. The Chemical Composition of Coffee Beans over the Plant Vertical Profile Is Dependent on PGRs or Water Regime
4. Discussion
4.1. The Use of Architectural Analysis in Determination of PGR or Water Regime Impacts on Entire Plant Photosynthesis, Plant Leaf Area and Berry Distribution over Branching Orders
4.2. The Use of PGGs to Increase Uniformity of Berry Maturation over the Vertical Plant Profile—Relations to Bean Chemical Quality
4.3. The Use of Irrigation Increases Coffee Yield but Delays Berry Maturation—Relations to Bean Chemical Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Leaf photosynthesis rate |
| A/Ci curves | Curves of leaf photosynthesis rate (A) versus CO2 inside the leaf (Ci) |
| Ap′ | Mean diurnal leaf photosynthesis of a plant |
| Ap″ | Whole plant photosynthesis |
| BE | Berry expansion phenophase |
| BM | Berry maturation phenophase |
| CAF | Caffeine |
| CFT | Cafestol |
| CGA | Total chlorogenic acid content |
| DM | Dry mass |
| E | Leaf transpiration |
| FM | Fresh mass |
| FSPM | Functional–structural plant modeling |
| GA3 | Gibberellic acid, C19H22O6 |
| gm | Mesophyll conductance |
| gs | Stomatal conductance |
| IRR | Irrigated conditions or control plants |
| Jcmax | Maximum rate of electron transport for the given light intensity |
| KAH | Kahweol |
| LA | Plant leaf area |
| LAI | Leaf area index |
| LIP | Lipids |
| MTGs | Multiscale tree graphs |
| NI | Not irrigated, rainfed conditions |
| PGR | Plant growth regulator(s) |
| PPFD | Photosynthetic photon flux density |
| PRO | Protein |
| Rd | Daily dark respiration |
| S1, S2, S3 | Stratum 1, Stratum 2, Stratum 3 |
| SUC | Sucrose |
| TPU | Maximum rate of triose phosphate use |
| TS | Total sugar content |
| Vcmax | Maximum carboxylation rate of RuBisCO |
| VPDleaf–air | Leaf-to-air vapor pressure deficit |
References
- Rena, A.B.; Barros, R.S. Critical aspects in the study of coffee flowering. In Efeitos da Irrigação Sobre a Qualidade e Produtividade do Café; Zambolim, L., Ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2004; pp. 149–172. [Google Scholar]
- Rakočević, M.; Scholz, M.B.S.; Good Kitzberger, C.S. Berry distributions on coffee trees cultivated under high densities modulate the chemical composition of respective coffee beans during one biannual cycle. Int. J. Fruit Sci. 2018, 18, 117–137. [Google Scholar] [CrossRef]
- Silva, F.B.; Tormena, C.D.; Pauli, E.D.; Almeida, A.G.; Berg, A.B.C.; Rakočević, M.; Bruns, R.E.; Scarminio, I.S.; Marcheafave, G.G. Time dependent berry maturation for planting density levels in Coffea arabica L. beans: Mixture design–fingerprinting using near–infrared transmittance spectroscopy. J. Food Compos. Anal. 2021, 97, 103795. [Google Scholar] [CrossRef]
- dos Santos Scholz, M.B.; Good Kitzberger, C.S.; Durand, N.; Rakočević, M. From the field to coffee cup: Impact of planting design on chlorogenic acid isomers and other compounds in coffee beans and sensory attributes of coffee beverage. Eur. Food Res. Technol. 2018, 244, 1793–1802. [Google Scholar] [CrossRef]
- Kazama, E.H.; da Silva, R.P.; Tavares, T.D.O.; Correa, L.N.; de Lima Estevam, F.N.; Nicolau, F.E.D.A.; Maldonado Júnior, W. Methodology for selective coffee harvesting in management zones of yield and maturation. Precis. Agric. 2020, 22, 711–733. [Google Scholar] [CrossRef]
- Bártholo, G.F.; Guimarães, P.T.G. Cuidados na colheita e preparo do café. Inf. Agropecu. 1997, 18, 33–42. [Google Scholar]
- Peña Quiñones, A.J.; Ramírez Builes, V.H.; Jaramillo Robledo, A.; Rendón Sáenz, J.R.; Arcila Pulgarín, J. Effects of daylength and soil humidity on the flowering of Coffee Coffea arabica L. in Colombia. Rev. Fac. Nac. Agron. Medellín 2011, 64, 5745–5754. [Google Scholar]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- López, M.E.; Santos, I.S.; de Oliveira, R.R.; Lima, A.A.; Cardon, C.H.; Chalfun–Junior, A. An overview of the endogenous and environmental factors related to the Coffea arabica flowering process. BPR Res. 2021, 1, 13. [Google Scholar] [CrossRef]
- Drinnan, J.E.; Menzel, C.M. Synchronization of anthesis and enhancement of vegetative growth in coffee (Coffea arabica L.) following water stress during floral initiation. J. Hortic. Sci. 1994, 69, 841–849. [Google Scholar] [CrossRef]
- Vicente, M.R.; Mantovani, E.C.; Fernandes, A.L.T.; Neves, J.C.L.; Delazari, F.T.; Figueredo, E.M. Effects of irrigation on the production and development of coffee in the West region of Bahia. Coffee Sci. 2017, 12, 544–551. [Google Scholar] [CrossRef]
- Rakočević, M.; Matsunaga, F.T.; Pazianotto, R.A.A.; Ramalho, J.C.; Costes, E.; Ribeiro, R.V. Drought responses in Coffea arabica as affected by genotype and phenophase. I—Leaf distribution and branching. Exp. Agric. 2024, 60, e7. [Google Scholar] [CrossRef]
- Camargo, A.P.; Camargo, M.B.P. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.V.; Araujo, W.L.; Fernie, A.R.; Moraes, G.A.B.K. In field–grown coffee trees source–sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Unigarro Muñoz, C.A.; Díaz Bejarano, L.M.; Acuña, J.R. Effect of fruit load of the first coffee harvests on leaf gas exchange. Pesq. Agropec. Trop. 2021, 51, e69865. [Google Scholar] [CrossRef]
- Rakočević, M.; Matsunaga, F.T.; Baroni, D.F.; Campostrini, E.; Costes, E. Multiscale analyses of growth and berry distributions along four branching orders and vertical profile of Coffea arabica L. cultivated under high–density planting systems. Sci. Hortic. 2021, 281, 109934. [Google Scholar] [CrossRef]
- Bote, A.D.; Vos, J. Tree management and environmental conditions affect coffee (Coffea arabica L.) bean quality. NJAS—Wagening. J. Life Sci. 2017, 83, 39–46. [Google Scholar] [CrossRef]
- Leroy, T.; Ribeyre, F.; Bertrand, B.; Charmetant, P.; Dufour, M.; Montagnon, C.; Marraccini, P.; Pot, D. Genetics of coffee quality. Braz. J. Plant Physiol. 2006, 18, 229–242. [Google Scholar] [CrossRef]
- Bi, X.; Yu, H.; Hu, F.; Fu, X.; Li, Y.; Li, Y.; Yang, Y.; Liu, D.; Li, G.; Shi, R.; et al. A systematic analysis of the correlation between flavor active differential metabolites and multiple bean ripening stages of Coffea arabica L. Molecules 2024, 29, 180. [Google Scholar] [CrossRef] [PubMed]
- Tolessa, K.; D’heer, J.; Duchateau, L.; Boeckx, P. Influence of growing altitude, shade and harvest period on quality and biochemical composition of Ethiopian specialty coffee. J. Sci. Food Agric. 2016, 97, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Vaast, P.; Bertrand, B.; Perriot, J.-J.; Guyot, B.; Génard, M. Fruit thinning and shade improve bean characteristics and beverage quality of coffee (Coffea arabica L.) under optimal conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Rakočević, M.; Scholz, M.B.S.; Pazianotto, R.A.A.; Matsunaga, F.T.; Ramalho, J.C. Variation in yield, berry distribution and chemical attributes of Coffea arabica beans among the canopy strata of four genotypes cultivated under contrasted water regimes. Horticulturae 2023, 9, 215. [Google Scholar] [CrossRef]
- Vinecky, F.; Davrieux, F.; Mera, A.C.; Alves, G.S.C.; Lavagnini, G.; Leroy, T.; Bonnot, F.; Rocha, O.C.; Bartholo, G.F.; Guerra, A.F.; et al. Controlled irrigation and nitrogen, phosphorous and potassium fertilization affect the biochemical composition and quality of Arabica coffee beans. J. Agricul. Sci. 2017, 155, 902–918. [Google Scholar] [CrossRef]
- Miranda, F.R.; Drumond, L.C.D.; Ronchi, C.P. Synchronizing coffee blossoming and fruit ripening in irrigated crops of the Brazilian Cerrado Mineiro Region. Aust. J. Crop Sci. 2020, 14, 605–613. [Google Scholar] [CrossRef]
- Mesquita, C.M.; Rezende, J.E.; Carvalho, J.S.; Fabri Júnior, M.A.; Moraes, N.C.; Dias, P.T.; Carvalho, R.M.; Araújo, W.G. Manual do café: Colheita e Preparo (Coffea arabica L.); EMATER–MG: Belo Horizonte, Brazil, 2016; 50p. [Google Scholar]
- Silva, E.A.; Brunini, O.; Sakai, E.; Arruda, F.B.; Pires, R.C. Influence of controlled water deficits on flowering synchronization and yield of coffee under three distinct edapho–climatic conditions of São Paulo State, Brazil. Bragantia 2009, 68, 493–501. [Google Scholar] [CrossRef]
- Somarriba, E.; Quesada, F. Modeling age and yield dynamics in Coffea arabica pruning systems. Agric. Syst. 2022, 201, 103450. [Google Scholar] [CrossRef]
- Masarirambi, M.T.; Shongwe, V.D.; Chingwara, V. The effect of GA3 and Ethephon on synchronization of coffee (Coffea arabica L.) flowering and berry ripening. Acta Hortic. 2010, 884, 573–580. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Masondo, N.A.; Gupta, S.; Moyo, M.; Aremu, A.O. Editorial: The application of phytohormones in plant biotechnology for sustainable agriculture. Front. Plant Sci. 2024, 15, 1382055. [Google Scholar] [CrossRef]
- Gupta, S.; Bhattacharyya, P.; Kulkarni, M.G.; Doležal, K. Editorial: Growth regulators and biostimulants: Upcoming opportunities. Front. Plant Sci. 2023, 14, 1209499. [Google Scholar] [CrossRef]
- Bacilieri, F.S.; DE Lima, L.C.; Lana, R.M.Q.; Guimarães, D.S.N.; Clemente, M.A. Efficacy of a plant growth regulator in the culture of coffee. Biosci. J. 2016, 32, 346–353. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Silva, P.C.; Junior, W.Q.R.; Ramos, M.L.G.; Rocha, O.C.; Veiga, A.D.; Silva, N.H.; Brasileiro, L.O.; Santana, C.C.; Soares, G.F.; Malaquias, J.V.; et al. Physiological changes of arabica coffee under different intensities and durations of water stress in the Brazilian Cerrado. Plants 2022, 11, 2198. [Google Scholar] [CrossRef] [PubMed]
- Rakočević, M.; Costes, E.; Campostrini, E.; Ramalho, J.C.; Ribeiro, R.V. Drought responses in Coffea arabica as affected by genotype and phenophase. I—photosynthesis at leaf and plant scales. Exp. Agric. 2024, 60, e22. [Google Scholar]
- Deuner, S.; Alves, J.D.; Zanandrea, I.; Goulart, P.F.P.; Silveira, N.M.; Henrique, P.C.; Mesquita, A.C. Stomatal behavior and components of the antioxidative system in coffee plants under water stress. Sci. Agric. 2011, 68, 7–85. [Google Scholar] [CrossRef]
- De Vos, D.; Dzhurakhalov, A.; Stijven, S.; Klosiewicz, P.; Beemster, G.T.S.; Broeckhove, J. Virtual plant tissue: Building blocks for next–generation plant growth simulation. Front. Plant Sci. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.P.; Barbier de Reuille, P.; Kuhlemeier, C. Toward a 3D model of phyllotaxis based on a biochemically plausible auxin–transport mechanism. PLoS Comput. Biol. 2019, 15, e1006896. [Google Scholar] [CrossRef]
- Aparecido, L.E.O.; Rolim, G.S.; Richetti, J.; Souza, P.S.; Johann, J.A. Köppen, Thornthwaite and Camargo climate classifications for climatic zoning in the State of Paraná, Brazil. Ciênc. Agrotec. 2016, 40, 405–417. [Google Scholar] [CrossRef]
- Rakočević, M.; Batista, E.R.; Pazianotto, R.A.A.; Scholz, M.B.S.; Souza, G.A.R.; Campostrini, E.; Ramalho, J.C. Leaf gas exchange and bean quality fluctuations over the whole canopy vertical profile of Arabic coffee cultivated under elevated CO2. Funct. Plant Biol. 2021, 48, 469–482. [Google Scholar] [CrossRef]
- Cesanelli, A.; Guarracino, L. Numerical modeling of actual evapotranspiration of a coffee crop. Sci. Agric. 2011, 68, 395–399. [Google Scholar] [CrossRef]
- Torres, J.D.; Espindula, M.C.; Araújo, L.F.B.D.; Marcolan, A.L.; Rocha, R.B. Grain yield of coffee plants fertilized with different doses of 20-00-20 NPK formulation under rainfed conditions. Rev. Caatinga 2021, 34, 486–493. [Google Scholar] [CrossRef]
- Rakočević, M. Coffee plant architecture. In Coffee—A Glimpse into the Future; DaMatta, F.M., Ramalho, J.C., Eds.; Elsevier: London, UK, 2025; pp. 29–62. [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Sharkey, T.D. What gas exchange data can tell us about photosynthesis? Plant Cell Environ. 2016, 39, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Godin, C.; Caraglio, Y. A multiscale model of plant topological structures. J. Theor. Biol. 1998, 191, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Godin, C.; Costes, E.; Sinoquet, H. A method for describing plant architecture which integrates topology and geometry. Ann. Bot. 1999, 84, 343–357. [Google Scholar] [CrossRef]
- Rakočević, M.; Costes, E.; Assad, E.D. Structural and physiological sexual dimorphism estimated from three dimensional virtual trees of yerba–mate (Ilex paraguariensis) is modified by cultivation environment. Ann. Appl. Biol. 2011, 159, 178–191. [Google Scholar] [CrossRef]
- Matsunaga, F.T.; Tosti, J.B.; Androcioli-Filho, A.; Brancher, J.D.; Costes, E.; Rakočević, M. Strategies to reconstruct 3D Coffea arabica L. plant structure. SpringerPlus 2016, 5, 2075. [Google Scholar] [CrossRef]
- Pradal, C.; Boudon, F.; Nouguier, C.; Chopard, J.; Godin, C. PlantGL: A Python–based geometric library for 3D plant modelling at different scales. Graph. Models 2009, 71, 1–21. [Google Scholar] [CrossRef]
- Adam, B.; Dones, N.; Sinoquet, H. VegeSTAR, Version 3.2; Software qui Calcule L’interception Lumineuse et la Photosynthèse; INRA: Paris, France, 2006; Available online: https://eng-piaf.clermont.hub.inrae.fr/methods-and-models/vegestar (accessed on 25 January 2025).
- Scholz, M.B.S.; Kitzberger, C.S.G.; Pereira, L.F.P.; Davrieux, F.; Pot, D.; Charmetant, P.; Leroy, T. Application of near infrared spectroscopy for green coffee biochemical phenotyping. J. Near Infrared Spectrosc. 2014, 22, 411–421. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Pagiatto, N.F.; Kitzberger, C.S.G.; Pereira, L.F.P.; Davrieux, F.; Charmetant, P.; Leroy, T. Validation of near–infrared spectroscopy for the quantification of cafestol and kahweol in green coffee. Food Res. Int. 2014, 61, 176–182. [Google Scholar] [CrossRef]
- R Core Team. 2022. Available online: https://www.r–project.org// (accessed on 1 December 2024).
- Furini, G.; Anese, R.D.O.; Reinehr, J.; da Silva, F.N.; Casa, R.T.; Rufato, L.; Rufato, D.P.; Bogo, A. Ethephon effect on defoliation of cluster zone, Botrytis bunch rot, and viticultural performance of ‘Cabernet Sauvignon’ grapevine in highland region of southern Brazil. Cienc. Rural 2024, 54, e20230207. [Google Scholar] [CrossRef]
- Hendgen, M.; Schubert, S.; Löhnertz, O. Ethephon–induced leaf senescence increases the concentration of yeast–assimilable nitrogen in grape must (Vitis vinifera cv. Riesling). Aust. J. Grape Wine Res. 2021, 27, 472–482. [Google Scholar] [CrossRef]
- Romano, L.S.; Filla, A.; Giomo, G.S.; Lemos, L.B. Characterization of Yellow Bourbon coffee strains for the production of differentiated specialty coffees. Bragantia 2022, 81, e2222. [Google Scholar] [CrossRef]
- Schuch, U.K.; Fuchigami, L.H.; Nagao, M.A. Gibberellic acid causes earlier flowering and synchronizes fruit ripening of coffee. Plant Growth Regul. 1990, 9, 59–64. [Google Scholar] [CrossRef]
- Scalco, M.S.; Alvarenga, L.A.; Guimarães, R.J.; Colombo, A.; Assis, G.A. Irrigated and non–irrigated coffee (Coffea arabica L.) under super dense conditions. Coffee Sci. 2012, 6, 193–202. [Google Scholar]
- Bisht, T.S.; Rawat, L.; Chakraborty, B.; Yadav, V. A recent advances in use of plant growth regulators (PGRs) in fruit crops—A review. Int. J. Cur. Microbiol. Appl. Sci. 2018, 7, 1307–1336. [Google Scholar] [CrossRef]
- Cocco, C.; Silvestre, W.P.; Schildt, G.W.; Tessaro, F.A. Effect of Ethephon application on fruit quality at harvest and post–harvest storage of Japanese plum (Prunus salicina) cv. Fortune. Braz. Arch. Biol. Technol. 2022, 65, e20210183. [Google Scholar] [CrossRef]
- Tormena, C.D.; Rutledge, D.N.; Rakočević, M.; Bruns, R.E.; Scarminio, I.S.; Marcheafave, G.G.; Pauli, E.D. Exogenous application of bioregulators in Coffea arabica beans during ripening: Investigation of UV–Visible and NIR mixture design–fingerprints using AComDim–ICA. Microchem. J. 2022, 181, 107702. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska–Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilization for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Hall, R.D.; Trevisan, F.; De Vos, R.C.H. Coffee berry and green bean chemistry—Opportunities for improving cup quality and crop circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.P.F.; Silva, J.S.; Carneiro, A.P.S.; Melo, E.C.; Silva, C.S.; Lisboa, C.F. Quality of coffee beans from peeled green berries after temporary immersion in water. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 713–718. [Google Scholar] [CrossRef]
- Barros, M.M.D.; Silva, F.M.D.; Costa, A.G.; Ferraz, G.A.E.S.; Silva, F.C.D. Use of classifier to determine coffee harvest time by detachment force. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 366–370. [Google Scholar] [CrossRef]
- Ferrão, R.G.; Fonseca, A.F.A.; Ferrão, M.A.G.; Verdim Filho, A.C.; Volpi, P.S.; Muner, L.H.; Lani, J.A.; Prezotti, L.C.; Ventura, J.A.; Marques, E.M.G.; et al. Café conilon: Técnicas de produção com variedades melhoradas. Circ. Téc. 2012, 03–I, 74. [Google Scholar]
- Nobre, G.W.; Borém, F.M.; Isquierdo, E.P.; Pereira, R.G.F.A.; Oliveira, P.D. Chemical composition of unripe Arabic coffee (Coffea arabica L.) processed under dry and wet conditions. Coffee Sci. 2011, 6, 107–113. [Google Scholar]
- Martello, M.; Molin, J.P.; Bazame, H.C. Obtaining and validating high–density coffee yield data. Horticulturae 2022, 8, 421. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakočević, M.; Batista, E.R.; Matsunaga, F.T.; Scholz, M.B.d.S. Plant Growth Regulators and Short-Term Irrigation for Berry Maturation Homogeneity and Increased Coffea arabica Bean Quality. Sustainability 2025, 17, 3803. https://doi.org/10.3390/su17093803
Rakočević M, Batista ER, Matsunaga FT, Scholz MBdS. Plant Growth Regulators and Short-Term Irrigation for Berry Maturation Homogeneity and Increased Coffea arabica Bean Quality. Sustainability. 2025; 17(9):3803. https://doi.org/10.3390/su17093803
Chicago/Turabian StyleRakočević, Miroslava, Eunice Reis Batista, Fabio Takeshi Matsunaga, and Maria Brígida dos Santos Scholz. 2025. "Plant Growth Regulators and Short-Term Irrigation for Berry Maturation Homogeneity and Increased Coffea arabica Bean Quality" Sustainability 17, no. 9: 3803. https://doi.org/10.3390/su17093803
APA StyleRakočević, M., Batista, E. R., Matsunaga, F. T., & Scholz, M. B. d. S. (2025). Plant Growth Regulators and Short-Term Irrigation for Berry Maturation Homogeneity and Increased Coffea arabica Bean Quality. Sustainability, 17(9), 3803. https://doi.org/10.3390/su17093803





