Regional Algorithm for Estimating High Coccolithophore Concentration in the Northeastern Part of the Black Sea
Abstract
1. Introduction
2. Materials and Methods
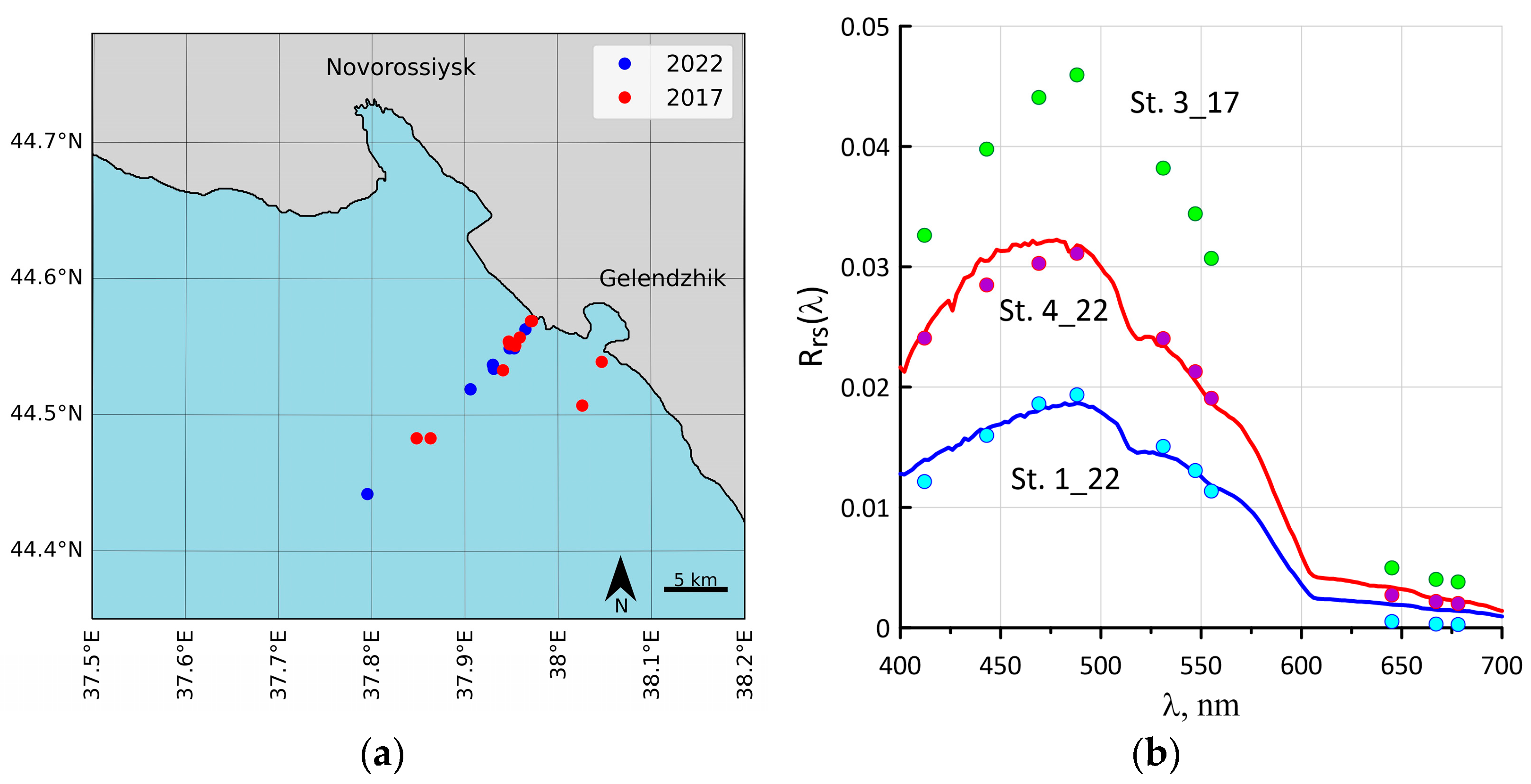
2.1. Study Area and Bio-Optical Measurements
2.2. Sampling
2.3. Satellite Data
2.4. Regional Algorithm 2014
2.5. Error Assessment
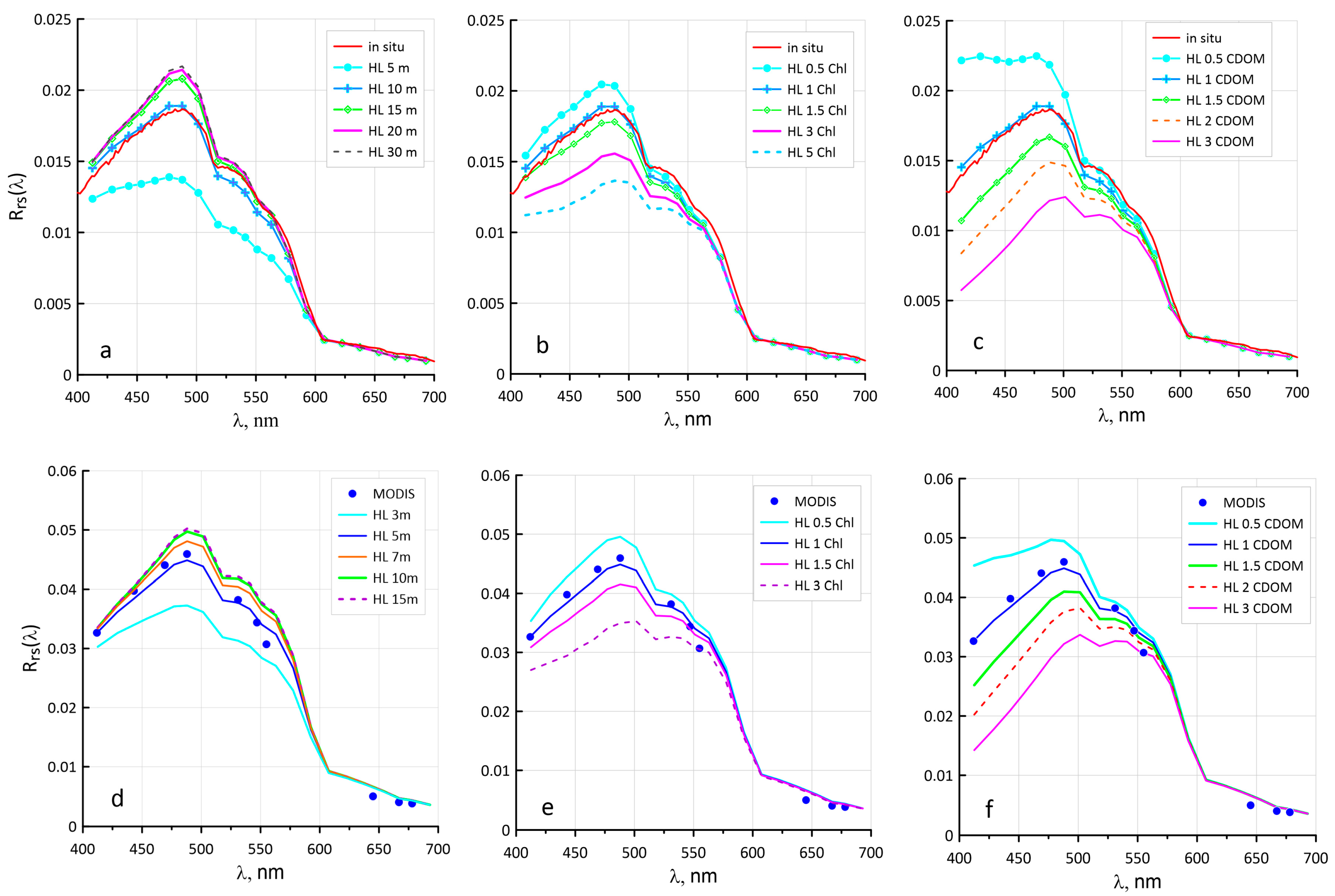
2.6. Tuning of the Hydrolight Model
3. Results
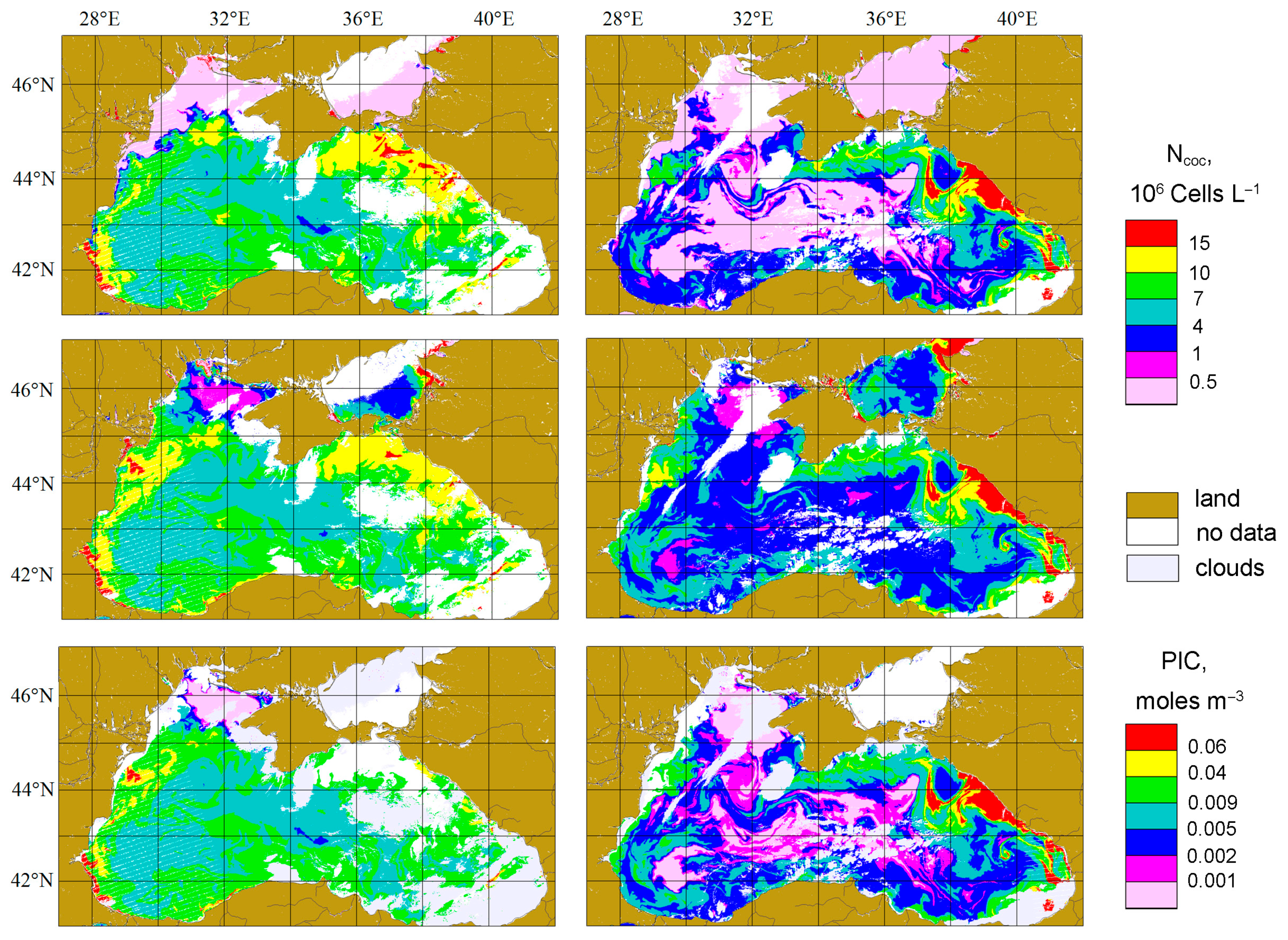
3.1. Coccolithophore Concentrations in the Northeastern Part of the Black Sea in 2017 and 2022
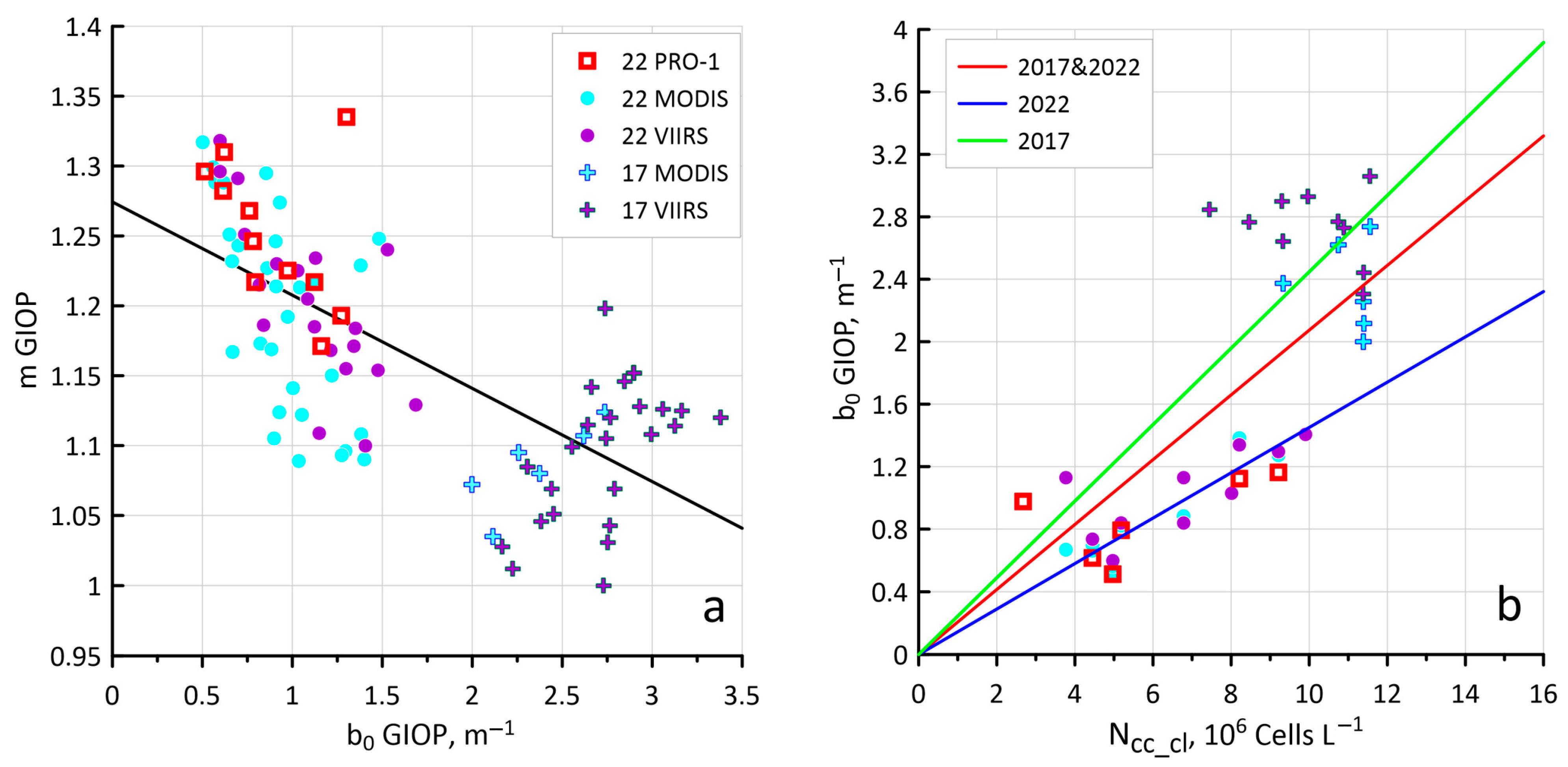
3.2. Sensitivity of the Ncoc Algorithm to Variations in Bio-Optical Characteristics
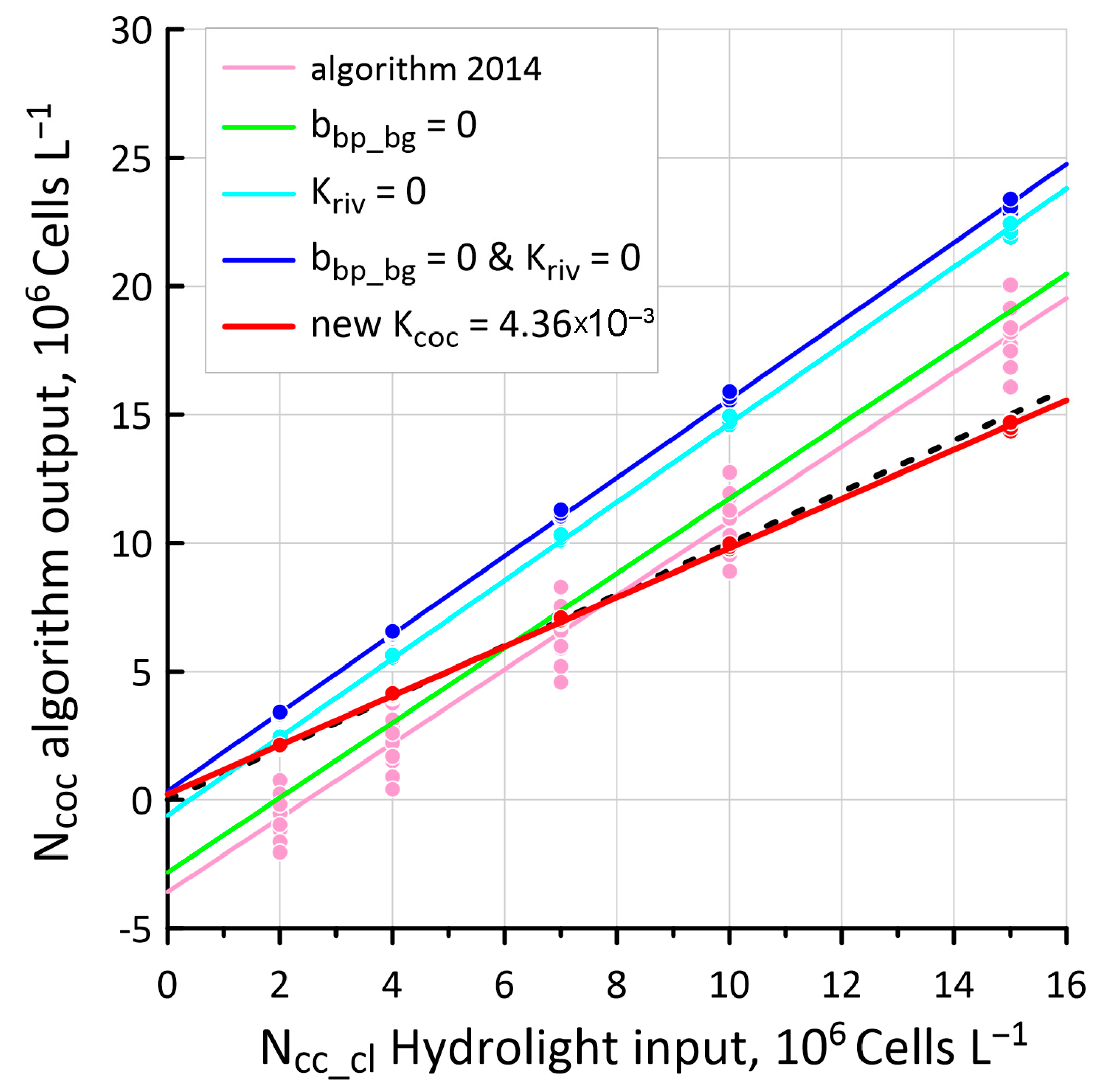
3.3. New Values of Kcoc as a Result of Hydrolight Modeling
3.4. Configuring Other Model Parameters
4. Discussion
4.1. Coccolithophore Blooms in the Black Sea
4.2. Sensitivity of the New Ncoc Algorithm
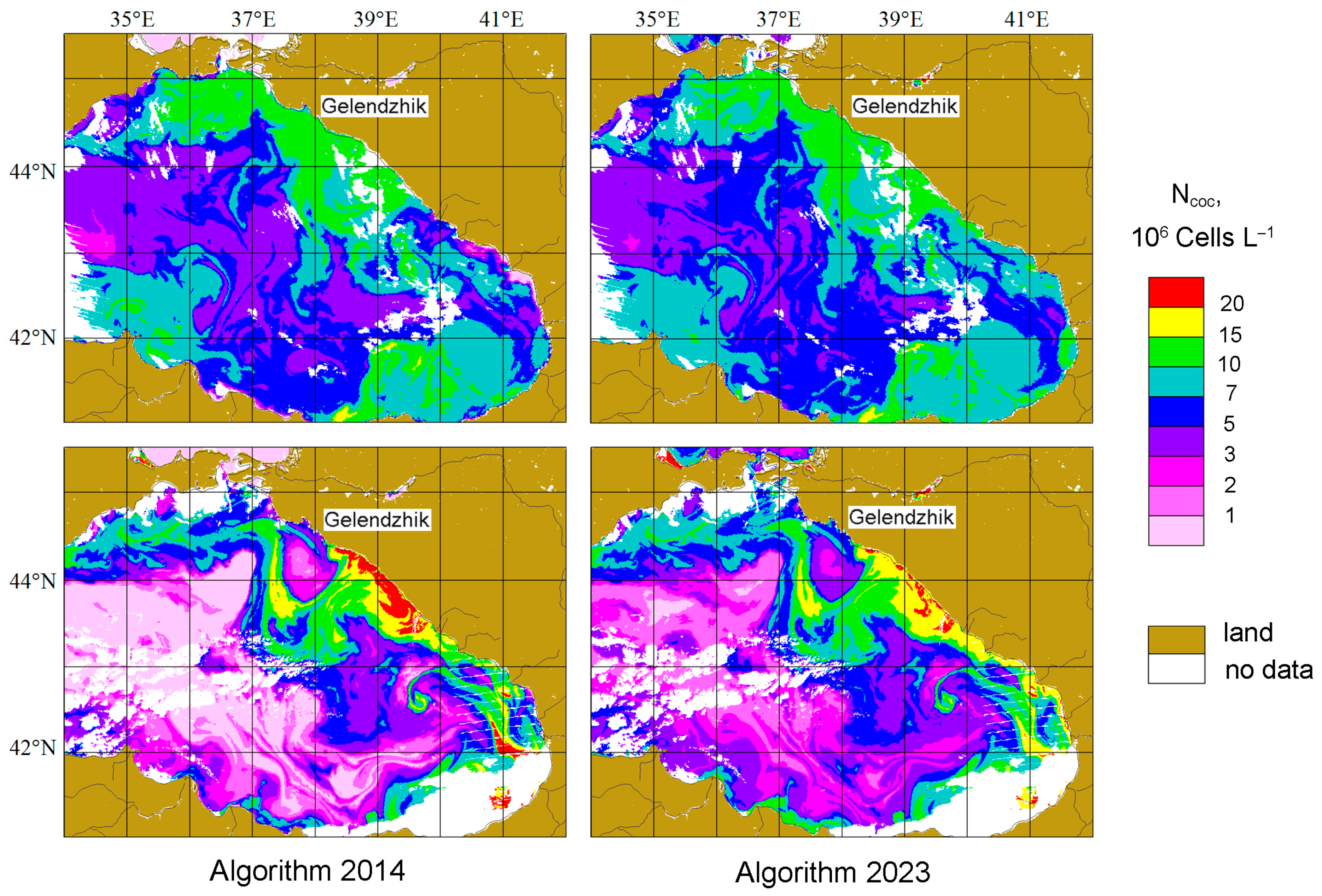
4.3. Comparison of Ncoc Distributions for 2014 and 2023 Algorithms
4.4. The Comparison with PIC Product
4.5. The Effect of the Bloom Phase (The Difference for Two Years)
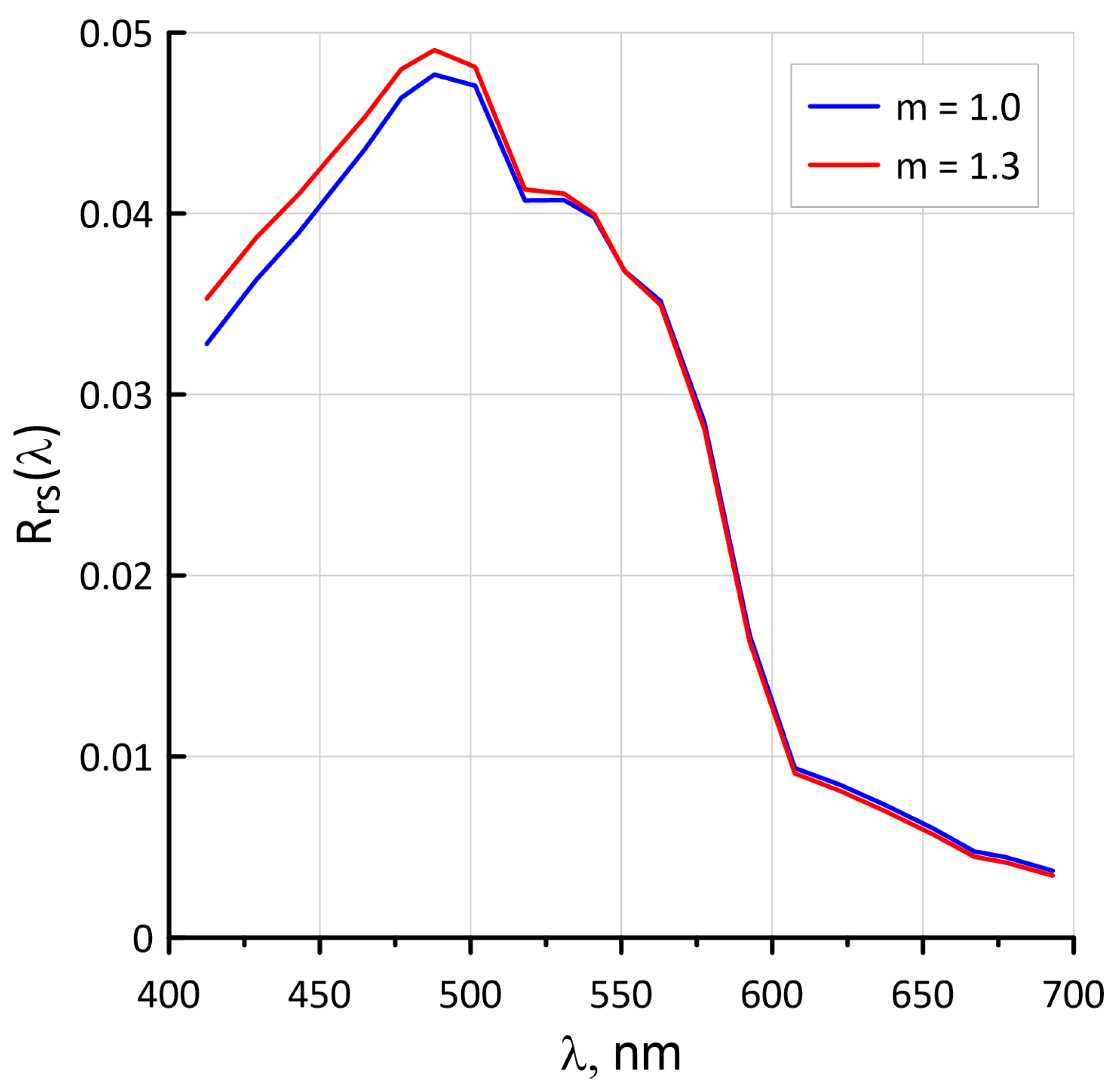
4.6. Influence of the Spectral Index m Values for the Suspended Matter on the Rrs Spectra
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis: Second Edition; Princeton University Press: Princeton, NJ, USA, 2013; ISBN 978-1-4008-4972-7. [Google Scholar]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Keppler, L.; Landschützer, P.; Gruber, N.; Lauvset, S.K.; Stemmler, I. Seasonal Carbon Dynamics in the Near-Global Ocean. Glob. Biogeochem. Cycles 2020, 34, e2020GB006571. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Volk, T.; Hoffert, M.I. Ocean Carbon Pumps: Analysis of Relative Strengths and Efficiencies in Ocean-Driven Atmospheric CO2 Changes. In Geophysical Monograph Series; Sundquist, E.T., Broecker, W.S., Eds.; American Geophysical Union: Washington, DC, USA, 2013; pp. 99–110. ISBN 978-1-118-66432-2. [Google Scholar]
- Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics; Princeton University Press: Princeton, NJ, USA, 2006; ISBN 978-0-691-01707-5. [Google Scholar]
- Henson, S.A.; Sanders, R.; Madsen, E.; Morris, P.J.; Le Moigne, F.; Quartly, G.D. A Reduced Estimate of the Strength of the Ocean’s Biological Carbon Pump: BIOLOGICAL CARBON PUMP STRENGTH. Geophys. Res. Lett. 2011, 38, L04606. [Google Scholar] [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global Assessment of Ocean Carbon Export by Combining Satellite Observations and Food-Web Models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Legendre, L.; Rivkin, R.B.; Weinbauer, M.G.; Guidi, L.; Uitz, J. The Microbial Carbon Pump Concept: Potential Biogeochemical Significance in the Globally Changing Ocean. Prog. Oceanogr. 2015, 134, 432–450. [Google Scholar] [CrossRef]
- Tréguer, P.J.; De La Rocha, C.L. The World Ocean Silica Cycle. Annu. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011; ISBN 978-0-521-15175-7. [Google Scholar]
- Milliman, J.D. Production and Accumulation of Calcium Carbonate in the Ocean: Budget of a Nonsteady State. Glob. Biogeochem. Cycles 1993, 7, 927–957. [Google Scholar] [CrossRef]
- Poulton, A.J.; Adey, T.R.; Balch, W.M.; Holligan, P.M. Relating Coccolithophore Calcification Rates to Phytoplankton Community Dynamics: Regional Differences and Implications for Carbon Export. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 538–557. [Google Scholar] [CrossRef]
- Balch, W.M. The Ecology, Biogeochemistry, and Optical Properties of Coccolithophores. Annu. Rev. Mar. Sci. 2018, 10, 71–98. [Google Scholar] [CrossRef]
- Kondrik, D.V.; Pozdnyakov, D.V.; Johannessen, O.M. Satellite Evidence that E. huxleyi Phytoplankton Blooms Weaken Marine Carbon Sinks. Geophys. Res. Lett. 2018, 45, 846–854. [Google Scholar] [CrossRef]
- Kondrik, D.V.; Kazakov, E.E.; Pozdnyakov, D.V.; Johannessen, O.M. Satellite evidence for enhancement of columnal mixing ratio of atmospheric CO2 over E. huxleyi blooms. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2019, 9, 125–135. [Google Scholar] [CrossRef]
- Morozov, E.A.; Kondrik, D.V.; Chepikova, S.S.; Pozdnyakov, D.V. Atmospheric columnar CO2 enhancement over E. huxleyi blooms: Case studies in the North Atlantic and Arctic waters. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2019, 3, 28–33. [Google Scholar] [CrossRef]
- Cermeño, P.; Dutkiewicz, S.; Harris, R.P.; Follows, M.; Schofield, O.; Falkowski, P.G. The Role of Nutricline Depth in Regulating the Ocean Carbon Cycle. Proc. Natl. Acad. Sci. USA 2008, 105, 20344–20349. [Google Scholar] [CrossRef]
- Holligan, P.M.; Fernández, E.; Aiken, J.; Balch, W.M.; Boyd, P.; Burkill, P.H.; Finch, M.; Groom, S.B.; Malin, G.; Muller, K.; et al. A Biogeochemical Study of the Coccolithophore, Emiliania huxleyi, in the North Atlantic. Glob. Biogeochem. Cycles 1993, 7, 879–900. [Google Scholar] [CrossRef]
- Young, J. Functions of coccoliths. In Coccolithophores; Winter, A., Siesser, W.G., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 63–82. [Google Scholar]
- Iglesias-Rodríguez, M.D.; Brown, C.W.; Doney, S.C.; Kleypas, J.; Kolber, D.; Kolber, Z.; Hayes, P.K.; Falkowski, P.G. Representing key phytoplankton functional groups in ocean carbon cycle models: Coccolithophorids. Glob. Biogeochem. Cycles 2002, 16, 47-1–47-20. [Google Scholar] [CrossRef]
- Paasche, E. A Review of the Coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with Particular Reference to Growth, Coccolith Formation, and Calcification-Photosynthesis Interactions. Phycologia 2001, 40, 503–529. [Google Scholar] [CrossRef]
- Tyrrell, T.; Merico, A. Emiliania huxleyi: Bloom Observations and the Conditions That Induce Them. In Coccolithophores; Thierstein, H.R., Young, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 75–97. ISBN 978-3-642-06016-8. [Google Scholar]
- Morozova-Vodyanitskaya, N.V.; Belogorskaya, E.V. On the Significance of Coccolithophores and, Especially, Pontosphere in the Black Sea Plankton. Tr. Sev. Biol. Stantsii. 1957, 9, 14–21. (In Russian) [Google Scholar]
- Pautova, L.A.; Mikaelyan, A.S.; Silkin, V.A. Structure of Plankton Phytocoenoses in the Shelf Waters of the Northeastern Black Sea during the Emiliania huxleyi Bloom in 2002–2005. Oceanology 2007, 47, 377–385. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Silkin, V.A.; Pautova, L.A. Coccolithophorids in the Black Sea: Their Interannual and Long-Term Changes. Oceanology 2011, 51, 39–48. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Giordano, M.; Chasovnikov, V.K.; Vostokov, S.V.; Podymov, O.I.; Pakhomova, S.V.; Moskalenko, L.V. Drivers of Phytoplankton Blooms in the Northeastern Black Sea. Mar. Pollut. Bull. 2019, 138, 274–284. [Google Scholar] [CrossRef]
- Kopelevich, O.; Burenkov, V.; Sheberstov, S.; Vazyulya, S.; Kravchishina, M.; Pautova, L.; Silkin, V.; Artemiev, V.; Grigoriev, A. Satellite Monitoring of Coccolithophore Blooms in the Black Sea from Ocean Color Data. Remote Sens. Environ. 2014, 146, 113–123. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Burenkov, V.I.; Vazyulya, S.V.; Sheberstov, S.V. Problems of detection of coccolithophore blooms from satellite data. Mod. Probl. Remote Sens. Earth Space 2012, 9, 241–250. [Google Scholar]
- Churilova, T.; Moncheva, S.; Suslin, V.; Kryvenko, O. Intensity, area extent and frequency of coccolithophores Emiliania huxleyi blooms in the Black Sea: Application of remote sensing approach. Int. Multidiscip. Sci. GeoConference SGEM 2017, 17, 871–879. [Google Scholar] [CrossRef]
- Pozdnyakov, D.V.; Pettersson, L.H.; Korosov, A.A. Exploring the Marine Ecology from Space; Springer Remote Sensing/Photogrammetry; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-30074-0. [Google Scholar]
- Pozdnyakov, D.V.; Gnatiuk, N.V.; Davy, R.; Bobylev, L.P. The Phenomenon of Emiliania huxleyi in Aspects of Global Climate and the Ecology of the World Ocean. GES 2021, 14, 50–62. [Google Scholar] [CrossRef]
- Cokacar, T.; Kubilay, N.; Oguz, T. Structure of Emiliania huxleyi Blooms in the Black Sea Surface Waters as Detected by SeaWIFS Imagery. Geophys. Res. Lett. 2001, 28, 4607–4610. [Google Scholar] [CrossRef]
- Kideys, A.E. Fall and Rise of the Black Sea Ecosystem. Science 2002, 297, 1482–1484. [Google Scholar] [CrossRef]
- Karabashev, G.S.; Sheberstov, S.V.; Yakubenko, V.G. The June maximum of normalized radiance and its relation to the hydrological conditions and coccolithophorid bloom in the Black Sea. Oceanology 2006, 46, 305–317. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Burenkov, V.I.; Sheberstov, S.V.; Vazulya, S.V.; Sahling, I.V. Coccolithophore Blooms in the North-Eastern Black Sea. In Proceedings of the Twelfth International Conference on the Mediterranean Coastal Environment, Varna, Bulgaria, 6–10 October 2015; MEDCOAST, Mediterranean Coastal Foundation: Dalyan, Turkey, 2015; Volume 1, pp. 363–374. [Google Scholar]
- Karabashev, G.S.; Evdoshenko, M.A. Manifestations of the rim current, coccolithophore blooms, and continental runoff in the long-term monthly mean distributions of satellite reflectance coefficients of the Black Sea. Oceanology 2015, 55, 36–46. [Google Scholar] [CrossRef]
- Karabashev, G.S. On the mesoscale structure of the Black Sea satellite images during coccolithophorid bloom. Remote Sens. Earth Space 2018, 15, 183–190. [Google Scholar] [CrossRef]
- Vostokov, S.V.; Vostokova, A.S.; Vazyulya, S.V. Seasonal and Long-Term Variability of Coccolithophores in the Black Sea According to Remote Sensing Data and the Results of Field Investigations. JMSE 2022, 10, 97. [Google Scholar] [CrossRef]
- Sahling, I.V.; Vazyulya, S.V.; Glukhovets, D.I.; Sheberstov, S.V.; Burenkov, V.I.; Yushmanova, A.V. Atlas of Bio-Optical Characteristics of the Russian Seas According to Satellite Ocean Color Scanners. Available online: http://optics.ocean.ru/ (accessed on 18 April 2023).
- Hedley, J.D.; Mobley, C.D. Hydrolight 6.0 Ecolight 6.0 Technical Documentation; Numerical Optics Ltd.: Tiverton, UK, 2019; Available online: https://scholar.google.com/scholar_lookup?title=HYDROLIGHT+6.0+ECOLIGHT+6.0+Technical+Documentation&author=Hedley,+J.D.&author=Mobley,+C.D.&publication_year=2019 (accessed on 18 April 2023).
- Artemiev, V.A.; Burenkov, V.I.; Vortman, M.I.; Grigoriev, A.V.; Kopelevich, O.V.; Khrapko, A.N. Sea-truth measurements of ocean color: A new floating spectroradiometer and its metrology. Oceanology 2000, 40, 139–145. [Google Scholar]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral Remote Sensing for Shallow Waters. I. A Semianalytical Model. Appl. Opt. 1998, 37, 6329–6338. [Google Scholar] [CrossRef]
- Burenkov, V.I.; Sheberstov, S.V.; Artemiev, V.A.; Taskaev, V.R. Estimation of Measurement Error of the Seawater Beam Attenuation Coefficient in Turbid Water of Arctic Seas. Light Eng. 2019, 27, 103–111. [Google Scholar] [CrossRef]
- Glukhovets, D.I.; Sheberstov, S.V.; Kopelevich, O.V.; Zaytseva, A.F.; Pogosyan, S.I. Measuring the sea water absorption factor using integrating sphere. Light Eng. 2018, 26, 120–126. [Google Scholar] [CrossRef]
- Yushmanova, A.V.; Kopelevich, O.V.; Vazyulya, S.V.; Sahling, I.V. Inter-annual variability of the seawater light absorption in surface layer of the northeastern Black Sea in connection with hydrometeorological factors. J. Mar. Sci. Eng. 2019, 7, 326. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a Determination: Improvements in Methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Arar, E.J.; Collins, G.B. Method 445.0 In Vitro Determination of Chlorophyll a and Pheophytin in Marine and Freshwater Algae by Fluorescence; United States Environmental Protection Agency: Washington, DC, USA, 1997.
- Tomas, C.R. Identifying Marine Phytoplankton; Academic Press: New York, NY, USA, 1997; p. 858. [Google Scholar]
- Throndsen, J.; Hasle, G.R.; Tangen, K.I. Phytoplankton of Norwegian Coastal Waters; Almater Forlorlag AS: Oslo, Norway, 2007; ISBN 978-82-7858-037-0. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Moncheva, S.; Parr, B. Manual for Phytoplankton Sampling and Analysis in the Black Sea. Phytoplankton Manual, UP-GRADE Black Sea Scene Project, FP7. 2010, p. 67. Available online: http://blacksea-commission.org/Downloads/Phytoplankton_%20Mannual-Final-1.pdf (accessed on 3 April 2023).
- Voss, K.J.; Balch, W.M.; Kilpatrick, K.A. Scattering and Attenuation Properties of Emiliania huxleyi Cells and Their Detached Coccoliths. Limnol. Oceanogr. 1998, 43, 870–876. [Google Scholar] [CrossRef]
- NASA’s OceanColor Web, Level 1&2 Brouser. Available online: https://oceancolor.gsfc.nasa.gov/cgi/browse.pl (accessed on 18 April 2023).
- Sheberstov, S.V. A system of batch processing of oceanological satellite data. Curr. Probl. Remote Sens. Earth Space 2015, 12, 154–161. Available online: http://jr.rse.cosmos.ru/article.aspx?id=1447&lang=eng (accessed on 18 April 2023).
- Cazzaniga, I.; Zibordi, G.; Mélin, F. Spectral Variations of the Remote Sensing Reflectance during Coccolithophore Blooms in the Western Black Sea. Remote Sens. Environ. 2021, 264, 112607. [Google Scholar] [CrossRef]
- Mobley, C.; Stramski, D.; Bissett, W.; Boss, E. Optical Modeling of Ocean Waters: Is the Case 1–Case 2 Classification Still Useful? Oceanography 2004, 17, 60–67. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption Spectrum (380–700 Nm) of Pure Water II Integrating Cavity Measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef]
- Röttgers, R.; McKee, D.; Utschig, C. Temperature and Salinity Correction Coefficients for Light Absorption by Water in the Visible to Infrared Spectral Region. Opt. Express 2014, 22, 25093–25108. [Google Scholar] [CrossRef]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Waters; Academic Press: San Diego, CA, USA, 1994; ISBN 978-0-12-502750-2. [Google Scholar]
- Loisel, H.; Morel, A. Light Scattering and Chlorophyll Concentration in Case 1 Waters: A Reexamination. Limnol. Oceanogr. 1998, 43, 847–858. [Google Scholar] [CrossRef]
- Morel, A.; Antoine, D.; Gentili, B. Bidirectional Reflectance of Oceanic Waters: Accounting for Raman Emission and Varying Particle Scattering Phase Function. Appl. Opt. 2002, 41, 6289–6306. [Google Scholar] [CrossRef]
- Werdell, P.J.; Franz, B.A.; Bailey, S.W.; Feldman, G.C.; Boss, E.; Brando, V.E.; Dowell, M.; Hirata, T.; Lavender, S.J.; Lee, Z.; et al. Generalized Ocean Color Inversion Model for Retrieving Marine Inherent Optical Properties. Appl. Opt. 2013, 52, 2019–2037. [Google Scholar] [CrossRef]
- Tyrrell, T.; Holligan, P.M.; Mobley, C.D. Optical Impacts of Oceanic Coccolithophore Blooms. J. Geophys. Res. 1999, 104, 3223–3241. [Google Scholar] [CrossRef]
- Kopelevich, O.; Sheberstov, S.; Vazyulya, S. Effect of a Coccolithophore Bloom on the Underwater Light Field and the Albedo of the Water Column. JMSE 2020, 8, 456. [Google Scholar] [CrossRef]
- Kopelevich, O.V.; Sahling, I.V.; Vazyulya, S.V.; Glukhovets, D.I.; Sheberstov, S.V.; Burenkov, V.I.; Karalli, P.G.; Yushmanova, A.V. Bio-Optical Characteristics of the Seas, Surrounding the Western Part of Russia, from Data of the Satellite Ocean Color Scanners of 1998–2017; VASh FORMAT, OOO: Moscow, Russia, 2018. [Google Scholar]
- Glukhovets, D.; Kopelevich, O.; Yushmanova, A.; Vazyulya, S.; Sheberstov, S.; Karalli, P.; Sahling, I. Evaluation of the CDOM Absorption Coefficient in the Arctic Seas Based on Sentinel-3 OLCI Data. Remote Sens. 2020, 12, 3210. [Google Scholar] [CrossRef]
- Arkhipkin, V.S.; Gippius, F.N.; Koltermann, K.P.; Surkova, G.V. Wind Waves in the Black Sea: Results of a Hindcast Study. Nat. Hazards Earth Syst. Sci. 2014, 14, 2883–2897. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Pautova, L.A.; Chasovnikov, V.K.; Mosharov, S.A.; Silkin, V.A. Alternation of Diatoms and Coccolithophores in the North-Eastern Black Sea: A Response to Nutrient Changes. Hydrobiologia 2015, 755, 89–105. [Google Scholar] [CrossRef]
- Drits, A.V.; Nikishina, A.B.; Sergeeva, V.M.; Solov’ev, K.A. Feeding, Respiration, and Excretion of the Black Sea Noctiluca Scintillans MacCartney in Summer. Oceanology 2013, 53, 442–450. [Google Scholar] [CrossRef]
- Burenkov, V.I.; Kopelevich, O.V.; Rat’kova, T.N.; Sheberstov, S.V. Satellite Observations of the Coccolithophorid Bloom in the Barents Sea. Oceanology 2011, 51, 766–774. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Mikaelyan, A.S.; Stanichny, S.V. Summer and Winter Coccolithophore Blooms in the Black Sea and Their Impact on Production of Dissolved Organic Matter from Bio-Argo Data. J. Mar. Syst. 2019, 199, 103220. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Mikaelyan, A.S.; Stanichny, S.V. Extremely Strong Coccolithophore Blooms in the Black Sea: The Decisive Role of Winter Vertical Entrainment of Deep Water. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2021, 173, 103554. [Google Scholar] [CrossRef]
- Kubryakova, E.A.; Kubryakov, A.A.; Mikaelyan, A.S. Winter Coccolithophore Blooms in the Black Sea: Interannual Variability and Driving Factors. J. Mar. Syst. 2021, 213, 103461. [Google Scholar] [CrossRef]
- Vostokov, S.V.; Lobkovskiy, L.I.; Vostokova, A.S.; Solov’ev, D.M. Seasonal and Interannual Variability of Phytoplankton in the Black Sea on the Basis of Remote Sensing Data and In Situ Measurements of Chlorophyll-a. Dokl. Earth Sci. 2019, 485, 293–297. [Google Scholar] [CrossRef]
- Vostokova, A.; Vostokov, S.; Saling, I. Regional Features of Seasonal Variability of Phytoplankton in the Black Sea Studied by Remote Sensing Data. In Proceedings of the 28th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Tomsk, Russia, 4–8 July 2022; Romanovskii, O.A., Matvienko, G.G., Eds.; SPIE: Tomsk, Russia, 2022; p. 281. [Google Scholar] [CrossRef]
- Gordon, H.R.; Balch, W.M. MODIS Detached Coccolith Concentration Algorithm Theoretical Basis Document, Version 4, Algorithm Theoretical Basis Document. 1999; p. 27. Available online: http://modis.gsfc.nasa.gov/data/atbd/atbd_mod23.pdf (accessed on 3 April 2023).
- Gordon, H.R.; Boynton, G.C.; Balch, W.M.; Groom, S.B.; Harbour, D.S.; Smyth, T.J. Retrieval of Coccolithophore Calcite Concentration from SeaWiFS Imagery. Geophys. Res. Lett. 2001, 28, 1587–1590. [Google Scholar] [CrossRef]
- Kalinskaya, D.V.; Papkova, A.S. Why Is It Important to Consider Dust Aerosol in the Sevastopol and Black Sea Region during Remote Sensing Tasks? A Case Study. Remote Sens. 2022, 14, 1890. [Google Scholar] [CrossRef]
- Konyukhov, I.V.; Glukhovets, D.I. A set of optical methods for studying marine phytoplankton. Oceanology 2017, 57, 419–423. [Google Scholar] [CrossRef]
- Glukhovets, D.I.; Goldin, Y.A. Express method for chlorophyll concentration assessment. J. Photochem. Photobiol. 2021, 8, 100083. [Google Scholar] [CrossRef]

| St. 1_22 | St. 3_17 | |||||||
|---|---|---|---|---|---|---|---|---|
| Zcoc | 5 | 10 | 15 | 20 | 3 | 5 | 7 | 10 |
| Ncoc | 1.4 | 3.1 | 3.6 | 3.8 | 11.0 | 14.3 | 15.7 | 16.4 |
| Δ | −53% | 1% | 19% | 24% | −16% | 10% | 21% | 26% |
| X * Chl | 0.5 | 1 | 1.5 | 3 | 0.5 | 1 | 1.5 | 3 |
| Ncoc | 3.5 | 3.1 | 2.8 | 2.0 | 15.2 | 14.3 | 13.6 | 11.8 |
| Δ | 14% | 1% | −10% | −36% | 17% | 10% | 4% | −9% |
| X CDOM | 0.5 | 1 | 1.5 | 2 | 0.5 | 1 | 1.5 | 2 |
| Ncoc | 3.8 | 3.1 | 2.4 | 1.7 | 15.0 | 14.3 | 13.6 | 12.9 |
| Δ | 25% | 1% | −22% | −45% | 15% | 10% | 5% | −1% |
| Kriv and bbp_bg * | 1.0_1.0 | 1.0_0.5 | 0.5_1.0 | 0.5_0.5 | 0.25_1.0 | 0.25_0.5 | 0.1_0.1 |
|---|---|---|---|---|---|---|---|
| Kcoc 103 | RMSE | ||||||
| 2.74 | 2.68 ** | 2.57 | 2.70 | 2.77 | 2.90 | 3.05 | 3.47 |
| 3.1 | 2.75 | 2.54 | 2.40 | 2.32 | 2.37 | 2.37 | 2.56 |
| 3.52 | 3.08 | 2.82 | 2.52 | 2.32 | 2.31 | 2.16 | 2.06 |
| 3.94 | 3.46 | 3.21 | 2.85 | 2.61 | 2.57 | 2.35 | 2.08 |
| 4.36 | 3.83 | 3.59 | 3.23 | 2.99 | 2.93 | 2.69 | 2.35 |
| 5.13 | 4.41 | 4.19 | 3.86 | 3.64 | 3.57 | 3.35 | 3.01 |
| Kcoc 103 | MAPE | ||||||
| 2.74 | 35% | 32% | 31% | 29% | 30% | 29% | 32% |
| 3.1 | 36% | 33% | 31% | 29% | 29% | 27% | 25% |
| 3.52 | 39% | 36% | 32% | 30% | 30% | 28% | 25% |
| 3.94 | 43% | 40% | 36% | 33% | 33% | 30% | 26% |
| 4.36 | 48% | 45% | 40% | 37% | 37% | 34% | 29% |
| 5.13 | 55% | 52% | 48% | 45% | 44% | 41% | 37% |
| St. 1_22 | St. 3_17 | |||||||
|---|---|---|---|---|---|---|---|---|
| Zcoc | 5 | 10 | 15 | 20 | 3 | 5 | 7 | 10 |
| Ncoc | 3.3 | 4.2 | 4.5 | 4.5 | 11.7 | 14.1 | 15.1 | 15.6 |
| Δ | −30% | −10% | −5% | −4% | −5% | 15% | 23% | 27% |
| X * Chl | 0.5 | 1 | 1.5 | 3 | 0.5 | 1 | 1.5 | 3 |
| Ncoc | 4.23 | 4.23 | 4.24 | 4.24 | 14.2 | 14.1 | 14.0 | 13.8 |
| Δ | −10% | −10% | −10% | −10% | 16% | 15% | 14% | 12% |
| X CDOM | 0.5 | 1 | 1.5 | 2 | 0.5 | 1 | 1.5 | 2 |
| Ncoc | 4.27 | 4.23 | 4.20 | 4.16 | 14.0 | 14.1 | 14.3 | 14.4 |
| Δ | −10% | −10% | −11% | −12% | −5% | 15% | 15% | 23% |
| 2017 | 2022 | |||||
|---|---|---|---|---|---|---|
| Kriv and bbp_bg | 1.0_1.0 | 0.5_0.5 | 0.1_0.1 | 1.0_1.0 | 0.5_0.5 | 0.1_0.1 |
| Kcoc 103 | RMSE | |||||
| 2.74 | 2.53 | 3.92 | 5.31 | 2.76 | 1.68 | 1.31 * |
| 3.1 | 1.94 | 2.60 | 3.68 | 3.17 | 2.11 | 1.43 |
| 3.52 | 2.16 | 1.86 | 2.39 | 3.54 | 2.58 | 1.83 |
| 3.94 | 2.76 | 1.95 | 1.79 | 3.85 | 2.96 | 2.24 |
| 4.36 | 3.38 | 2.45 | 1.88 | 4.09 | 3.29 | 2.61 |
| 5.13 | 4.35 | 3.46 | 2.76 | 4.44 | 3.75 | 3.16 |
| Kcoc 103 | MAPE | |||||
| 2.74 | 23% | 35% | 50% | 42% | 26% | 21% |
| 3.1 | 16% | 23% | 33% | 48% | 33% | 21% |
| 3.52 | 17% | 16% | 21% | 53% | 39% | 27% |
| 3.94 | 22% | 16% | 15% | 57% | 44% | 33% |
| 4.36 | 28% | 19% | 15% | 61% | 49% | 38% |
| 5.13 | 38% | 29% | 22% | 67% | 55% | 46% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazyulya, S.; Deryagin, D.; Glukhovets, D.; Silkin, V.; Pautova, L. Regional Algorithm for Estimating High Coccolithophore Concentration in the Northeastern Part of the Black Sea. Remote Sens. 2023, 15, 2219. https://doi.org/10.3390/rs15092219
Vazyulya S, Deryagin D, Glukhovets D, Silkin V, Pautova L. Regional Algorithm for Estimating High Coccolithophore Concentration in the Northeastern Part of the Black Sea. Remote Sensing. 2023; 15(9):2219. https://doi.org/10.3390/rs15092219
Chicago/Turabian StyleVazyulya, Svetlana, Dmitriy Deryagin, Dmitry Glukhovets, Vladimir Silkin, and Larisa Pautova. 2023. "Regional Algorithm for Estimating High Coccolithophore Concentration in the Northeastern Part of the Black Sea" Remote Sensing 15, no. 9: 2219. https://doi.org/10.3390/rs15092219
APA StyleVazyulya, S., Deryagin, D., Glukhovets, D., Silkin, V., & Pautova, L. (2023). Regional Algorithm for Estimating High Coccolithophore Concentration in the Northeastern Part of the Black Sea. Remote Sensing, 15(9), 2219. https://doi.org/10.3390/rs15092219






