Numerical Simulation Study on the Dynamic Diffusion Characteristics of Ammonia Leakage in Ship Engine Room
Abstract
:1. Introduction
2. Numerical Model
2.1. Mathematical Model
- (1)
- Continuity Equation
- (2)
- Momentum Conservation Equations
- (3)
- Species Transport Equation
- (4)
- Energy Equation
- (5)
- Turbulence Model
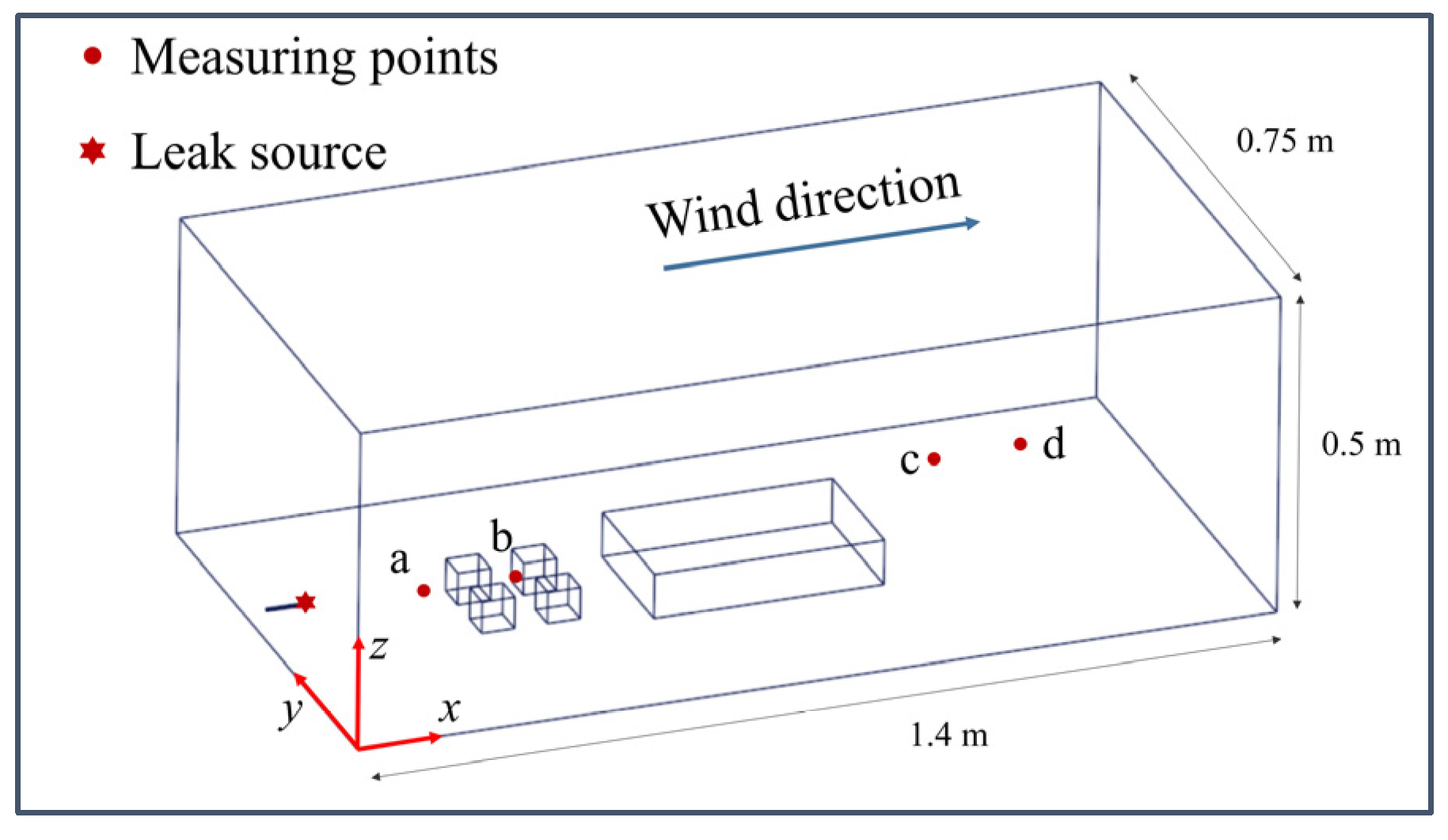
2.2. Computational Domain and Boundary Conditions
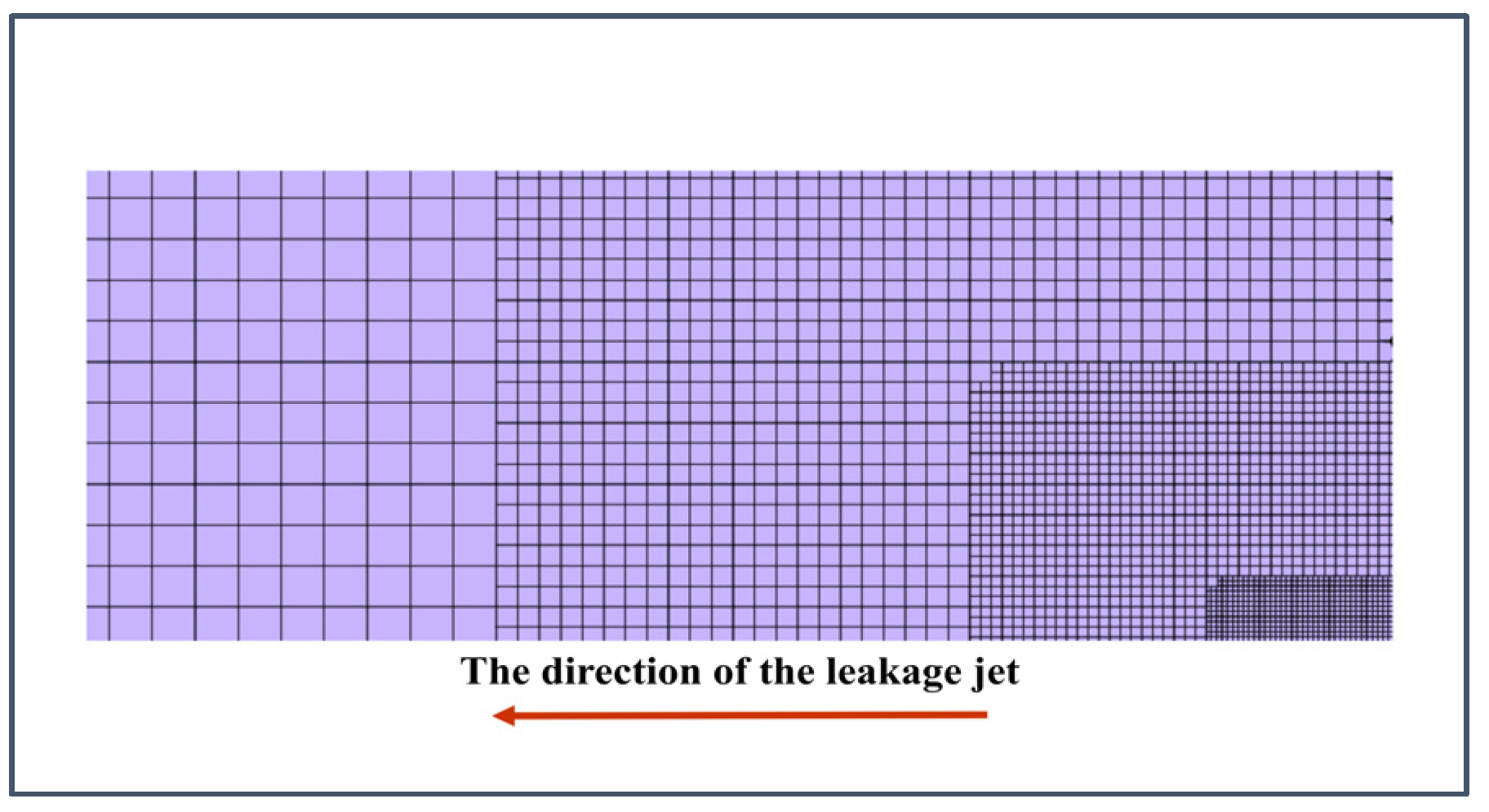
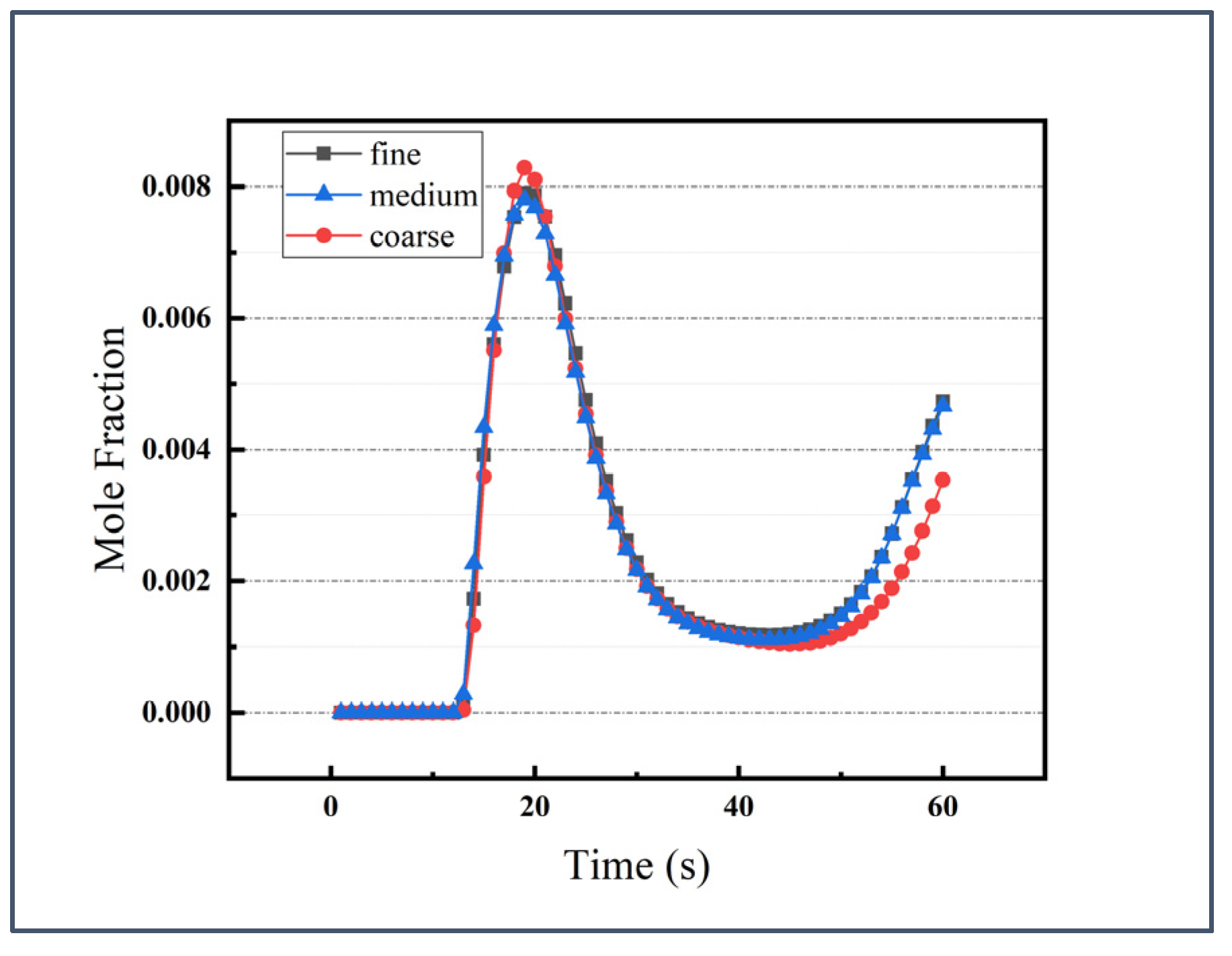
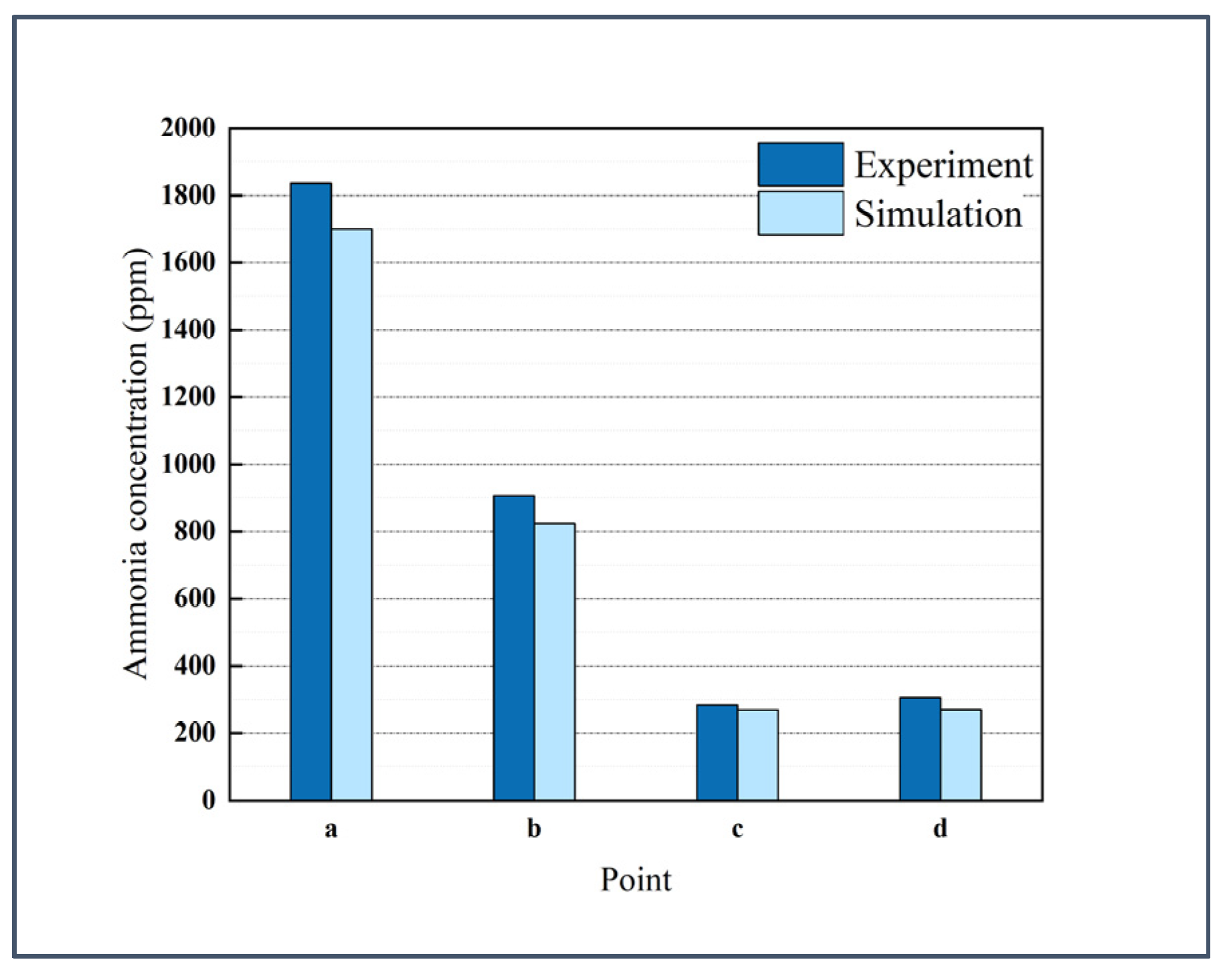
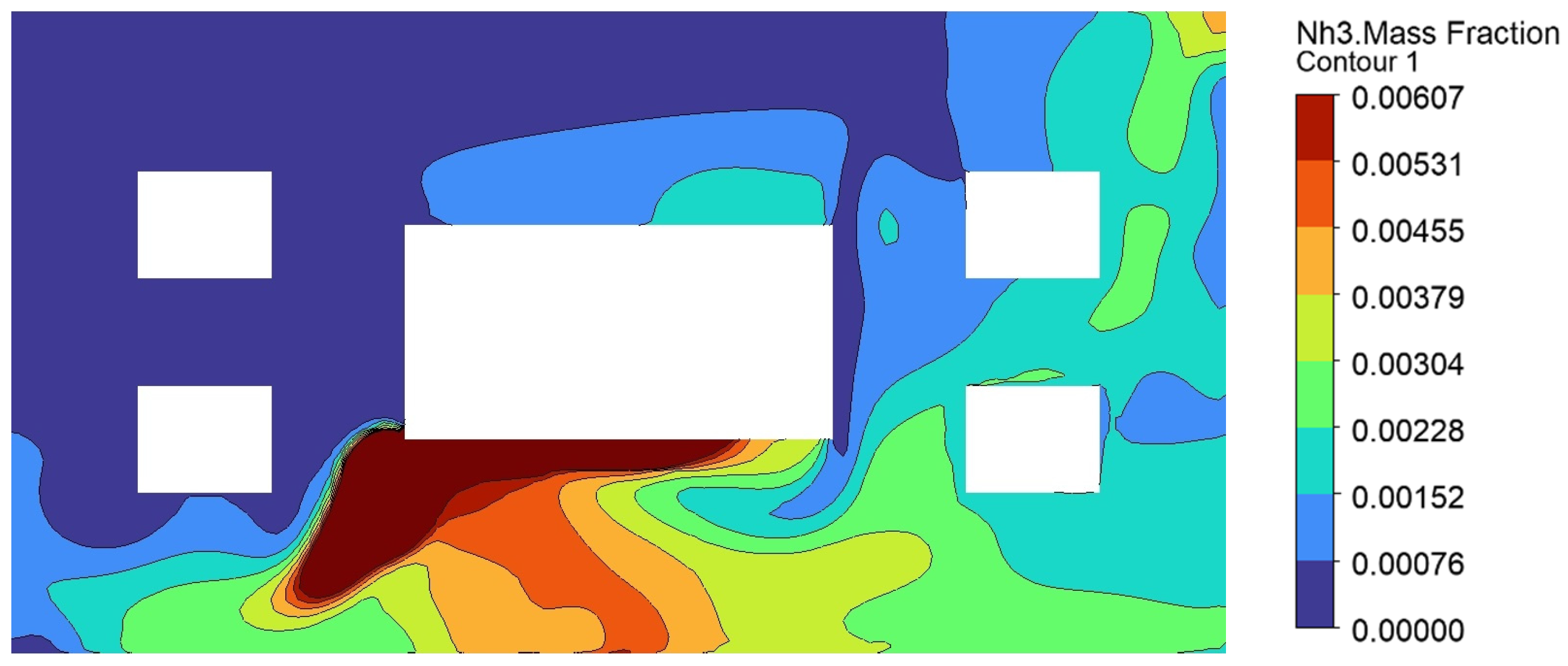
2.3. Grid Independence Verification and Model Validation
3. Results and Discussion
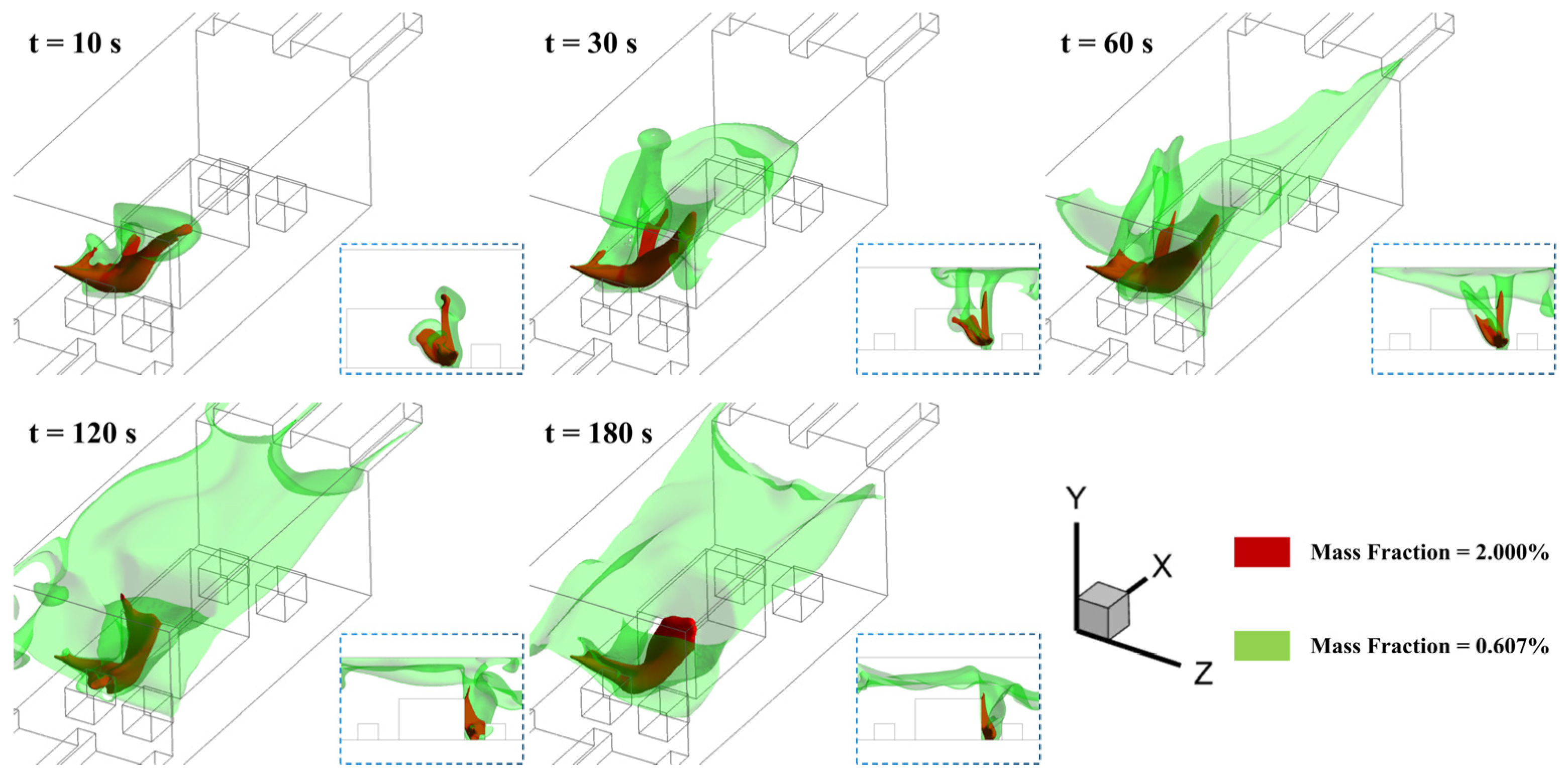
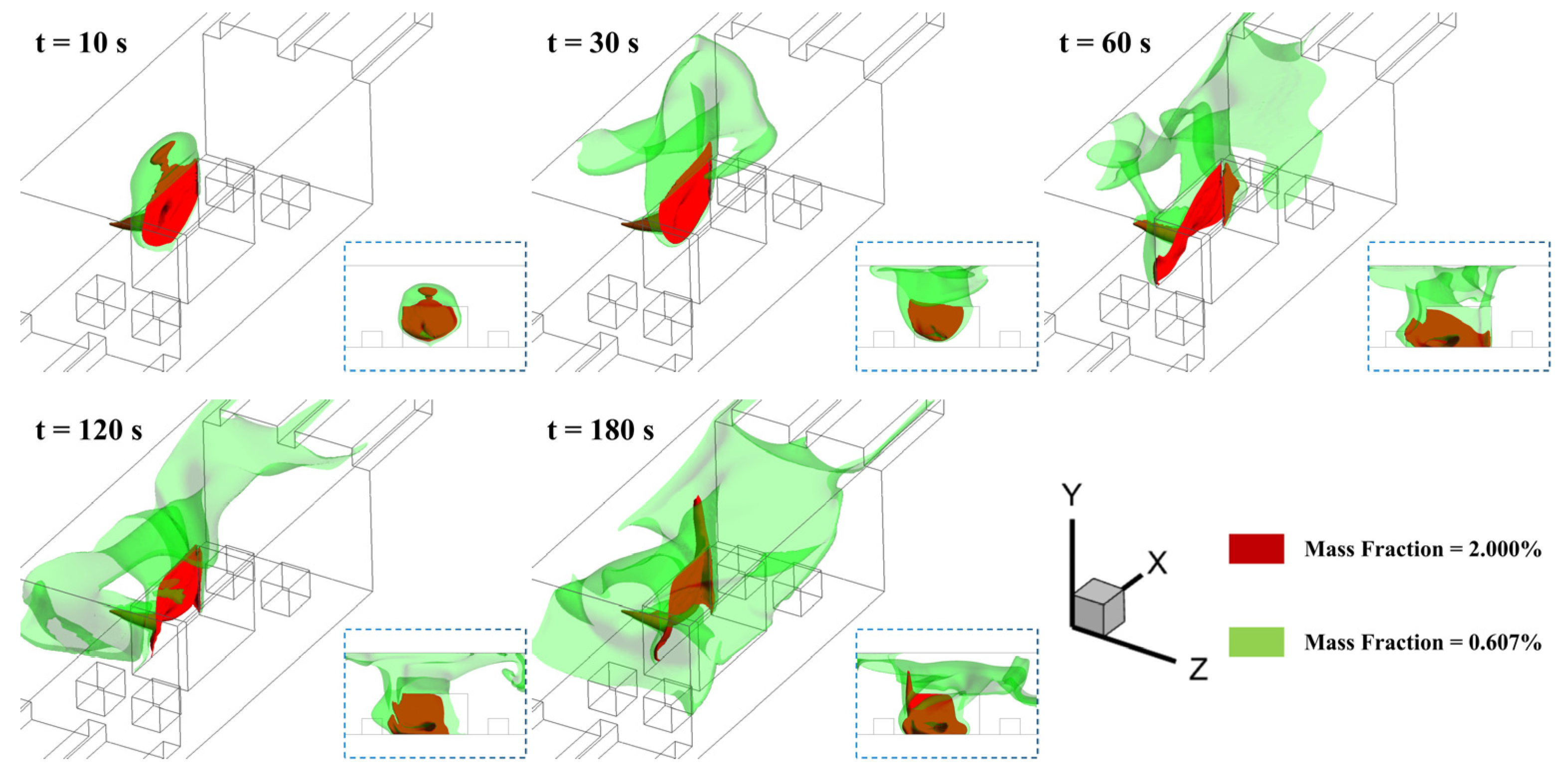
3.1. Effect of Leakage Location
3.2. Effect of Leakage Rate
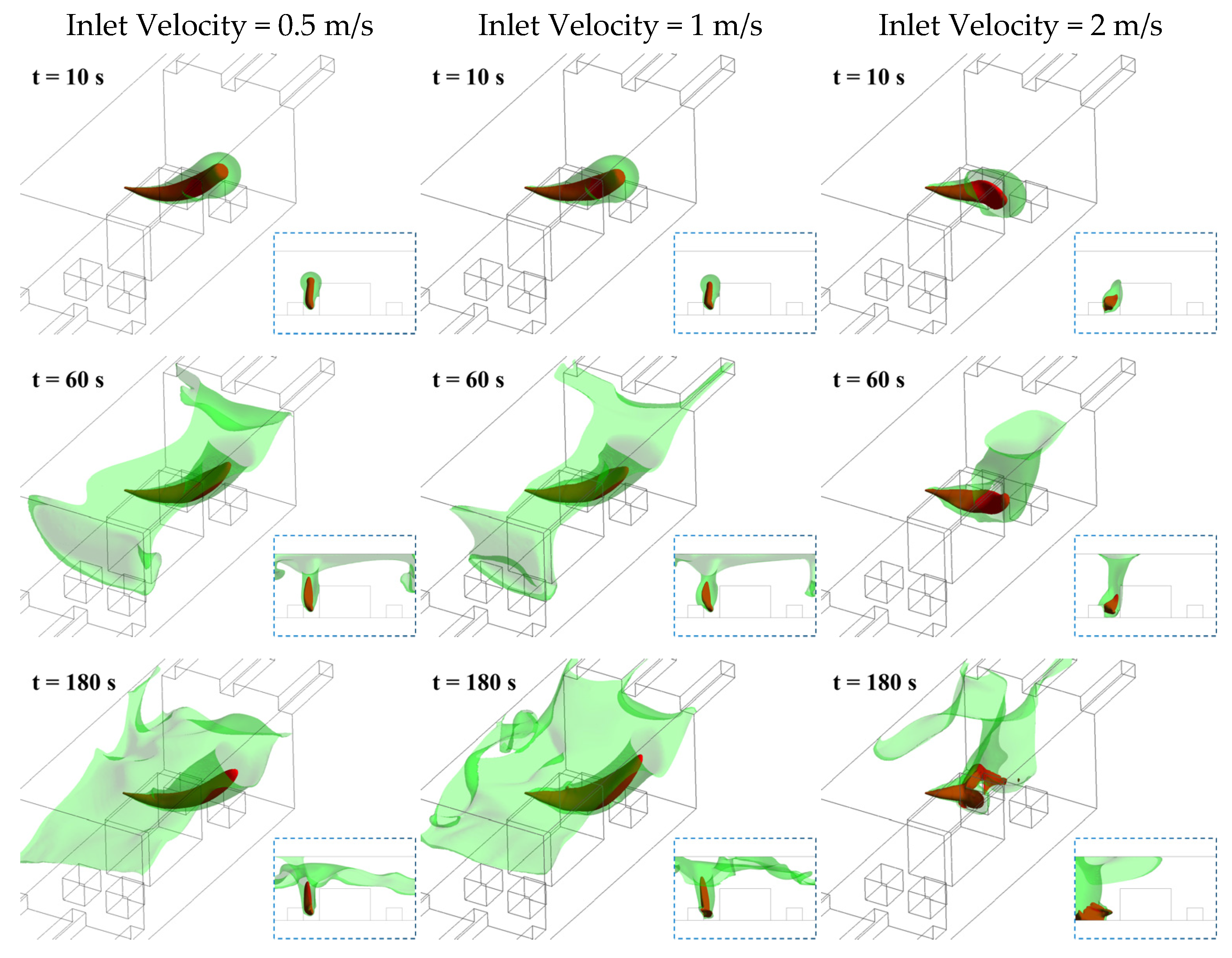
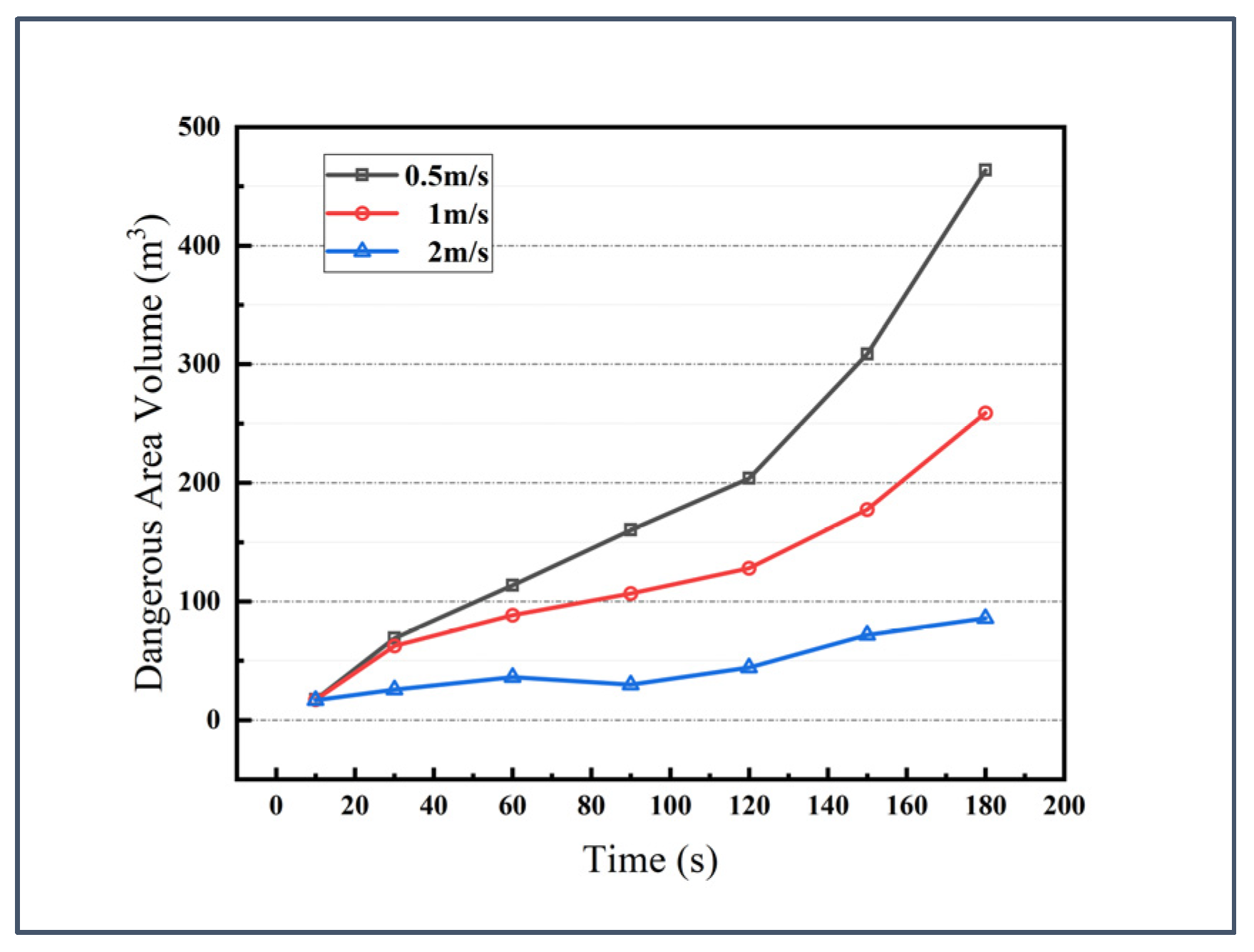
3.3. Effect of Ventilation Rate
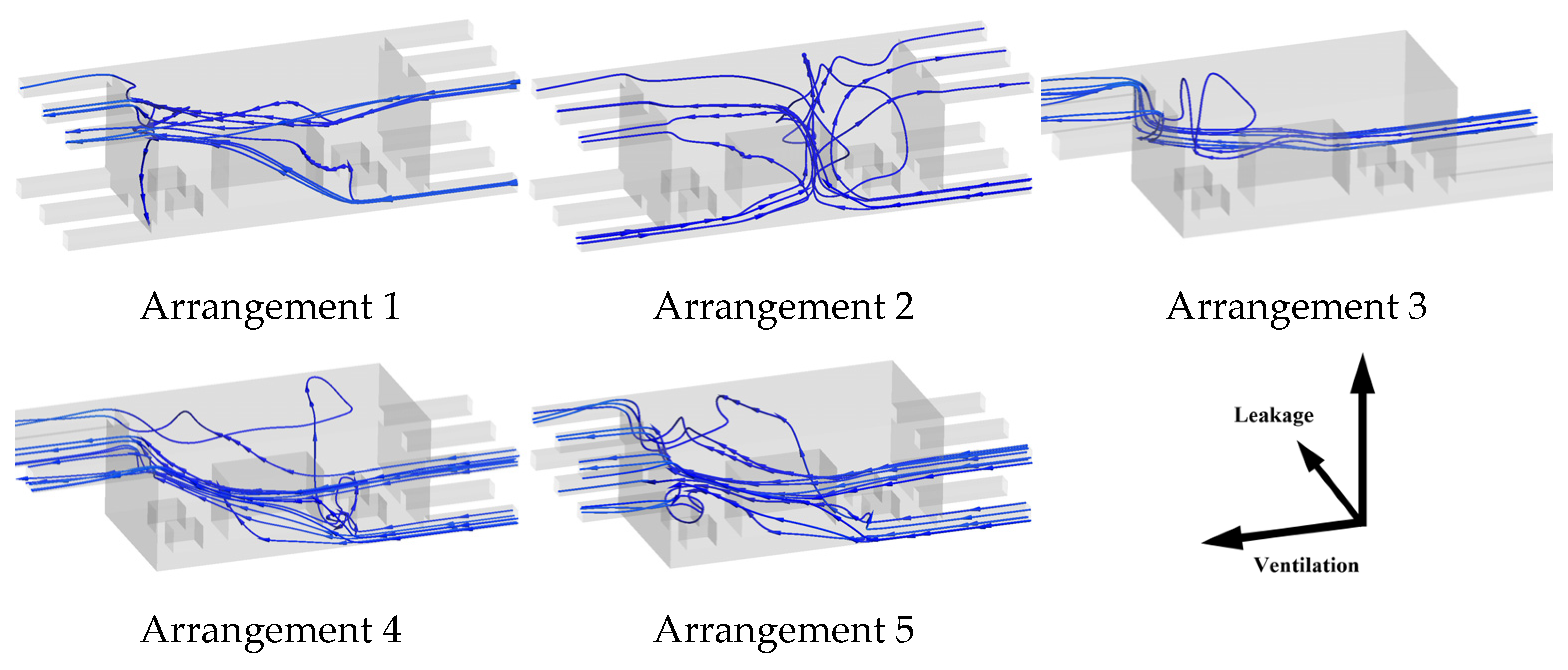
3.4. Adjustment of Ventilation Configuration
- A1 enhances horizontal ventilation in near-floor zones while maintaining ceiling ventilation;
- A2 adopts bilateral floor inlets and bilateral ceiling exhaust vents to establish upward airflow, promoting ammonia ascent and intensifying upper-space exhaust;
- A3 increases the cross-sectional area of all ventilation openings compared to the original design;
- A4 relocates a portion of the inlet area to the mid-upper cabin to strengthen top-space inflow, building on A3;
- A5 further shifts part of the exhaust area downward from A4.
- (1)
- The momentum carried by the inlet airflow from both sides at the base suppresses the lateral diffusion of ammonia in the near-surface space;
- (2)
- Upward airflow accelerates vertical dispersion;
- (3)
- Dual ceiling exhaust vents enable localized ammonia removal, minimizing ceiling accumulation.
4. Conclusions
- The horizontal ammonia dispersion process in confined engine rooms comprises three distinct phases: (1) jet phase dominated by initial momentum; (2) buoyancy-dominated ascent phase; and (3) downward dispersion phase driven by concentration gradients after the cloud occupies the ceiling space;
- Jets at different leakage locations generate varying degrees of near-floor high-concentration accumulation due to obstacle distributions and ventilation airflow. Obstacle-induced turbulence intensifies near-wall interactions, resulting in the largest high-concentration zones when jets directly impact walls;
- When leakage rates exceed the ventilation system’s capacity, the growth rate of hazardous zone volume increases disproportionately relative to the leakage rate;
- During the initial 10 s of leakage, the hazardous zone volumes show negligible variation across different wind speeds. Increasing the airflow significantly enhances ventilation efficiency—at the 180 s mark, the hazardous zone volume at 2 m/s inlet wind speed is 65% smaller than that at 1 m/s. However, this accelerated ventilation may paradoxically intensify other risks. Elevated ammonia concentration accumulation near leakage sources and enhanced downward dispersion of ceiling-level gas clouds. Both mechanisms could expand immediately lethal zones in near-ground areas. Furthermore, considering the cubic proportionality between fan power consumption and rotational speed, coupled with accelerated wear of ventilation components under high-speed operation, these factors may collectively compromise the vessel’s operational economy;
- Compared to horizontal ventilation enhancements, a bilateral inlet–outlet configuration (inlets on opposite floor sides, exhausts on opposite ceiling sides) reduces hazardous zone volume by 50% and minimizes near-floor hazards. For horizontal layouts, optimal performance requires maximizing vent opening areas, positioning inlets as low as possible and exhausts as high as possible. Optimizing ventilation layout design can significantly enhance near-ground ventilation efficiency without requiring substantial increases in fan rotational speed. This approach demonstrates superior feasibility and merits priority consideration in engineering implementations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IMO. Marine Environment Protection Committee (MEPC 80), 3–7 July 2023. Available online: https://www.imo.org/en/MediaCentre/MeetingSummaries/Pages/MEPC-80.aspx (accessed on 18 March 2025).
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to Decarbonise International Shipping: Options for Fuels, Technologies and Policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Guo, H.; Xue, S.; Wang, X.; Wang, Z.; Chen, T.; Yang, L.; Zeng, X.; Su, P. Technical Requirements for 2023 IMO GHG Strategy. Sustainability 2024, 16, 2766. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Roh, G.; Lee, S.; Choung, C.; Kang, H. Comparative Life Cycle Assessments and Economic Analyses of Alternative Marine Fuels: Insights for Practical Strategies. Sustainability 2024, 16, 2114. [Google Scholar] [CrossRef]
- Braun, A.; Gierenz, N.; Braun, S.; Kubach, H.; Bernhardt, S.; Prehn, S.; Müller, M.; Engelmeier, L.; Fehlemann, L.; Steffen, M.; et al. Aspects of Ammonia as Green Fuel for Propulsion Systems of Inland Water Vessels. Energy Technol. 2024, 13, 2301648. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, J. Preliminary Assessment of the Potential for Rapid Combustion of Pure Ammonia in Engine Cylinders Using the Multiple Spark Ignition Strategy. Int. J. Hydrogen Energy 2024, 55, 375–385. [Google Scholar] [CrossRef]
- Park, C.; Jang, I.; Lee, J.; Kim, M.; Park, C.; Kim, Y.; Choi, Y. Effect of a Compression Ratio Increase and High-Flow-Rate Injection on the Combustion Characteristics of an Ammonia Direct Injection Spark-Ignited Engine. J. Mar. Sci. Eng. 2025, 13, 268. [Google Scholar] [CrossRef]
- Al-Aboosi, F.Y.; El-Halwagi, M.M.; Moore, M.; Nielsen, R.B. Renewable Ammonia as an Alternative Fuel for the Shipping Industry. Curr. Opin. Chem. Eng. 2021, 31, 100670. [Google Scholar] [CrossRef]
- Chen, X.; Hu, R.; Luo, K.; Wu, H.; Biancardo, S.A.; Zheng, Y.; Xian, J. Intelligent Ship Route Planning via an A* Search Model Enhanced Double-Deep Q-Network. Ocean. Eng. 2025, 327, 120956. [Google Scholar] [CrossRef]
- Dong, T.; Alamoush, A.; Schönborn, A.; Masodzadeh, P.G.; Park, C.; Park, H.-S.; Vakili, S.; Bilgili, L.; Ballini, F.; Ölcer, A.I. Roadmap for the Decarbonization of Domestic Passenger Ferries in the Republic of Korea. Sustainability 2025, 17, 1545. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Zhang, Y.; Wang, Z.; Song, Z.; Zan, G.; Wang, Z.; Guo, H.; Zhang, H.; Zhu, J.; et al. Economic Assessment of Maritime Fuel Transformation for GHG Reduction in the International Shipping Sector. Sustainability 2024, 16, 10605. [Google Scholar] [CrossRef]
- Ejder, E.; Arslanoğlu, Y. Evaluation of Ammonia Fueled Engine for a Bulk Carrier in Marine Decarbonization Pathways. J. Clean. Prod. 2022, 379, 134688. [Google Scholar] [CrossRef]
- Otsason, R.; Laasma, A.; Gülmez, Y.; Kotta, J. Ulla Tapaninen Comparative Analysis of the Alternative Energy: Case of Reducing GHG Emissions of Estonian Pilot Fleet. J. Mar. Sci. Eng. 2025, 13, 305. [Google Scholar] [CrossRef]
- Jang, H.; Mujeeb-Ahmed, M.P.; Wang, H.; Park, C.; Hwang, I.; Jeong, B.; Zhou, P.; Mickeviciene, R. Regulatory Gap Analysis for Risk Assessment of Ammonia-Fuelled Ships. Ocean. Eng. 2023, 287, 115751. [Google Scholar] [CrossRef]
- China CDC. Technical Plan for Health Emergency Response to Acute Ammonia Poisoning Incidents. Available online: https://niohp.chinacdc.cn/zdyyjzt/zdyajjsfa/201309/P020130910594811572280.pdf (accessed on 18 March 2025).
- Hu, L.; Niu, J.; Wu, W.; Zhao, Z.; Du, M.; Chen, L.; Zheng, Q.; Cao, H. Quantitative Analysis of Toxicity Risks in the Operation of Ammonia-Fueled Tugboats. Ocean. Eng. 2024, 310, 118759. [Google Scholar] [CrossRef]
- Joseph Sekhar, S.; Samuel, M.S.; Glivin, G.; Le, T.; Mathimani, T. Production and Utilization of Green Ammonia for Decarbonizing the Energy Sector with a Discrete Focus on Sustainable Development Goals and Environmental Impact and Technical Hurdles. Fuel 2024, 360, 130626. [Google Scholar] [CrossRef]
- Wong, A.Y.H.; Selin, N.E.; Eastham, S.D.; Mounaïm-Rousselle, C.; Zhang, Y.; Allroggen, F. Climate and Air Quality Impact of Using Ammonia as an Alternative Shipping Fuel. Environ. Res. Lett. 2024, 19, 084002. [Google Scholar] [CrossRef]
- Theobald, M.R.; Sanz-Cobena, A.; Vallejo, A.; Sutton, M.A. Suitability and Uncertainty of Two Models for the Simulation of Ammonia Dispersion from a Pig Farm Located in an Area with Frequent Calm Conditions. Atmos. Environ. 2015, 102, 167–175. [Google Scholar] [CrossRef]
- Scotto di Perta, E.; Agizza, M.A.; Sorrentino, G.; Boccia, L.; Pindozzi, S. Study of Aerodynamic Performances of Different Wind Tunnel Configurations and Air Inlet Velocities, Using Computational Fluid Dynamics (CFD). Comput. Electron. Agric. 2016, 125, 137–148. [Google Scholar] [CrossRef]
- Nielsen, M.; Ott, S.; Jørgensen, H.E.; Bengtsson, R.; Nyrén, K.; Winter, S.; Ride, D.; Jones, C. Field Experiments with Dispersion of Pressure Liquefied Ammonia. J. Hazard. Mater. 1998, 56, 59–105. [Google Scholar] [CrossRef]
- Hanna, S.; Britter, R.; Argenta, E.; Chang, J. The Jack Rabbit Chlorine Release Experiments: Implications of Dense Gas Removal from a Depression and Downwind Concentrations. J. Hazard. Mater. 2012, 213–214, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.J. Comparison of Chlorine and Ammonia Concentration Field Trial Data with Calculated Results from a Gaussian Atmospheric Transport and Dispersion Model. J. Hazard. Mater. 2013, 254–255, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Dharmavaram, S.; Carroll, M.J.; Lutostansky, E.M.; McCormack, D.; Chester, A.D. Allason Red Squirrel Tests: Air Products’ Ammonia Field Experiments. Process Saf. Prog. 2023, 42, 481–498. [Google Scholar] [CrossRef]
- Labovský, J.; Jelemenský, Ľ. CFD Simulations of Ammonia Dispersion Using “Dynamic” Boundary Conditions. Process Saf. Environ. Prot. 2010, 88, 243–252. [Google Scholar] [CrossRef]
- Galeev, A.D.; Salin, A.A.; Ponikarov, S.I. Consequence Analysis of Aqueous Ammonia Spill Using Computational Fluid Dynamics. J. Loss Prev. Process Ind. 2013, 26, 628–638. [Google Scholar] [CrossRef]
- Ohba, R.; Kouchi, A.; Hara, T.; Vieillard, V.; Nedelka, D. Validation of Heavy and Light Gas Dispersion Models for the Safety Analysis of LNG Tank. J. Loss Prev. Process Ind. 2004, 17, 325–337. [Google Scholar] [CrossRef]
- Tan, W.; Du, H.; Liu, L.; Su, T.; Liu, X. Experimental and Numerical Study of Ammonia Leakage and Dispersion in a Food Factory. J. Loss Prev. Process Ind. 2017, 47, 129–139. [Google Scholar] [CrossRef]
- Tan, W.; Lv, D.; Guo, X.; Du, H.; Liu, L.; Wang, Y. Accident Consequence Calculation of Ammonia Dispersion in Factory Area. J. Loss Prev. Process Ind. 2020, 67, 104271. [Google Scholar] [CrossRef]
- Yadav, A.; Jeong, B. Safety Evaluation of Using Ammonia as Marine Fuel by Analysing Gas Dispersion in a Ship Engine Room Using CFD. J. Int. Marit. Saf. Environ. Aff. Shipp. 2022, 6, 99–116. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Hao, Z.; Xu, Z.; Qin, J.; Zhu, J. Numerical Simulation and Experimental Study of Gas Diffusion in a Ship Engine Room. Ocean Eng. 2023, 271, 113638. [Google Scholar] [CrossRef]
- Mei, Y.; Shuai, J. Research on Natural Gas Leakage and Diffusion Characteristics in Enclosed Building Layout. Process Saf. Environ. Prot. 2022, 161, 247–262. [Google Scholar] [CrossRef]
- Gao, Q. A Study on the Simulation of Ammonia Leakage and Diffusion Based on CFD. Master’s Thesis, University of Petroleum, Beijing, China, 2017. [Google Scholar]











| Symbol | Meaning | Unit |
|---|---|---|
| τ | Viscous stress | Pa |
| F | Body forces | N |
| ρ | Density | kg/m3 |
| t | Time | s |
| u | Velocity vector | m/s |
| p | Pressure | Pa |
| Cs | Volume concentration | % |
| Ds | Diffusion coefficient | m2/s |
| E | Total energy | J |
| hi | Specific enthalpy of substance i | J/kg |
| ji | Diffusion flux of substance i | kg/(m2·s) |
| k | Turbulent kinetic energy | m2/s2 |
| μ | Molecular viscosity coefficient | kg/(m·s) |
| μt | Turbulent viscosity coefficient | kg/(m·s) |
| σk | Turbulent kinetic energy diffusion constant | - |
| Gb | Turbulent kinetic energy generation term due to buoyancy | kg/(m·s3) |
| Gk | Turbulent kinetic energy generation term due to mean velocity gradient | kg/(m·s3) |
| ε | Turbulent kinetic energy dissipation rate | m2/s3 |
| σε | Constant related to the diffusion of turbulent kinetic energy dissipation rate | - |
| C1 | Constant of the generation term of the turbulent kinetic energy dissipation rate | - |
| C2 | Constant of the dissipation term of the turbulent kinetic energy dissipation rate | - |
| C1ε | Constant of the buoyancy effect term on the turbulent kinetic energy dissipation rate | - |
| Option | Value |
|---|---|
| Air inlet | Velocity-inlet |
| Ammonia inlet | Mass-flow-inlet |
| Outlet | Outflow |
| Temperature | 35 °C |
| Method | SIMPLEC |
| Residual | 10−5 |
| Time Step | 0.05 s |
| Point | Location (m) | ||
|---|---|---|---|
| x | y | z | |
| leak source | 0.05 | 0.375 | 0.05 |
| a | 0.35 | 0.375 | 0.05 |
| b | 0.475 | 0.375 | 0.05 |
| c | 1.1 | 0.45 | 0.05 |
| d | 1.3 | 0.445 | 0.05 |
| Point | a | b | c | d |
|---|---|---|---|---|
| Deviation | −7.35% | −9.16% | −5.28% | −11.76% |
| Variables as Research Subjects | Parameter Settings | ||
|---|---|---|---|
| Leakage Location | Leakage Rate (kg/s) | Ventilation Rate (m/s) | |
| Leakage Location | A, B, C | 0.05 | 1 |
| Leakage Rate | A | 0.025, 0.05, 0.075, 0.1 | 1 |
| Ventilation Rate | C | 0.05 | 0.5, 1, 2 |
| Ventilation Configuration | A | 0.05 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, G.; Sun, B.; Li, J.; Sun, Y. Numerical Simulation Study on the Dynamic Diffusion Characteristics of Ammonia Leakage in Ship Engine Room. Sustainability 2025, 17, 3826. https://doi.org/10.3390/su17093826
Liu X, Yang G, Sun B, Li J, Sun Y. Numerical Simulation Study on the Dynamic Diffusion Characteristics of Ammonia Leakage in Ship Engine Room. Sustainability. 2025; 17(9):3826. https://doi.org/10.3390/su17093826
Chicago/Turabian StyleLiu, Xinyu, Guogang Yang, Baixun Sun, Jihui Li, and Yinhui Sun. 2025. "Numerical Simulation Study on the Dynamic Diffusion Characteristics of Ammonia Leakage in Ship Engine Room" Sustainability 17, no. 9: 3826. https://doi.org/10.3390/su17093826
APA StyleLiu, X., Yang, G., Sun, B., Li, J., & Sun, Y. (2025). Numerical Simulation Study on the Dynamic Diffusion Characteristics of Ammonia Leakage in Ship Engine Room. Sustainability, 17(9), 3826. https://doi.org/10.3390/su17093826





