Invasive weeds have a large impact on natural ecosystems, as well as urban, peri-urban and agricultural environments. In natural ecosystems, they are responsible for substantial biodiversity loss [
1], while in productive systems they substantially reduce agricultural productivity [
2]. Weed management, through biosecurity programs that facilitate detection, monitoring and eradication, play a critical role in minimising these impacts. Weed detection and mapping is critical for management and eradication programs. Increasingly, remote sensing (RS) is being utilised in these applications, providing a cost-effective and efficient means of mapping weed distributions at multiple spatial and temporal scales.
1.1. Alligator Weed
Alligator weed (
Alternanthera philoxeroides (Mart.) Griseb.) has invaded over 30 countries across Asia, Europe, North America, South America and Oceania [
3]. It is native to South America and was first detected in Australia in the 1940s. Alligator weed is an aquatic plant that forms dense, monotypic stands throughout wetlands and open waterbodies. It also grows in terrestrial habitats. In Australia, the growing season is typically between November and May, with leaf area and biomass peaking during January (mid-summer) [
4]. Alligator weed reproduces and disperses vegetatively through stem fragments [
5]. It has not been observed to produce viable seeds in Australia [
4]. Alligator weed can grow very rapidly [
6], and can double in biomass in 41 days [
4]. It is identified by hollow stems, small white flowers and bright green leaves growing in opposite pairs [
6,
7,
8]. Alligator weed flowers over summer (December–February in Australia) [
4].
Weeds are often managed in a biosecurity context, which aims to protect the environment, economy and community from the impacts of weeds [
9]. Measures are enacted to control weeds based on a strategic approach where priority weeds are selected (often through government legislated noxious weed lists). Control programs are enacted with clear aims to either eradicate, contain or suppress the target weed, depending on the stage of weed invasion and the perceived potential impacts [
10,
11,
12,
13]. Alligator weed is declared a weed of national significance in Australia. It is declared a prohibited weed in Victoria, where it is subject of an ongoing weed biosecurity eradication program implemented by the State Government.
A critical component of any weed biosecurity program are surveillance activities to find the location and extent of the weed, particularly during the early stages of colonisation. The probability of eradication is highest, and eradication is achievable at lowest cost, when a weed invasion is detected at a stage when the species is neither abundant nor widely distributed [
14,
15,
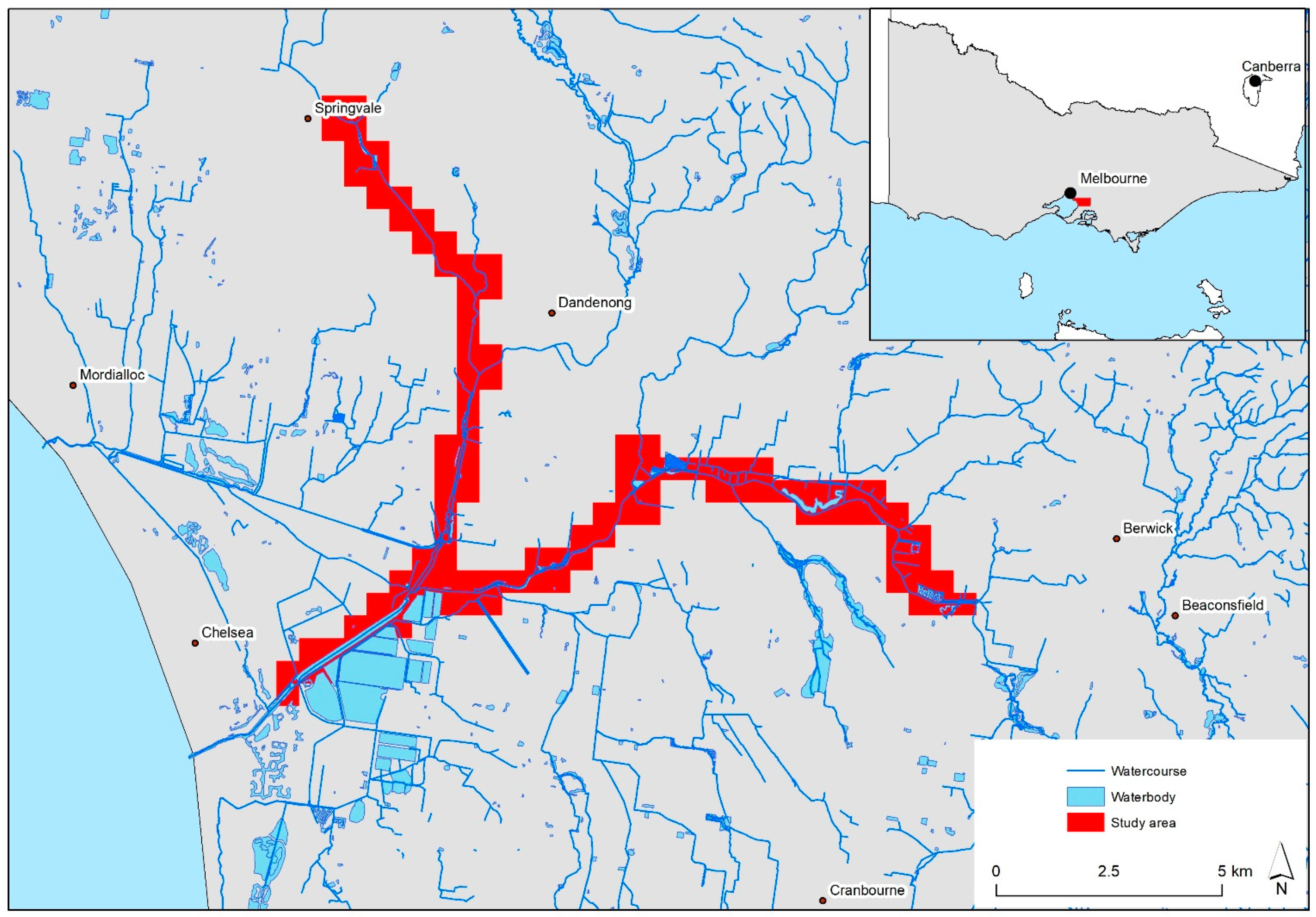
16]. Current approaches within Victoria rely heavily on ground surveys and community reporting. While ground surveys are effective, they do not provide a systematic approach to surveillance across landscapes or regions. Therefore, infestations may go undetected for years since being introduced to a site, compromising eradication programs. For example, in Springvale, Melbourne, Victoria, alligator weed was first reported in 2009; a subsequent study utilising historical aerial photography (AP) showed alligator weed was present at this site for at least five years before being reported [
6]. During this time, the infestation had become much larger (>6000 m
2) and would have produced many propagules capable of downstream dispersal. This example highlights the need for new, innovative approaches for weed detection and surveillance.
1.2. Weed Detection Utilising Remote Sensing
A wide range of RS data and analytical techniques are used to detect weed species, including data from unmanned aerial vehicles (UAVs), aircraft and satellites. Many applications have focused on agricultural weeds, with weed detection from RS being used to improve crop management activities such as herbicide spraying [
17,
18,
19]. In aquatic and riparian environments, RS has been utilised in a range of applications, including mapping vegetation communities [
20], weed abundance [
21,
22] or target species such as Phragmites [
23,
24,
25], water hyacinth (
Eichhornia crassipes (Mart.) Solms) [
26,
27,
28,
29] or giant salvinia (
Salvinia molesta D.S.Mitchell) [
30].
RS offers a range of spectral features which can be used to identify weed species [
17,
31,
32]. In some studies, image texture features are used to enhance discrimination [
31,
33]. Image classification approaches adopted are often specific to the particular study area. Examples used to detect aquatic weeds include random forests (RF) [
28], support vector machines (SVM) [
24], classification and regression tree (CART) [
21], and neural networks (NN) [
25]. Some studies, such as Singh et al. [
29] use multiple classification approaches as part of a multistep process. The choice, and performance, of different classification approaches is largely study-specific. Mukarugwiro et al. [
28] found that, compared with an SVM approach, an RF classification provided better discrimination of water hyacinth, an aquatic weed, from other vegetation. There is an increasing use of machine learning and deep learning in RS weed detection applications [
34]. RF is one such example. Reviews considering the use of RF for RS applications have been published by Pal [
35] and Belgiu and Drăguţ [
36].
RF [
37] is an ensemble classification approach based on a large cohort of decision trees. These trees are formed using random samples of training data, generated by bagging. Each tree provides an independent class vote, with the aggregated results used to determine probabilities for each class per unit (e.g., pixel). The final class is assigned based on the highest probability. The large number of trees generated in this approach improves the classification accuracy [
37]. The use of a large number of trees, and use of randomly selected subsets of training data and input variables to split nodes within these trees, mean the RF approach is considered less sensitive to overfitting than other machine learning classifiers [
36,
37]. Training data is a crucial consideration for RS classification applications, including RF. It has been noted that RF tend to be more robust against data noise and data quality issues such as mislabelling [
36,
37,
38]. Pal [
35] found RFs are also able to deal with unbalanced training data. Colditz [
39] also compared the impact of different class training data allocations, and found the use of proportional training data based on area produced the best results. This is supported by [
40]. However, these findings have not been consistent in the literature, with a different study finding a balanced training data set performed better [
38].
Whilst RS has been used in a range of applications to detect aquatic weeds, there have been limited studies published utilising RS technologies to specifically identify alligator weed.
Göktoǧan et al. [
41] used an SVM algorithm to detect alligator weed from UAV imagery. Clements et al. [
6] published a study documenting the change in alligator weed patches (size and number) within a lake using AP. The extent of alligator weed patches was manually delineated on each of the photos. A study published by Clements et al. [
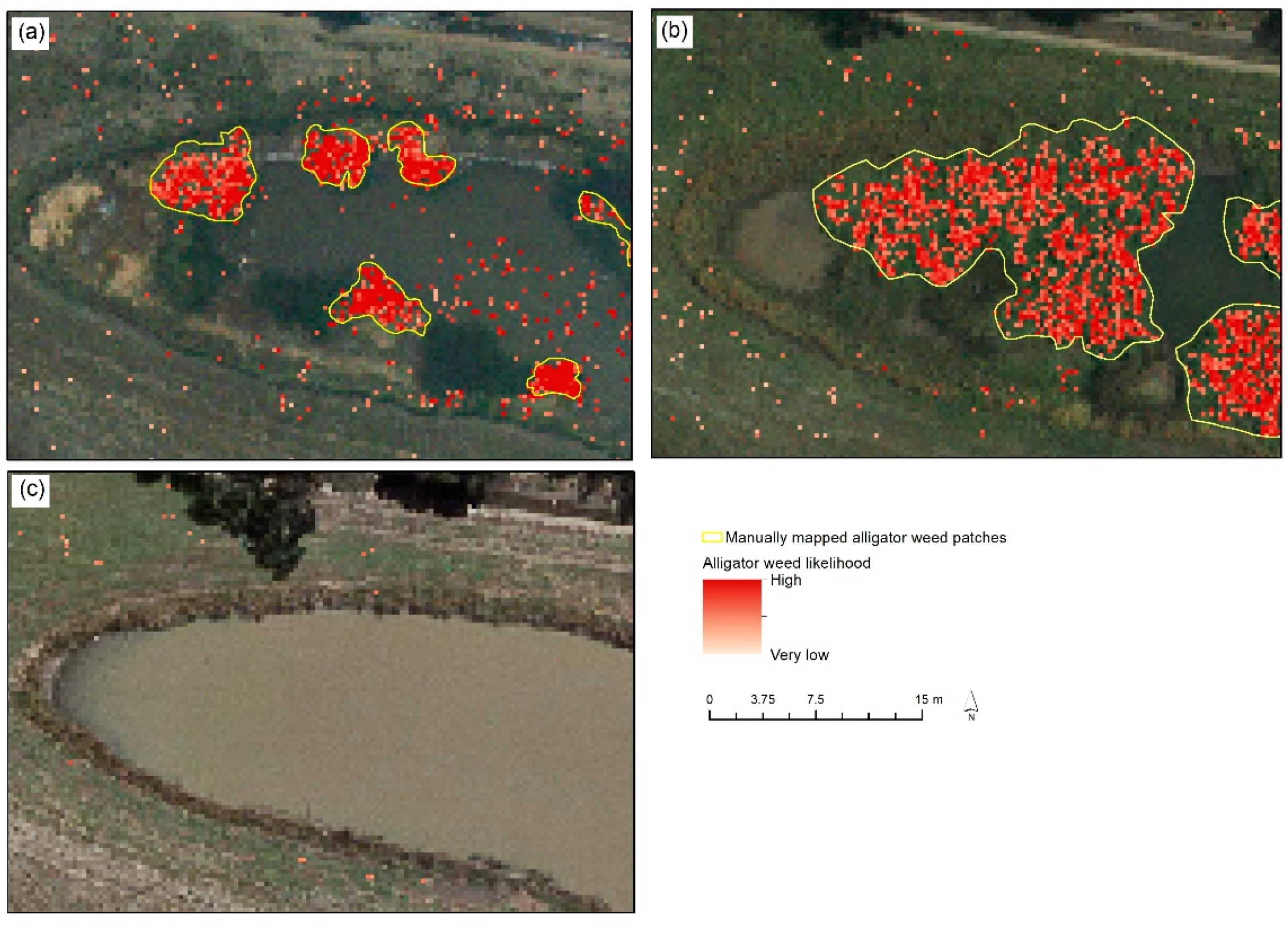
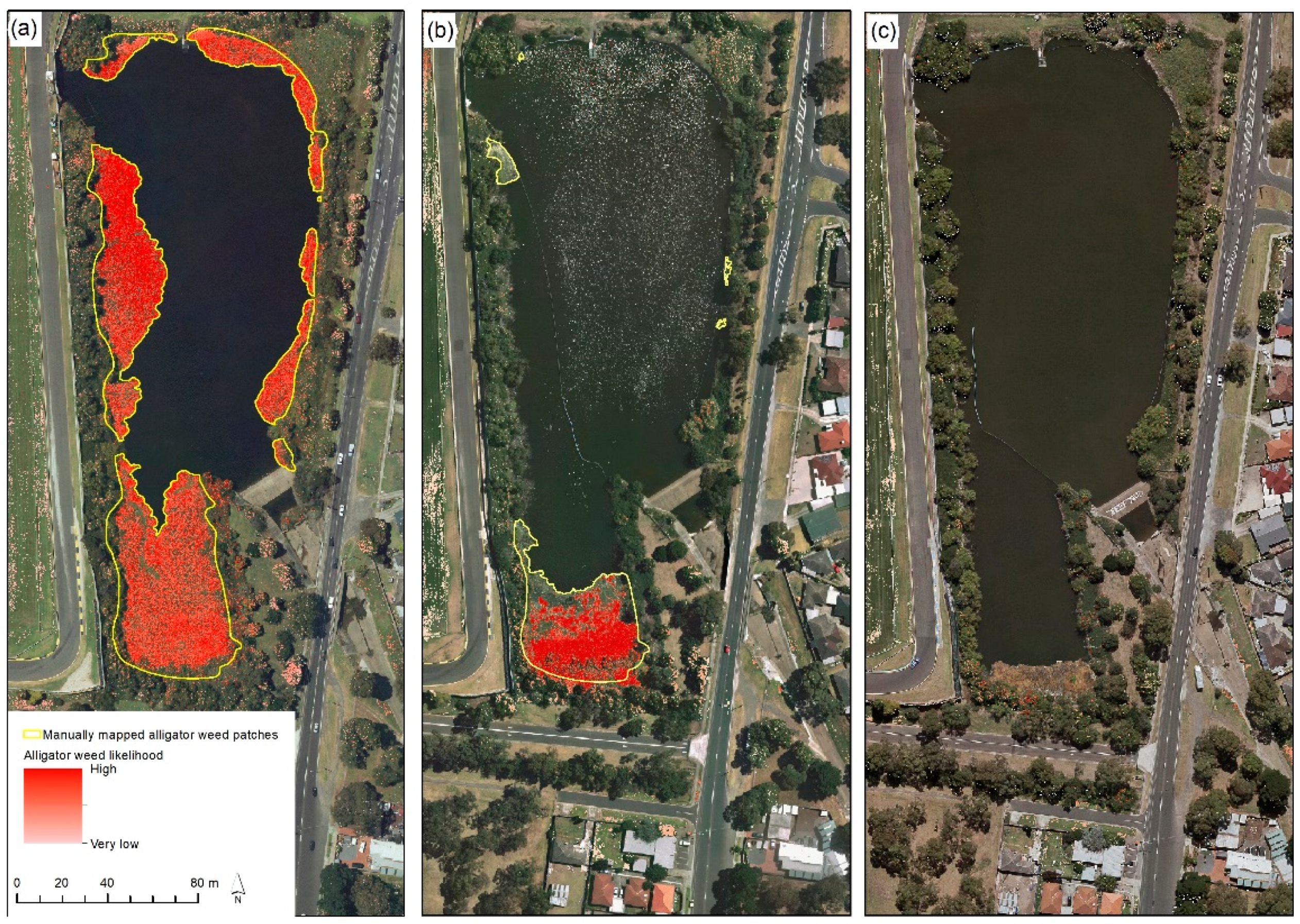
42] compared three approaches to identifying alligator weed along waterways in Victoria, Australia: (1) physically measuring patches of alligator weed in the field and recording the location of these patches with a high-spatial accuracy GPS, (2) manually inspecting UAV imagery and mapping patches of alligator weed in a desktop visual image interpretation exercise and (3) a supervised classification of UAV imagery using a machine learning approach. The training data used for the machine learning was assembled by manually annotating subsets of the UAV imagery. The on-ground surveys were deemed to detect 100% of alligator weed present. The authors found the visual image interpretation exercise to manually map patches of alligator weed identified more patches and smaller areas of alligator weed compared with the machine learning classification approach.
RS can provide valuable data for monitoring changes in vegetation and weeds associated with waterways [
21,
26,
33] and landscapes more generally [
31,
43,
44]. Brinkhoff et al. [
21] used RS to characterise weed growth in irrigation channels and subsequently monitor changes in weed abundance. Khanna et al. [
26] used image classifications from multiple years to asses changes in weed abundance, and then used those outputs to quantify changes in area and the direction of change (increase or decrease) of target species. Vegetation phenology is an important consideration for monitoring applications. While it can impact the accuracy of RS classifications, knowledge of vegetation growth patterns can also be used to aid discrimination and the interpretation of results [
31].
The integration of RS technologies with existing biosecurity weed programs presents opportunities to:
Increase weed detection capacity,
Improve the timeliness of data availability, and
Increase the cost effectiveness of surveillance and management efforts.
This potential has been recognised in a number of studies [
32,
45,
46,
47]. Spring et al. [
48] demonstrated the benefit of complementing existing on-ground survey and management work with AP surveillance in a fire ant (
Solenopsis invicta) eradication program. Brinkhoff et al. [
21] suggested use of their weed monitoring approach to instigate weed removal in irrigation channel maintenance programs. Mukarugwiro et al. [
28] also noted the potential utility of their results to support water hyacinth management.
While the use of RS for weed detection and monitoring is widely accepted, there is a large diversity in both the imagery and approaches taken. In recognition of this, Sheffield and Dugdale [
32] proposed the Weed Aerial Surveillance Program (WASP) as a conceptual framework to guide the development of RS approaches and outputs at multiple spatial scales to complement existing biosecurity programs.
1.3. Aims and Objectives
The studies highlighted above demonstrate the potential for utilising RS imagery to support detection and surveillance programs for alligator weed in waterways. This study aimed to develop an approach utilising regularly acquired AP to support existing weed biosecurity programs to:
Detect alligator weed within urban aquatic landscapes, with a known level of accuracy,
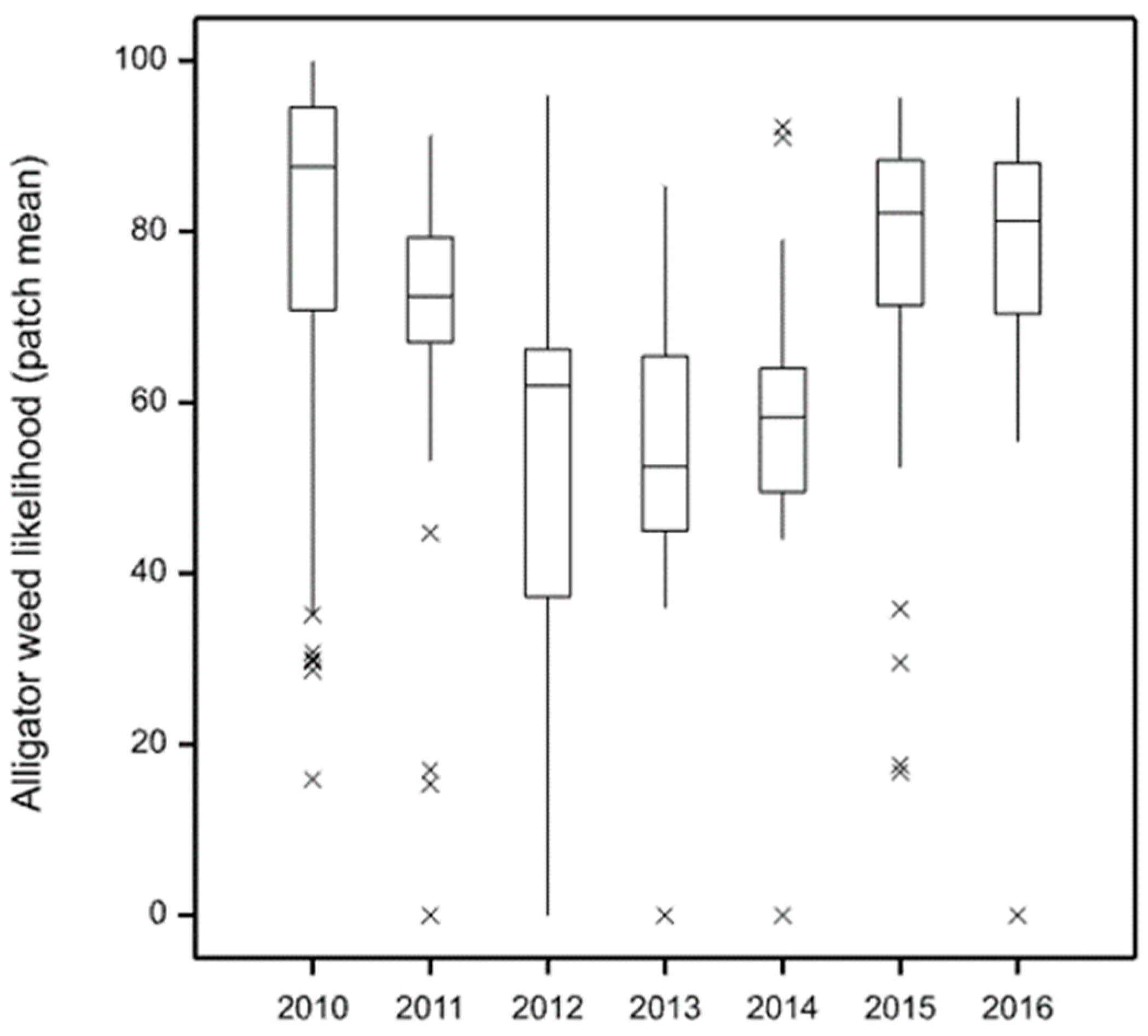
Produce repeatable results over a series of years (2010–2016), and
Be used to support monitoring operations at localised sites, as well as surveillance across landscapes.
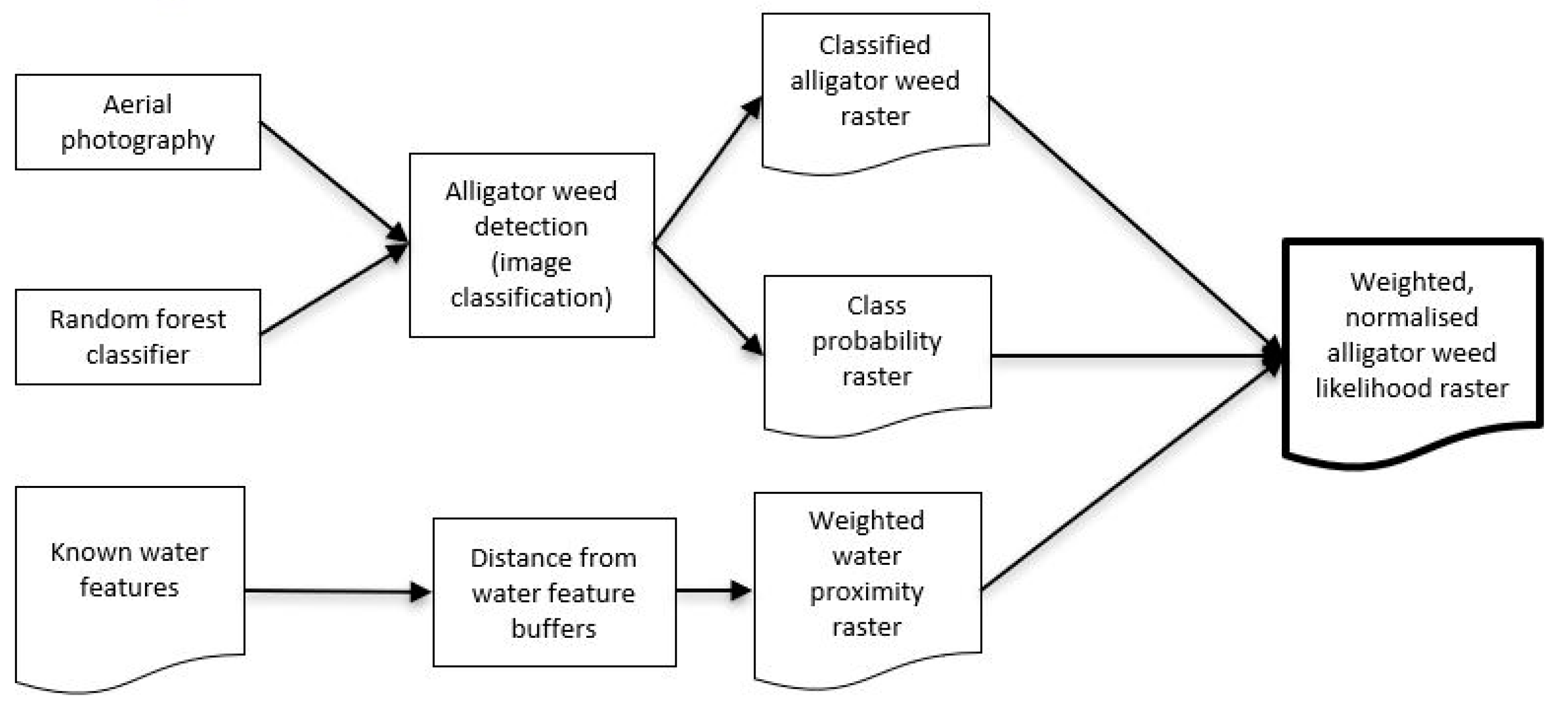
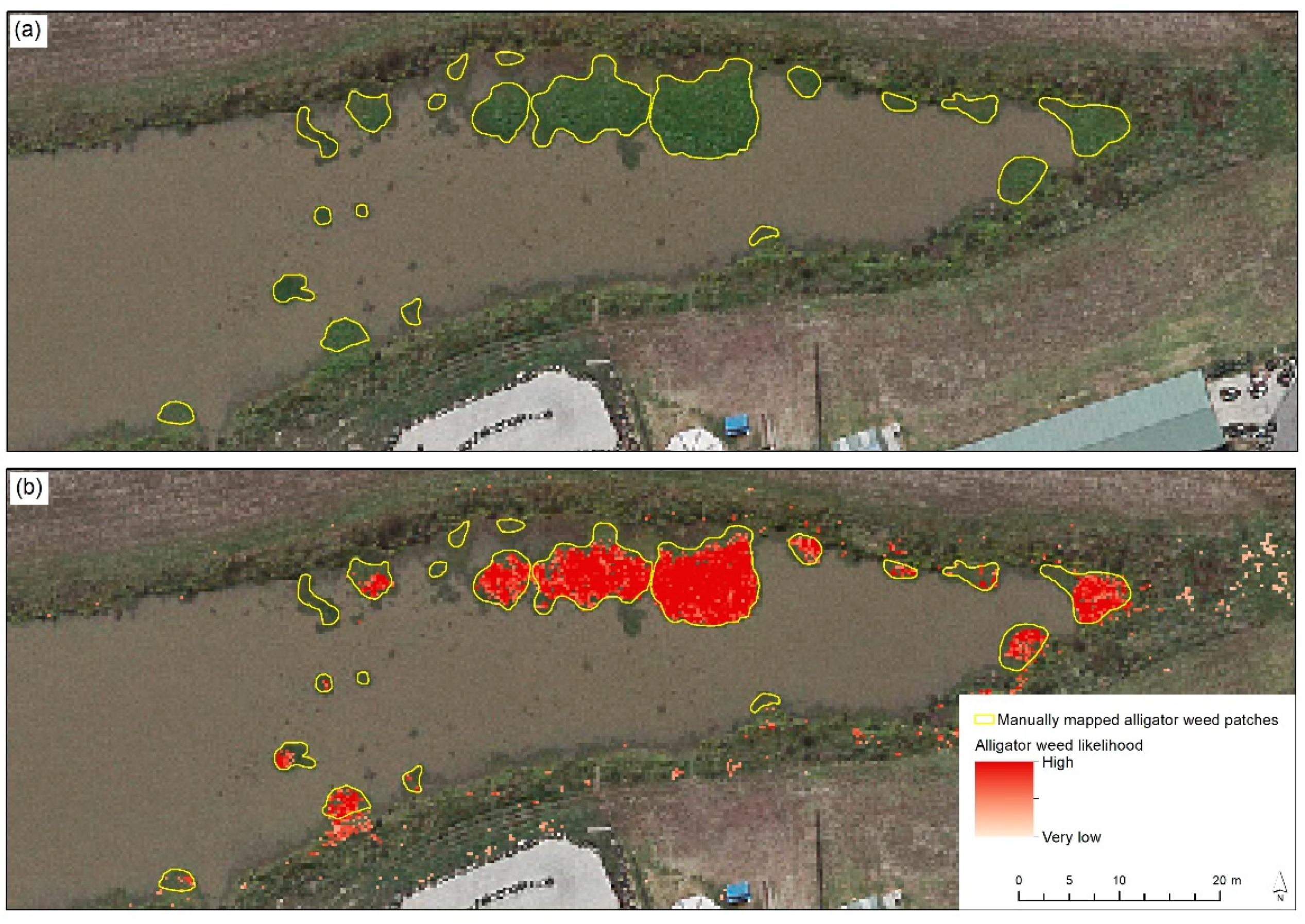
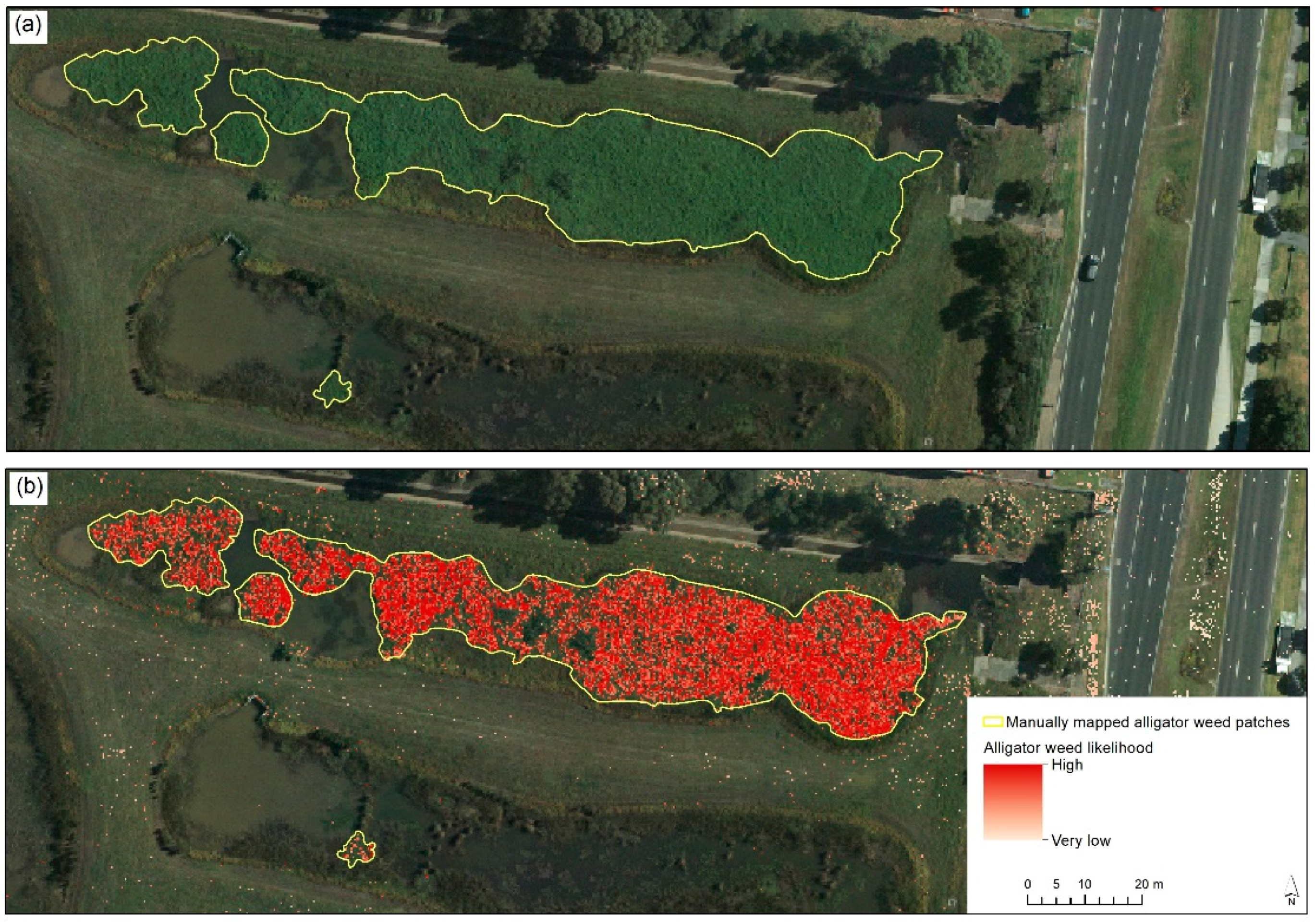
To achieve this, we explored the use of RF, combined with additional spatial data, for detecting aquatic alligator weed, as outlined below. While there are multiple classification approaches available, we used RF, as it is less sensitive to overfitting than some other approaches [
36,
37], is robust against training data mislabelling [
36,
37,
38] and has been shown to support imbalanced training data classes [
35,
39,
40], which are important considerations when dealing with a target species of limited abundance. The ability to examine underlying class probabilities, in addition to the nominated categorical class within the RF classification output, also proved beneficial for this study.