Mapping the Habitat Suitability of West Nile Virus Vectors in Southern Quebec and Eastern Ontario, Canada, with Species Distribution Modeling and Satellite Earth Observation Data
Abstract
:1. Introduction
2. Materials and Methods
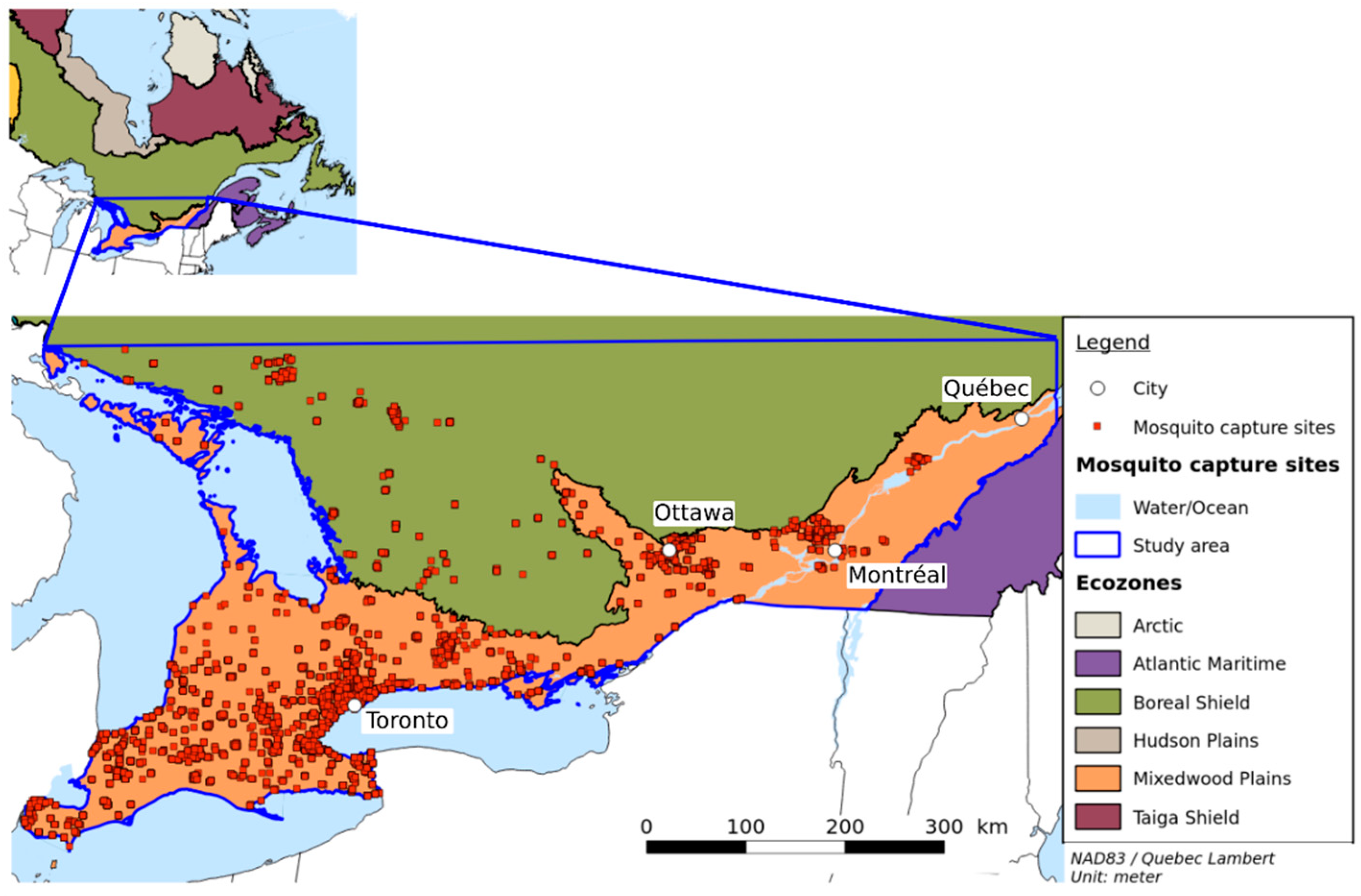
2.1. Study Area
2.2. Maxent
2.3. Entomological Data
2.4. Environmental Data
2.4.1. Existing Geographical Data
2.4.2. Land Use Land Cover
2.4.3. Geographical Derived Data
2.5. Model Calibration and Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| References | |||||
|---|---|---|---|---|---|
| Classes | Paved | Tree Cover | Grass Cover | Mixed Area | User Accuracy |
| Paved | 432 | 0 | 9 | 1 | 97.73% |
| Tree cover | 0 | 550 | 3 | 4 | 98.74% |
| Grass cover | 5 | 1 | 132 | 4 | 92.95% |
| Mixed area | 0 | 2 | 6 | 511 | 98.46% |
| Producer accuracy | 98.85% | 99.46% | 98.27% | 88% | |

Appendix B
| Geographical Layers | Providers | Date | Initial Spatial Resolution | Classes or Value Ranges | Derived Layers | Final Spatial Resolution |
|---|---|---|---|---|---|---|
| Annual crop inventory (ACI) | AAFC 1 | 2016 | 30 m | In total 71 different classes were defined: four forest classes, 60 agriculture classes, and seven other classes (urban, shrubland, grassland, wetlands, bare soil, water and cloud) |
| 30 m |
| National Hydro Network (NHN) | NRCan 2 | 2004 | 1:50,000 | - | ||
| National Roads Network (NRN) | NRCan | 2007 | 1:10,000 | - | ||
| Wetlands | Ontario Ministry of Natural Resources and Forestry | 2011–Present | 1:10,000 | Wetlands are classified into six classes: bog, fen, marsh, swamp, open water and unknown | ||
| Unlimited Ducks Canada and Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs Québec | 2009–Present | 1:20,000 | Wetlands are classified into seven classes: marsh, swamp, peatland fen, peatland bog, peatland bog, peatland forested, shallow water and wet meadow. | |||
| Landsat-8 OLI | United States Geological Survey | 2014–2017 | 30 m | - | ||
| Canadian Digital Elevation Model (CDEM) | NRCan | 1945–2011 | 20 m | 0–1128 m |
| 30 m |
| Soils of Canada, Derived | NRCan | Originally produced in 1980 | 1:5,000,000 | The drainage is classified into five classes: Unclassified, Poorly drained, Imperfectly drained, Moderately/Well drained and Rapidly drained | Drainage (DRA) | 30 m |
References
- World Health Organization. Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 21 September 2020).
- World Health Organization. Mosquito-Borne Diseases. Available online: http://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/ (accessed on 21 September 2020).
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The Outbreak of West Nile Virus Infection in the New York City Area in 1999. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, N.E.; McGeer, A.; Mazzulli, T.; et al. West Nile virus infection in 2002: Morbidity and mortality among patients admitted to hospital in southcentral Ontario. Can. Med. Assoc. J. 2003, 168, 1399–1405. [Google Scholar]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex pipiens(Diptera: Culicidae): A Bridge Vector of West Nile Virus to Humans. J. Med. Èntomol. 2008, 45, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Apperson, C.S.; Hassan, H.K.; Harrison, B.A.; Savage, H.M.; Aspen, S.E.; Farajollahi, A.; Crans, W.; Daniels, T.J.; Falco, R.C.; Benedict, M.; et al. Host Feeding Patterns of Established and Potential Mosquito Vectors of West Nile Virus in the Eastern United States. Vector-Borne Zoonotic Dis. 2004, 4, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Kramer, L.D.; Campbell, S.R.; Alleyne, E.O.; Dobson, A.P.; Daszak, P. West Nile Virus Risk Assessment and the Bridge Vector Paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Brown, H.E.; Andreadis, T.G.; Fish, D. Modeling the Spatial Distribution of Mosquito Vectors for West Nile Virus in Connecticut, USA. Vector-Borne Zoonotic Dis. 2006, 6, 283–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trawinski, P.R.; Mackay, D.S. Identification of Environmental Covariates of West Nile Virus Vector Mosquito Population Abundance. Vector-Borne Zoonotic Dis. 2010, 10, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.M.; Lampman, R.L.; Muturi, E.J. Land Use Patterns and the Risk of West Nile Virus Transmission in Central Illinois. Vector-Borne Zoonotic Dis. 2014, 14, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.; Diuk-Wasser, M.; Andreadis, T.; Fish, D. Remotely-Sensed Vegetation Indices Identify Mosquito Clusters of West Nile Virus Vectors in an Urban Landscape in the Northeastern United States. Vector-Borne Zoonotic Dis. 2008, 8, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, D.M.; Dang, P.T.; Ellis, R.A. The Mosquitoes of Canada: Diptera, Culicidae; The Insects and Arachnids of Canada; Agriculture Canada; Canadian Govt. Pub. Centre, Supply and Services Canada: Hull, QC, Canada, 1979; ISBN 978-0-660-10402-7. [Google Scholar]
- Jackson, M.J.; Gow, J.L.; Evelyn, M.J.; Meikleham, N.E.; McMahon, T.S.; Koga, E.; Howay, T.J.; Wang, L.; Yan, E. Culex Mosquitoes, West Nile Virus, and the Application of Innovative Management in the Design and Management of Stormwater Retention Ponds in Canada. Water Qual. Res. J. 2009, 44, 103–110. [Google Scholar] [CrossRef]
- Mackay, A.J.; Muturi, E.J.; Ward, M.P.; Allan, B.F. Cascade of ecological consequences for West Nile virus transmission when aquatic macrophytes invade stormwater habitats. Ecol. Appl. 2016, 26, 219–232. [Google Scholar] [CrossRef]
- Deichmeister, J.M.; Telang, A. Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J. Vector Ecol. 2011, 36, 75–85. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Alimi, T.O.; Fuller, D.O.; Qualls, W.A.; Herrera, S.V.; Arevalo-Herrera, M.; Quinones, M.L.; Lacerda, M.V.G.; Beier, J.C. Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population. Parasites Vectors 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeilhofer, P.; Dos Santos, E.S.; Ribeiro, A.L.; Miyazaki, R.D.; Dos Santos, M.A. Habitat suitability mapping of Anopheles darlingi in the surroundings of the Manso hydropower plant reservoir, Mato Grosso, Central Brazil. Int. J. Health Geogr. 2007, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moua, Y.; Roux, E.; Girod, R.; Dusfour, I.; De Thoisy, B.; Seyler, F.; Briolant, S. Distribution of the Habitat Suitability of the Main Malaria Vector in French Guiana Using Maximum Entropy Modeling. J. Med. Èntomol. 2016, 54, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Leite, R.; Vilarinho, A.C.; Novaes, M.C.; Tonetto, A.F.; Vilardi, G.C.; Guillermo-Ferreira, R. Recent and future environmental suitability to dengue fever in Brazil using species distribution model. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.H.; Atif, S.; Rasheed, S.B.; Zaidi, F.; Hussain, E. Species Distribution Modelling ofAedes aegyptiin two dengue-endemic regions of Pakistan. Trop. Med. Int. Health 2016, 21, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Meneses, B.M. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop. 2017, 168, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Sánchez-Cordero, V.; Beard, C.B.; Ramsey, J.M. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg. Infect. Dis. 2002, 8, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Strutz, S.E.; Frank, D.M.; Rivaldi, C.; Sissel, B.; Sánchez–Cordero, V. Chagas Disease Risk in Texas. PLoS Negl. Trop. Dis. 2010, 4, e836. [Google Scholar] [CrossRef]
- Darsie, R.F.; Ward, R.A.; Chang, C.C.; Litwak, T. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico; University Press of Florida: Gainesville, FL, USA, 1983; ISBN 978-0-8130-6233-4. [Google Scholar]
- Hongoh, V.; Hoen, A.G.; Aenishaenslin, C.; Waaub, J.-P.; Bélanger, D.; Michel, P. The Lyme-MCDA Consortium Spatially explicit multi-criteria decision analysis for managing vector-borne diseases. Int. J. Health Geogr. 2011, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Kotchi, S.O.; Brazeau, S.; Ludwing, A.; Aube, G.; Berthiaume, P. Earth Observation and Indicators Pertaining to Determinants of Health—An Approach to Support Local Scale Characterization of Environmental Determinants of Vector-Borne Diseases. In Proceedings of the ESA Communications, ESTEC, Noordwijk, The Netherlands, 9 May 2016; Volume 740, p. 9. [Google Scholar]
- Statistics Canada Census in Brief: Municipalities in Canada with the Largest and Fastest-Growing Populations between 2011 and 2016. Available online: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/98-200-x/2016001/98-200-x2016001-eng.cfm (accessed on 28 October 2020).
- ESTR Secretariat Mixedwood. Plains ecozone + evidence for key finding summary. In Biodiversity: Ecosystem Status and Trends 2010; Evidence for Key Findings Summary Report No7; Canadian Councils of Resource Ministers: Ottawa, ON, Canada, 2016; ISBN 978-1-100-25867-6. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2010, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudia, W.D.; Chamberlain, R.W. Battery-operated light trap, an improved model. By W. D. Sudia and R. W. Chamberlain, 1962. J. Am. Mosq. Control. Assoc. 1988, 4, 126–129. [Google Scholar]
- Fay, R.W.; Prince, W.H. A Modified Visual Trap for Aedes Aegypti. Mosq. News 1970, 30, 20–23. [Google Scholar]
- Wood, D.M. Clés des Genres et des Espèces de Moustiques du Canada: Diptera:Culicidae; Agriculture Canada: Ottawa, ON, Canada, 1983; ISBN 978-0-660-91096-3. [Google Scholar]
- Ciota, A.T.; Drummond, C.L.; Ruby, M.A.; Drobnack, J.; Ebel, G.D.; Kramer, L.D. Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J. Med. Èntomol. 2012, 49, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GRASS Development Team. Geographic Resources Analysis Support System (GRASS) Software, Version 7.2. Available online: https://grass.osgeo.org/ (accessed on 21 September 2020).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [Green Version]
- Varela, S.; Anderson, R.P.; García-Valdés, R.; Fernández-González, F. Environmental filters reduce the effects of sampling bias and improve predictions of ecological niche models. Ecography 2014, 37, 1084–1091. [Google Scholar] [CrossRef]
- Pagès, J. Analyse Factorielle de Données Mixtes. Rev. Stat. Appl. 2004, 52, 93–111. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity forMaxentecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Myer, M.H.; Campbell, S.R.; Johnston, J.M. Spatiotemporal modeling of ecological and sociological predictors of West Nile virus in Suffolk County, NY, mosquitoes. Ecosphere 2017, 8, e01854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreadis, T.G. The Contribution of Culex pipiens Complex Mosquitoes to Transmission and Persistence of West Nile Virus in North America. J. Am. Mosq. Control Assoc. 2012, 28, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Hunter, F.F. Influence of elevation and avian or mammalian hosts on attraction of Culex pipiens (Diptera: Culicidae) in southern Ontario. Can. Èntomol. 2010, 142, 250–255. [Google Scholar] [CrossRef]
- Taieb, L.; Ludwig, A.; Ogden, N.H.; Lindsay, R.L.; Iranpour, M.; Gagnon, C.A.; Bicout, D.J. Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec. Int. J. Res. Public Health 2020, 17, 4517. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.L.; Wilson, L.K.; Elliott, J.E.; Bishop, C.A.; Tomlin, A.D.; Henning, K.V. Transfer of DDT and metabolites from fruit orchard soils to American robins (Turdus migratorius) twenty years after agricultural use of DDT in Canada. Arch. Contam. Toxicol. 2000, 39, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding Behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef] [PubMed]
- Irwin, P.; Arcari, C.; Hausbeck, J.; Paskewitz, S. Urban Wet Environment as Mosquito Habitat in the Upper Midwest. EcoHealth 2008, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Doran, B.R.; Lewis, D.J. The species composition and seasonal distribution of mosquitoes in vernal pools in suburban Montreal, Quebec. J. Am. Mosq. Control Assoc. 2003, 19, 339–346. [Google Scholar]
- Yoo, E.-H.; Chen, D.; Diao, C.; Russell, C. The Effects of Weather and Environmental Factors on West Nile Virus Mosquito Abundance in Greater Toronto Area. Earth Interact. 2016, 20, 1–22. [Google Scholar] [CrossRef]
- Yee, N.A. Tires as Habitats for Mosquitoes: A Review of Studies within the Eastern United States: Table 1. J. Med. Èntomol. 2008, 45, 581–593. [Google Scholar] [CrossRef] [PubMed]



| Providers | Dates | Number of Capture Sites/Number of Capture Sites Where Cx. pipiens-restuans Were Present | Traps |
|---|---|---|---|
| PHAC 1 | 2006–2016 | 120/111 | CDC 2 |
| PHAC | 2014–2016 | 24/24 | CDC |
| PHAC | 2017–2018 | 94/91 | CDC |
| PHO 1 | 2012–2019 | 5001/4406 | CDC, OmniDirectional 2, BGS 2 |
| Classes | Train (75%) | Test (25%) |
|---|---|---|
| Forest/tree cover | 1289 | 553 |
| Grass vegetation/herbaceous | 1214 | 520 |
| Mixed | 351 | 150 |
| Paved | 1020 | 437 |
| Classes | Abbreviations | Description |
|---|---|---|
| Agriculture | AG | Annual crops, pastures |
| Water | WA | Rivers, lakes, reservoirs |
| Wetlands | WE | Bogs, fens, marshes, swamps, shallow water |
| Shrubland | SH | Grassland and shrubland |
| Forest | FO | Coniferous, broadleaf, mixed wood In urban areas: may be parks with a high cover of trees |
| Herbaceous | HB | Urban and peri-urban areas with grass cover, such as park without tree, golf courses, garden |
| Mixed area | MA | Mix of building and vegetation, such as residential areas with gardens, industrial sites with vegetation |
| Paved area | PA | Built-up lands with paved surface without vegetation |
| Bare soil | BS | Bare soil |
| Cloud | CL | Cloud |
| Environmental Variables | Percentage of Contribution |
|---|---|
| MIX_PER | 53.5 |
| PA_PER | 25.5 |
| MIX_DIST | 13.7 |
| LULC | 2.2 |
| DRA | 1.3 |
| SHR_DIST | 1.2 |
| HERB_PER | 0.9 |
| FOR_DIST | 0.9 |
| ALT | 0.5 |
| FOR_PER | 0.2 |
| WET_DIST | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moua, Y.; Kotchi, S.O.; Ludwig, A.; Brazeau, S. Mapping the Habitat Suitability of West Nile Virus Vectors in Southern Quebec and Eastern Ontario, Canada, with Species Distribution Modeling and Satellite Earth Observation Data. Remote Sens. 2021, 13, 1637. https://doi.org/10.3390/rs13091637
Moua Y, Kotchi SO, Ludwig A, Brazeau S. Mapping the Habitat Suitability of West Nile Virus Vectors in Southern Quebec and Eastern Ontario, Canada, with Species Distribution Modeling and Satellite Earth Observation Data. Remote Sensing. 2021; 13(9):1637. https://doi.org/10.3390/rs13091637
Chicago/Turabian StyleMoua, Yi, Serge Olivier Kotchi, Antoinette Ludwig, and Stéphanie Brazeau. 2021. "Mapping the Habitat Suitability of West Nile Virus Vectors in Southern Quebec and Eastern Ontario, Canada, with Species Distribution Modeling and Satellite Earth Observation Data" Remote Sensing 13, no. 9: 1637. https://doi.org/10.3390/rs13091637






