Developing a Semi-Automated Near-Coastal, Water Quality-Retrieval Process from Global Multi-Spectral Data: South-Eastern Australia
Abstract
:1. Introduction and Background
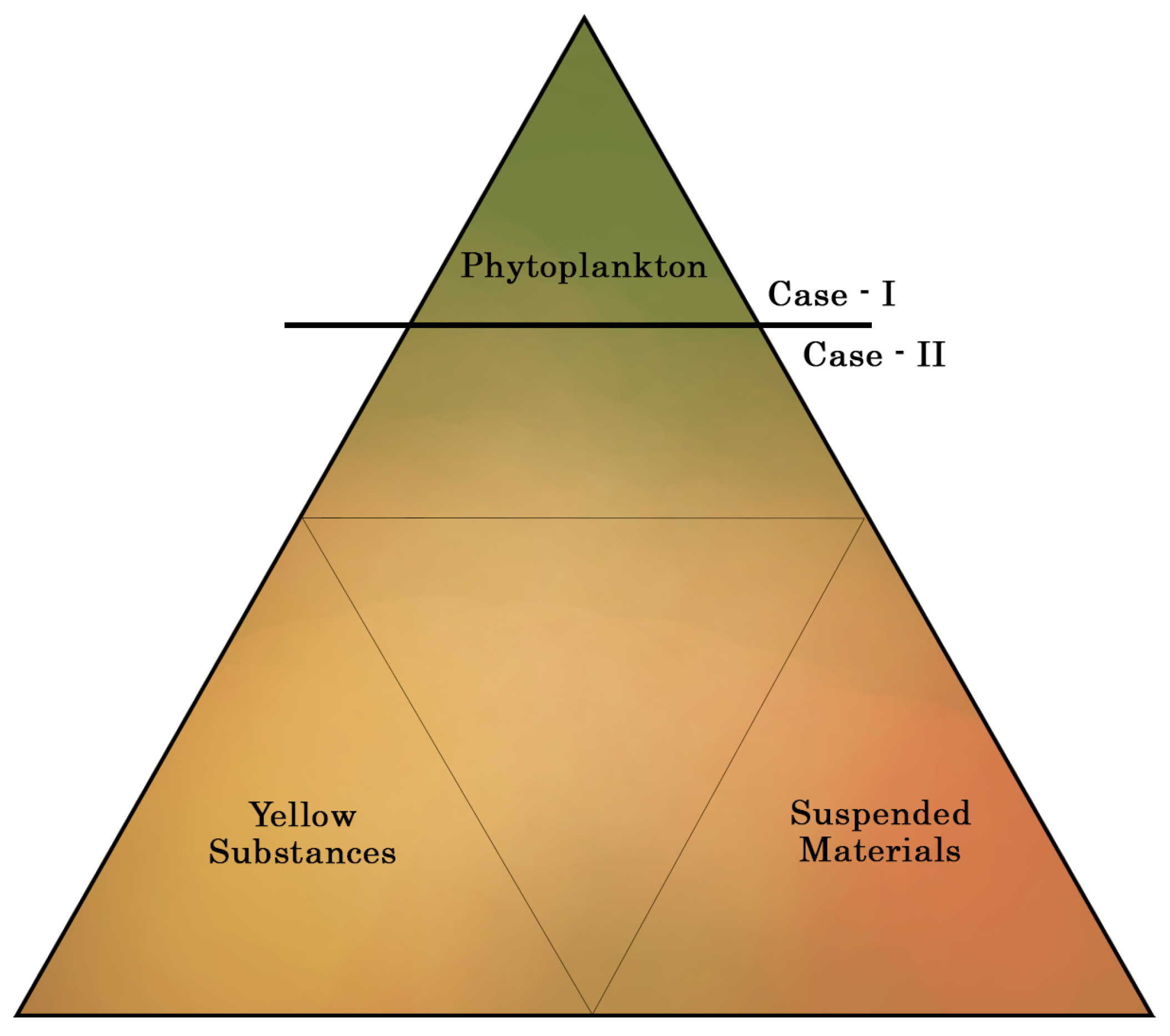
1.1. Water Quality-Estimation Approaches from Satellite Multi-Spectral Data
1.2. Aims
2. Data and Methodology
2.1. Field Data Collection
2.2. Satellite and Field Data to Estimate Chlorophyll a Concentration
2.3. Extract Data from Satellite Images at Field Study Sites to Link with In Situ Data
2.4. Develop Models to Estimate Chlorophyll a Concentration from L8 Satellite Reflectance and Field Measurements
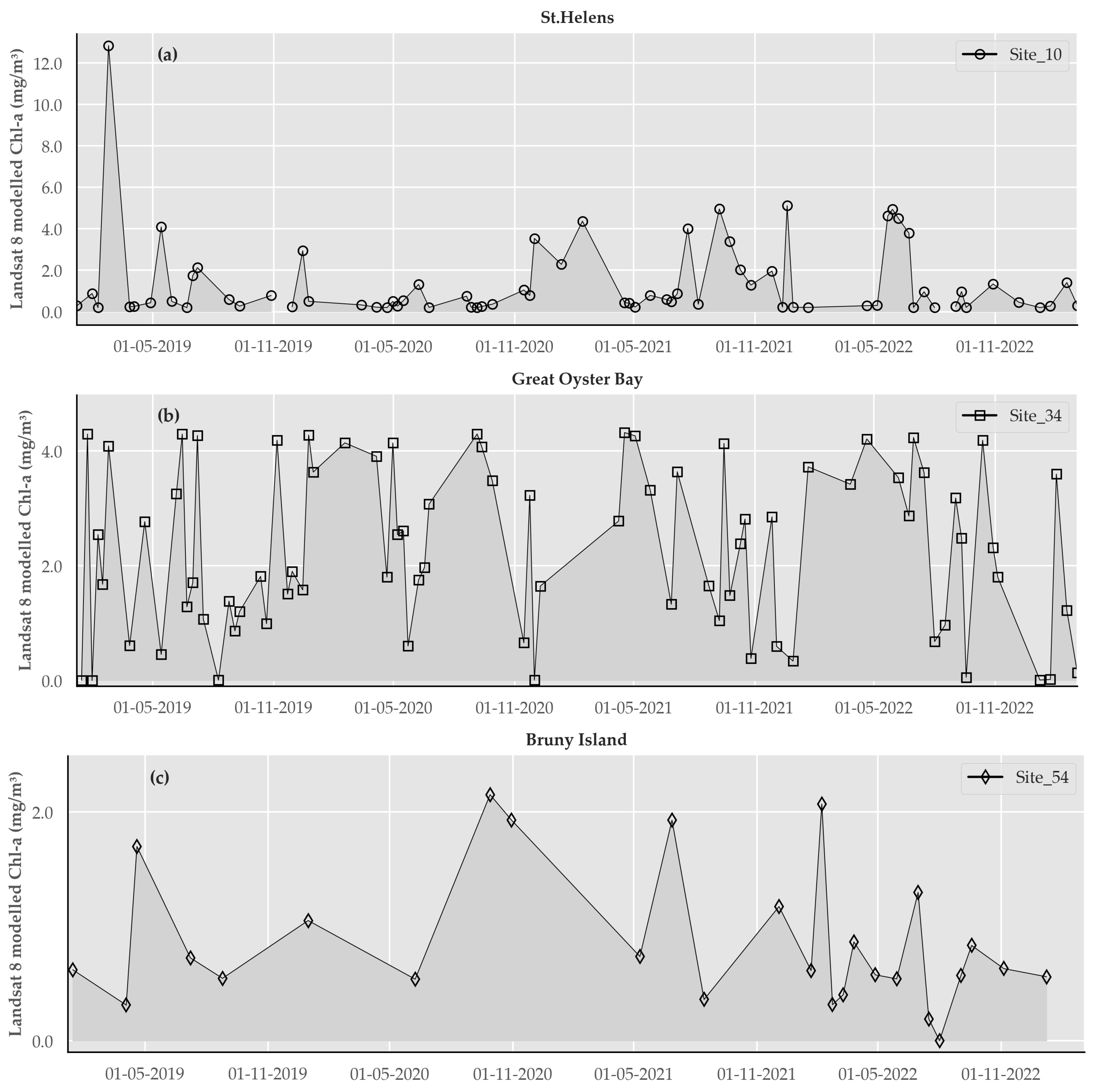
2.5. Produce Time-Series of Output Chlorophyll a Maps at Each Study Site
2.6. Validate Estimated Chlorophyll Levels against Field Data Not Used in Model Development
3. Results
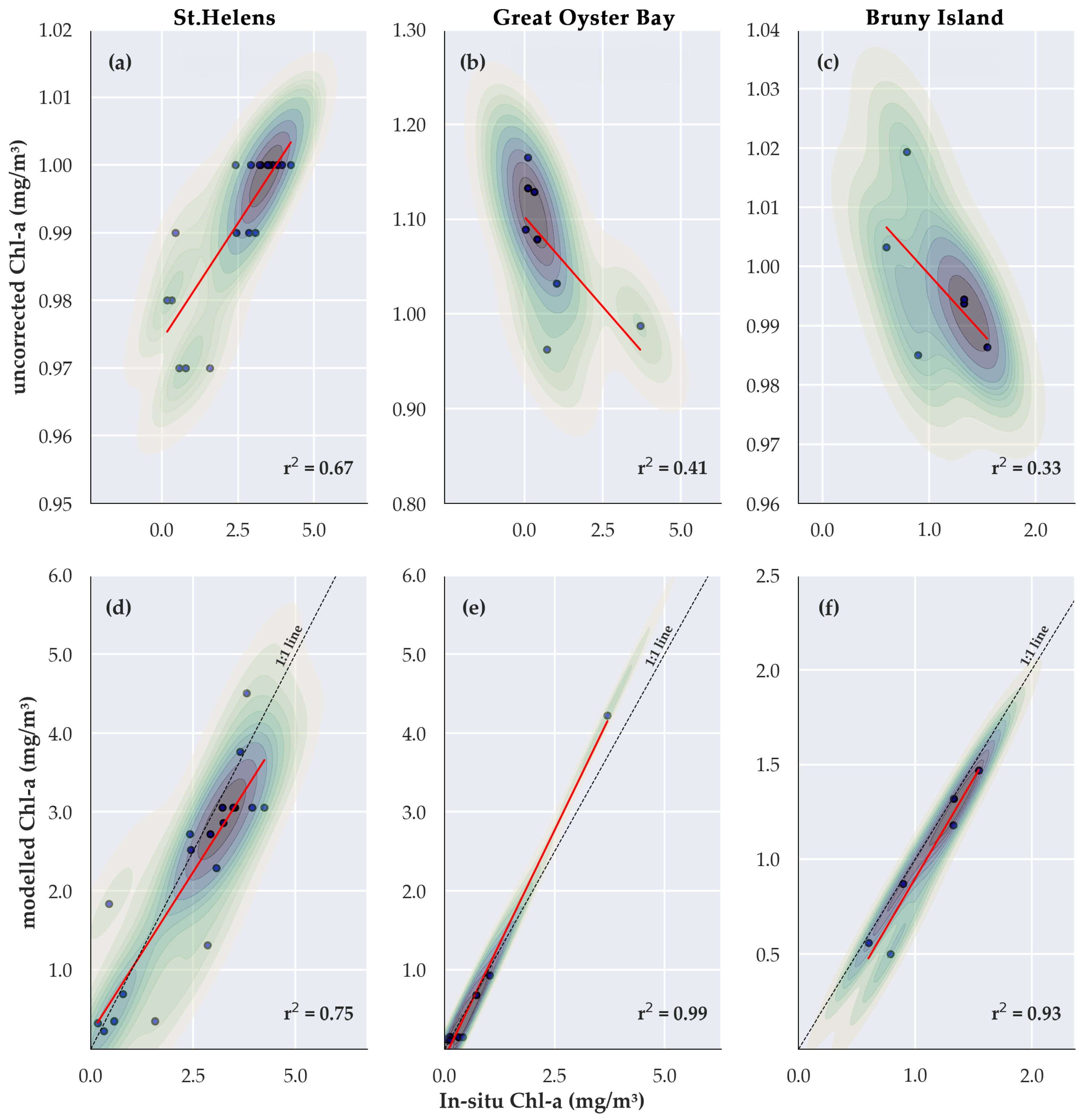
3.1. Linking Satellite and Field Data to Estimate Chlorophyll a Concentration
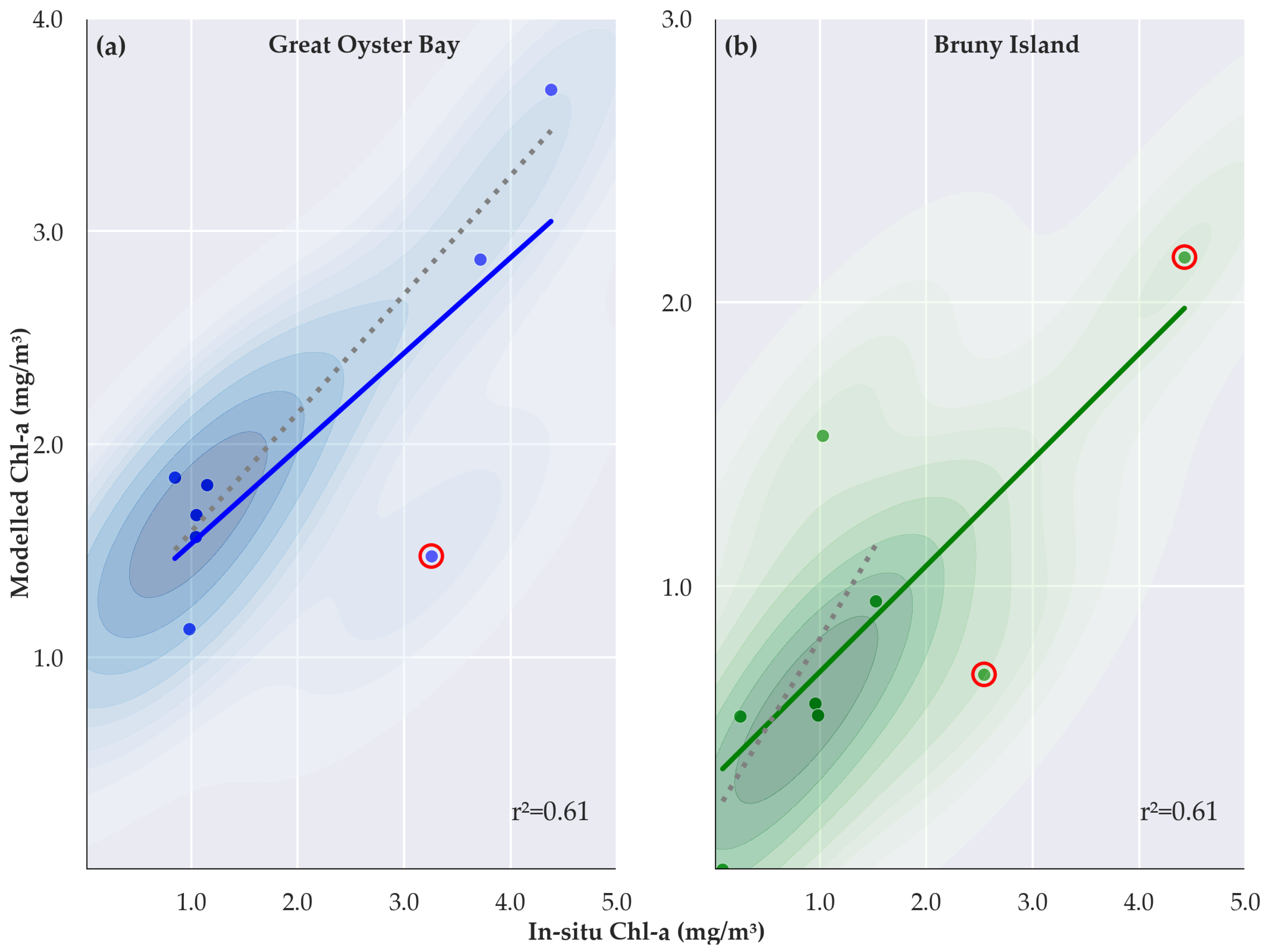
3.2. Validation of Estimate Chlorophyll a Concentration
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AODN | Australian Ocean Data Network |
| ARD | Analysis ready data |
| BNI | Bruny Island |
| CDOM | Coloured dissolved organic matter |
| Chl-a | Chlorophyll a |
| CSIRO | Commonwealth Scientific and Industrial Research Organisation |
| GEE | Google Earth engine |
| GLORIA | Global reflectance community dataset for imaging and optical sensing of aquatic environments |
| GOB | Great Oyster bay |
| HAB | Harmful algae bloom |
| IMOS | Integrated Marine Observing System |
| IOPs | Inherent optical properties |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| NAP | Non-algae particles |
| NASA | National Aeronautics and Space Administration |
| NOMAD | NASA bio-optical marine algorithm dataset |
| PACE | Plankton, Aerosol, Cloud, ocean Ecosystem |
| SeaWiFS | Sea-viewing Wide Field-of-view Sensor |
| SST | Sea surface temperature |
| STH | St. Helens |
| TSS | Total suspended solids |
| USGS | United States Geological Survey |
| VIIRS | Visible Infrared Imaging Radiometer Suite |
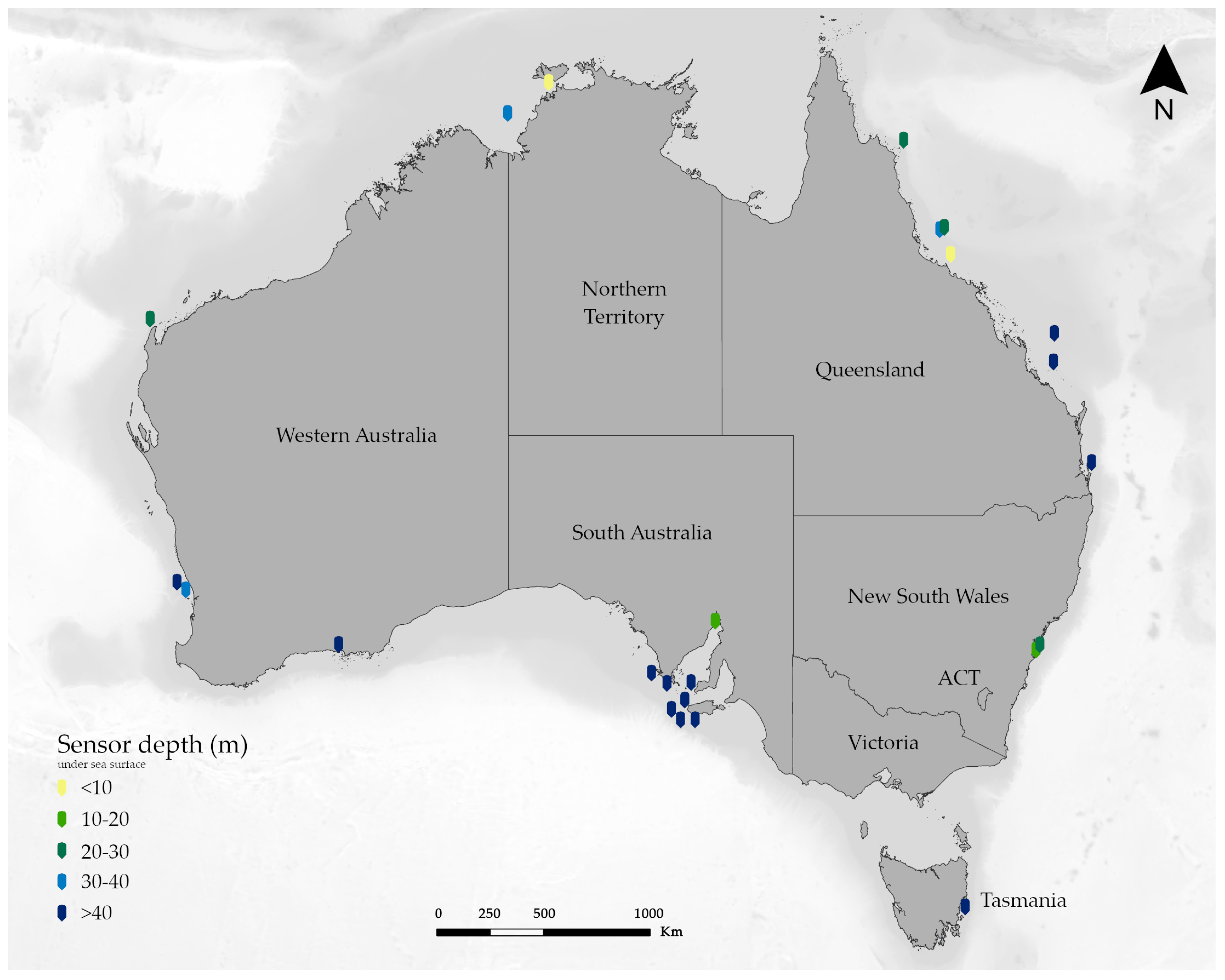
Appendix A. IMOS Sensors around Australia
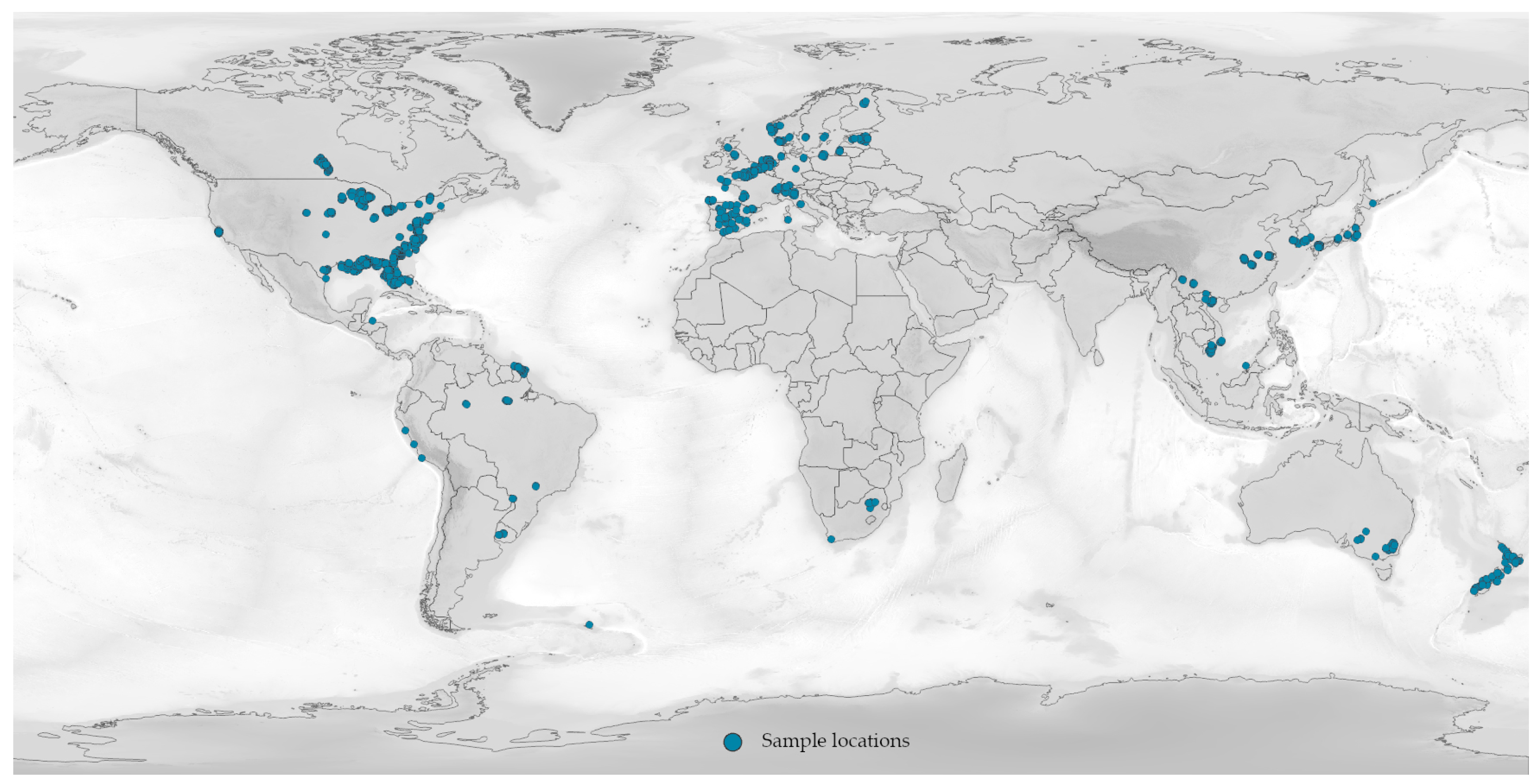
Appendix B. NOMAD Dataset Availability

Appendix C. GLORIA Dataset
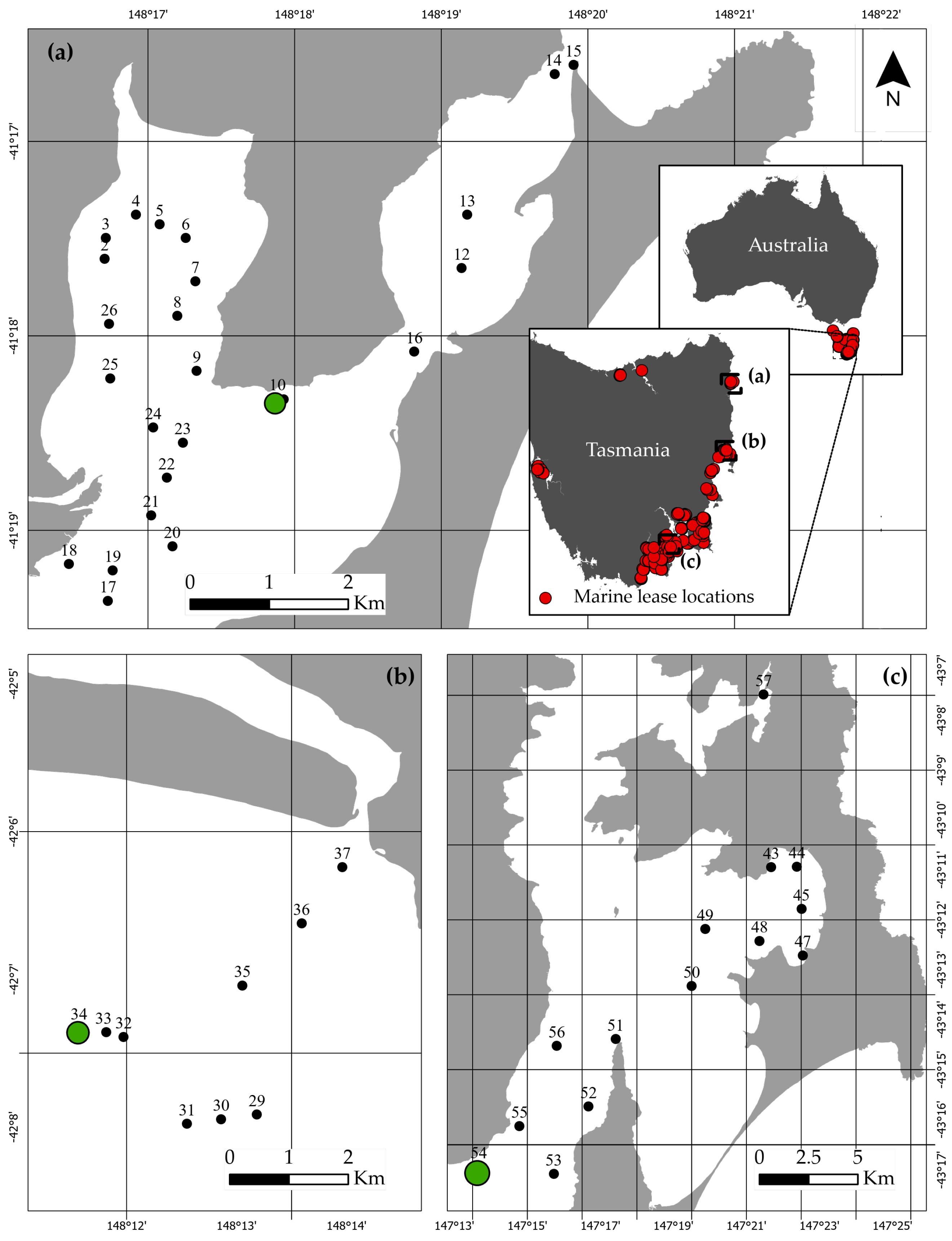
Appendix D. Bottom Depth
| St.Helens | Great Oyster Bay | ||||
|---|---|---|---|---|---|
| Survey Location ID | Location Coordinates | Bottom Depth (m) | Survey Location ID | Location Coordinates | Bottom Depth (m) |
| 2 | 148.278421, −41.293399 | 1.2 | 29 | 148.219753, −42.131927 | 9.3 |
| 3 | 148.278547, −41.291595 | 1.6 | 30 | 148.214318, −42.132448 | 9.6 |
| 4 | 148.281969, −41.289609 | 1.4 | 31 | 148.209152, −42.132973 | 9.7 |
| 5 | 148.284655, −41.29045 | 2.8 | 32 | 148.199544, −42.123159 | 8.6 |
| 6 | 148.287617, −41.291611 | 5.7 | 33 | 148.196921, −42.122631 | 9 |
| 7 | 148.28871, −41.295325 | 7.5 | 34 | 148.192777, −42.122149 | 9.3 |
| 8 | 148.286674, −41.298296 | 9.5 | 35 | 148.217547, −42.117361 | 7.8 |
| 9 | 148.288877, −41.302993 | 16.2 | 36 | 148.226515, −42.110353 | 7.2 |
| 10 | 148.298046, −41.305381 | 5.1 | 37 | 148.232691, −42.104013 | 1.8 |
| 11 | 148.298752, −41.305432 | 4.2 | |||
| 12 | 148.318981, −41.294184 | 2 | |||
| 13 | 148.319617, −41.289626 | 1.9 | Bruny Island | ||
| 14 | 148.329573, −41.277549 | 2.7 | |||
| 15 | 148.331688, −41.276767 | 4 | 49 | 147.331163, −43.20305 | 13 |
| 16 | 148.313606, −41.301362 | 3.7 | 50 | 147.32499, −43.222123 | 12 |
| 17 | 148.278786, −41.322706 | 10.9 | 51 | 147.2903, −43.239789 | 12.5 |
| 18 | 148.274344, −41.319546 | 0.6 | 52 | 147.277944, −43.262333 | 13.3 |
| 19 | 148.279323, −41.320089 | 2.5 | 53 | 147.262045, −43.284649 | 14.5 |
| 20 | 148.286096, −41.31803 | 14.5 | 54 | 147.227832, −43.282213 | 11.2 |
| 21 | 148.283693, −41.315374 | 1 | 55 | 147.24639, −43.268818 | 10.6 |
| 22 | 148.285471, −41.312168 | 18.7 | 56 | 147.263409, −43.24208 | 5.8 |
| 23 | 148.287293, −41.309146 | 21.1 | 57 | 147.357791, −43.124773 | 13.9 |
| 24 | 148.283931, −41.307852 | 20.4 | |||
| 25 | 148.279043, −41.303636 | 5.1 | |||
| 26 | 148.27892, −41.298971 | 1.2 | |||
References
- Le, C.; Li, Y.; Zha, Y.; Sun, D.; Huang, C.; Lu, H. A four-band semi-analytical model for estimating chlorophyll a in highly turbid lakes: The case of Taihu Lake, China. Remote Sens. Environ. 2009, 113, 1175–1182. [Google Scholar]
- Gitelson, A.A.; Gurlin, D.; Moses, W.J.; Barrow, T. A bio-optical algorithm for the remote estimation of the chlorophyll a concentration in case 2 waters. Environ. Res. Lett. 2009, 4, 045003. [Google Scholar]
- Matthews, M.W. A current review of empirical procedures of remote sensing in inland and near-coastal transitional waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Koponen, S.; Attila, J.; Pulliainen, J.; Kallio, K.; Pyhälahti, T.; Lindfors, A.; Rasmus, K.; Hallikainen, M. A case study of airborne and satellite remote sensing of a spring bloom event in the Gulf of Finland. Cont. Shelf Res. 2007, 27, 228–244. [Google Scholar]
- Gordon, H.R. Diffuse reflectance of the ocean: The theory of its augmentation by chlorophyll a fluorescence at 685 nm. Appl. Opt. 1979, 18, 1161–1166. [Google Scholar] [PubMed]
- Yu, Y.; Chen, S.; Qin, W.; Lu, T.; Li, J.; Cao, Y. A Semi-Empirical Chlorophyll a Retrieval Algorithm Considering the Effects of Sun Glint, Bottom Reflectance, and Non-Algal Particles in the Optically Shallow Water Zones of Sanya Bay Using SPOT6 Data. Remote Sens. 2020, 12, 2765. [Google Scholar] [CrossRef]
- Matsushita, B.; Yang, W.; Chang, P.; Yang, F.; Fukushima, T. A simple method for distinguishing global Case-1 and Case-2 waters using SeaWiFS measurements. ISPRS J. Photogramm. Remote Sens. 2012, 69, 74–87. [Google Scholar]
- Morel, A.; Prieur, L. Analysis of variations in ocean color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Sathyendranath, S. Remote Sensing of Ocean Colour in Coastal, and Other Optically-Complex, Waters; Report 3; International Ocean Colour Coordinating Group (IOCCG): Dartmouth, NS, Canada, 2000. [Google Scholar]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A comprehensive review on water quality parameters estimation using remote sensing techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef]
- Ogashawara, I.; Kiel, C.; Jechow, A.; Kohnert, K.; Ruhtz, T.; Grossart, H.P.; Hölker, F.; Nejstgaard, J.C.; Berger, S.A.; Wollrab, S. The use of Sentinel-2 for chlorophyll a spatial dynamics assessment: A comparative study on different lakes in northern Germany. Remote Sens. 2021, 13, 1542. [Google Scholar] [CrossRef]
- Ansper, A.; Alikas, K. Retrieval of chlorophyll a from Sentinel-2 MSI data for the European Union water framework directive reporting purposes. Remote Sens. 2018, 11, 64. [Google Scholar] [CrossRef]
- Kuhn, C.; de Matos Valerio, A.; Ward, N.; Loken, L.; Sawakuchi, H.O.; Kampel, M.; Richey, J.; Stadler, P.; Crawford, J.; Striegl, R.; et al. Performance of Landsat-8 and Sentinel-2 surface reflectance products for river remote sensing retrievals of chlorophyll a and turbidity. Remote Sens. Environ. 2019, 224, 104–118. [Google Scholar] [CrossRef]
- Watanabe, F.; Alcantara, E.; Rodrigues, T.; Rotta, L.; Bernardo, N.; Imai, N. Remote sensing of the chlorophyll a based on OLI/Landsat-8 and MSI/Sentinel-2A (Barra Bonita reservoir, Brazil). An. Acad. Bras. Ciências 2017, 90, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Jarreau, B.; Jolliff, J.; Ladner, S.; Lawson, T.A.; McCarthy, S.; Martinolich, P.; Montes, M. Assessing Planet Nanosatellite Sensors for Ocean Color Usage. Remote Sens. 2023, 15, 5359. [Google Scholar] [CrossRef]
- Yang, H.; Kong, J.; Hu, H.; Du, Y.; Gao, M.; Chen, F. A review of remote sensing for Water Quality Retrieval: Progress and challenges. Remote Sens. 2022, 14, 1770. [Google Scholar] [CrossRef]
- Greb, S.; Dekker, A.; Binding, C. (Eds.) Earth Observations in Support of Global Water Quality Monitoring; Number 17 in IOCCG Report Series; International Ocean Colour Coordinating Group: Dartmouth, NS, Canada, 2018. [Google Scholar]
- Bohn, V.Y.; Carmona, F.; Rivas, R.; Lagomarsino, L.; Diovisalvi, N.; Zagarese, H.E. Development of an empirical model for chlorophyll a and Secchi Disk Depth estimation for a Pampean shallow lake (Argentina). Egypt. J. Remote Sens. Space Sci. 2018, 21, 183–191. [Google Scholar] [CrossRef]
- Poddar, S.; Chacko, N.; Swain, D. Estimation of chlorophyll a in Northern Coastal Bay of Bengal using Landsat-8 OLI and Sentinel-2 MSI sensors. Front. Mar. Sci. 2019, 6, 598. [Google Scholar] [CrossRef]
- Song, K.; Wang, Z.; Blackwell, J.; Zhang, B.; Li, F.; Zhang, Y.; Jiang, G. Water quality monitoring using Landsat Themate Mapper data with empirical algorithms in Chagan Lake, China. J. Appl. Remote Sens. 2011, 5, 053506. [Google Scholar] [CrossRef]
- Härmä, P.; Vepsäläinen, J.; Hannonen, T.; Pyhälahti, T.; Kämäri, J.; Kallio, K.; Eloheimo, K.; Koponen, S. Detection of water quality using simulated satellite data and semi-empirical algorithms in Finland. Sci. Total Environ. 2001, 268, 107–121. [Google Scholar] [CrossRef]
- Dekker, A.G.; Vos, R.; Peters, S.W. Comparison of remote sensing data, model results and in situ data for total suspended matter (TSM) in the southern Frisian lakes. Sci. Total Environ. 2001, 268, 197–214. [Google Scholar] [CrossRef]
- Chang, K.W.; Shen, Y.; Chen, P.C. Predicting algal bloom in the Techi reservoir using Landsat TM data. Int. J. Remote Sens. 2004, 25, 3411–3422. [Google Scholar] [CrossRef]
- Kutser, T.; Pierson, D.C.; Kallio, K.Y.; Reinart, A.; Sobek, S. Mapping lake CDOM by satellite remote sensing. Remote Sens. Environ. 2005, 94, 535–540. [Google Scholar] [CrossRef]
- Mayo, M.; Gitelson, A.; Yacobi, Y.; Ben-Avraham, Z. Chlorophyll distribution in lake Kinneret determined from Landsat Thematic Mapper data. Remote Sens. 1995, 16, 175–182. [Google Scholar] [CrossRef]
- Tamiminia, H.; Salehi, B.; Mahdianpari, M.; Quackenbush, L.; Adeli, S.; Brisco, B. Google Earth Engine for geo-big data applications: A meta-analysis and systematic review. ISPRS J. Photogramm. Remote Sens. 2020, 164, 152–170. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, H.; Wang, L.; Huang, B.; Ranjan, R.; Zomaya, A.; Jie, W. Remote sensing big data computing: Challenges and opportunities. Future Gener. Comput. Syst. 2015, 51, 47–60. [Google Scholar] [CrossRef]
- Acheampong, C. Deriving algal concentration from Sentinel-2 through a downscaling technique: A case near the intake of a desalination plant. J. Geophys. Res. 2018, 103, 24937–24953. [Google Scholar]
- Binding, C.; Greenberg, T.; Bukata, R. The MERIS Maximum Chlorophyll Index; its merits and limitations for inland water algal bloom monitoring. J. Great Lakes Res. 2013, 39, 100–107. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Fu, R.; Wang, C.; Li, D.; Peng, Y.; Wang, L.; Jiang, H.; Zheng, Q. Remote sensing estimation of chlorophyll A in case-II waters of coastal areas: Three-band model versus genetic algorithm–artificial neural networks model. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 3640–3658. [Google Scholar] [CrossRef]
- Dekker, A.; Hestir, E. Evaluating the Feasibility of Systematic Inland Water Quality Monitoring with Satellite Remote Sensing; Commonwealth Scientific and Industrial Research Organization: Canberra, Australia, 2012. [Google Scholar]
- Gitelson, A.A.; Gurlin, D.; Moses, W.J.; Yacobi, Y.Z. Remote estimation of chlorophyll a concentration in inland, estuarine, and coastal waters. In Advances in Environmental Remote Sensing: Sensors, Algorithms, and Applications; Weng, Q., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2011; Chapter 18. [Google Scholar]
- Kallio, K.; Koponen, S.; Pulliainen, J. Feasibility of airborne imaging spectrometry for lake monitoring—A case study of spatial chlorophyll a distribution in two meso-eutrophic lakes. Int. J. Remote Sens. 2003, 24, 3771–3790. [Google Scholar] [CrossRef]
- Kravitz, J.; Matthews, M.; Bernard, S.; Griffith, D. Application of Sentinel 3 OLCI for chl-a retrieval over small inland water targets: Successes and challenges. Remote Sens. Environ. 2020, 237, 111562. [Google Scholar] [CrossRef]
- Lins, R.C.; Martinez, J.M.; Motta Marques, D.D.; Cirilo, J.A.; Fragoso, C.R., Jr. Assessment of chlorophyll a remote sensing algorithms in a productive tropical estuarine-lagoon system. Remote Sens. 2017, 9, 516. [Google Scholar] [CrossRef]
- Malthus, T.J.; Lehmann, E.; Ho, X.; Botha, E.; Anstee, J. Implementation of a satellite based inland water algal bloom alerting system using analysis ready data. Remote Sens. 2019, 11, 2954. [Google Scholar] [CrossRef]
- Neil, C.; Spyrakos, E.; Hunter, P.D.; Tyler, A.N. A global approach for chlorophyll a retrieval across optically complex inland waters based on optical water types. Remote Sens. Environ. 2019, 229, 159–178. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean color chlorophyll algorithms for SeaWiFS. J. Geophys. Res. Ocean. 1998, 103, 24937–24953. [Google Scholar] [CrossRef]
- Pahlevan, N.; Smith, B.; Schalles, J.; Binding, C.; Cao, Z.; Ma, R.; Alikas, K.; Kangro, K.; Gurlin, D.; Hà, N.; et al. Seamless retrievals of chlorophyll a from Sentinel-2 (MSI) and Sentinel-3 (OLCI) in inland and coastal waters: A machine-learning approach. Remote Sens. Environ. 2020, 240, 111604. [Google Scholar] [CrossRef]
- Gitelson, A. The peak near 700 nm on radiance spectra of algae and water: Relationships of its magnitude and position with chlorophyll concentration. Int. J. Remote Sens. 1992, 13, 3367–3373. [Google Scholar] [CrossRef]
- Gower, J.; Doerffer, R.; Borstad, G. Interpretation of the 685nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens. 1999, 20, 1771–1786. [Google Scholar] [CrossRef]
- Gilerson, A.A.; Gitelson, A.A.; Zhou, J.; Gurlin, D.; Moses, W.; Ioannou, I.; Ahmed, S.A. Algorithms for remote estimation of chlorophyll a in coastal and inland waters using red and near infrared bands. Opt. Express 2010, 18, 24109–24125. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of chlorophyll a concentration in case II waters using MODIS and MERIS data—successes and challenges. Environ. Res. Lett. 2009, 4, 045005. [Google Scholar] [CrossRef]
- Power, M.E.; Brozović, N.; Bode, C.; Zilberman, D. Spatially explicit tools for understanding and sustaining inland water ecosystems. Front. Ecol. Environ. 2005, 3, 47–55. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Saprygin, V.; Povazhnyi, V. Operational MERIS-based NIR-red algorithms for estimating chlorophyll a concentrations in coastal waters—The Azov Sea case study. Remote Sens. Environ. 2012, 121, 118–124. [Google Scholar] [CrossRef]
- Dogliotti, A.I.; Schloss, I.R.; Almandoz, G.O.; Gagliardini, D.A. Evaluation of SeaWiFS and MODIS chlorophyll a products in the Argentinean Patagonian continental shelf (38 S–55 S). Int. J. Remote Sens. 2009, 30, 251–273. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Lee, Z.; Franz, B.A.; Bailey, S.W.; Werdell, P.J.; Proctor, C.W. Improving satellite global chlorophyll a data products through algorithm refinement and data recovery. J. Geophys. Res. Ocean. 2019, 124, 1524–1543. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll algorithms for ocean color sensors-OC4, OC5 & OC6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar]
- Winarso, G.; Marini, Y. MODIS standard (OC3) chlorophyll a algorithm evaluation in Indonesian seas. Int. J. Remote Sens. Earth Sci. 2014, 11, 11–20. [Google Scholar] [CrossRef]
- Cherukuru, N.; Brando, V.E.; Schroeder, T.; Clementson, L.A.; Dekker, A.G. Influence of river discharge and ocean currents on coastal optical properties. Cont. Shelf Res. 2014, 84, 188–203. [Google Scholar] [CrossRef]
- Menken, K.D.; Brezonik, P.L.; Bauer, M.E. Influence of chlorophyll and colored dissolved organic matter (CDOM) on lake reflectance spectra: Implications for measuring lake properties by remote sensing. Lake Reserv. Manag. 2006, 22, 179–190. [Google Scholar] [CrossRef]
- Werdell, P.J.; Bailey, S.W. An improved in-situ bio-optical dataset for ocean color algorithm development and satellite data product validation. Remote Sens. Environ. 2005, 98, 122–140. [Google Scholar] [CrossRef]
- SeaBASS. NOMAD: NASA bio-Optical Marine Algorithm Dataset. 2012. Available online: https://seabass.gsfc.nasa.gov/wiki/NOMAD (accessed on 2 February 2023).
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll aalgorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Ocean. 2012, 117, C01011. [Google Scholar] [CrossRef]
- Schroeder, T.; Brando, V.; Cherukuru, N.; Clementson, L.; Blondeau-Patissier, D.; Dekker, A.; Schaale, M.; Fischer, J. Remote sensing of apparent and inherent optical properties of Tasmanian coastal waters: Application to MODIS data. In Proceedings of the XIX Ocean Optics Conference, Barga, Italy, 3–4 October 2008; pp. 6–10. [Google Scholar]
- Yacobi, Y.Z. From Tswett to identified flying objects: A concise history of chlorophyll a use for quantification of phytoplankton. Isr. J. Plant Sci. 2012, 60, 243–251. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, D.; Zhu, Y.; Peng, H.; Xie, H. A review of forest carbon cycle models on spatiotemporal scales. J. Clean. Prod. 2022, 339, 130692. [Google Scholar] [CrossRef]
- Amani, M.; Ghorbanian, A.; Ahmadi, S.A.; Kakooei, M.; Moghimi, A.; Mirmazloumi, S.M.; Moghaddam, S.H.A.; Mahdavi, S.; Ghahremanloo, M.; Parsian, S.; et al. Google Earth engine cloud computing platform for remote sensing big data applications: A comprehensive review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 5326–5350. [Google Scholar] [CrossRef]
- Department of Natural Resources and Environment Tasmania. LIST Marine Leases; Department of Natural Resources and Environment Tasmania: Tasmania, Australia, 2022. Available online: https://maps.thelist.tas.gov.au/listmap/app/list/map (accessed on 2 September 2022).
- Tasmanian Government. Department of Natural Resources and Environment Tasmania: Aquaculture. Available online: https://nre.tas.gov.au/aquaculture/aquaculture-species-in-tasmania/salmon-farming (accessed on 20 May 2024).
- Oysters Tasmania—Our Industry. Available online: https://www.oysterstasmania.org/ourindustry.html (accessed on 8 May 2024).
- Department of Agriculture, Fisheries and Forestry. Aquaculture Industry in Australia; 2020. Available online: https://www.agriculture.gov.au/agriculture-land/fisheries/aquaculture/aquaculture-industry-in-australia (accessed on 2 September 2022).
- Franz, B.A.; Bailey, S.W.; Kuring, N.; Werdell, P.J. Ocean color measurements with the Operational Land Imager on Landsat-8: Implementation and evaluation in SeaDAS. J. Appl. Remote Sens. 2015, 9, 096070. [Google Scholar] [CrossRef]
- Nazeer, M.; Bilal, M.; Nichol, J.E.; Wu, W.; Alsahli, M.M.; Shahzad, M.I.; Gayen, B.K. First experiences with the Landsat-8 aquatic reflectance product: Evaluation of the regional and ocean color algorithms in a coastal environment. Remote Sens. 2020, 12, 1938. [Google Scholar] [CrossRef]
- Wu, Q. geemap: A Python package for interactive mapping with Google Earth Engine. J. Open Source Softw. 2020, 5, 2305. [Google Scholar] [CrossRef]
- Cardille, J.A.; Crowley, M.A.; Saah, D.; Clinton, N.E. Cloud-Based Remote Sensing with Google Earth Engine: Fundamentals and Applications; Springer Nature Switzerland AG: Cham, Switzerland, 2023. [Google Scholar]
- Engine, G.E. LANDSAT/LC08/C02/T1_L2—Landsat 8 Collection 2, Tier 1, Level-2 Surface Reflectance. Available online: https://developers.Google.com/earth-engine/datasets/catalog/LANDSAT_LC08_C02_T1_L2 (accessed on 4 April 2022).
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of shallow water bathymetry, hydro-optics, and benthos mapping techniques in Australian and Caribbean coastal environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Cherukuru, N.; Ford, P.W.; Matear, R.J.; Oubelkheir, K.; Clementson, L.A.; Suber, K.; Steven, A.D. Estimating dissolved organic carbon concentration in turbid coastal waters using optical remote sensing observations. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 149–154. [Google Scholar] [CrossRef]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Weeks, S.; Werdell, P.J.; Schaffelke, B.; Canto, M.; Lee, Z.; Wilding, J.G.; Feldman, G.C. Satellite-derived photic depth on the Great Barrier Reef: Spatio-temporal patterns of water clarity. Remote Sens. 2012, 4, 3781–3795. [Google Scholar] [CrossRef]
- Tasmanian Tide Tables. Bureau of Meteorology, Australian Government. 2024. Available online: http://www.bom.gov.au/oceanography/projects/ntc/tas_tide_tables.shtml (accessed on 12 May 2024).
- Olmanson, L.G.; Brezonik, P.L.; Finlay, J.C.; Bauer, M.E. Comparison of Landsat 8 and Landsat 7 for regional measurements of CDOM and water clarity in lakes. Remote Sens. Environ. 2016, 185, 119–128. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, J.; Wang, K.; Wei, J.; Mu, B.; Ma, Y.; Zhu, J.; Liu, R.; Chen, X. Remote sensing of chlorophyll a concentration in turbid coastal waters based on a global optical water classification system. ISPRS J. Photogramm. Remote Sens. 2020, 163, 187–201. [Google Scholar] [CrossRef]
- IMOS. AODN Open Access to Ocean Data. 2021. Available online: https://portal.aodn.org.au/search?uuid=8964658c-6ee1-4015-9bae-2937dfcc6ab9https://portal.aodn.org.au/search (accessed on 9 April 2022).
- Gordon, H.R.; McCluney, W. Estimation of the depth of sunlight penetration in the sea for remote sensing. Appl. Opt. 1975, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.K.; Gurlin, D.; Pahlevan, N.; Alikas, K.; Anstee, J.; Balasubramanian, S.V.; Barbosa, C.C.; Binding, C.; Bracher, A.; Bresciani, M.; et al. GLORIA-A globally representative hyperspectral in situ dataset for optical sensing of water quality. Sci. Data 2023, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.; Duan, H.; Baek, S.; Kim, M.S.; Jeon, T.; Kwon, Y.S.; Lee, H.; Cho, K.H. A convolutional neural network regression for quantifying cyanobacteria using hyperspectral imagery. Remote Sens. Environ. 2019, 233, 111350. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R. Normalized difference chlorophyll index: A novel model for remote estimation of chlorophyll a concentration in turbid productive waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A simple semi-analytical model for remote estimation of chlorophyll a in turbid waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Bright, C.; Ardila, D.; Hestir, E.; Malthus, T.; Matthews, M.; Thompson, D.; Carter, N.; Dekker, A.; Frasson, R.; Green, R.; et al. The AquaSat-1 Mission Concept: Actionable Information on Water Quality and Aquatic Ecosystems for Australia and Western USA. In Proceedings of the IGARSS 2023—2023 IEEE International Geoscience and Remote Sensing Symposium, Pasadena, CA, USA, 16–21 July 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 4590–4593. [Google Scholar]
- Lehmann, M.K.; Gurlin, D.; Pahlevan, N.; Alikas, K.; Anstee, J.M.; Balasubramanian, S.V.; Barbosa, C.C.F.; Binding, C.; Bracher, A.; Bresciani, M.; et al. GLORIA—A global dataset of remote sensing reflectance and water quality from inland and coastal waters. Sci. Data 2022, 10, 100. [Google Scholar] [CrossRef]

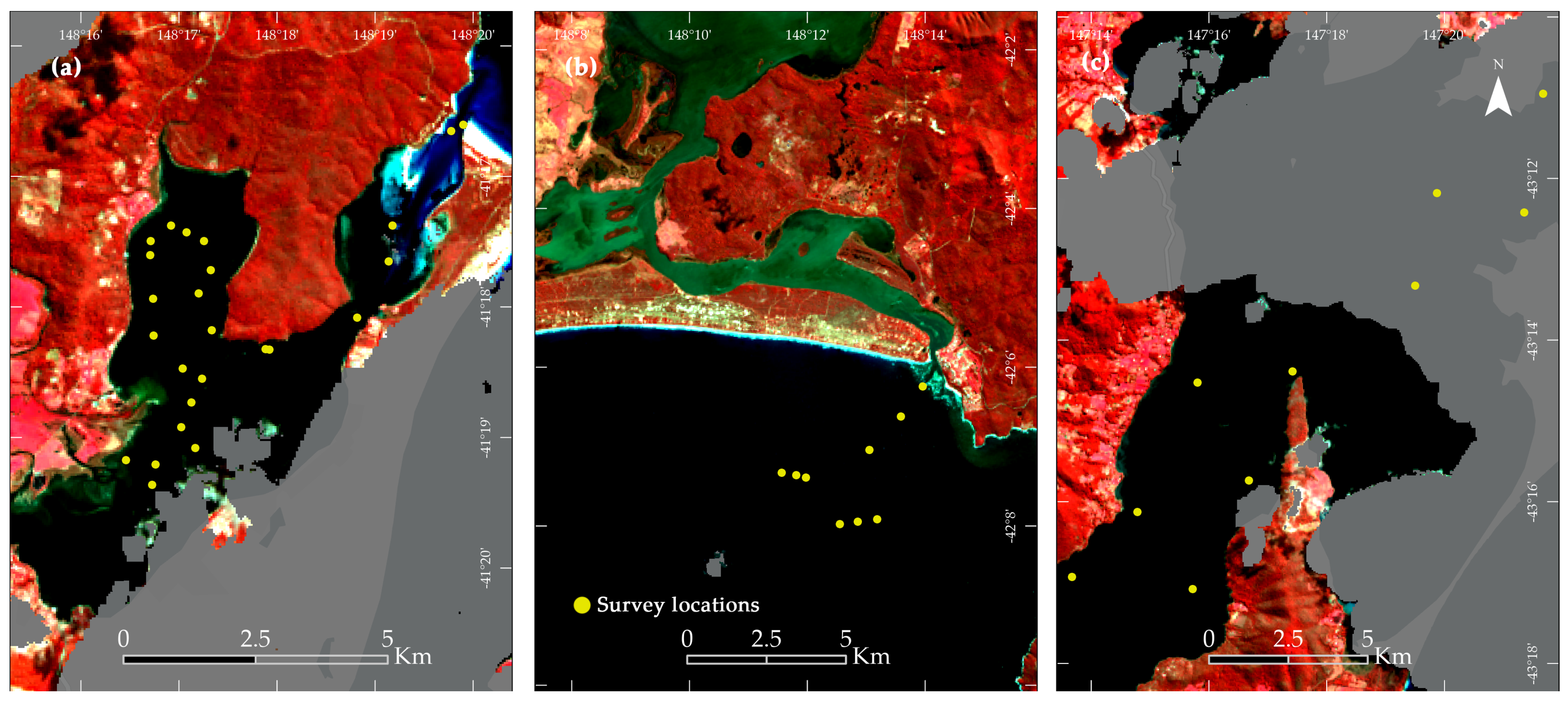

| Sensor | Map Label | Location | Survey Date | Image Capture Date | Image |
|---|---|---|---|---|---|
| Landsat 8 | a | STH | 13-SEP-22 | 11-SEP-22 | LANDSAT/LC08/C02/T1_L2/LC08_089089_20220911 |
| b | GOB | 15-SEP-22 | 18-SEP-22 | LANDSAT/LC08/C02/T1_L2/LC08_089089_20220918 | |
| c | BNI | 16-SEP-22 | 18-SEP-22 | LANDSAT/LC08/C02/T1_L2/LC08_089090_20220918 |
| Sensor | Location | Location | Installation Date |
|---|---|---|---|
| Multi-parameter | 147.363586, −43.207112 | BNI | 21 October |
| Xylem Exo 2 | 148.210739, −42.131580 | GOB | 23 May |
| Location | Sites | n | RMSE (mg/m3) | Remarks | |
|---|---|---|---|---|---|
| STH | All depths * | 25 | 0.39 | uncorrected ** | |
| Depth m * | 19 | 0.59 | uncorrected | ||
| 0.75 | 0.74 | modelled ** | |||
| GOB | All depths | 8 | 0.41 | uncorrected | |
| 0.99 | 0.22 | modelled | |||
| BNI | All depths | 6 | 0.33 | uncorrected | |
| 0.93 | 0.14 | modelled | |||
| Combined | Depth m | 33 | 0.10 | uncorrected | |
| 0.10 | modelled |
| Dataset | Availability | Depth | Australian Waters | |
|---|---|---|---|---|
| Coastal Waters | Inland Waters | |||
| NOMAD | 1991–2008 | unknown | X | X |
| IMOS | 2008–to date | 12–500 m | ✓ | X |
| GLORIA | till 2022 | 0–500 m | X | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandy, A.; Phinn, S.; Grinham, A.; Albert, S. Developing a Semi-Automated Near-Coastal, Water Quality-Retrieval Process from Global Multi-Spectral Data: South-Eastern Australia. Remote Sens. 2024, 16, 2389. https://doi.org/10.3390/rs16132389
Nandy A, Phinn S, Grinham A, Albert S. Developing a Semi-Automated Near-Coastal, Water Quality-Retrieval Process from Global Multi-Spectral Data: South-Eastern Australia. Remote Sensing. 2024; 16(13):2389. https://doi.org/10.3390/rs16132389
Chicago/Turabian StyleNandy, Avik, Stuart Phinn, Alistair Grinham, and Simon Albert. 2024. "Developing a Semi-Automated Near-Coastal, Water Quality-Retrieval Process from Global Multi-Spectral Data: South-Eastern Australia" Remote Sensing 16, no. 13: 2389. https://doi.org/10.3390/rs16132389







