Drone-Based Assessment of Marine Megafauna off Wave-Exposed Sandy Beaches
Abstract
:1. Introduction
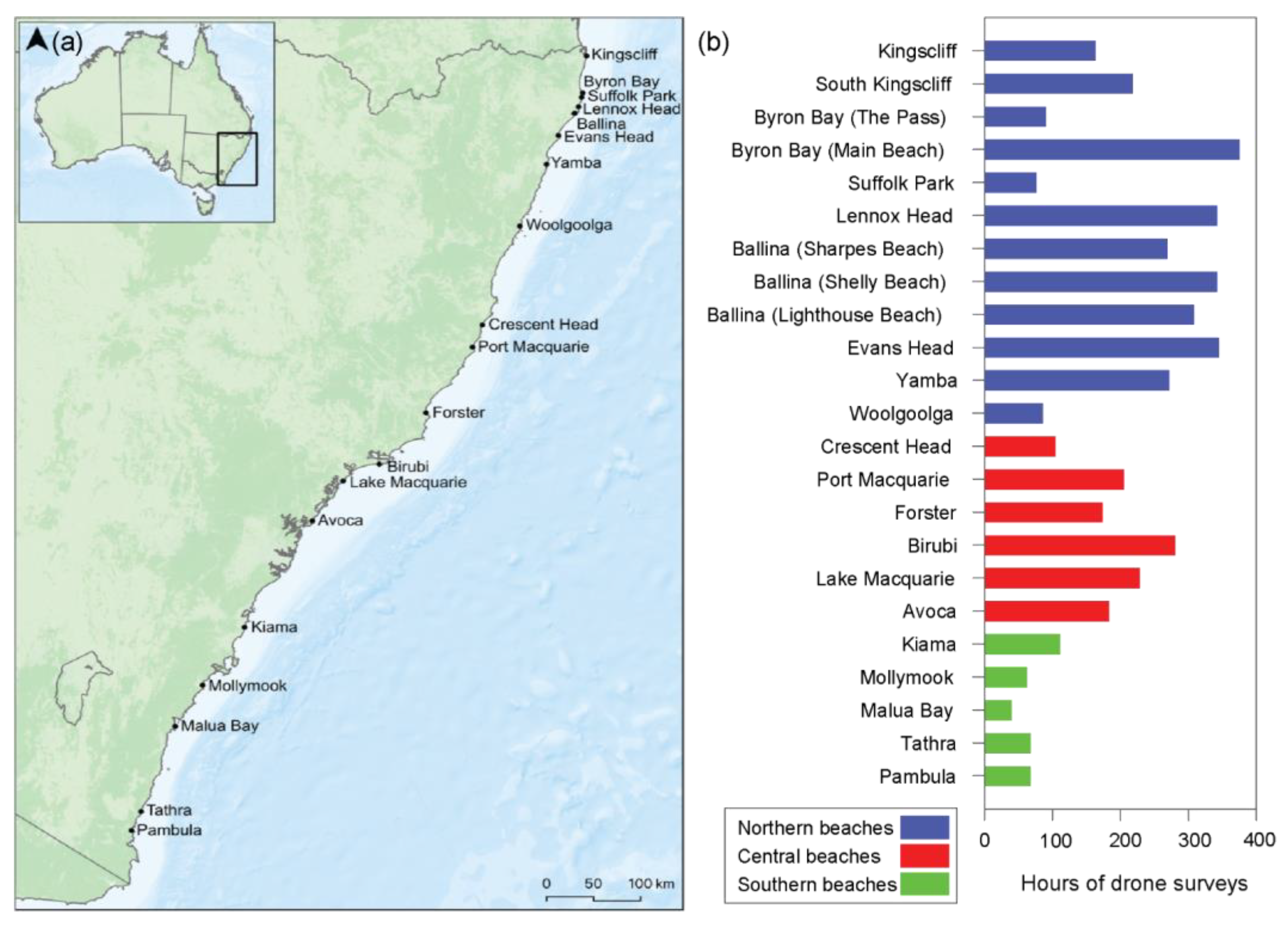
2. Materials and Methods
2.1. Sampling Methodology
2.2. Analysis of Data
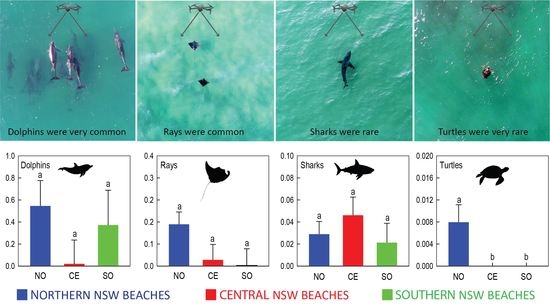
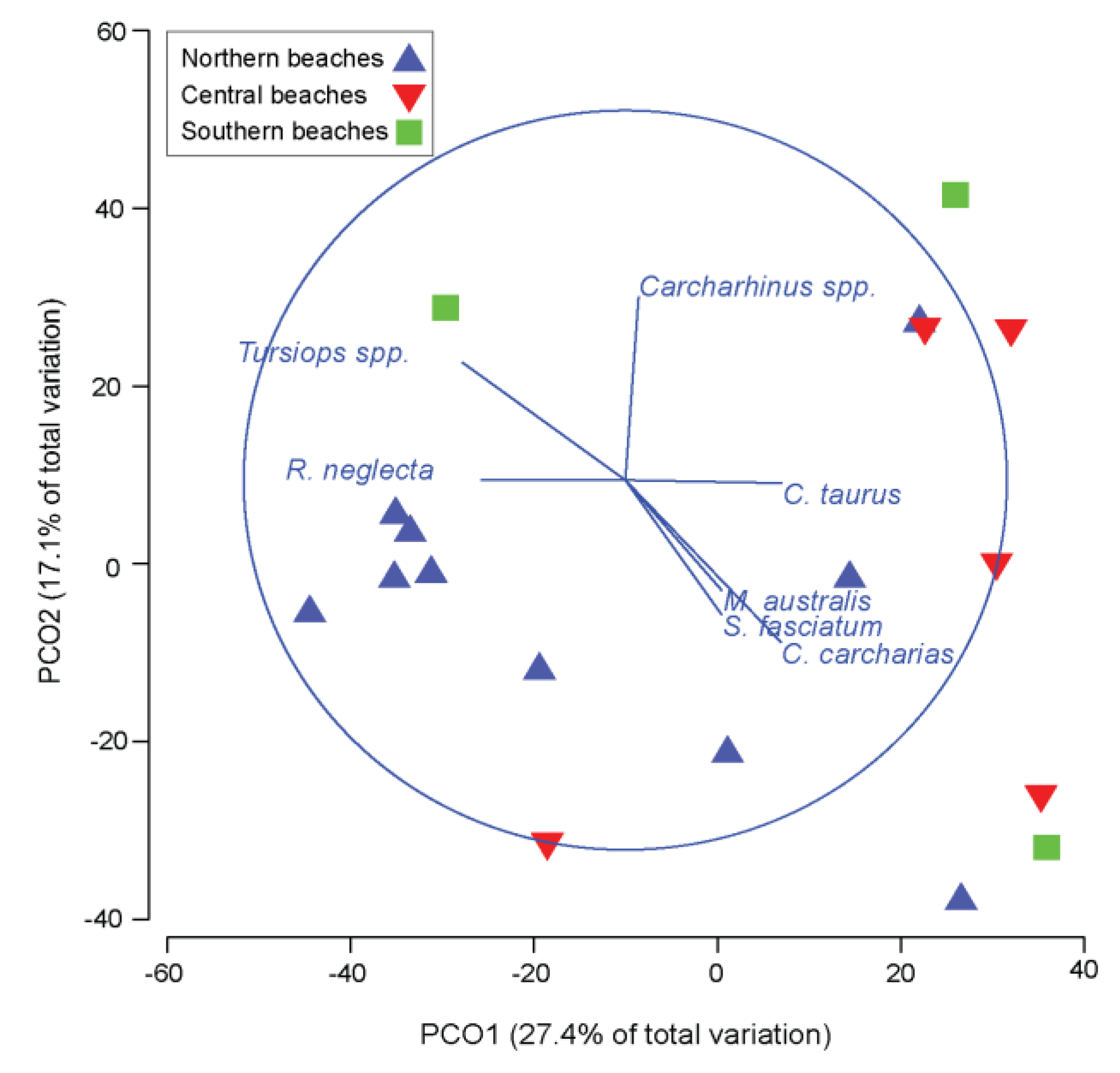
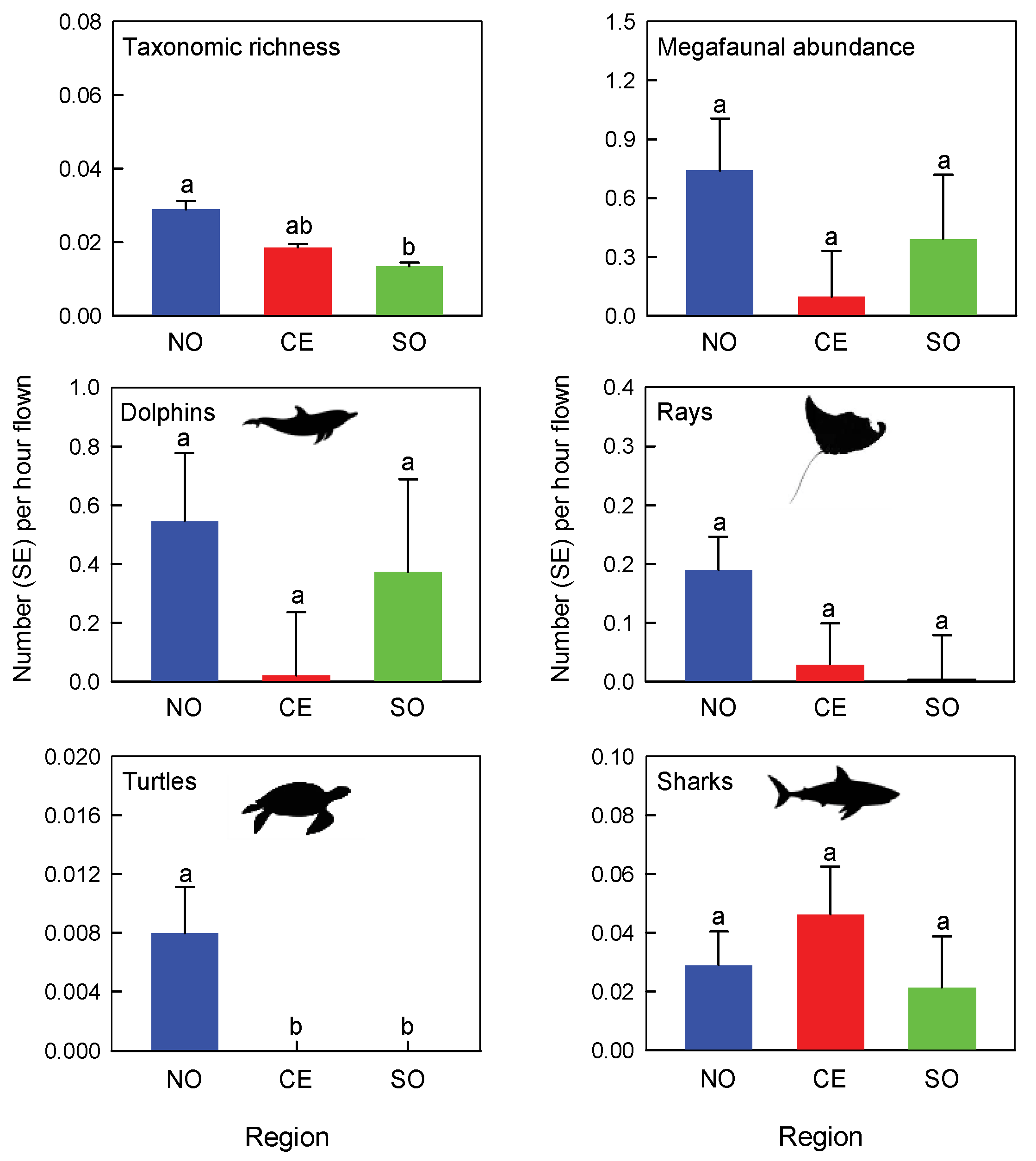
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luijendijk, A.; Hagenaars, G.; Ranasinghe, R.; Baart, F.; Donchyts, G.; Aarninkhof, S. The state of the world’s beaches. Sci. Rep. 2018, 8, 6641. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.L.; Musick, J.A. The Biology of Sea Turtles, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Jones, A.R.; Schlacher, T.A.; Schoeman, D.S.; Weston, M.A.; Withycombe, G.M. Ecological research questions to inform policy and the management of sandy beaches. Ocean. Coast. Manag. 2017, 148, 158–163. [Google Scholar] [CrossRef]
- Nel, R.; Campbell, E.E.; Harris, L.; Hauser, L.; Schoeman, D.S.; McLachlan, A.; du Preez, D.R.; Bezuidenhout, K.; Schlacher, T.A. The status of sandy beach science: Past trends, progress, and possible futures. Estuar. Coast. Shelf Sci. 2014, 150, 1–10. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Dugan, J.; Schoeman, D.S.; Lastra, M.; Jones, A.; Scapini, F.; McLachlan, A.; Defeo, O. Sandy beaches at the brink. Divers. Distrib. 2007, 13, 556–560. [Google Scholar] [CrossRef] [Green Version]
- McLachlan, A.; Defeo, O. The Ecology of Sandy Shores, 3rd ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Guinet, C. Intentional stranding apprenticeship and social play in killer whales (Orcinus orca). Can. J. Zool. 1991, 69, 2712–2716. [Google Scholar] [CrossRef]
- Le Boeuf, B.J.; Peterson, R.S. Social status and mating activity in elephant seals. Science 1969, 163, 91–93. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Colefax, A.P.; Tagliafico, A.; Bishop, M.J.; Giles, A.; Butcher, P.A. Assessing variation in assemblages of large marine fauna off ocean beaches using drones. Mar. Freshw. Res. 2020, 71, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Kelaher, B.P.; Peddemors, V.M.; Hoade, B.; Colefax, A.P.; Butcher, P.A. Comparison of sampling precision for nearshore marine wildlife using unmanned and manned aerial surveys. J. Unmanned Veh. Syst. 2020, 8, 30–43. [Google Scholar] [CrossRef]
- Giles, A.B.; Butcher, P.A.; Colefax, A.P.; Pagendam, D.E.; Mayjor, M.; Kelaher, B.P. Responses of bottlenose dolphins (Tursiops spp.) to small drones. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 677–684. [Google Scholar] [CrossRef]
- Ferguson, G.F.; Ward, T.M.; Ivey, A.R.; Barnes, T.C. Life history of Argyrosomus japonicus, a large sciaenid at the southern part of its global distribution: Implications for fisheries management. Fish. Res. 2014, 151, 148–157. [Google Scholar] [CrossRef]
- Olds, A.D.; Vargas-Fonseca, E.; Connolly, R.M.; Gilby, B.L.; Huijbers, C.M.; Hyndes, G.A.; Layman, C.A.; Whitfield, A.K.; Schlacher, T.A. The ecology of fish in the surf zones of ocean beaches: A global review. Fish Fish. 2018, 19, 78–89. [Google Scholar] [CrossRef]
- Butcher, P.A.; Colefax, A.P.; Gorkin, R.A., III; Kajiura, S.M.; Lopez, N.A.; Mourier, J.; Purcell, C.R.; Skomal, G.B.; Tucker, J.P.; Walsh, A.J.; et al. The drone revolution of shark science: A review. Drones 2021, 5, 8. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Reliability of marine faunal detections in drone-based monitoring. Ocean Coast. Manag. 2019, 174, 108–115. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, X.; Dedman, S.; Rosso, M.; Zhu, J.; Yang, J.; Xia, Y.; Tian, Y.; Zhang, G.; Wang, J. UAV remote sensing applications in marine monitoring: Knowledge visualization and review. Sci. Total Environ. 2022, 838, 155939. [Google Scholar] [CrossRef]
- Pirotta, V.; Hocking, D.P.; Iggleden, J.; Harcourt, R. Drone observations of marine life and human and wildlife Interactions off Sydney, Australia. Drones 2022, 6, 75. [Google Scholar] [CrossRef]
- Fiori, L.; Martinez, E.; Bader, M.K.-F.; Orams, M.B.; Bollard, B. Insights into the use of an unmanned aerial vehicle (UAV) to investigate the behavior of humpback whales (Megaptera novaeangliae) in Vava’u, Kingdom of Tonga. Mar. Mammal Sci. 2020, 36, 209–223. [Google Scholar] [CrossRef]
- Russell, G.; Colefax, A.; Christiansen, F.; Fowler, Z.; Cagnazzi, D. Body condition and migratory timing of east Australian humpback whales. Mar. Ecol. Prog. Ser. 2022, 692, 169–183. [Google Scholar] [CrossRef]
- Brennecke, D.; Siebert, U.; Kindt-Larsen, L.; Midtiby, H.S.; Egemose, H.D.; Ortiz, S.T.; Knickmeier, K.; Wahlberg, M. The fine-scale behavior of harbor porpoises towards pingers. Fish. Res. 2022, 255, 106437. [Google Scholar] [CrossRef]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned aerial vehicles (UAVs) for surveying marine fauna: A dugong case study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef] [Green Version]
- Yauri, S.; Ramos, E.; Castelblanco-Martínez, N.; Niño Torres, C.; Searle, L. Using small drones to photo-identify Antillean manatees: A novel method for monitoring an endangered marine mammal in the Caribbean Sea. Endanger. Species Res. 2020, 41, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Dickson, L.C.D.; Negus, S.R.B.; Eizaguirre, C.; Katselidis, K.A.; Schofield, G. Aerial drone surveys reveal the efficacy of a protected area network for marine megafauna and the value of sea turtles as umbrella species. Drones 2022, 6, 291. [Google Scholar] [CrossRef]
- Dunstan, A.; Robertson, K.; Fitzpatrick, R.; Pickford, J.; Meager, J. Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island. PLoS ONE 2020, 15, e0228524. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing white shark (Carcharodon carcharias) behavior along coastal beaches for conservation-focused shark mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Monteforte, K.I.P.; Butcher, P.A.; Morris, S.G.; Kelaher, B.P. The relative abundance and occurrence of sharks off ocean Beaches of New South Wales, Australia. Biology 2022, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Allan, B.M.; Ierodiaconou, D.; Hoskins, A.J.; Arnould, J.P.Y. A rapid UAV method for assessing body condition in fur seals. Drones 2019, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Fudala, K.; Bialik, R.J. Breeding colony dynamics of southern elephant seals at Patelnia Point, King George Island, Antarctica. Remote Sens. 2020, 12, 2964. [Google Scholar] [CrossRef]
- McIntosh, R.R.; Holmberg, R.; Dann, P. Looking without landing—Using remote piloted aircraft to monitor fur seal populations without disturbance. Front. Mar. Sci. 2018, 5, 202. [Google Scholar] [CrossRef] [Green Version]
- Krause, D.J.; Hinke, J.T.; Goebel, M.E.; Perryman, W.L. Drones minimize Antarctic predator responses relative to ground survey methods: An appeal for context in policy advice. Front. Mar. Sci. 2021, 8, 648772. [Google Scholar] [CrossRef]
- Oleksyn, S.; Tosetto, L.; Raoult, V.; Joyce, K.E.; Williamson, J.E. Going batty: The challenges and opportunities of using drones to monitor the behaviour and habitat use of rays. Drones 2021, 5, 12. [Google Scholar] [CrossRef]
- Tagliafico, A.; Butcher, P.A.; Colefax, A.P.; Clark, G.F.; Kelaher, B.P. Variation in cownose ray Rhinoptera neglecta abundance and group size on the central east coast of Australia. J. Fish Biol. 2020, 96, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ezat, M.; Fritsch, C.; Downs, C. Use of an unmanned aerial vehicle (drone) to survey Nile crocodile populations: A case study at Lake Nyamithi, Ndumo game reserve, South Africa. Biol. Conserv. 2018, 223, 76–81. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Provost, E.J.; Coleman, M.A.; Butcher, P.A.; Colefax, A.; Schlacher, T.A.; Bishop, M.J.; Connolly, R.M.; Gilby, B.L.; Henderson, C.J.; Jones, A.; et al. Quantifying human use of sandy shores with aerial remote sensing technology: The sky is not the limit. Ocean Coast. Manag. 2021, 211, 105750. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- R-Development-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- McCulloch, S.; Meynecke, J.-O.; Franklin, T.; Franklin, W.; Chauvenet, A.L.M. Humpback whale (Megaptera novaeangliae) behaviour determines habitat use in two Australian bays. Mar. Freshw. Res. 2021, 72, 1251–1267. [Google Scholar] [CrossRef]
- Read, T.C.; Wantiez, L.; Werry, J.M.; Farman, R.; Petro, G.; Limpus, C.J. Migrations of green turtles (Chelonia mydas) between nesting and foraging grounds across the Coral Sea. PLoS ONE 2014, 9, e100083. [Google Scholar] [CrossRef] [Green Version]
- Smoothey, A.F.; Lee, K.A.; Peddemors, V.M. Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia. Sci. Rep. 2019, 9, 18864. [Google Scholar] [CrossRef] [Green Version]
- Frusher, S.; Jennings, S.; Haward, M.; Holbrook, N.; Pecl, G.; Hobday, A.; Creighton, C.; D’Silva, D.; Nursey-Bray, M. The short history of research in a marine climate change hotspot: From anecdote to adaptation in south-east Australia. Rev. Fish Biol. Fish. 2013, 24, 593–611. [Google Scholar] [CrossRef]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin: Social relationships in a fission-fusion society. In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Connor, R.C., Tyack, P.L., Whitehead, H., Eds.; The University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. [Google Scholar]
- Hawkins, E.R.; Gartside, D.F. Social and behavioural characteristics of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in northern New South Wales, Australia. Aust. Mammal. 2008, 30, 71–82. [Google Scholar] [CrossRef]
- Grattarola, C.; Minoia, L.; Giorda, F.; Consales, G.; Capanni, F.; Ceciarini, I.; Franchi, E.; Ascheri, D.; Garibaldi, F.; Dondo, A.; et al. Health status of stranded common bottlenose dolphins (Tursiops truncatus) and contamination by immunotoxic pollutants: A threat to the Pelagos Sanctuary—Western Mediterranean Sea. Diversity 2023, 15, 569. [Google Scholar] [CrossRef]
- Bearzi, G.; Fortuna, C.M.; Reeves, R.R. Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea. Mammal Rev. 2009, 39, 92–123. [Google Scholar] [CrossRef]
- Sørensen, P.M.; Haddock, A.; Guarino, E.; Jaakkola, K.; McMullen, C.; Jensen, F.H.; Tyack, P.L.; King, S.L. Anthropogenic noise impairs cooperation in bottlenose dolphins. Curr. Biol. 2023, 33, 749–754.e744. [Google Scholar] [CrossRef] [PubMed]
- Barratclough, A.; Wells, R.S.; Schwacke, L.H.; Rowles, T.K.; Gomez, F.M.; Fauquier, D.A.; Sweeney, J.C.; Townsend, F.I.; Hansen, L.J.; Zolman, E.S.; et al. Health assessments of common bottlenose dolphins (Tursiops truncatus): Past, present, and potential conservation applications. Front. Vet. Sci. 2019, 6, 444. [Google Scholar] [CrossRef] [Green Version]
- Schwacke, L.H.; Twiner, M.J.; De Guise, S.; Balmer, B.C.; Wells, R.S.; Townsend, F.I.; Rotstein, D.C.; Varela, R.A.; Hansen, L.J.; Zolman, E.S.; et al. Eosinophilia and biotoxin exposure in bottlenose dolphins (Tursiops truncatus) from a coastal area impacted by repeated mortality events. Environ. Res. 2010, 110, 548–555. [Google Scholar] [CrossRef]
- Vail, C.S. An overview of increasing incidents of bottlenose dolphin harassment in the Gulf of Mexico and possible dolutions. Front. Mar. Sci. 2016, 3, 110. [Google Scholar] [CrossRef] [Green Version]
- Kassamali-Fox, A.; Christiansen, F.; May-Collado, L.J.; Ramos, E.A.; Kaplin, B.A. Tour boats affect the activity patterns of bottlenose dolphins (Tursiops truncatus) in Bocas del Toro, Panama. PeerJ 2020, 8, e8804. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.; Broad, A.; Ruiz-García, D.; Davis, A.R. Continuous wildlife monitoring using blimps as an aerial platform: A case study observing marine megafauna. Aust. Zool. 2020, 40, 407–415. [Google Scholar] [CrossRef]
- Giles, A.B.; Davies, J.E.; Ren, K.; Kelaher, B. A deep learning algorithm to detect and classify sun glint from high-resolution aerial imagery over shallow marine environments. ISPRS J. Photogramm. Remote Sens. 2021, 181, 20–26. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelaher, B.P.; Monteforte, K.I.; Morris, S.G.; Schlacher, T.A.; March, D.T.; Tucker, J.P.; Butcher, P.A. Drone-Based Assessment of Marine Megafauna off Wave-Exposed Sandy Beaches. Remote Sens. 2023, 15, 4018. https://doi.org/10.3390/rs15164018
Kelaher BP, Monteforte KI, Morris SG, Schlacher TA, March DT, Tucker JP, Butcher PA. Drone-Based Assessment of Marine Megafauna off Wave-Exposed Sandy Beaches. Remote Sensing. 2023; 15(16):4018. https://doi.org/10.3390/rs15164018
Chicago/Turabian StyleKelaher, Brendan P., Kim I. Monteforte, Stephen G. Morris, Thomas A. Schlacher, Duane T. March, James P. Tucker, and Paul A. Butcher. 2023. "Drone-Based Assessment of Marine Megafauna off Wave-Exposed Sandy Beaches" Remote Sensing 15, no. 16: 4018. https://doi.org/10.3390/rs15164018
APA StyleKelaher, B. P., Monteforte, K. I., Morris, S. G., Schlacher, T. A., March, D. T., Tucker, J. P., & Butcher, P. A. (2023). Drone-Based Assessment of Marine Megafauna off Wave-Exposed Sandy Beaches. Remote Sensing, 15(16), 4018. https://doi.org/10.3390/rs15164018