Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case
Abstract
1. Introduction
2. Materials and Methods
2.1. Hyperspectral Technique Setup
2.2. Stomatal Conductance Datasets
2.3. Pre-Processing of Data for Important Features Selection
2.4. Wavelength Selection
2.5. Validation of Features Selected
2.6. Statistical Packages
3. Results
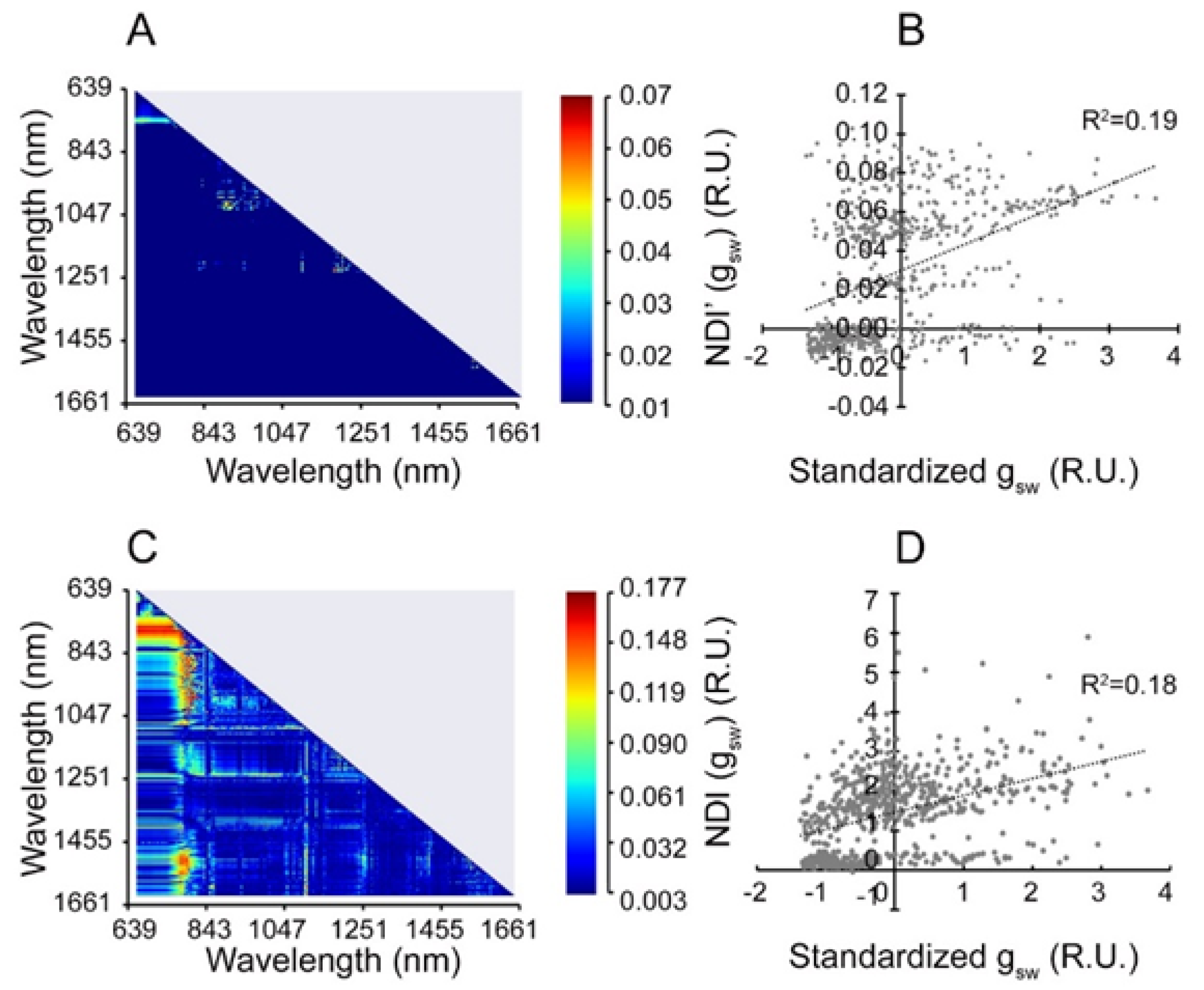
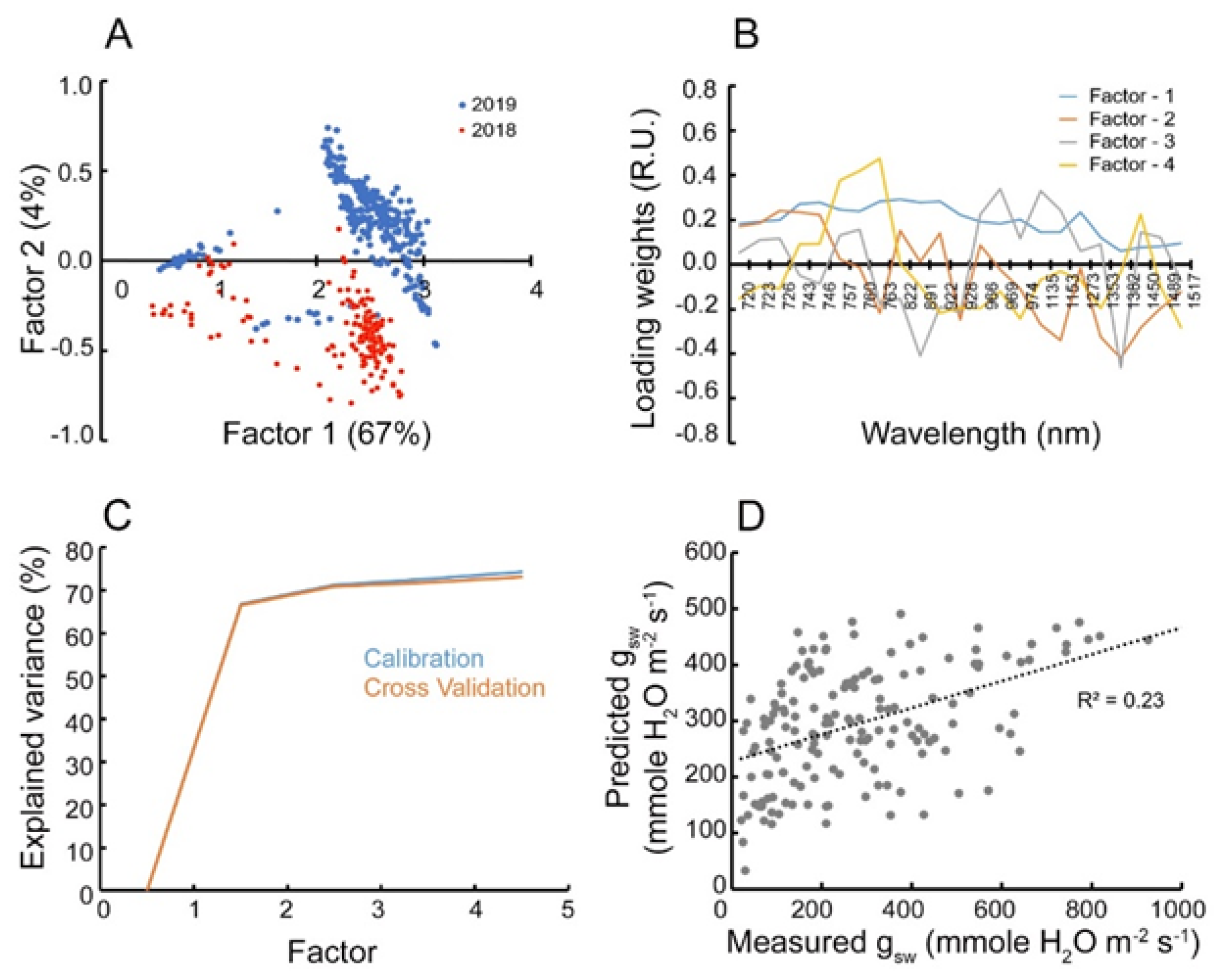
3.1. Wavelength Selection
3.2. Validation of Feature Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collatz, G.J.; Ball, J.T.; Grivet, C.; Berry, J.A. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agric. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Buckley, T.N. Modeling Stomatal Conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A. Reflectance Wavebands and Indices for Remote Estimation of Photosynthesis and Stomatal Conductance in Pine Canopies. Remote Sens. Environ. 1998, 63, 61–72. [Google Scholar] [CrossRef]
- Field, C.B.; Randerson, J.T.; Malmström, C.M. Global net primary production: Combining ecology and remote sensing. Remote Sens. Environ. 1995, 51, 74–88. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J., Jr. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.-M. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P.; Biggs, T.; Post, K. Hyperspectral narrowband and multispectral broadband indices for remote sensing of crop evapotranspiration and its components (transpiration and soil evaporation). Agric. For. Meteorol. 2016, 218–219, 122–134. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, W.; Zhou, D.; Gong, H. Sensitivity Analysis of Vegetation Reflectance to Biochemical and Biophysical Variables at Leaf, Canopy, and Regional Scales. IEEE Trans. Geosci. Remote Sens. 2014, 52, 4014–4024. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Ustin, S.L.; Riaño, D. Contributions of imaging spectroscopy to improve estimates of evapotranspiration. Hydrol. Process. 2011, 25, 4069–4081. [Google Scholar] [CrossRef]
- Burkart, A.; Cogliati, S.; Schickling, A.; Rascher, U. A Novel UAV-Based Ultra-Light Weight Spectrometer for Field Spectroscopy. IEEE Sens. J. 2014, 14, 62–67. [Google Scholar] [CrossRef]
- Rascher, U.; Alonso, L.; Burkart, A.; Cilia, C.; Cogliati, S.; Colombo, R.; Damm, A.; Drusch, M.; Guanter, L.; Hanus, J.; et al. Sun-induced fluorescence—A new probe of photosynthesis: First maps from the imaging spectrometer HyPlant. Glob. Chang. Biol. 2015, 21, 4673–4684. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.R.; Wang, M. Influence of oceanic whitecaps on atmospheric correction of ocean-color sensors. Appl. Opt. AO 1994, 33, 7754–7763. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trac Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Cochran, W.G. The distribution of the largest of a set of estimated variances as a fraction of their total. Ann. Eugen. 1941, 11, 47–52. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Van Rossum, G. Python Programming Language. In Proceedings of the USENIX Annual Technical Conference, Santa Clara, CA, USA, 17–22 June 2007; Volume 41, p. 36. [Google Scholar]
- Powell, M.J. A view of algorithms for optimization without derivatives. Math. Today-Bull. Inst. Math. Its Appl. 2007, 43, 170–174. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Nan, F.; Wang, J.; Saligrama, V. Pruning random forests for prediction on a budget. In Proceedings of the Advances in Neural Information Processing Systems, Barcelona, Spain, 5–10 December 2016; pp. 2334–2342. [Google Scholar]
- Andrews, D.F. A robust method for multiple linear regression. Technometrics 1974, 16, 523–531. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Sousa, S.I.V.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Pereira, M.C. Multiple linear regression and artificial neural networks based on principal components to predict ozone concentrations. Environ. Model. Softw. 2007, 22, 97–103. [Google Scholar] [CrossRef]
- Cybenko, G. Approximation by superpositions of a sigmoidal function. Math. Control Syst. 1989, 2, 303–314. [Google Scholar] [CrossRef]
- Hecht-Nielsen, R. Theory of the backpropagation neural network. In Neural Networks for Perception; Elsevier: Amsterdam, The Netherlands, 1992; pp. 65–93. [Google Scholar]
- Jones, C.L.; Weckler, P.R.; Maness, N.O.; Stone, M.L.; Jayasekara, R. Estimating water stress in plants using hyperspectral sensing. In Proceedings of the 2004 ASAE Annual Meeting, Ottawa, ON, Canada, 1–4 August 2004; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2004; p. 1. [Google Scholar]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Peñuelas, J.; Valentini, R. Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis, and transpiration, II. The role of biophysics in the linearity of their interdependence. Remote Sens. Environ. 1987, 21, 143–183. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random forest regression and spectral band selection for estimating sugarcane leaf nitrogen concentration using EO-1 Hyperion hyperspectral data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Horning, N. Random Forests: An algorithm for image classification and generation of continuous fields data sets. In Proceedings of the International Conference on Geoinformatics for Spatial Infrastructure Development in Earth and Allied Sciences, Osaka, Japan, 9–11 December 2010. [Google Scholar]
- Smith, K.L.; Steven, M.D.; Colls, J.J. Use of hyperspectral derivative ratios in the red-edge region to identify plant stress responses to gas leaks. Remote Sens. Environ. 2004, 92, 207–217. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Earth Resources Technology Satellite Symposium; NASA SP-351; NASA: Washington, DC, USA, 1973; Volume 1, pp. 309–317. [Google Scholar]
- Lee, Y.J.; Yang, C.M.; Chang, K.W.; Shen, Y. A simple spectral index using reflectance of 735 nm to assess nitrogen status of rice canopy. Agron. J. 2008, 100, 205–212. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, J.; Huang, W.; Li, C.; Xu, X.; Ding, X.; Shi, J.; Zhou, B. Hyperspectral discrimination and response characteristics of stressed rice leaves caused by rice leaf folder. In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Beijing, China, 29–31 October 2011; Springer: Berlin, Germany, 2011; pp. 528–537. [Google Scholar]
- Zhao, J.; Zhang, D.; Luo, J.; Dong, Y.; Yang, H.; Huang, W. Characterization of the rice canopy infested with brown spot disease using field hyperspectral data. Wuhan Univ. J. Nat. Sci. 2012, 17, 86–92. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Mehrotra, R.; Siesler, H.W. Application of mid infrared/near infrared spectroscopy in sugar industry. Appl. Spectrosc. Rev. 2003, 38, 307–354. [Google Scholar] [CrossRef]
- Peet, M.M.; Huber, S.C.; Patterson, D.T. Acclimation to high CO2 in monoecious cucumbers: II. Carbon exchange rates, enzyme activities, and starch and nutrient concentrations. Plant Physiol. 1986, 80, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.H.; Kelm, B.M.; Masuch, R.; Himmelreich, U.; Bachert, P.; Petrich, W.; Hamprecht, F.A. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinform. 2009, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, S. Geospatial Modeling of Canopy Chlorophyll Content Using High Spectral Resolution Satellite Data in Himalayan Forests. Clim. Chang. Environ. Sustain. 2018, 6, 20–34. [Google Scholar] [CrossRef]
- Yang, C.-M.; Cheng, C.-H.; Chen, R.-K. Changes in spectral characteristics of rice canopy infested with brown planthopper and leaffolder. Crop Sci. 2007, 47, 329–335. [Google Scholar] [CrossRef]
- Yi, Q.; Huang, J.; Wang, F.; Wang, X. Quantifying biochemical variables of corn by hyperspectral reflectance at leaf scale. J. Zhejiang Univ. Sci. B 2008, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Skidmore, A.K.; Darvishzadeh, R.; Wang, T. Mapping forest canopy nitrogen content by inversion of coupled leaf-canopy radiative transfer models from airborne hyperspectral imagery. Agric. For. Meteorol. 2018, 253, 247–260. [Google Scholar] [CrossRef]
- Deel, L.N. Assessing the cumulative impact of disturbance on canopy structure and chemistry in Appalachian forests. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2010. [Google Scholar]
- Dvořáček, V.; Štěrbová, L.; Matějová, E.; Bradová, J.; Hermuth, J. Reflectance Spectrometry as a Screening Tool for Prediction of Lutein Content in Diverse Wheat Species (Triticum spp.). Food Anal. Methods 2018, 11, 2579–2589. [Google Scholar] [CrossRef]
- Lobato, A.K.S.; Gonçalves-Vidigal, M.C.; Vidigal Filho, P.S.; Andrade, C.A.B.; Kvitschal, M.V.; Bonato, C.M. Relationships between leaf pigments and photosynthesis in common bean plants infected by anthracnose. N. Z. J. Crop Hortic. Sci. 2010, 38, 29–37. [Google Scholar] [CrossRef]
- Junhua, B.; Shaokun, L.; Keru, W. The response of canopy reflectance spectrum for the cotton LAI and LAI inversion. Sci. Agric. Sin. 2007, 40, 63–69. [Google Scholar]
- Kusumo, B.H.; Hedley, M.J.; Hedley, C.B.; Tuohy, M.P. Measuring carbon dynamics in field soils using soil spectral reflectance: Prediction of maize root density, soil organic carbon and nitrogen content. Plant Soil 2011, 338, 233–245. [Google Scholar] [CrossRef]
- Clevers, J.; Van der Heijden, G.; Verzakov, S.; Schaepman, M.E. Estimating grassland biomass using SVM band shaving of hyperspectral data. Photogramm. Eng. Remote Sens. 2007, 73, 1141–1148. [Google Scholar] [CrossRef]
- Rollin, E.M.; Milton, E.J. Processing of high spectral resolution reflectance data for the retrieval of canopy water content information. Remote Sens. Environ. 1998, 65, 86–92. [Google Scholar] [CrossRef]
- Chatani, E.; Tsuchisaka, Y.; Masuda, Y.; Tsenkova, R. Water molecular system dynamics associated with amyloidogenic nucleation as revealed by real time near infrared spectroscopy and aquaphotomics. PLoS ONE 2014, 9, e101997. [Google Scholar] [CrossRef] [PubMed]
- Somdatta, C.; Chakrabarti, S. Pre-processing of hyperspectral data: A case study of Henry and Lothian Islands in Sunderban Region, West Bengal, India. Int. J. Geomat. Geosci. 2011, 2, 490. [Google Scholar]
- Mullen, K.E. Early Detection of Mountain Pine Beetle Damage in Ponderosa Pine Forests of the Black Hills Using Hyperspectral and WorldView-2 Data. Master’s Thesis, Minnesota State University, Mankato, MN, USA, 2016. [Google Scholar]
- Liu, Z.-Y.; Huang, J.-F.; Tao, R.-X. Characterizing and estimating fungal disease severity of rice brown spot with hyperspectral reflectance data. Rice Sci. 2008, 15, 232–242. [Google Scholar] [CrossRef]
- Basayigit, L.; Albayrak, S. Reflectance measurement of N, P and K content of wollypod vetch under different N, P and K fertilization. Asian J. Chem. 2007, 19, 5609. [Google Scholar]
- Kuska, M.; Wahabzada, M.; Leucker, M.; Dehne, H.-W.; Kersting, K.; Oerke, E.-C.; Steiner, U.; Mahlein, A.-K. Hyperspectral phenotyping on the microscopic scale: Towards automated characterization of plant-pathogen interactions. Plant Methods 2015, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Carter, G.A. Corn (Zea mays L.) growth, leaf pigment concentration, photosynthesis and leaf hyperspectral reflectance properties as affected by nitrogen supply. Plant Soil 2003, 257, 205–218. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The Genetics of Lignin Biosynthesis: Connecting Genotype to Phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Lindenthal, M.; Steiner, U.; Dehne, H.-W.; Oerke, E.-C. Effect of Downy Mildew Development on Transpiration of Cucumber Leaves Visualized by Digital Infrared Thermography. Phytopathology 2005, 95, 233–240. [Google Scholar] [CrossRef]
- Li, P.; Liu, W.; Zhang, Y.; Xing, J.; Li, J.; Feng, J.; Su, X.; Zhao, J. Fungal canker pathogens trigger carbon starvation by inhibiting carbon metabolism in poplar stems. Sci. Rep. 2019, 9, 10111. [Google Scholar] [CrossRef] [PubMed]
- An, G. The effects of adding noise during backpropagation training on a generalization performance. Neural Comput. 1996, 8, 643–674. [Google Scholar] [CrossRef]
- Berk, A.; Bernstein, L.S.; Anderson, G.P.; Acharya, P.K.; Robertson, D.C.; Chetwynd, J.H.; Adler-Golden, S.M. MODTRAN Cloud and Multiple Scattering Upgrades with Application to AVIRIS. Remote Sens. Environ. 1998, 65, 367–375. [Google Scholar] [CrossRef]
- Vermote, E.; Tanré, D.; Deuzé, J.L.; Herman, M.; Morcrette, J.J.; Kotchenova, S.Y. Second simulation of a satellite signal in the solar spectrum-vector (6SV). 6S User Guide Version 2006, 3, 1–55. [Google Scholar]
- Verrelst, J.; Rivera, J.P.; van der Tol, C.; Magnani, F.; Mohammed, G.; Moreno, J. Global sensitivity analysis of the SCOPE model: What drives simulated canopy-leaving sun-induced fluorescence? Remote Sens. Environ. 2015, 166, 8–21. [Google Scholar] [CrossRef]





| # | Year | Date | Time | VPD Ϯ (kPa) | Wind Speed (ms−1) | Light Intensity (Wm−2) | RH † (%) | Average Air Temp. (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2018 | 25.06 | 11:00 | 2.88 | 2 | 994 | 39.9 | 32.1 |
| 12:30 | 3.26 | 3.7 | 1030 | 36.6 | 33.4 | |||
| 2 | 26.06 | 10:30 | 2.52 | 2 | 993 | 46.7 | 31.9 | |
| 12:00 | 3.18 | 4.4 | 1049 | 37.8 | 33.3 | |||
| 3 | 02.07 | 10:30 | 2.37 | 1.0 | 938.5 | 48.1 | 31.2 | |
| 13:00 | 3.95 | 2.2 | 1003 | 33.3 | 35.9 | |||
| 4 | 03.07 | 10:30 | 2.51 | 1.6 | 973 | 46.7 | 31.8 | |
| 13:00 | 3.25 | 1.8 | 997 | 40.5 | 34.4 | |||
| 5 | 09.07 | 10:30 | 2.91 | 1.3 | 990 | 42.7 | 33.2 | |
| 12:00 | 3.63 | 1.4 | 1036.0 | 36 | 35.2 | |||
| 6 | 10.07 | 10:30 | 3.12 | 1.4 | 988 | 38.3 | 33.1 | |
| 11:30 | 3.68 | 2.9 | 1051 | 31.6 | 34.2 | |||
| 7 | 16.07 | 10:30 | 2.88 | 1.1 | 952 | 42.1 | 32.8 | |
| 13:00 | 3.92 | 2.3 | 983 | 36.7 | 36.7 | |||
| 8 | 17.07 | 10:30 | 2.53 | 1.8 | 946 | 49 | 32.7 | |
| 13:00 | 3.09 | 3.7 | 999 | 45.1 | 35 | |||
| 9 | 30.07 | 10:30 | 2.53 | 3.1 | 742.3 | 46.5 | 31.9 | |
| 13:00 | 3.01 | 4.5 | 1028 | 40.8 | 33.2 | |||
| 10 | 09.08 | 10:30 | 2.63 | 1.7 | 920 | 49.6 | 33.7 | |
| 11:30 | 3.43 | 1.8 | 954 | 40.7 | 35.5 | |||
| 1 | 2019 | 05.08 | 10:30 | 1.77 | 1.4 | 820 | 60.2 | 30.9 |
| 2 | 07.08 | 10:30 | 2.38 | 1 | 785.1 | 47.7 | 31.3 | |
| 12:30 | 3.1 | 1.3 | 930 | 41.4 | 33.9 | |||
| 3 | 14.08 | 10:30 | 2.7 | 1.2 | 735.7 | 44.3 | 32.4 | |
| 4 | 15.08 | 10:30 | 2.16 | 2.2 | 766.2 | 52.7 | 31.3 | |
| 12:30 | 2.89 | 4.1 | 903 | 44.1 | 33.5 | |||
| 5 | 21.08 | 10:30 | 2.67 | 2.3 | 766.5 | 44.6 | 32.2 | |
| 12:00 | 3.1 | 4.2 | 875 | 39.3 | 33.3 | |||
| 6 | 26.08 | 10:30 | 2 | 1.2 | 740.5 | 55.6 | 31 | |
| 12:30 | 3.64 | 2.2 | 874 | 38.2 | 35.8 | |||
| 7 | 28.08 | 10:30 | 2.12 | 0.6 | 695.6 | 55 | 31.9 | |
| 12:30 | 3.31 | 1.7 | 846 | 42.3 | 35.4 | |||
| 8 | 02.09 | 10:30 | 2.18 | 0.7 | 739.5 | 52.3 | 31.3 | |
| 12:30 | 4.14 | 3.9 | 876 | 30.4 | 36 | |||
| 9 | 04.09 | 10:30 | 1.83 | 0.5 | 737 | 54.3 | 29 | |
| 12:30 | 2.6 | 1 | 834 | 46.3 | 32.3 | |||
| 10 | 09.09 | 10:30 | 1.8 | 0.7 | 704.3 | 57.6 | 30 | |
| 12:30 | 2.85 | 2 | 795 | 44.2 | 33.3 |
| Performance Parameter | Train | Test |
|---|---|---|
| Correlation (R) | 0.75 | 0.71 |
| Over/Under estimation of model (MBE Ϯ) | 0.02 | 0.03 |
| Absolute Error (MAE †) | 0.43 | 0.59 |
| RMSE ‡ | 0.66 | 0.73 |
| “Error-Free” results (d2 connectivity) | 0.93 | 0.82 |
| Feature (nm) | Importance (R.U.) ‡ | Meaning | Reference |
|---|---|---|---|
| 757 | 3.13 | Chlorophyll content, pest related, atmospheric oxygen absorption feature | [50,51] [46] |
| 760 | 2.98 | Atmospheric oxygen absorption feature, chlorophyll content, nitrogen concentration | [52] |
| 726 | 2.65 | Red-edge, chlorophyll content | [45,53] |
| 1353 | 2.29 | Biochemical process, lutein content, water content | [54,55] |
| 922 | 2.06 | Moisture, potassium level, oil level | [46] |
| 754 | 1.95 | Disease related band (anthracnose), atmospheric oxygen absorption feature | [56] |
| 822 | 1.84 | Disease related band (brown spot disease in rice) | [45] |
| 723 | 1.71 | Leaf Area Index, red-edge | [57] |
| 1455 | 1.43 | Lignin, water content | [58] |
| 1135 | 1.40 | Lignin, dry matter, chlorophyll content | [59] |
| 974 | 1.29 | Water content | [60] |
| 1382 | 1.25 | Water content, water molecules | [61] |
| 1517 | 1.25 | Water vapor, protein, nitrogen | [62] |
| 1152 | 1.21 | Pest response related, lignin | [63] |
| 1273 | 1.14 | Water, lignin, cellulose | [46] |
| 746 | 1.13 | Fungal disease response-related | [64] |
| 968 | 1.12 | Water content | [8,46] |
| 891 | 1.11 | Disease-related | [31] |
| 1489 | 1.04 | Cellulose, sugar | [46] |
| 762 | 0.97 | Nitrogen level, fluorescence level | [41,65] |
| 743 | 0.97 | Plant-pathogen interactions | [66] |
| 928 | 0.92 | Oil | [46] |
| 1433 | 0.90 | Unknown | |
| 721 | 0.89 | Chlorophyll related, nitrogen-related | [67] |
| 966 | 0.87 | water, starch | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitrack-Tamam, S.; Holtzman, L.; Dagan, R.; Levi, S.; Tadmor, Y.; Azizi, T.; Rabinovitz, O.; Naor, A.; Liran, O. Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case. Remote Sens. 2020, 12, 2213. https://doi.org/10.3390/rs12142213
Vitrack-Tamam S, Holtzman L, Dagan R, Levi S, Tadmor Y, Azizi T, Rabinovitz O, Naor A, Liran O. Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case. Remote Sensing. 2020; 12(14):2213. https://doi.org/10.3390/rs12142213
Chicago/Turabian StyleVitrack-Tamam, Snir, Lilach Holtzman, Reut Dagan, Shai Levi, Yuval Tadmor, Tamir Azizi, Onn Rabinovitz, Amos Naor, and Oded Liran. 2020. "Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case" Remote Sensing 12, no. 14: 2213. https://doi.org/10.3390/rs12142213
APA StyleVitrack-Tamam, S., Holtzman, L., Dagan, R., Levi, S., Tadmor, Y., Azizi, T., Rabinovitz, O., Naor, A., & Liran, O. (2020). Random Forest Algorithm Improves Detection of Physiological Activity Embedded within Reflectance Spectra Using Stomatal Conductance as a Test Case. Remote Sensing, 12(14), 2213. https://doi.org/10.3390/rs12142213





