Abstract
Landsat images from 1979, 1988, 1999, 2008, and 2013 were used to analyze the landscape area change of salt pans lying on the coast of Tianjin. While initially (1979–1988), the area of Tianjin’s salt pan increased, later (1988–2013) it declined dramatically. In the first phase (1979–1988) of the studied period the primary roll-in landscape of the salt pan wasbarren land with an area of 60.0 km2. By 1988, the area of Tianjin’s salt pan rose to 457.8 km2. The main roll-out landscape of the salt pan during 1988–2013 was urban, barren land, village/town, harbor, and road whose area amounted to 69.8, 35.9, 27.3, 25.5 and 18.4 km2 respectively. The roll-out barren land will be transformed to construction land ultimately. By 2013, the total loss reached 167.3 km2, which was 36.5% of the salt pan area of Tianjin in 1988. With the development of coastal economy, the salterns with a lower economic value were transformed to and replaced by land use types with a higher economic value. This trend would influence the production of sea salt and the development of sodium hydroxide and sodium carbonate industries. Seawater desalination provides an opportunity for the restoration and compensation of salt production capacity. Based on the theory of circular economy and industrial symbiosis, in this article an industrial symbiosis model for sea salt production and sea water desalination is explored: “mariculture–power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical industry”. Through the application of this process sustainable development of the sea salt production in Tianjin could be achieved.
1. Introduction
With the development of its economy, China is being faced with an increasing number of severe problems with resource availability and other environmental and ecological issues. The adjustment of the economic growth to environmental protection in order to achieve sustainable development is an urgent and important concern for the Chinese government.
Salt is needed for human health maintenance; furthermore, it is also a development determining factor as a raw material utilized by the chemical industry in processes, such as the production of sodium hydroxide and sodium carbonate. In general, this substance plays an exceedingly important role, and its stable provision serves as a guarantee and implies normal functioning of the social system. In China sea salt is the most important salt type and represents the largest production portion [1]. Of all salterns in China, Changlu, lying on the Tianjin and Hebei coast, is the greatest one because of its maximum production capacity.
The main facility for sea salt production is the salt pan, one of the dominant coast landscape types in China. Sea salt production needs a large area of coastal mudflat for the construction of evaporation ponds with different salinity levels and crystallization ponds. In this multi-pond system, water evaporates and salinity increases from the normal level of seawater up to the sodium chloride saturation point. The concentrated water is pumped or directed by gravity to the next pond, until it ultimately crystallizes and precipitates in the crystallization pond [2,3].
The instability of sea salt production would affect the sustainable development of the salt chemical industry, which is in the downstream of sea salt production. Along with the economic development and the decline of the comparative benefits of salterns all over the world, there is a trend towards a gradual loss of the saltern landscape and the transformation of its land utilization to other land use types with comparatively higher economic benefits per unit area, such as fishery and tourism [4,5,6]. This tendency is obvious in China and is caused by its high-speed economic growth. Moreover, the non-agricultural nature of the saltern land additionally aggravates its loss. In Tianjin, this trend is even more drastic because of national policy guidance. On 9 November 2009, the State Council agreed that Tianjin City can adjust its administrative regionalization, integrating the whole District of Tanggu, Hangu, Dagang, and Wuxia Street in Dongli District, and the town of Gegu in Jinnan District into the Binhai New Area. The foundation of Binhai New Area has promoted the economic growth of Tianjin dramatically and will undoubtedly affect the coastal salt pans. So far, large proportions of salt pans in Tianjin Municipality have disappeared [7,8,9], which inevitably will influence the development of the local salt chemical industry. Therefore, finding out a pathway to compensate and restore the production capacity of sea salt, threatened by the rapid economic development, is the focus of our concern.
Is there an answer to this discrepancy? Fortunately, seawater desalination provides a possible solution. The whole world’s seawater desalination capacity was 4.5 × 108 m3/day by the end of 2005, and this technology was widely used in the countries in the Persian Gulf, and by the Mediterranean and Red Sea [10]. One concern related to seawater desalination addresses the disposal of the concentrated saline, resulting from seawater desalination, which could cause serious environmental problems in the marine ecosystem [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. However, because the proportion of the constant chemical elements contained in the concentrated saline from seawater desalination and the seawater concentrated saline are almost the same, the usage of the concentrated liquid obtained from seawater desalination for the production of sea salt can be achieved [27]. The integration of seawater desalination and sea salt production has been reported in China [27,28,29,30,31,32,33,34,35,36] and elsewhere in the world [37,38,39,40,41,42]. Moreover, owing to the higher salt level of the concentrated saline stemming from seawater desalination, which is normally twice that of seawater [12,18], the needed volume of the concentrated saline originating from the desalination is just half that of the seawater necessary to acquire the same quantity of sea salt [27,29]. Thus, the combination of seawater desalination and sea salt production, and driving the concentrated saline from the desalination in salt pans for salt production instead of immediately disposing it to the sea, will generate a win-win situation. In other words, a solution is gained to both the environmental problem caused by the disposal of concentrated saline, resulting from seawater desalination, and the loss of the capacity for sea salt production.
The area change of Tianjin saltern landscape during period 1979–2013 was analyzed through the use of Landsat images of five different periods. Based on the principles of circular economy and industrial symbiosis, this article aims to establish an industrial symbiosis model for seawater desalination and sea salt production in agreement with the local reality of Tianjin for the achievement of their sustainable development as well as that of the related industries in this area.
2. Materials and Method
2.1. Study Area
Tianjin Municipality (longitude ranging from 116°42′5″ to 118°3′31″E, latitude ranging from 38°33′57″ to 40°14′57″N) lies on the northeastern edge of the North China Plain, at the downstream of Haihe River. Bohai Bay is situated on the east of the Municipality, and the coast type of Tianjin is silt. The area of Tianjin Municipality is 11,917.3 km2, and its coast length is 153 km. The coastal area belongs to the Tianjin Binhai New Area, which consists of the three former districts—Hangu, Tanggu, and Dagang, and part of Dongli and Jinan District, namely Wuxia Street in Dongli District, and the town of Gegu in Jinnan District, respectively. The terrain is low and flat, and there are abundant wetland typesin Tianjin Municipality, such as mudflat, marsh, estuary, reservoir, pool, paddy field, and salt pans. The climate is a warm, semi-humid continental monsoon climate, and the average yearly temperature is 12 °C. The annual precipitation is about 500–700 mm, while the annual evaporation capacity is 1750–1840 mm, which is three times that of precipitation. The annual sunshine duration is 2160–3090 h [43,44,45]. The sea salt production is a conventional industry in Tianjin, and there are two large saltworks located in the coastal area of Tianjin Municipality, namely the Hangu and Tanggu saltworks, which are parts of Changlu Saltern. Consideringthat the saltpans all lie in the Hangu, Tanggu, and Dagang Districts, these former three administrative districts have been chosen as the study area (Figure 1).
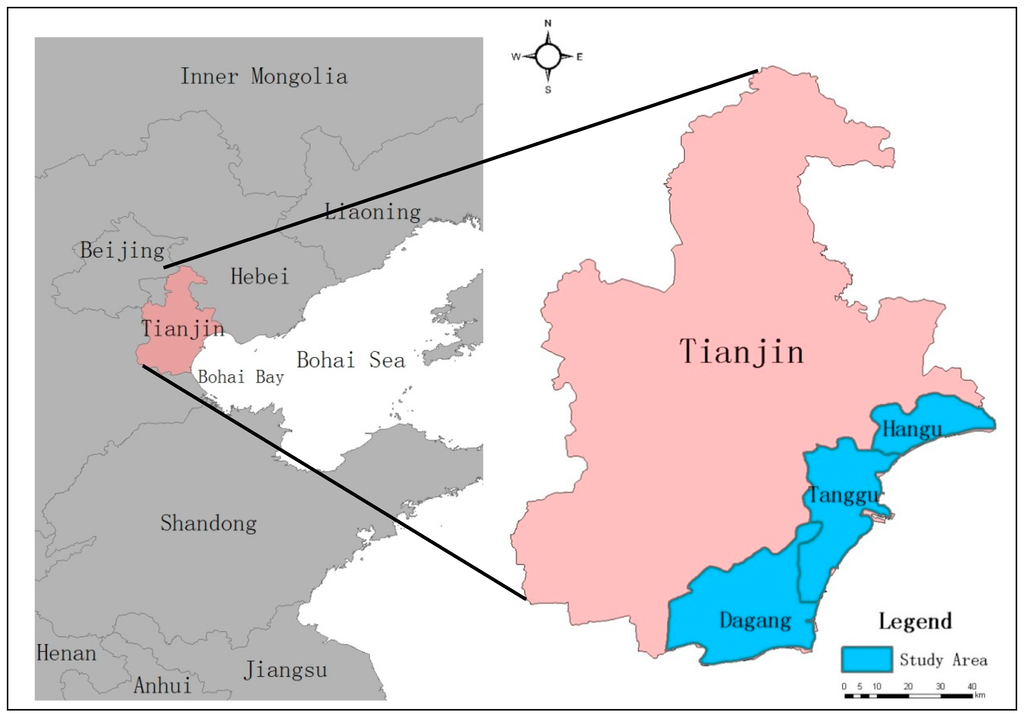
Figure 1.
Location of the study area.
Figure 1.
Location of the study area.
2.2. Data and Methods for Analysis of the Landscape Area Changes of Salt Pans
Based on the remote sensing data of MSS/TM/ETM/OLI in 1979, 1988, 1999, 2008, and 2013, a study was conducted on the landscape area change of the salt pans in the Tianjin Binhai New Area. Most of the satellite images, acquired in summer or autumn, were cloud-free. The UTM zone 50N and WGS 84 datum was chosen as the project standard. ERDAS Imagine (version 9.2) and ArcGIS Desktop (version 9.3) were used for data preparation. Salterns have specific textural features and geographical positions. For example, the sedimentation and evaporation ponds normally have square shapes, whereas the crystallization ponds are bright and white in the Landsat images when there is salt in this kind of pond [46]. So they were distinguished by manual visual interpretation after supervised classification by a maximum likelihood classifier. The landscape type system used is a two-level system, based on the National Land Classification System of China (2001), including seven first-class types (cultivated land, wood land/grass land, settlement, transportation land, saltwater body, fresh water body, and unused land) and 17 second-class types (paddy field, dry land, wood land, grass land, urban land, village/town, road, harbor, salt pan, shrimp farm, river/channel, reservoir/lake, pond, reed bed, barren land, reclamation land, and intertidal beach).
2.3. Bases of Proposing Sustainable Processes for Sea Salt Production with the Related Industries
2.3.1. Principles of Circular Economy and Industrial Symbiosis
Based on the principles of circular economy and industrial symbiosis, the designed combination of seawater desalination and sea salt production may be the final measure to tackle the problems faced by both of them.
The concept of circular economy (CE) was first raised by the American economist Boulding in 1960s, and the term of CE was initially used by two British environmental economists Pearce and Turner in 1990s [47]. A circular economy can be defined as an economy type with a closed-loop of material flows, which is opposite to the traditional open-ended economy [47]. The latter economy is a linear development model that results in severe problems such as environmental degradation and resource scarcity [48], whereas CE will help to maintain harmony between humanity and the environment by the closed-loop utilization of input material. The essence of CE is a “resource–production–waste–renewable resource” material flow model which features “low exploitation, high exploiting and low effluents” [49]. The principles of CE can be concluded as a reduction, reuse, and recycling, namely the “3R” [47,48,49]. Reduction refers to minimizing the input of original energy and raw materials through the improvement of production efficiency. Reuse suggests using the by-products and wastes stemming from one firm as resources for other firms or industries. Recycling encourages reprocessing the recyclable materials into new products, so that the consumption of the original materials can be reduced [47]. The implementation of the “3R” principles is always carried out in different corporations and industries, which involves the characteristics of circular economy.
A comprehensive definition of industrial symbiosis (IS) is offered by Chertow: industrial symbiosis unites traditionally separate but geographically close industries as a collective entirety for a competitive advantage through physical exchanges of materials, energy, water, and/or by-products [50]. One of the most paradigmatic examples of IS development is in Kalundborg (Denmark), which has been recurrently used as a model of IS networks in the literature [51]. The key to IS is the exchange of waste and by-products. By exchanging materials and energy among different organizational units, IS networks can reduce the intake of virgin raw materials and the production of waste [52]. This would ultimately alleviate environmental pressure.
In China, the CE strategy has been applied and further developed in various areas, and the enacted 12th five-year plan (2011–2015) for China’s economic and social development suggests the continuous implementation and further development of this strategy [47]. For example, some industries, especially the chemical industry, have already practiced the principles of CE and IS. The corporation of Shandong Haihua, for example, combines the related production processes, such as seawater cultivation, Artemia culture, bromide extraction, sea salt production, and the salt chemical industry into a comprehensive chain by gradually taking advantage of seawater and using the wastes of a process as feeding resources for the next. This material chain of the gradual, multistage use of seawater would obviously benefit the environment because of the reduction in the quantity of original seawater needed and the final waste produced [53]. This circular economy and industrial symbiosis model have also been established in the Shandong Lubei Chemical company in Shandong province [54].
Based on the existing examples, the salt production and the salt-related chemical industry can be united as an IS chain. The relative usage of seawater also includes seawater cultivation, Artemia culture, and bromide extraction. These processes can be added to the “salt-salt chemical” chain to make it a much more comprehensive IS model. In this article, we aim to devise a model, bringing together seawater desalination, sea salt production, and all other types of related usage to better understand the problems faced by them in Tianjin. As a result of the analysis, a gradual material usage chain of seawater has been proposed according to the principles of CE and IS.
2.3.2. Seawater Desalination and Environmental Problems Caused by Brine Disposal
Water scarcity is one of the most critical environmental issues of our time, expected to become an ever-increasing problem in the future. At present, over one third of the world’s population lives in countries without access to adequate water supply [10]. Therefore, we need to find unconventional fresh water sources to alleviate the fresh water scarcity problem, and among all these sources, seawater desalination is deemed as an exceedingly important pathway.
Seawater desalination is a technology for the production of fresh water by removing dissolved salts from seawater [21,55]. The essence of the desalination process is the separation of fresh water from the concentrated brine containing a high level of dissolved salt. Based on the technology used, seawater desalination processes can be divided into two broad categories: thermal evaporation and membrane separation. The thermal approach includes mainly multistage flash (MSF) distillation, multiple-effect distillation (MED), vapor compression distillation (VC), and freezing, whereas the membrane type consists primarily of reverse osmosis (RO) and electro-dialysis (ED) [21,56,57]. At present, the thermal methods are being replaced by membrane separation techniques, whose share in the desalination industry is expanding. However, there is a problem with membrane fouling that limits the continuous spread of this seawater desalination technique. Melián-Martel et al. reported that the chemical constituent causing RO membrane fouling [58] and the cleaning of RO membrane would generate large amounts of acidic wastewater [59]. To overcome this problem, the use of the electro-dialysis reversal (EDR) procedure was initiated and promoted since it could reverse the electrode polarity automatically, and thus had the capacity to backwash the membrane and inhibit membrane fouling [59,60,61,62]. Other methods that are still undergoing research and development include forward osmosis (FO), membrane distillation (MD), capacitance deionization (CDI), gas hydrates (GH), humidification-dehumidification (HDH), and solar stills desalination. Besides, the normally used technologies for pretreatment membrane separation include ultra-filtration (UF), nano-filtration (NF), and ionic filtration (IF) [56]. The most widely used desalination technologies are MSF, MED, and RO, of which MSF and MED are adopted mainly in the Middle East because of the vast amount of fossil energy required to drive the thermal desalination process, whereas RO is the predominant technique employed in the other regions where only electricity is needed [21]. With relative technology innovation, seawater desalination has experienced rapid progress. As a result, this technology has been widely used in the Persian Gulf, Mediterranean, and Red Sea, and its application could also be observed in other regions, such as California (USA) and Australia [10]. The whole world’s seawater desalination capacity was about 4.5 × 108m3/day by the end of 2005 [10], and this figure is expected to increase to 1 × 1011 m3/day in 2016 [63].
Tianjin is located in the downstream of Haihe River, but its per capita water resource is just 160 m3, which is equal to one-fifteenth of the national average [64]. Given this reality, research on seawater desalination started in 1980s, making Tianjin the leader in practicing this technology all over the country. In 1989, a seawater desalination project with MSF leeching on Tianjin Dagang Power Station was implemented to provide 6000 m3/d of fresh water, and its capacity was expanded to 7200 m3/day in 1997 [55]. By the end of 2013, the total seawater desalination capacity of Tianjin amounted to 31.7 × 104 m3/day, accounting for 35% of the whole desalination potential of China, out of which, 21.6 × 104 m3/day was provided by MED, 6000 m3/day through MSF, and 10.1 × 104 m3/day by RO. Moreover, when the planned seawater desalination projects are completed, the magnitude of the whole desalination volume in Tianjin will reach 81.7 × 104 m3/day [65].The operation of all these projects will undoubtedly help alleviate the fresh water shortage situation in Tianjin.
Although there has been much progress in seawater desalination technology in the world, and the success of this technology is obvious, there are still concerns about the environmental impact that would be exerted by their application, including losses of aquatic organisms and their habitats due to the open seawater intake [10,23], decreased concentration of dissolved oxygen [22,23], and the rise in water salinity, heat, and turbidity caused by saline discharge [10,13,15,17,18,22,23,25,26]. Adverse influence is caused also by the greenhouse gas emissions generated during the thermo-electric desalination process [17,19,23], the chemical additives used as antiscalants, antifoulants, corrosion inhibitors, defoamers, chlorine, and acids [10,13,15,16,17,18,21,22,23,24] and their reaction products [16,23], increased level of metals such as copper [10,13,15,16,23]. The leakage of concentrated saline and seawater from the pipes leads to groundwater contamination [17,18,20,24]; in addition, noise pollution is inflicted in the RO process [17,20]. Among these problems resulting from seawater desalination, much attention has been focused on the disposal of the concentrated saline, whose inappropriate treatment would undoubtedly cause severe environmental problems. The most frequently used methods for disposal of concentrated saline include submerged or surface discarding in water bodies (seas, lakes, or rivers); placement at the front or at the end of the wastewater treatment plant; land incorporation or deep well injection; disposal in evaporation ponds, brine concentrators, or the application of zero liquid discharge treatments [21,24]. The most widely utilized approach is the direct discharge into the sea, which causes a series of problems to the ambient seawater body by its influence on the salinity, temperature, turbidity, dissolved oxygen rates, and metal concentration. The release of concentrated saline with a high salt level, normally twice that of seawater [12,18], would result in dramatic ecological degradation, such as a substantial detriment to sea grass, plankton, invertebrates, and fish [10,11,12,13,14,15,22,25,26].
The increase of salinity can lead to a rise of water turbidity, which would prevent the penetration of light, disrupt the photosynthesis process [13], and cause growth retardation, tissue necrosis, and premature senescence of sea grass [14]. Salinity of 40–45 g/L appeared to induce severe mortality of the exposed plants, epifaunal mysids, and echinoderms [14]. After 96-hour exposure to 58‰ brine dilution, ascidians were the most sensitive species with more than 50% mortality, whereas a similar mortality rate was established in echinoids subjected to 85‰ brine dilutions for the same time period. Sea grass photosynthesis declined by 50% after 24-hour exposure to 120‰ brine dilution [66]. The larvae of crustaceans and other invertebrates were more vulnerable to the variations of salinity than the fully developed adults and most of them would not survive at high salinity levels [13]. This phenomenon was also found in fish [10]. Salinity of 55, 60, and 70 g/L has been found to be acutely toxic to juveniles of sea bream and clam, and larval flounders respectively [67]. The concentrated brine was also acutely toxic to developing cuttlefish embryos, and fewer eggs of the giant Australian cuttlefish (Sepia apama) developed to term when exposed to brine with salinity over 45 g/L. Moreover, the surviving individuals in these concentrated liquids displayed behavior degeneration, such as slow response to stimulation and reduced ink-jet defense response [68]. Behavior avoidance was also noticed at salinity of 45 g/L [40]. The high weight of concentrated saline stemming from seawater desalination always causes its sinking to the bottom of sea [13,18,22], which prevents mixing with other water bodies and reduces the organic species and qualities and dissolved oxygen level [18,69]. All these factors lead to mortality of benthic organisms [15,22,70], ultimately resulting in degradation to a salt desert status of the ambient water body [13].
Owing to its features, such as the gradual changes in the bottom level, shallow water depth, sealing sea-basin, low flow velocity, and poor exchange capacity, the Bohai Bay is more sensitive to variations of the salt concentration level than the open water bodies [13,71,72]. Therefore, the unreasonable disposal of concentrated saline stemming from seawater desalination would lead to the extinction of some specific species more easily. With the rapid progress and the bright prospect of seawater desalination in Tianjin, this approach would undoubtedly generate increasingly higher amounts of concentrated saline water in the future, and dealing with its disposal is becoming an intricate problem.
3. Result Analyses
3.1. Coastal Landscape Changes and Salt Pan Area Decrease
The land use/cover of Tianjin Binhai New Area (The coverage does not include Wuxia Street in Dongli District, and the town of Gegu in Jinnan District) in 1979, 1988, 1999, 2008, and 2013 is shown in Figure 2, while the area change of all landscape types in this region during the period 1979–2013 is presented in Table 1.
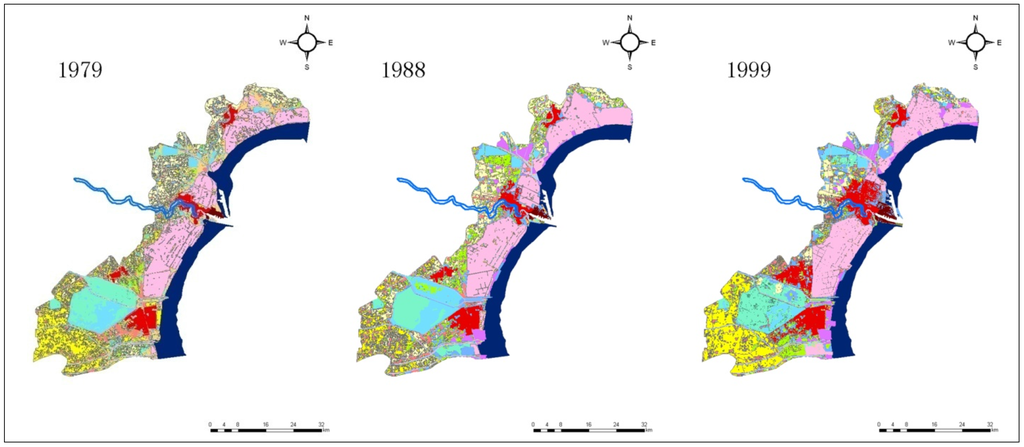
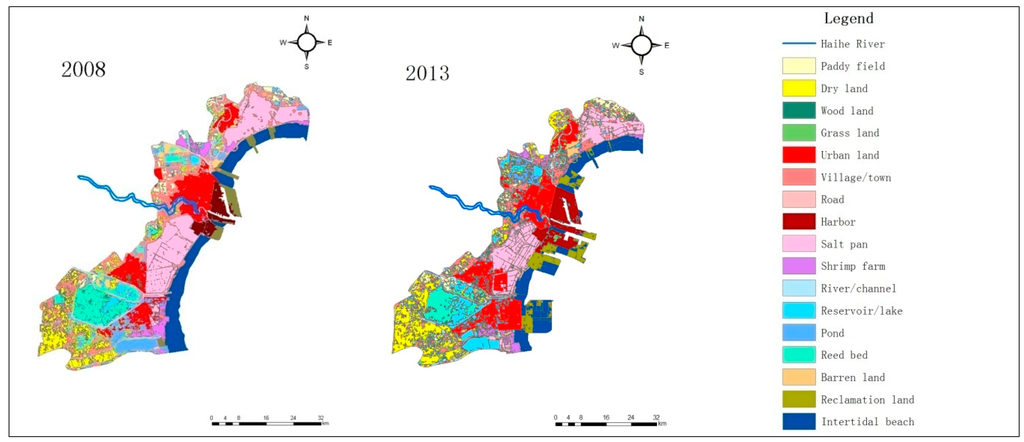
Figure 2.
Coastal landscape changes in Tianjin Binhai New Area during the period 1979–2013. (The coverage does not include Wuxia Street in Dongli District, and the town of Gegu in Jinnan District.)
Figure 2.
Coastal landscape changes in Tianjin Binhai New Area during the period 1979–2013. (The coverage does not include Wuxia Street in Dongli District, and the town of Gegu in Jinnan District.)
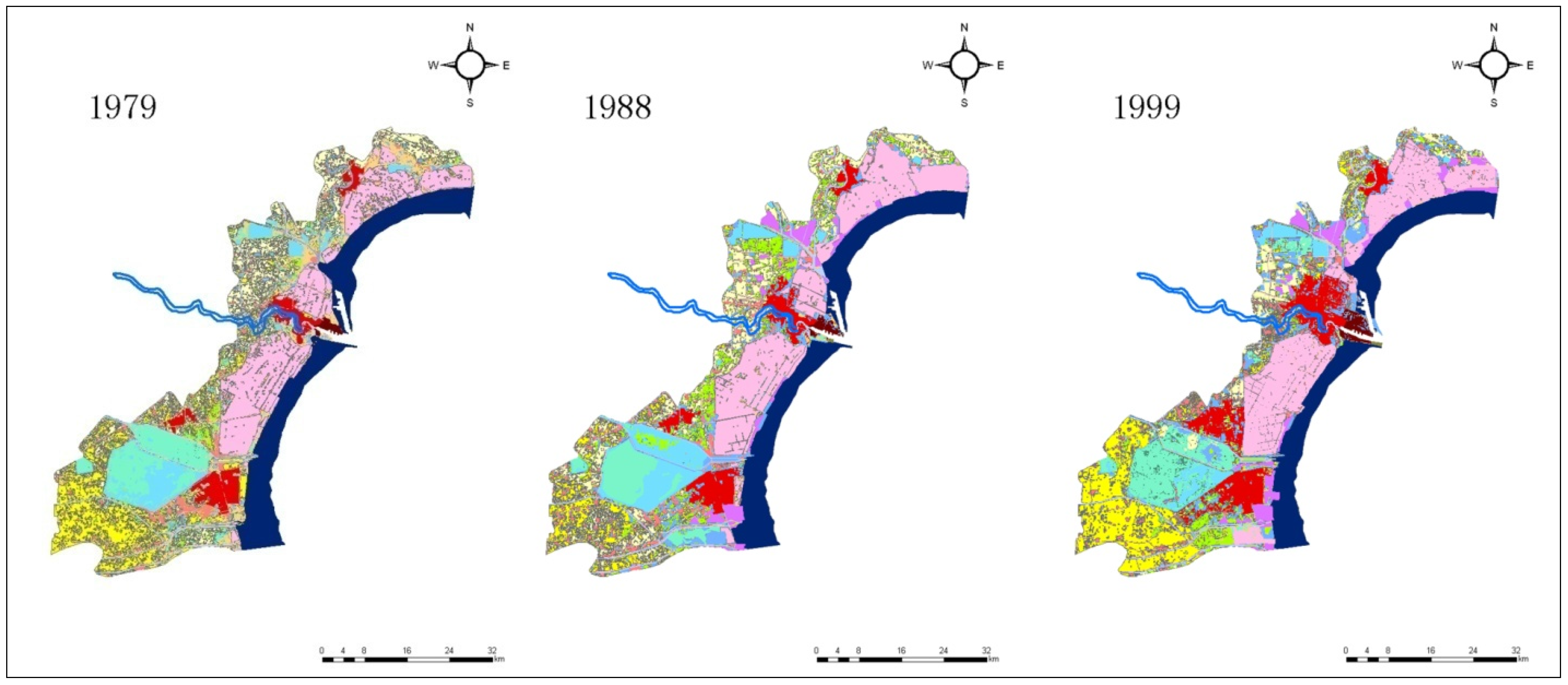
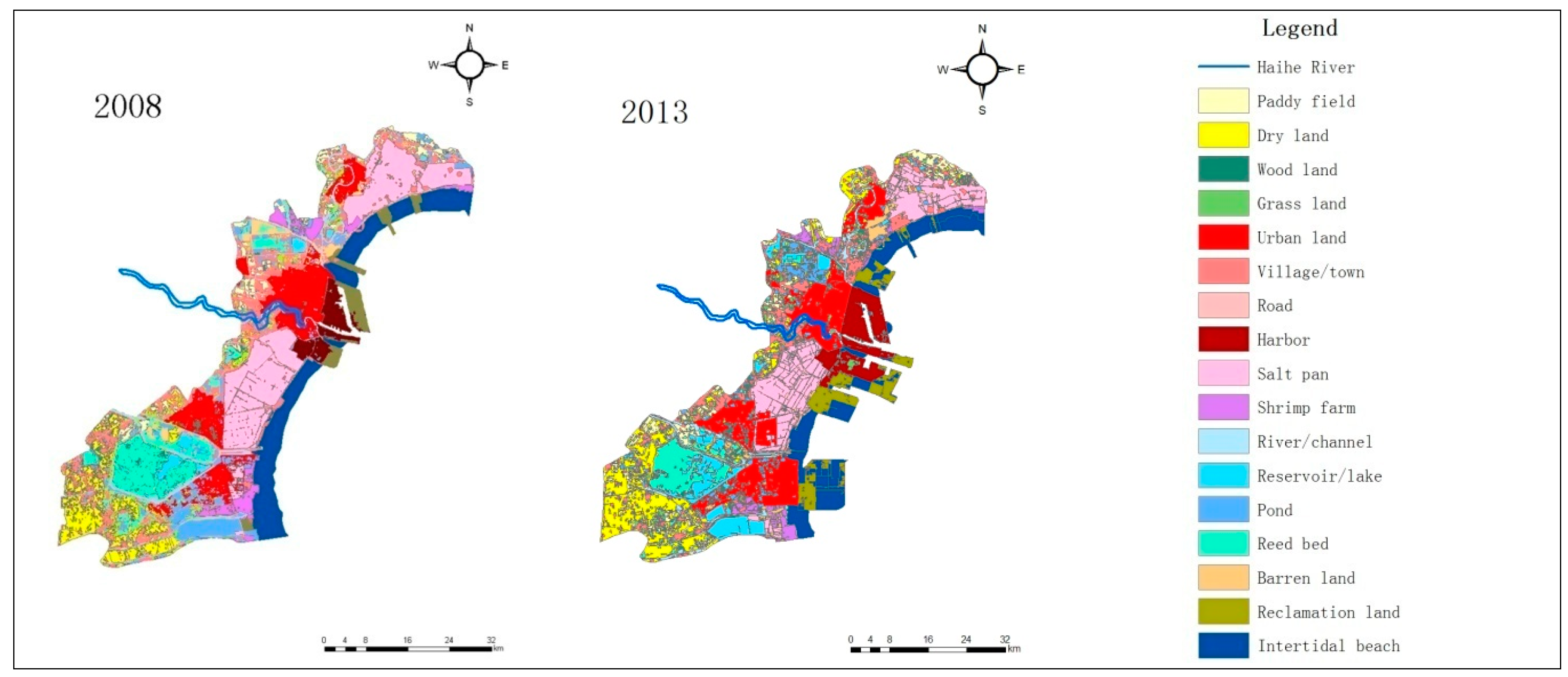

Table 1.
The area change of all landscape types in Tianjin Binhai New Area during the period 1979–2013 (km2).
| Land Use/Cover Type | 1979 | 1988 | 1999 | 2008 | 2013 |
|---|---|---|---|---|---|
| Paddy field | 471.9 | 532.9 | 141.4 | 151.2 | 119.7 |
| Dry land | 443.4 | 203.9 | 423.7 | 246.8 | 283.9 |
| Wood land | 9.1 | 10.6 | 30.8 | 36 | 64.3 |
| Grass land | 86.7 | 124.6 | 82.4 | 56.5 | 87.3 |
| Urban land | 95.8 | 120 | 211 | 279.3 | 369.3 |
| Village/town | 57.8 | 93.3 | 166 | 326 | 213.0 |
| Road | 37.7 | 42.1 | 38.9 | 45.3 | 38.1 |
| Harbor | 8.7 | 11 | 19.4 | 68.7 | 140.0 |
| Salt pan | 391.8 | 457.8 | 401.1 | 365.3 | 290.5 |
| Shrimp farm | 0 | 82.9 | 108.4 | 84.3 | 52.8 |
| River/channel | 70.1 | 97 | 89.4 | 82.6 | 71.1 |
| Reservoir/lake | 108.2 | 151 | 63.5 | 48.5 | 137.6 |
| Pond | 65.5 | 138.2 | 198.8 | 187 | 92.8 |
| Reed bed | 169.6 | 114.3 | 161.9 | 184.1 | 132.0 |
| Barren land | 211.9 | 13.8 | 17.1 | 48.6 | 79.1 |
| Reclamation land | 0 | 0.7 | 1.3 | 64.6 | 126.7 |
| Intertidal beach | 1435.9 | 1286.9 | 1088.8 | 497.3 | 281.1 |
Note: The coverage does not include Wuxia Street in Dongli District, and the town of Gegu in Jinnan District.
As seen in Figure 2 and Table 1, the area of the salt pans increased from 1979 to 1988, and then decreased considerably. In 1979, the total salt pan area of Tianjin was about 391.8 km2, whereas this figure was about 457.8 km2 in 1988, accounting for a rise by 66 km2. From 1988 onwards, there was a decline, and in 2013, this area was only 290.5 km2, while the total loss of salt pan zones reached 167.3 km2, which was 36.5% of Tianjin’s total salt pan territory in 1988.
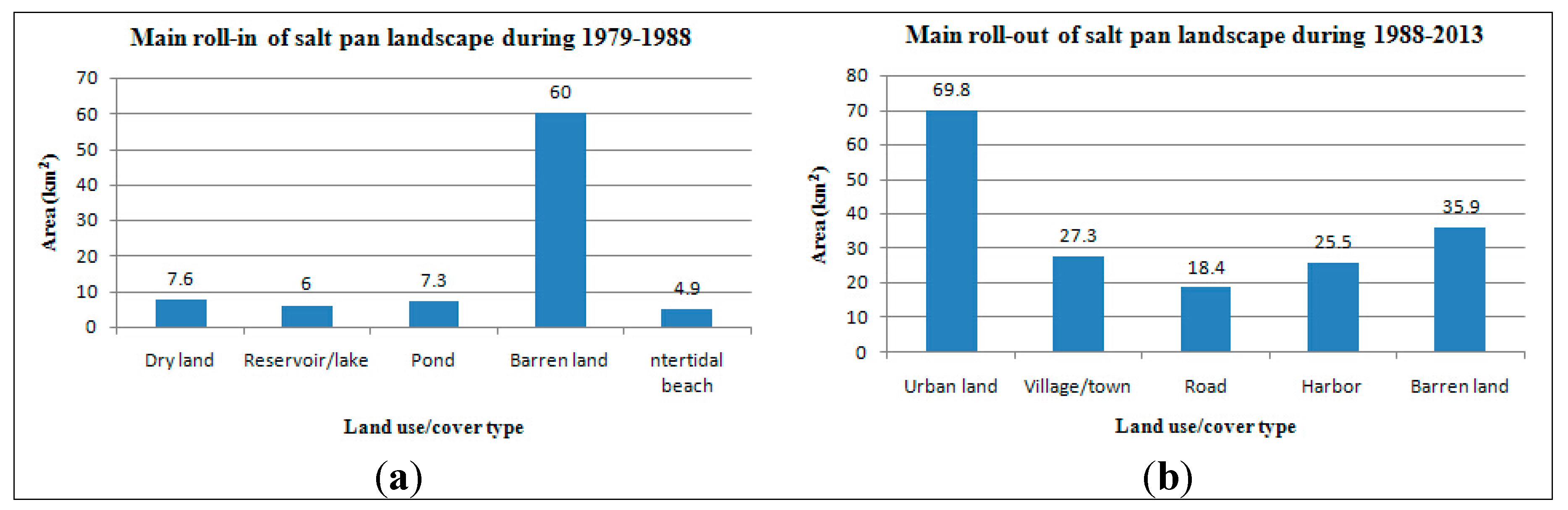
The roll-in of salt pan landscape during 1979–1988 and the roll-out of this landscape type during 1988–2013 were studied. Figure 2 illustrates the roll-in of salt pan space during 1979–1988 which occurred mainly in Hangu District. According to the analysis, the primary roll-in landscape of salt pans was barren land, and its area amounted to 60.0 km2 during 1979–1988. Other sources of the roll-in during this period also included dry land, reservoir/lake, pond, intertidal beach, etc. The main roll-out of salt pan lands during 1988–2013 was urban, barren land, village/town, harbor, and road territories, whose areas amounted to 69.8, 35.9, 27.3, 25.5, and 18.4 km2, respectively. The area of the roll-out barren land was large, and the reason for this phenomenon was mostly that the barren land, reclaimed from the salt pans, would ultimately be used as a construction land, such as urban or village/town location. The main roll-in (1979–1988) and roll-out (1988–2013) landscape types of salt pan are shown in Figure 3.
Figure 2 depicts a large territory of salt pans in Tanggu District lying to the north of Haihe River which disappeared gradually during 1988–2013. The area of this part was approximately 60.5 km2, and the deserted salt pan terrain was eventually transformed into urban, harbor, and village/town construction land. This transition of Tanggu Saltern to urban land took place mainly to the north of the Haihe River, and the area of this kind of transformation was 33.7 km2, while the shift to harbor land happened on both sides of the Haihe River. Among the whole range of changes, the transition of Tanggu Saltern, lying to the north of the Haihe River, to harbor land was about 12.2 km2, whereas the decreased area due to the transformation to village/town location in this direction was 8.2 km2.
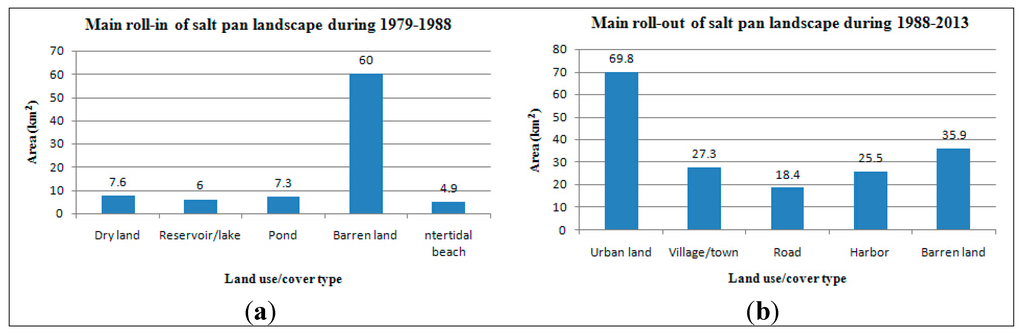
Figure 3.
The main roll-in (1979–1988) and roll-out (1988–2013) of salt pan landscape in the Tianjin Binhai New Area.
Figure 3.
The main roll-in (1979–1988) and roll-out (1988–2013) of salt pan landscape in the Tianjin Binhai New Area.
The salt pan of Tanggu District, situated to the south of the Haihe River occupied a large portion of the total saltern area in Tianjin Municipality, and in 1988 it spanned a territory of 208.1 km2, which accounted for 45.5% of the total salt pan area of Tianjin. In 2013, this region encompassed 165.8 km2 (57.1%), and while its area declined in an absolute value, its relative portion increased. The lost land was transformed predominantly to urban (24.2 km2) and harbor (13.3 km2) land.
The area increase of Tianjin’s salt pan during 1979–1988 happened primarily in the Hangu District, coming mainly from the conversion of barren land. The area of the Hangu saltern was about 128.3 km2 in 1979, while it was 178.2 km2 in 1988, accounting for an expansion of 49.9 km2. In the total enlargement, the area changed from barren land was about 37.1 km2. The Hangu saltern territory decreased consistently since 1988, and its span was about 115.4 km2 in 2013, while the total loss during the period (1988–2013) was 62.8 km2. The prevalent roll-out landscape types identified in Hangu District were barren land and village/town locations. The areas transformed to urban and village/town spaces were about 30.6 and 18.4 km2, respectively.
3.2. Sustainable Approach for Sea Salt Production Combined with Seawater Desalination and Other Relative Industries according to CE and IS
Based on the area change analysis of the saltern landscape in Tianjin Municipality, a dramatic decline in the sea salt production area has been revealed. However, with the development of the sodium hydroxide and sodium carbonate industries, the demand for sea salt is envisaged to continue its rise. This discrepancy between the decline in the production capacity and the higher commercial needs is a serious challenge for the sustainable development of sea salt production. Fortunately, seawater desalination may provide a feasible approach to recover the capacity of sea salt production in Tianjin.
The combination of seawater desalination and sea salt production can be realized through several approaches, such as the traditional saltworks method [27,28,29,30,31,32,35,38], the electrodialysis-based technique [40], the multieffect evaporation-based procedure [73,74],the multistage flash distillation-based system [42], etc. The method utilizing the existing saltpan facilities and chemical equipment is most economically sound and is capable of implementing well-developed technologies. In this manner the demand for dealing with concentrated saline resulting from seawater desalination and providing adequate sea salt quantities can be satisfied [27,28]. The specific process can be described as follows: First, the initial seawater has to be clarified in settling ponds. The Bohai Bay area is occupied by a shallow muddy coast with a mild slope and a high sediment concentration which needs to be diminished before the utilization of water as an input for desalination. In the Tianjin Beijiang Power Plant, for example, the settling ponds were constructed as a two-step scheme and after the clarification of the settling ponds, the seawater sediment concentration was reduced from 500–1000 mg/L to 100 mg/L [75]. Then the clean seawater is transported to a seawater desalination plant for the production of fresh water. The process of bromide extraction normally occurs before the sea salt production [53,54], so it is the next step of the process. The concentrated saline obtained after the bromide extraction is stored in evaporation ponds, waiting for its subsequent exploitation. The next step is salt production in crystallization ponds. The NaCl crystal is formed when the total salt concentration reaches a value above 300 g/L. After most of the NaCl precipitates, the remaining concentrated brine (the “bittern”) contains mainly the ions Mg2+, K+, Cl−, and SO42− [76]. Finally, the waste bittern from the salt production is delivered to salt chemical industries to utilize the remaining mineral compounds, including KCl, MgCl2, and MgSO4. The entire industrial symbiosis chain is shown in Figure 4. Renato De Medeiros Rocha et al. reported the practice of magnesium and potassium exploitation in Brazil [77]. The continually progressing bittern chemical industry is a reliable guarantee for the existence and functioning of this symbiosis chain [29].
Seawater desalination can also be combined with power plant cooling and Artemia culture. The energy necessary to separate the fresh water, usually containing low concentrations of mineral ions, from the concentrated brine with high salt levels is normally provided by a thermal power station [29], commonly acquiring fresh water as a cooling liquid. If the water used for lowering the temperature in the power station can be replaced by seawater, large amounts of fresh water would be saved, achieving both economical and environmental benefits. In China, seawater has been conventionally used as power station cooling water for over 70 years, and this technology has advanced substantially. Hundreds of enterprises in the coastal regions of China have applied the practice of utilizing seawater as a cooling agent, and 90% of the entire usage of cooling seawater is occupied by power enterprises [78]. As a result from its exploitation as a cooling fluid, the raw seawater is heated, and afterwards it is used as a feeding material for the thermal desalination process, which otherwise requires intentional costly heating of the feeding water for the successful separation of the fresh water from the concentrated saline. Since this approach can obviously save great amounts of the energy, a win-win situation is achieved between the seawater desalination and the thermal power station productivity.
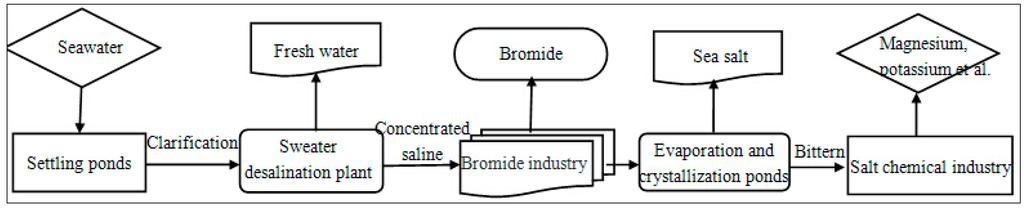
Figure 4.
Block diagram for the seawater usage in “Seawater desalination–bromide extraction–sea salt production–salt chemical” process.
Figure 4.
Block diagram for the seawater usage in “Seawater desalination–bromide extraction–sea salt production–salt chemical” process.
Cultivation of Artemia, a kind of small-sized crustacean (1.2–1.5 cm in length), living in salty water bodies (salt pans, salt lakes, etc.), is also a related area [29,77]. These miniature organisms can be found in the reservoir and evaporation ponds of salterns, and the water with a medium salt concentration offers the most suitable conditions for their living. Artemia are widespread in China, but they are most abundant in the coastal salt pans in Liaoning, Hebei, Shandong Province, and Tianjin Municipality [79]. The cultivation of this crustacean has a great economic value, and due to the convenience of usage and the high nutritional value (yolk, protein, fat, etc.), Artemia are commonly used as bait and feed for the larvae of fish, shrimp, and crab [77,80]. Along with the development of fish and shellfish hatchery aquaculture, the utilization of Artemia biomass in the diet of the larval cultures of many species has become widespread all over the world [81]. Moreover, this organism is ecologically beneficial to salt production, and this indispensability is caused by its capacity to clear brine from particles up to 50 micrometers diameter, which influences light penetration. The micro crustacean biologically controls the excessive toxic blooms of cyanobacteria, which can produce massive amounts of polysaccharide slime and negatively affect the salt production process, and also provides highly suitable food for the Halobacterium populations in the downstream ponds [76,82]. The crystallization ponds are featured by the red coloration caused mainly by Halobacterium which lives on decomposing Artemia corpses. By trapping solar radiation, these microorganisms reduce the viscosity of the brine and elevate its temperature, which will ultimately enhance the rate of evaporation and increases the salt production [77,83]. The salt level of the concentrated saline stemming from seawater desalination is about 60 g/L, while the suitable salt level for Artemia is in the range 70–180 g/L, thus the saline water coming from seawater desalination can be used safely to cultivate the crustacean [29]. The combined model, including power plant cooling and Artemia culture is shown in Figure 5.
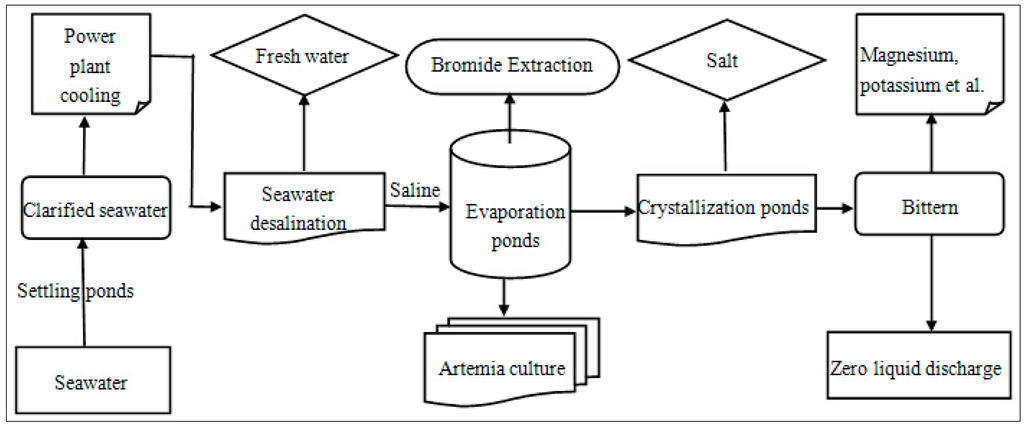
Figure 5.
Block diagram for the seawater usage in “Power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” process.
Figure 5.
Block diagram for the seawater usage in “Power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” process.
However, there is a problem in this design. The seawater desalination process would cause the death of the algae living in evaporation ponds [27], and this would undoubtedly influence the growth of Artemia dwelling on these algae. Furthermore, because the Artemia corpses are food for Halobacterium populations, the reduction of algae availability will ultimately impact the quantity and quality of sea salt products. In view of this reality, some evaporation ponds with natural seawater should be retained [29]. Seawater with an original salt concentration level could be utilized to mix with the saline resulting from seawater desalination as feeding water for the “power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” model. The results of earlier investigations indicate that when the ratio of concentrated saline stemming from seawater desalination and natural seawater is 1.5, the environmental and economic benefits are the most significant [84].
Some reports demonstrate the possibility for the exploitation of the evaporation ponds with natural seawater for fish cultivation [38,77]. In Australia, for example, the species growing well in water bodies with a high salinity level are Barramundi (Lates calcarifer), Black Bream (Acanthopagrus butcheri), Red Snapper (Pagrus auratus), Milk Fish (Chanos chanos), Mullet (Mugil cephalus), and Tilapia (Oreochromis mossambicus) [38]. Accordingly, the model with the added mariculture is illustrated in Figure 6.
Based on the above analysis, the “seawater desalination–sea salt production” industrial symbiosis model could include an exceedingly greater number of related areas, such as mariculture, power plant cooling, Artemia culture, bromide extraction, and salt chemical industry. This comprehensive model would lead to considerably more benefits both economically and environmentally. In the economic aspect, sea salt enterprises can acquire much more sea salt production because of higher salt level of brine from desalination, which is normally twice that of seawater [12,18].Furthermore, the brine disposal problem, which is normally a limitation factor, has been addressed. Thus, seawater desalination can be developed in a much faster and stable manner. In addition, the combination of other relative industries could secure additional economic benefits. Finally, the agglomeration of relative enterprises could bring a pool of production factors such as labor, capital, and energy, thereby decreasing factor prices, raising productivity [85], and contributing to Tianjin’s higher regional competitiveness. In the environmental aspect, the proposed “seawater desalination–sea salt production” IS model will relieve the rise of salinity, temperature, and turbidity, and the decline of dissolved oxygen level in the ambient seawater body of brine outlet. Thus, the environment degradation issue from brine disposal in Bohai Bay could be improved.
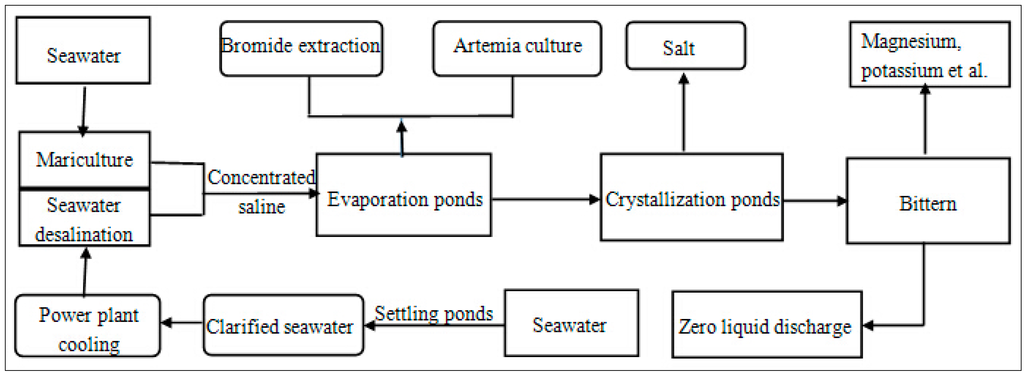
Figure 6.
Block diagram for the seawater usage in “Mariculture–power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” process.
Figure 6.
Block diagram for the seawater usage in “Mariculture–power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” process.
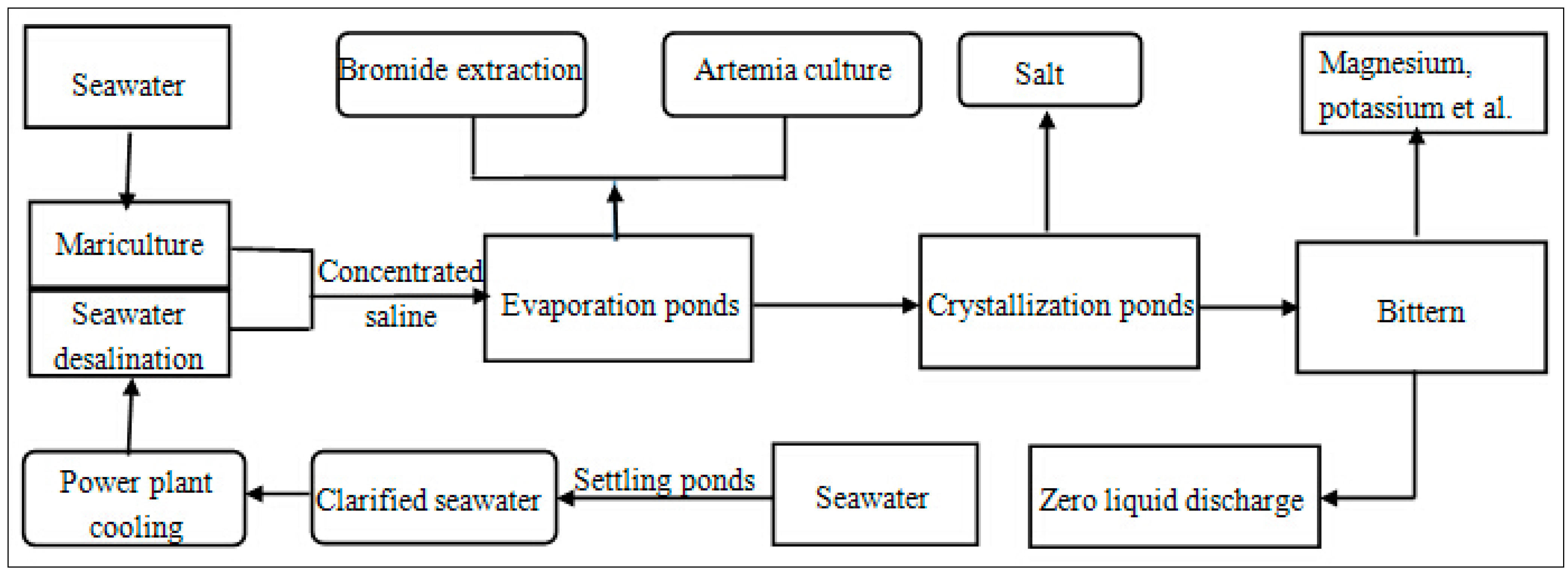
In terms of CE, using brine from seawater desalination for sea salt extraction, as well as bittern from sea salt production for salt chemical, is a reflection of the CE recycling principle. Furthermore, the combination of power plant cooling and seawater desalination, by which both fresh water and energy could be saved, is a practical example of the CE reduction principle. Finally, including as many relative domains of seawater use as possible into the final IS model manifests the CE reuse principle. The closed-loop use of seawater will eventually be realized by the proposed IS model.
To date, the combination of seawater desalination, sea salt production, and other relative areas such as power plant cooling, bromide extraction, and salt chemical has been practiced in Tianjin. Such a combination may also be observed in the Beijiang Power Plant, whose “power generation–seawater desalination–sea salt production–salt chemical–waste reuse” CE model has been chosen as a pilot project in the national first patch of CE [29]. This CE model comprises a thermal power plant, MED desalination plant [34] with 20 × 104 m3/day fresh water manufacture capacity [30], the Hangu saltern [86], and salt chemical enterprises providing KCl, MgCl2, and MgSO4 [34]. These models supply plentiful water, salt, raw chemical material, and energy to the development area, bonded area, and the Sino-Singapore Eco-Town in the Tianjin Bianhai New Area [29,86].
At present, the Tianjin seawater desalination industry and power plant enterprises are witnessing prosperity. Desalinated fresh water has been used as boiler feed water or other industrial purposes, such as ethylene manufacture [65]. Part of the desalinated fresh water of Beijiang Power Plant has been put into municipal water supply pipes [65]. This flourishing development is because of the rapid expansion of economic and population scale of the municipality of Tianjin. In the future, the considerable potential in this field could be exploited. Furthermore, power plant cooling water has already been primarily replaced by seawater, whereas sea salt production and salt chemical industry already comprise strong productivity and industrial foundation in Tianjin. By comparison, bromide extraction, Artemia culture, bittern chemical, and mariculture through evaporation ponds with low salinity concentration level must be reinforced in the coming years.
Additionally, limitations remain on the further promotion of this IS chain. With respect to technology, the use of chemical additives such as antiscalants, antifoulants, corrosion inhibitors, and defoamers may influence the efficiency of sea salt production. The extraction capacity of bittern chemical materials must be confirmed. Economically, perhaps the use of chemical additives in desalination could enhance the purification cost of sea salt and salt chemicals. Furthermore, the profitability of the participation of other relative industries must be verified. Socially, the lack of public awareness about CE and poor enforceability of relative legislation will impede the application of the proposed IS chain.
Considering these limitations and challenges, countermeasures could be adopted. At the micro level, the improvement of technological feasibility of relative fields is necessary for the success of this IS model. At the meso-level, an industrial park could be acquired to promote the implementation of the combination of seawater desalination, sea salt production, and relative fields, similar to the Kalundborg (Denmark). At the macro level, economic incentive policy such as providing rewards and subsidies for enterprises acquiring seawater usage gradually may be useful for the development of this IS chain.
4. Discussion and Conclusions
In China, sea salt is the most important type of salt with the largest yield capacity. Because of the continuous occupation of the coastal salt pans, the quantity of sea salt produced from seawater has declined dramatically, and this trend is especially obvious in Tianjin. The findings from the area change analysis of the saltpan landscape provide evidence that the total loss of Tianjin’s salt pan reached 167.3 km2 from 1988 to 2013, which was about 36.5% of the salt pan area in 1988, and these lost salt pans were ultimately transformed to urban, village/town, harbor, and road lands. The reduction of sea salt production capacity will eventually threaten the sustainable development of salt chemical industry. Fortunately, seawater desalination provides an opportunity to recover and compensate for the capacity decline. This combination of seawater desalination and sea salt production is the practice of circular economy and industrial symbiosis principles and will effectively solve the problem with the disposal of concentrated saline stemming from seawater desalination. The final symbiosis chain can be concluded as a “mariculture–power plant cooling–seawater desalination–Artemia culture–bromide extraction–sea salt production–salt chemical” process. This symbiosis will undoubtedly help to build the sustainable development capacity in Tianjin Municipality. However, this work provides just a possibly conceptual IS model for the combination of seawater desalination and sea salt production industries. In the future, the validity and reasonability of this prototype should be tested. For example, whether the chemical additives utilized in the desalination process would impact the production of salt should be verified.
Acknowledgments
This research is financially supported by the Project (No. 41271102) from National Natural Science Foundation of China. The authors kindly acknowledge the support.
Author Contributions
Hui Wang contributed to the development of the idea and the composition of this manuscript and also wrote it. Xuegong Xu contributed to the direction of the idea and the revision of this manuscript. Gaoru Zhu and Hui Wang were mainly responsible for the Landsat image interpretation. All authors have given approval to the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, Z.Y.; Liu, C.H. Preliminary analysis on salt making industry structure in China and its development trend. China Well Rock Salt. 2007, 38, 3–9. [Google Scholar]
- Davis, J. Structure, function, and management of the biological system for seasonal solar saltworks. Glob. Nest. 2000, 2, 217–226. [Google Scholar]
- Masero, J.A. Assessing alternative anthropogenic habitats for conserving waterbirds: Salinas as buffer areas against the impact of natural habitat loss for shorebirds. Biodivers. Conserv. 2003, 12, 1157–1173. [Google Scholar] [CrossRef]
- Dias, M.P. Use of salt ponds by wintering shorebirds throughout the tidal cycle. Waterbirds 2009, 32, 531–537. [Google Scholar] [CrossRef]
- Ecological Management of Salinas. http://www.docin.com/p-431942722.html (accessed on 23 July 2015).
- Weber, T.P.; Houston, A.I.; Ens, B.J. Consequences of habitat loss at migratory stopover sites: A theoretical investigation. J. Avian Biol. 1999, 30, 416–426. [Google Scholar] [CrossRef]
- Hu, H.J. Current situation and development trend of the Chinese marine salt industry and the challenges it is confronted with. Mar. Econ. 2012, 2, 35–39. [Google Scholar]
- Li, S.S.; Zhao, S.F.; Zhou, X.Y.; Li, H. The analysis of environmental situation of Changlu Saltern in North China. Tianjin Sci. Technol. 2011, 2011, 114–116. [Google Scholar]
- Liu, S.C. The study of intensive use and protection of land resource in Tianjin Binhai New Area. Constr. Sci. Technol. 2009, 2009, 85–87. [Google Scholar]
- Lattemann, S.; Hopner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Latorre, M. Environmental impact of brine disposal on Posidonia seagrasses. Desalination 2005, 182, 517–524. [Google Scholar] [CrossRef]
- Al-Agha, M.R.; Mortaja, R.S. Desalination in the Gaza Strip: Drinking water supply and environmental impact. Desalination 2005, 173, 157–171. [Google Scholar] [CrossRef]
- Miri, R.; Chouikhi, A. Ecotoxicological marine impacts from seawater desalination plants. Desalination 2005, 182, 403–410. [Google Scholar] [CrossRef]
- Sa´nchez-Lizaso, J.L.; Romero, J.; Ruiz, J.; Gacia, E.; Buceta, J.; Invers, O.; Torquemada, Y.; Mas, J.; Ruiz-Mateo, A.; Manzanera, M. Salinity tolerance of the Mediterranean seagrass Posidonia oceanica: Recommendations to minimize the impact of brine discharges from desalination plants. Desalination 2008, 221, 602–607. [Google Scholar] [CrossRef]
- Roberts, D.A.; Johnston, E.L.; Knott, N.A. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Res. 2010, 44, 5117–5128. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, T. A procedure for environmental impact assessments (EIA) for seawater desalination plants. Desalination 1999, 124, 1–12. [Google Scholar] [CrossRef]
- Sadhwani, J.J.; Veza, J.M.; Santana, C. Case studies on environmental impact of seawater desalination. Desalination 2005, 185, 1–8. [Google Scholar] [CrossRef]
- Baalousha, H. Dasalination status in the Gaza Strip and its environmental impact. Desaliantion 2006, 196, 1–12. [Google Scholar] [CrossRef]
- Meerganz, G.L.; Medeazza, V. “Direct” and socially-induced environmental impacts of desalination. Desalination 2005, 185, 57–70. [Google Scholar] [CrossRef]
- Einav, R.; Lokiec, F. Environmental aspects of a desalination plants in Ashkelon. Desalination 2003, 156, 79–85. [Google Scholar] [CrossRef]
- Tularam, G.A.; Ilahee, M. Environmental concerns of desalinating seawater using reverse osmosis. J. Environ. Monitor. 2007, 9, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.; Hajjaj, M. Impact of desalination plants fluid effluents on the integrity of seawater, with the Arabian Gulf in perspective. Desalination 2005, 182, 373–393. [Google Scholar] [CrossRef]
- Dawoud, M.A.; Al-Mulla, M.M. Environmental impacts of seawater desalination: Arabian Gulf case study. Int. J. Environ. Sustain. 2012, 1, 22–37. [Google Scholar]
- Younos, T. Environmental issues of desalination. J. Contemporary Water Res. Educ. 2005, 132, 11–18. [Google Scholar] [CrossRef]
- Huang, Y.J.; Chen, Q.Z.; Zeng, J.N.; Jiang, Z.B. Influences of the high salinity wastewater from desalination plants on the marine ecological environment. J. Mar. Sci. 2009, 27, 103–110. [Google Scholar]
- Nie, L.H.; Liu, X.B.; Tian, S.Y.; Yu, P. Effect of the waste liquid drainage of high salinity from seawater desalination on the marine ecosystem. J. Salt Chem. Ind. 2008, 37, 50–53. [Google Scholar]
- Feng, J.J.; Wang, X.S.; Zhai, Y.J. Research on a joint process type for both seawater desalination & salt production. J. Salt Chem. Ind. 2005, 34, 4–6. [Google Scholar]
- Cui, S.J.; Han, H.R.; Deng, H.N.; Yuan, J.S. Discussion on comprehensive utilization of concentrated brine from seawater desalination. J. Salt Chem. Ind. 2007, 3, 36–42. [Google Scholar]
- Wu, Q.; Wang, X.K. Preliminary analysis on two-way joint process model for salt production. J. Salt Chem. Ind. 2010, 39, 38–41. [Google Scholar]
- Xing, L.Q.; Chen, Y.H. Forecast of technology development for electricity generation–sea water desalination–salt and alt chemical engineering. J. Salt Chem. Ind. 2012, 41, 1–3. [Google Scholar]
- Zhang, H.C.; Jin, H.B.; Wang, B.; Yu, X.F.; Lu, A.D.; Yu, G.D. Experimental study on salt producing in solar salt—ponds by concentrated seawater of RO desalination. J. Salt Chem. Ind. 2014, 43, 18–21. [Google Scholar]
- Ouyang, Q.M. Desalination of sea water and analysis on the recycling economy project of salt production. China Well Rock Salt. 2014, 45, 40–43. [Google Scholar]
- Wu, Z.S. Technology study on salt production by halogen method with concentrated water from seawater desalination. J. Salt Chem. Ind. 2012, 41, 10–11. [Google Scholar]
- Wang, H.J.; Zhang, L.; Yan, Y.M. Study on zero discharge technology of concentrated seawater from desalination. J. Salt Chem. Ind. 2014, 43, 11–15. [Google Scholar]
- Qu, F.C.; Wu, X.F. Economic development model of seawater desalination project in coastal chemical industry park. Chem. Ind. 2012, 30, 1–6. [Google Scholar]
- Zhang, J.K.; Cai, R.H.; Liu, L.F.; Wang, Y.Q.; Wang, L.C.; Zhang, Y.S. Study on new technique of multipurpose utilization of desalinated water. J. Salt Chem. Ind. 2012, 41, 23–27. [Google Scholar]
- Al-Mutaz, I. By-product recovery from Saudi desalination plants. Desalination 1987, 64, 97–110. [Google Scholar] [CrossRef]
- Ahmed, M.; Arakel, A.; Hoey, D.; Coleman, M. Integrated power, water and salt generation: A discussion paper. Desalination 2001, 134, 37–45. [Google Scholar] [CrossRef]
- Ravizky, A.; Nadav, N. Salt production by the evaporation of SWRO brine in Eilat: A success story. Desalination 2007, 205, 374–379. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ehara, R.; Itoi, S.; Goto, T. Ion-exchange membrane electrodialytic salt production using brine discharged from a reverse osmosis seawater desalination plant. J. Membr. Sci. 2003, 222, 71–86. [Google Scholar] [CrossRef]
- Turek, M. Dual-purpose desalination-salt production electrodialysis. Desalination 2002, 153, 377–381. [Google Scholar] [CrossRef]
- Turek, M. Seawater desalination and salt production in a hybrid membrane-thermal process. Desalination 2002, 153, 173–177. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.L. Analysis on variation characteristics of Tianjin wetland area and its main controlling facts during 1976–2009. J. Tianjin Norm. Univ. (Nat. Sci. Ed.) 2013, 33, 32–38. [Google Scholar]
- Li, H.Y. Analysis of 30-year ecological land use variation in the Binhai New District of Tianjin based on remote sensing and GIS. South. -North. Water Transf. Water Sci. Technol. 2013, 11, 75–80. [Google Scholar]
- Liu, D.Y.; Wang, Q.; Du, L.F.; Huang, X.L.; Feng, Z.K. Changes of wetland landscape pattern and water birds’ habitat in Tianjin during 1999–2007. Wetl. Sci. 2012, 10, 350–358. [Google Scholar]
- Yu, Y.G.; Ma, Y. Spot-5 image marks for interpreting the types of land use/cover in the coastal zone of the Jiaozhou Bay. Coast. Eng. 2011, 30, 61–70. [Google Scholar]
- Su, B.W.; Heshmati, A.; Geng, Y.; Yu, X.M. A review of the circular economy in China: Moving from rhetoric to implementation. J. Clean. Prod. 2013, 42, 215–227. [Google Scholar] [CrossRef]
- Han, J.; He, X. Development of circular economy is a fundamental way to achieve agriculture sustainable development in China. Energy Procedia 2011, 5, 1530–1534. [Google Scholar]
- Li, R.H.; Su, C.H. Evaluation of the circular economy development level of Chinese chemical enterprises. Procedia Environ. Sci. 2012, 13, 1595–1601. [Google Scholar] [CrossRef]
- Chertow, M.R. Industrial symbiosis: Literature and taxonomy. Annu. Rev. Energy Environ. 2000, 25, 313–337. [Google Scholar] [CrossRef]
- Jacobsen, N.B. Industrial symbiosis in Kalundborg, Denmark: A quantitative assessment of economic and environmental aspects. J. Ind. Ecol. 2006, 10, 239–55. [Google Scholar] [CrossRef]
- Domenech, T.; Davies, M. Structure and morphology of industrial symbiosis networks: The case of Kalundborg. Procedia Soc. Behav. Sci. 2011, 10, 79–89. [Google Scholar] [CrossRef]
- Jiang, X. The comprehensive utilization of brine water in Shandong Haihua Group. Soda Ind. 2000. [Google Scholar] [CrossRef]
- Yu, C.X.; Wu, C.Y.; Wang, W.Z. Pattern selection of LuBei eco-industry of China based on circular economy. China Soft Sci. 2007. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, L.; Lin, H.; Gao, C. Progress and prospects of seawater desalination in China. Desalination 2005, 182, 13–18. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. Desalination 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Kim, D.H. A review of desalting process techniques and economic analysis of the recovery of salts from retentates. Desalination 2011, 270, 1–8. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.J.; Malamis, S.; Ochsenkühn-Petropoulou, M. Structural and chemical characterization of long-term reverse osmosis membrane fouling in a full scale desalination plant. Desalination 2012, 305, 44–53. [Google Scholar] [CrossRef]
- Pilat, B. Practice of water desalination by electrodialysis. Desalination 2001, 139, 385–392. [Google Scholar] [CrossRef]
- Valero, F.; Arbós, R. Desalination of brackish river water using Electrodialysis Reversal (EDR) Control of the THMs formation in the Barcelona (NE Spain) area. Desalination 2010, 253, 170–174. [Google Scholar] [CrossRef]
- Ruggieri, F.; Fernandez-Turiel, J.L.; Gimeno, D.; Valero, F.; García, J.C.; Medina, M.E. Limestone selection criteria for EDR water remineralization. Desalination 2008, 227, 314–326. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Wang, Q.Z.; Liu, P. The discussion of seawater desalination approach in Tianjin. China Water Resour. 2007, 23, 20–21. [Google Scholar]
- Li, L.; Liu, S.J. Development potential and patterns of seawater desalination in Binhai New Area of Tianjin. J. Econ. Water Resour. 2015, 33, 48–50. [Google Scholar]
- Chesher, R. Biological impact of a large-scale desalination plant at Key West, Florida. Elsevier Oceanogr. Ser. 1975, 12, 99–153. [Google Scholar]
- Iso, S.; Suizu, S.; Maejima, A. The lethal effect of hypertonic solutions and avoidance of marine organisms in relation to discharged brine from a desalination plant. Desalination 1994, 97, 389–399. [Google Scholar] [CrossRef]
- Dupavillon, J.L.; Gillanders, B.M. Impacts of seawater desalination on the giant Australian cuttlefish Sepia apama in the upper Spencer Gulf, South Australia. Mar. Environ. Res. 2009, 67, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Okely, P.; Antenucci, J.P.; Imberger, J.; Marti, C.L. Field Investigations into the Impact of the Perth Seawater Desalination Plant Discharge on Cockburn Sound; Centre for Water Research, University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Hodges, B.R.; Furnans, J.E.; Kulis, P.S. Thin-layer gravity current with implications for desalination brine disposal. J. Hydraul. Eng. 2001, 137, 356–371. [Google Scholar] [CrossRef]
- Hoepner, T.; Windelberg, J. Elements of environmental impact studies on coastal desalination plants. Desalination 1996, 108, 11–18. [Google Scholar] [CrossRef]
- Nie, H.T.; Tao, J.H. Eco-environment status of the Bohai Bay and the impact of coastal exploitation. Mar. Sci. Bull. 2009, 11, 81–96. [Google Scholar]
- Melián-Martel, N.; Sadhwani, J.J.; Pérez, S.O. Saline waste disposal reuse for desalination plants for the chlor-alkali industry The particular case of pozo izquierdo SWRO desalination plant. Desalination 2011, 281, 35–41. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.J.; Pérez, S.O. Reuse and management of brine in sustainable SWRO desalination plants. Desalin. Water Treat. 2013, 51, 560–566. [Google Scholar] [CrossRef]
- Sun, B.; Sun, L.Y.; Yu, H.M.; Han, X.; Liu, J.J. Key seawater utilization and intake techniques at the Tianjin Beijiang Power Plant. Mar. Econ. 2011, 1, 29–34. [Google Scholar]
- Oren, A. Saltern evaporation ponds as model systems for the study of primary production processes under hypersaline conditions. Aquat. Microb. Ecol. 2009, 56, 193–204. [Google Scholar] [CrossRef]
- Rocha, R.M.; Costa, D.F.S.; Lucena-Filho, M.A.; Bezerra, R.M.; Medeiros, D.H.M.; Azevedo-Silva, A.M.; Araújo, C.N.; Xavier-Filho, L. Brazilian solar saltworks-ancient uses and future possibilities. Aquatic Biosyst. 2012. [Google Scholar] [CrossRef]
- Shen, M.Q.; Zhou, L.; Hao, Y. The study of current situation and development tendency of seawater comprehensive usage in China. Ocean. Dev. Manag. 2010, 27, 23–27. [Google Scholar]
- Liu, D.S.; Wang, H.T.; Li, Y.Y. The study of cultivation and exploitation of improved varieties of Artemia in Bohai Bay. Shandong Fish. 2010, 27, 13–14. [Google Scholar]
- Barata, C.; Hontoria, F.; Amat, F. Life history, resting egg, formation and hatching may explain the temporal geographical distribution of Artemia strains in the Mediterranean basin. Hydrobiologia 1995, 298, 295–305. [Google Scholar] [CrossRef]
- Zmora, O.; Avital, E.; Gordin, H. Results of an attempt for mass production of Artemia in extensive ponds. Aquaculture 2002, 213, 395–400. [Google Scholar] [CrossRef]
- Rocha, R.M.; Câmara, M.R. Prediction, monitoring and management of detrimental algal blooms on solar salt production in north-east Brazil. Seventh Symp. Salt 1993, 1, 657–660. [Google Scholar]
- Oren, A.; Stambler, N.; Dubinsky, Z. On the red coloration of saltern crystallizer ponds. Int. J. Salt Lake Res. 1992, 1, 77–89. [Google Scholar] [CrossRef]
- Li, N. Study on Physico-Chemical Zoology Characteristic of Desalinated Seawater and Effect on Salt Technics. Master’s Thesis, Hebei Industrial University, Tianjin, China, 2006. [Google Scholar]
- Anderson, G. Industry clustering for economic development. Econ. Dev. Rev. 1994, 12, 26–33. [Google Scholar]
- Yu, H.M.; Li, C.R.; Zhao, P. Application and extension of the major marine industries circular economy model-a case study of circular economy project of Beijiang Power Plant. Mar. Econ. 2011, 1, 46–51. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
