Post-Logging Canopy Gap Dynamics and Forest Regeneration Assessed Using Airborne LiDAR Time Series in the Brazilian Amazon with Attribution to Gap Types and Origins
Abstract
:1. Introduction
2. Materials and Methods
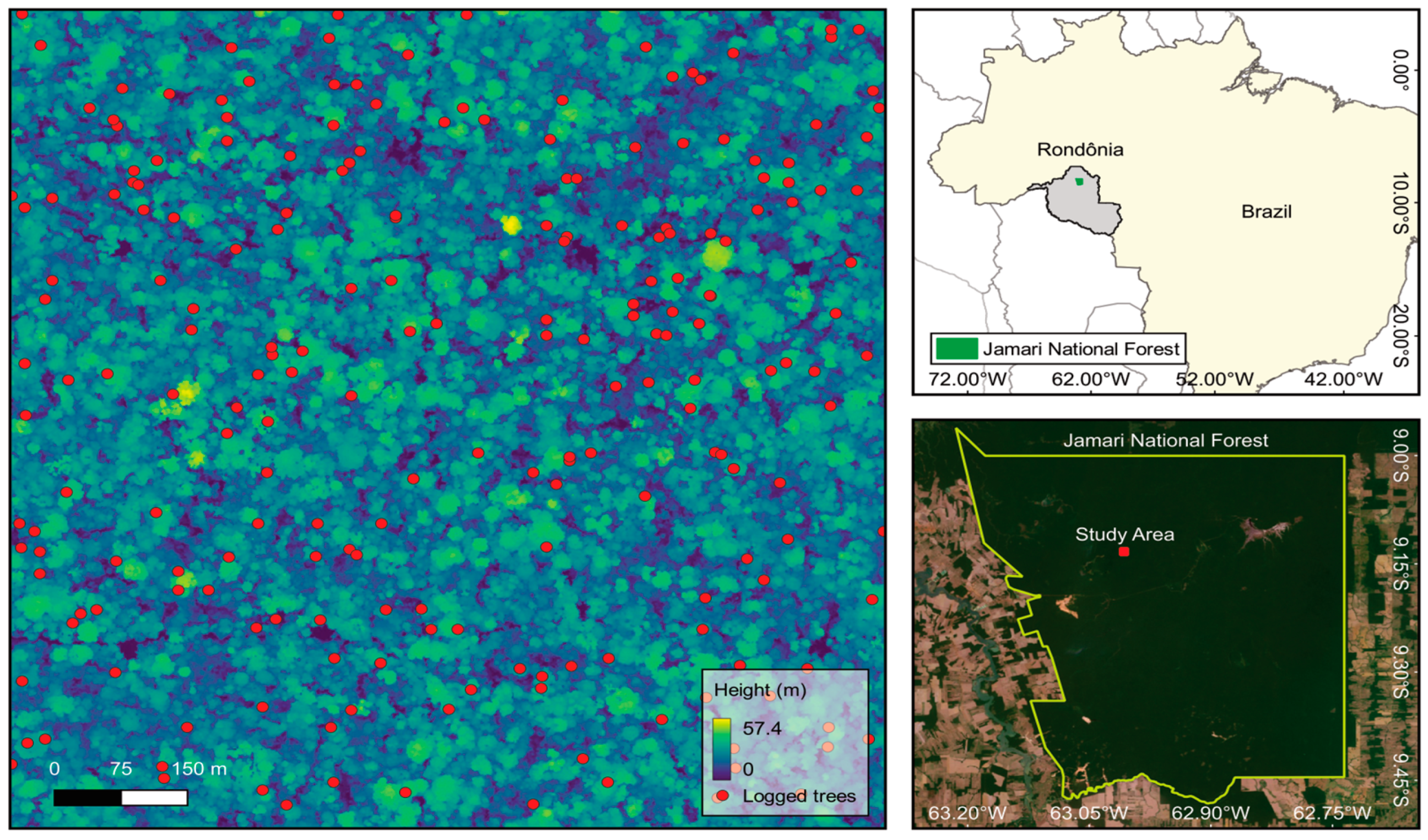
2.1. Study Area
2.2. Multi-Temporal LiDAR Data
2.3. Gap Delineation
2.4. Data Analysis
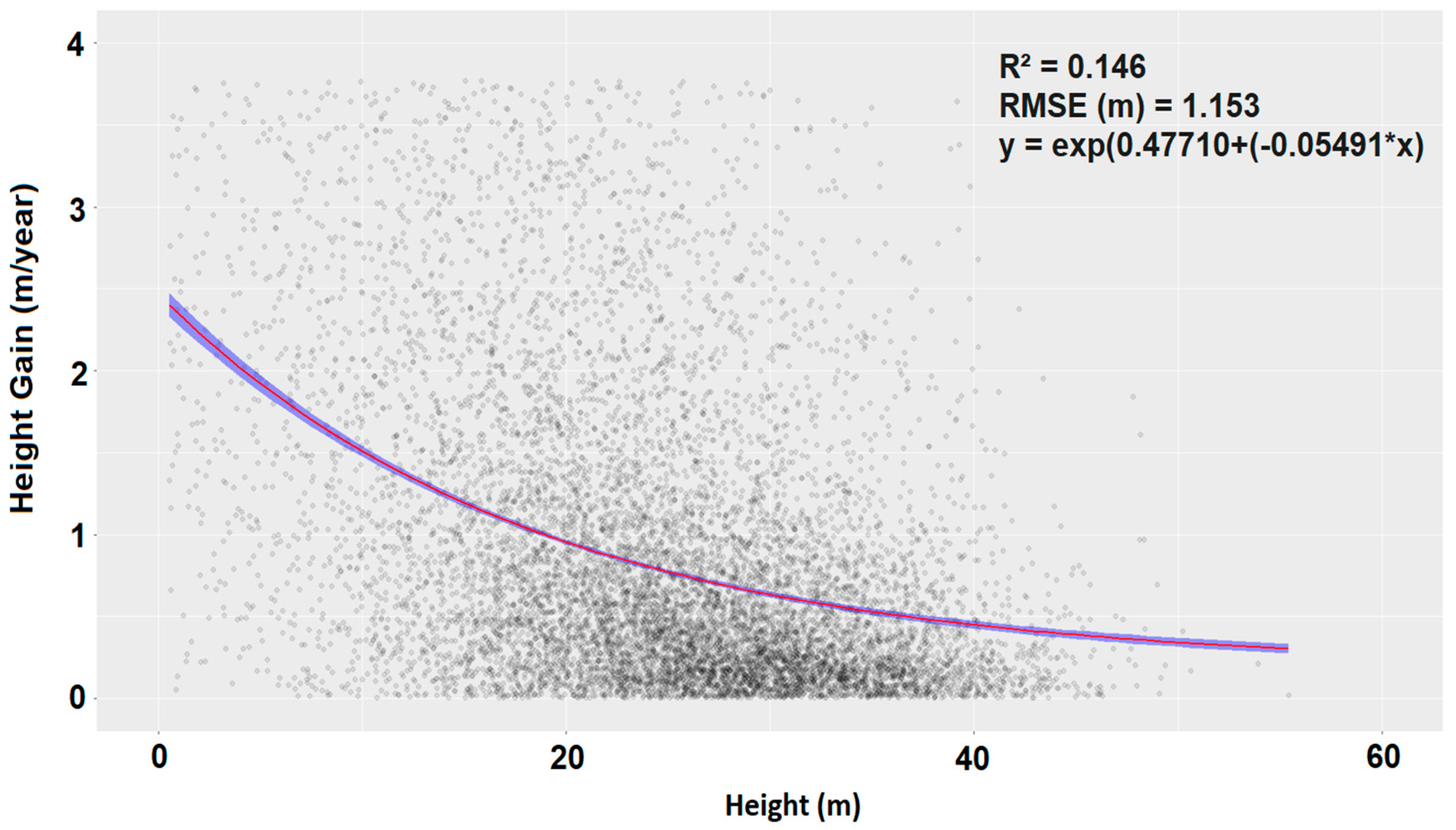
2.4.1. Lateral and Vertical Growth
2.4.2. Growth Model
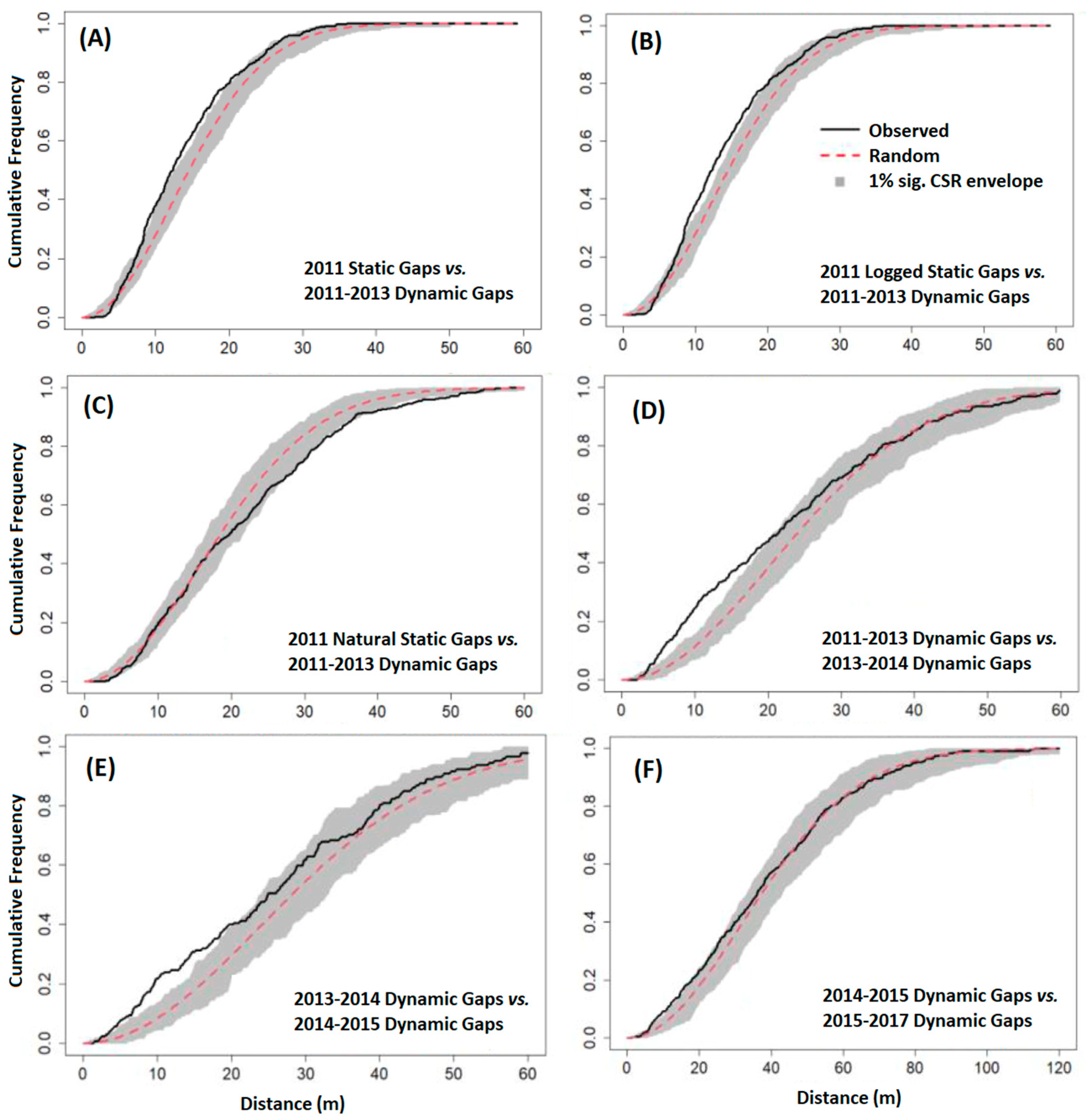
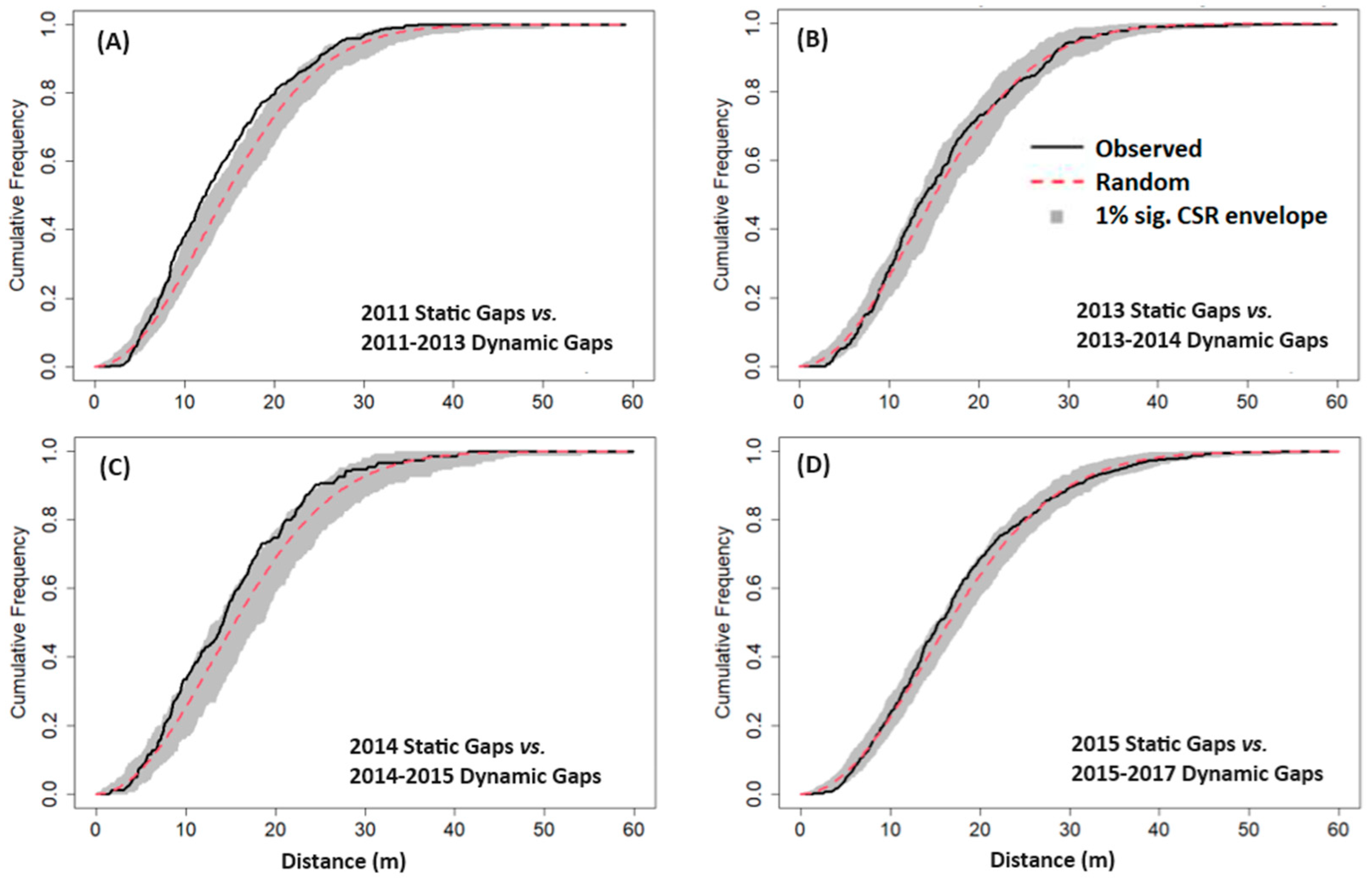
2.4.3. Gap Contagiousness
3. Results
3.1. Gap Opening
3.2. Mode of Gap Closure and Tree Growth
3.3. Gap Contagiousness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomao, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F.; et al. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.V.; Basso, L.S.; Miller, J.B.; Gloor, M.; Gatti Domingues, L.; Cassol, H.L.G.; Tejada, G.; Aragão, L.E.O.C.; Nobre, C.; Peters, W.; et al. Amazonia as a carbon source linked to deforestation and climate change. Nature 2021, 595, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lapola, D.M.; Pinho, P.; Barlow, J.; Aragão, L.E.O.C.; Berenguer, E.; Carmenta, R.; Liddy, H.M.; Seixas, H.; Silva, C.V.J.; Silva-Junior, C.H.L.; et al. The Drivers and Impacts of Amazon Forest Degradation. Science 2023, 379, 349. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Aragão, L.E.O.C.; Anderson, L.O.; Fonseca, M.G.; Shimabukuro, Y.E.; Vancutsem, C.; Achard, F.; Beuchle, R.; Numata, I.; Silva, C.A.; et al. Persistent collapse of biomass in Amazonian forest edges following deforestation leads to unaccounted carbon losses. Sci. Adv. 2020, 6, eaaz8360. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-term forest degradation surpasses deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.; Silva, J.N. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Yamamoto, S.-I. Forest Gap Dynamics and Tree Regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Brokaw, N.V.L. The Definition of Treefall Gap and Its Effect on Measures of Forest Dynamics. Biotropica 1982, 14, 158–160. [Google Scholar] [CrossRef]
- Negrón-Juárez, R.I.; Chambers, J.Q.; Marra, D.M.; Ribeiro, G.H.P.M.; Rifai, S.W.; Higuchi, N.; Roberts, D. Detection of Subpixel Treefall Gaps with Landsat Imagery in Central Amazon Forests. Remote Sens. Environ. 2011, 115, 3322–3328. [Google Scholar] [CrossRef]
- Gora, E.M.; Burchfield, J.C.; Muller-Landau, H.C.; Bitzer, P.M.; Yanoviak, S.P. Pantropical Geography of Lightning-Caused Disturbance and Its Implications for Tropical Forests. Glob. Chang. Biol. 2020, 26, 5017–5026. [Google Scholar] [CrossRef] [PubMed]
- Dalagnol, R.; Wagner, F.H.; Galvão, L.S.; Streher, A.S.; Phillips, O.L.; Gloor, E.; Pugh, T.A.M.; Ometto, J.P.H.B.; Aragão, L.E.O.C. Large-scale variations in the dynamics of Amazon forest canopy gaps from airborne lidar data and opportunities for tree mortality estimates. Sci. Rep. 2021, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, N.V.L. Gap-Phase Regeneration in a Tropical Forest. Ecology 1985, 66, 682–687. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Treefall Gaps and the Maintenance of Species Diversity in a Tropical Forest. Ecology 2001, 82, 913–919. [Google Scholar] [CrossRef]
- Hartshorn, G. Tree falls and tropical forest dynamics Book reviews View project Tropical forest vegetation descriptions View project. In Tropical Trees as Living Systems; Cambridge University Press: Cambridge, UK, 1978; pp. 617–638. [Google Scholar]
- Espírito-Santo, F.D.B.; Keller, M.M.; Linder, E.; Oliveira Junior, R.C.; Pereira, C.; Oliveira, C.G. Gap formation and carbon cycling in the Brazilian Amazon: Measurement using high-resolution optical remote sensing and studies in large forest plots. Plant Ecol. Divers. 2013, 7, 305–318. [Google Scholar] [CrossRef]
- Hunter, M.O.; Keller, M.; Morton, D.; Cook, B.; Lefsky, M.; Ducey, M.; Saleska, S.; de Oliveira, R.C.; Schietti, J. Structural Dynamics of Tropical Moist Forest Gaps. PLoS ONE 2015, 10, e0132144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. Identification of gaps in mangrove forests with airborne LIDAR. Remote Sens. Environ. 2008, 112, 2309–2325. [Google Scholar] [CrossRef]
- Almeida, D.R.A.; Broadbent, E.N.; Zambrano, A.M.A.; Wilkinson, B.E.; Ferreira, M.E.; Chazdon, R.; Meli, P.; Gorgens, E.B.; Silva, C.A.; Stark, S.C.; et al. Monitoring the structure of forest restoration plantations with a drone-lidar system. Int. J. Appl. Earth Obs. Geoinf. 2019, 79, 192–198. [Google Scholar] [CrossRef]
- Almeida, D.R.A.; Stark, S.C.; Chazdon, R.; Nelson, B.W.; Cesar, R.G.; Meli, P.; Gorgens, E.B.; Duarte, M.M.; Valbuena, R.; Moreno, V.S.; et al. The effectiveness of lidar remote sensing for monitoring forest cover attributes and landscape restoration. For. Ecol. Manag. 2019, 438, 34–43. [Google Scholar] [CrossRef]
- Jucker, T. Deciphering the Fingerprint of Disturbance on the Three-Dimensional Structure of the World’s Forests. New Phytol. 2022, 233, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Koukoulas, S.; Blackburn, G.A. Quantifying the spatial properties of forest canopy gaps using LiDAR imagery and GIS. Int. J. Remote Sens. 2004, 25, 3049–3072. [Google Scholar] [CrossRef]
- Espírito-Santo, F.D.B.; Gloor, M.; Keller, M.; Malhi, Y.; Saatchi, S.; Nelson, B.; Junior, R.C.O.; Pereira, C.; Lloyd, J.; Frolking, S.; et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat. Commun. 2014, 5, 3434. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, R.; Malthus, T.J. LiDAR mapping of canopy gaps in continuous cover forests: A comparison of canopy height model and point cloud based techniques. Int. J. Remote Sens. 2010, 31, 1193–1211. [Google Scholar] [CrossRef]
- Leitold, V.; Morton, D.C.; Longo, M.; dos-Santos, M.N.; Keller, M.; Scaranello, M. El Niño drought increased canopy turnover in Amazon forests. New Phytol. 2018, 219, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Dalagnol, R.; Phillips, O.L.; Gloor, E.; Galvão, L.S.; Wagner, F.H.; Locks, C.J.; Aragão, L.E.O.C. Quantifying Canopy Tree Loss and Gap Recovery in Tropical Forests under Low-Intensity Logging Using VHR Satellite Imagery and Airborne LiDAR. Remote Sens. 2019, 11, 817. [Google Scholar] [CrossRef]
- Asner, G.P.; Keller, M.; Silva, J.N.M. Spatial and temporal dynamics of forest canopy gaps following selective logging in the eastern Amazon. Glob. Chang. Biol. 2004, 10, 765–783. [Google Scholar] [CrossRef]
- Vaughn, N.R.; Asner, G.P.; Giardina, C.P. Long-term fragmentation effects on the distribution and dynamics of canopy gaps in a tropical montane forest. Ecosphere 2015, 6, art271. [Google Scholar] [CrossRef]
- Jansen, P.A.; Meer, P.J.V.; der Bongers, F. Spatial Contagiousness of Canopy Disturbance in Tropical Rain Forest: An Individual-Tree-Based Test. Ecology 2008, 89, 3490–3502. [Google Scholar] [CrossRef] [PubMed]
- Vepakomma, U.; St-Onge, B.; Kneeshaw, D. Spatially Explicit Characterization of Boreal Forest Gap Dynamics Using Multi-Temporal Lidar Data. Remote Sens. Environ. 2008, 112, 2326–2340. [Google Scholar] [CrossRef]
- Pryke, J.S.; Samways, M.J. Conservation management of complex natural forest and plantation edge effects. Landsc. Ecol. 2011, 27, 73–85. [Google Scholar] [CrossRef]
- Lenz, B.B.; Jack, K.M.; Spironello, W.R. Edge effects in the primate community of the biological dynamics of forest fragments project, Amazonas, Brazil. Am. J. Phys. Anthropol. 2014, 155, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Cysneiros, V.C.; Machado, S.d.A.; Pelissari, A.L.; Figueiredo Filho, A.; Urbano, E. Modeling of the Commercial Volume Stock in an Ombrophilous Forest in the Southwest of the Amazon. Cerne 2016, 22, 457–464. [Google Scholar] [CrossRef]
- ICMBio Plano de Manejo da Floresta Nacional do Jamari; Diagnóstico: Brasília, Brazil, 2005.
- Leitold, V.; Keller, M.; Morton, D.C.; Cook, B.D.; Shimabukuro, Y.E. Airborne lidar-based estimates of tropical forest structure in complex terrain: Opportunities and trade-offs for REDD+. Carbon Balance and Management 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, M. GitHub—Kheaactua/LAStools: Efficient Tools for LiDAR Processing. GitHub, 2015. Available online: https://github.com/kheaactua/LAStools#readme (accessed on 27 July 2021).
- Jean-Romain. GitHub—Jean-Romain/lidR: R Package for Airborne LiDAR Data Manipulation and Visualisation for Forestry Application. GitHub, 2021. Available online: https://github.com/Jean-Romain/lidR (accessed on 27 July 2021).
- R Core Team. R: A Language and Environment for Statistical ## Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 3 February 2021).
- Baddeley, A.; Ruban, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Routledge & CRC Press: London, UK, 2015; Available online: https://www.routledge.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/9781482210200/ (accessed on 24 August 2021).
- Baddeley, A.; Turner, R.; Rubak, E. Spatstat—Spatstat Website. Spatstat.org, 2014. Available online: https://spatstat.org/ (accessed on 27 July 2021).
- Bonnesoeur, V.; Constant, T.; Moulia, B.; Fournier, M. Forest trees filter chronic wind-signals to acclimate to high winds. New Phytol. 2016, 210, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.R.; Tapley, I.; Milne, A.; Williams, M.L.; Zhou, Z.; Lehmann, E.; Caccetta, P.; Lowell, K.; Held, A. C- and L-band SAR interoperability: Filling the gaps in continuous forest cover mapping in Tasmania. Remote Sens. Environ. 2014, 155, 58–68. [Google Scholar] [CrossRef]
- Aleixo, I.; Norris, D.; Hemerik, L.; Barbosa, A.; Prata, E.; Costa, F.; Poorter, L. Amazonian rainforest tree mortality driven by climate and functional traits. Nat. Clim. Change 2019, 9, 384–388. [Google Scholar] [CrossRef]
- Kamimura, B.A.; Magnani, M.; Luciano, W.A.; Campagnollo, F.B.; Pimentel, T.C.; Alvarenga, V.O.; Pelegrino, B.O.; Cruz, A.G. Brazilian Artisanal Cheeses: An Overview of their Characteristics, Main Types and Regulatory Aspects. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1636–1657. [Google Scholar] [CrossRef] [PubMed]
- Vepakomma, U.; St-Onge, B.; Kneeshaw, D. Response of a boreal forest to canopy opening: Assessing vertical and lateral tree growth with multi-temporal lidar data. Ecol. Appl. 2011, 21, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Clark, D.B. Getting to the Canopy: Tree Height Growth in a Neotropical Rain Forest. Ecology 2001, 82, 1460–1472. [Google Scholar] [CrossRef]
- Krüger, K.; Send, C.; Jucker, T.; Pflugmacher, D.; Seidl, R. Gap Expansion Is the Dominant Driver of Canopy Openings in a Temperate Mountain Forest Landscape. J. Ecol. 2024, 1365–2745, 14320. [Google Scholar] [CrossRef]
- Kent, R.; Lindsell, J.; Laurin, G.; Valentini, R.; Coomes, D. Airborne LiDAR Detects Selectively Logged Tropical Forest Even in an Advanced Stage of Recovery. Remote Sens. 2015, 7, 8348–8367. [Google Scholar] [CrossRef]
- Felton, A.; Felton, A.M.; Wood, J.; Lindenmayer, D.B. Vegetation structure, phenology, and regeneration in the natural and anthropogenic tree-fall gaps of a reduced-impact logged subtropical Bolivian forest. For. Ecol. Manag. 2006, 235, 186–193. [Google Scholar] [CrossRef]
- Génin, A.; Majumder, S.; Sankaran, S.; Danet, A.; Guttal, V.; Schneider, F.D.; Kéfi, S. Monitoring ecosystem degradation using spatial data and the R package spatial warnings. Methods Ecol. Evol. 2018, 9, 2067–2075. [Google Scholar] [CrossRef]
- Heinrich, V.H.A.; Dalagnol, R.; Cassol, H.L.G.; Rosan, T.M.; de Almeida, C.T.; Silva Junior, C.H.L.; Campanharo, W.A.; House, J.I.; Sitch, S.; Hales, T.C.; et al. Large Carbon Sink Potential of Secondary Forests in the Brazilian Amazon to Mitigate Climate Change. Nat. Commun. 2021, 12, 1785. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, V.H.A.; Vancutsem, C.; Dalagnol, R.; Rosan, T.M.; Fawcett, D.; Silva-Junior, C.H.L.; Cassol, H.L.G.; Achard, F.; Jucker, T.; Silva, C.A.; et al. The Carbon Sink of Secondary and Degraded Humid Tropical Forests. Nature 2023, 615, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.; Sitch, S.; Ciais, P.; Wigneron, J.P.; Silva-Junior, C.H.L.; Heinrich, V.; Vancutsem, C.; Achard, F.; Bastos, A.; Yang, H.; et al. Declining Amazon Biomass due to Deforestation and Subsequent Degradation Losses Exceeding Gains. Glob. Chang. Biol. 2023, 29, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, D.I.; Morton, D.C.; Longo, M.; Keller, M.; Dubayah, R.; Dos-Santos, M.N. Quantifying long-term changes in carbon stocks and forest structure from Amazon forest degradation. Environ. Res. Lett. 2018, 13, 065013. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J. Forest regeneration in logged and unlogged forests of Kibale National Park, Uganda. Biotropica 1997, 29, 396–412. [Google Scholar] [CrossRef]
- Kyei, E.; Bondzie-Mensah, M. Effect of Canopy Gaps on Composition and Diversity of Natural Regeneration Following Selective Logging in a Moist Evergreen Forest in Ghana; ResearchGate: Berlin, Germany, 2020; Available online: https://www.researchgate.net/publication/339428624_Effect_of_canopy_gaps_on_composition_and_diversity_of_natural_regeneration_following_selective_logging_in_a_moist_evergreen_forest_in_Ghana/link/5ebea607299bf1c09abc9381/download (accessed on 11 August 2021).
- Arellano, G.; Medina, N.G.; Tan, S.; Mohamad, M.; Davies, S.J. Crown Damage and the Mortality of Tropical Trees. New Phytol. 2019, 221, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Zweede, J. Canopy dynamics in unlogged and logged forest stands in the eastern Amazon. For. Ecol. Manag. 2006, 236, 56–64. [Google Scholar] [CrossRef]
- de Moura, Y.M.; Balzter, H.; Galvão, L.S.; Dalagnol, R.; Espírito-Santo, F.; Santos, E.G.; Garcia, M.; Bispo, P.d.C.; Oliveira, R.C.; Shimabukuro, Y.E. Carbon Dynamics in a Human-Modified Tropical Forest: A Case Study Using Multi-Temporal LiDAR Data. Remote Sens. 2020, 12, 430. [Google Scholar] [CrossRef]
- Dalagnol, R.; Wagner, F.H.; Galvão, L.S.; Braga, D.; Osborn, F.; Sagang, L.B.; Da Conceição Bispo, P.; Payne, M.; Silva Junior, C.; Favrichon, S.; et al. Mapping Tropical Forest Degradation with Deep Learning and Planet NICFI Data. Remote Sens. Environ. 2023, 298, 113798. [Google Scholar] [CrossRef]
- Mills, M.; Malhi, Y.; Ewers, R.M.; Kho, L.K.; Teh, Y.A.; Both, S.; Burslem, D.F.R.P.; Majalap, N.; Nilus, R.; Huasco, W.H.; et al. Tropical forests post-logging are a persistent net carbon source to the atmosphere. Proc. Natl. Acad. Sci. USA 2023, 120, e2214462120. [Google Scholar] [CrossRef] [PubMed]


| Information | 2011 | 2013 | 2014 | 2015 | 2017 |
|---|---|---|---|---|---|
| Laser Scan Sensor | Optech 3100 | Optech, Orion | Trimble, Harrier 68i | Optech 3100 | Optech ALTM Gemini |
| Acquisition date | 17 November 2011 | 20 September 2013 | 10 September 2014 | 21 September 2015 | 20 April 2017 |
| Acquisition altitude (m) | 850 | 853 | 500 | 750 | 700 |
| Off-nadir angle (°) | 11.1 | 11.1 | 15 | 15 | 15 |
| Scan frequency (kHz) | 59.8 | 67.5 | 400 | 100 | 100 |
| Average point cloud density (ppm2) | 15.4 | 15.5 | 30.4 | 33.6 | 12 |
| First Spatial Dataset | Second Spatial Dataset |
|---|---|
| Experiment 1—Static gaps vs. subsequent dynamic gaps: | |
| 2011 Static Gaps | 2011–2013 Dynamic Gaps |
| 2013 Static Gaps | 2013–2014 Dynamic Gaps |
| 2014 Static Gaps | 2014–2015 Dynamic Gaps |
| 2015 Static Gaps | 2015–2017 Dynamic Gaps |
| Experiment 2—Static gaps from 2011 stratified by type vs. subsequent dynamic gaps: | |
| 2011 Logged Gaps | 2011–2013 Dynamic Gaps |
| 2011 Natural Gaps | 2011–2013 Dynamic Gaps |
| Experiment 3—Dynamic gaps vs. subsequent dynamic gaps: | |
| 2011–2013 Dynamic Gaps | 2013–2014 Dynamic Gaps |
| 2013–2014 Dynamic Gaps | 2014–2015 Dynamic Gaps |
| 2014–2015 Dynamic Gaps | 2015–2017 Dynamic Gaps |
| Year | Area (m2) | Number of Static Gaps | Mean Gap Area (m2) | Gap Fraction (%) |
|---|---|---|---|---|
| 2011 | 83,806 | 1156 | 72.5 | 8.4 |
| 2013 | 56,563 | 1069 | 52.9 | 5.6 |
| 2014 | 56,366 | 1074 | 52.5 | 5.6 |
| 2015 | 43,138 | 939 | 45.9 | 4.3 |
| 2017 | 43,908 | 1017 | 43.2 | 4.4 |
| Newly Open Dynamic Gaps (Gaps per Year) | Closed Dynamic Gaps (Gaps per Year) | |
|---|---|---|
| 2011–2013 | 230.1 | 212.5 |
| 2013–2014 | 306.7 | 260.0 |
| 2014–2015 | 191.8 | 321.5 |
| 2015–2017 | 352.4 | 200.9 |
| Year | Gap Closed Vertically (m2) | Gaps Closed Laterally (m2) | Gaps Remain Open (m2) |
|---|---|---|---|
| 2013 | 13,061 | 21,583 | 48,664 |
| 2014 | 8754 | 6922 | 32,988 |
| 2015 | 7365 | 3917 | 21,706 |
| 2017 | 6770 | 4493 | 10,443 |
| Sum | 35,950 | 36,915 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winstanley, P.; Dalagnol, R.; Mendiratta, S.; Braga, D.; Galvão, L.S.; Bispo, P.d.C. Post-Logging Canopy Gap Dynamics and Forest Regeneration Assessed Using Airborne LiDAR Time Series in the Brazilian Amazon with Attribution to Gap Types and Origins. Remote Sens. 2024, 16, 2319. https://doi.org/10.3390/rs16132319
Winstanley P, Dalagnol R, Mendiratta S, Braga D, Galvão LS, Bispo PdC. Post-Logging Canopy Gap Dynamics and Forest Regeneration Assessed Using Airborne LiDAR Time Series in the Brazilian Amazon with Attribution to Gap Types and Origins. Remote Sensing. 2024; 16(13):2319. https://doi.org/10.3390/rs16132319
Chicago/Turabian StyleWinstanley, Philip, Ricardo Dalagnol, Sneha Mendiratta, Daniel Braga, Lênio Soares Galvão, and Polyanna da Conceição Bispo. 2024. "Post-Logging Canopy Gap Dynamics and Forest Regeneration Assessed Using Airborne LiDAR Time Series in the Brazilian Amazon with Attribution to Gap Types and Origins" Remote Sensing 16, no. 13: 2319. https://doi.org/10.3390/rs16132319






