Predicting Carbohydrate Concentrations in Avocado and Macadamia Leaves Using Hyperspectral Imaging with Partial Least Squares Regressions and Artificial Neural Networks
Abstract
:1. Introduction
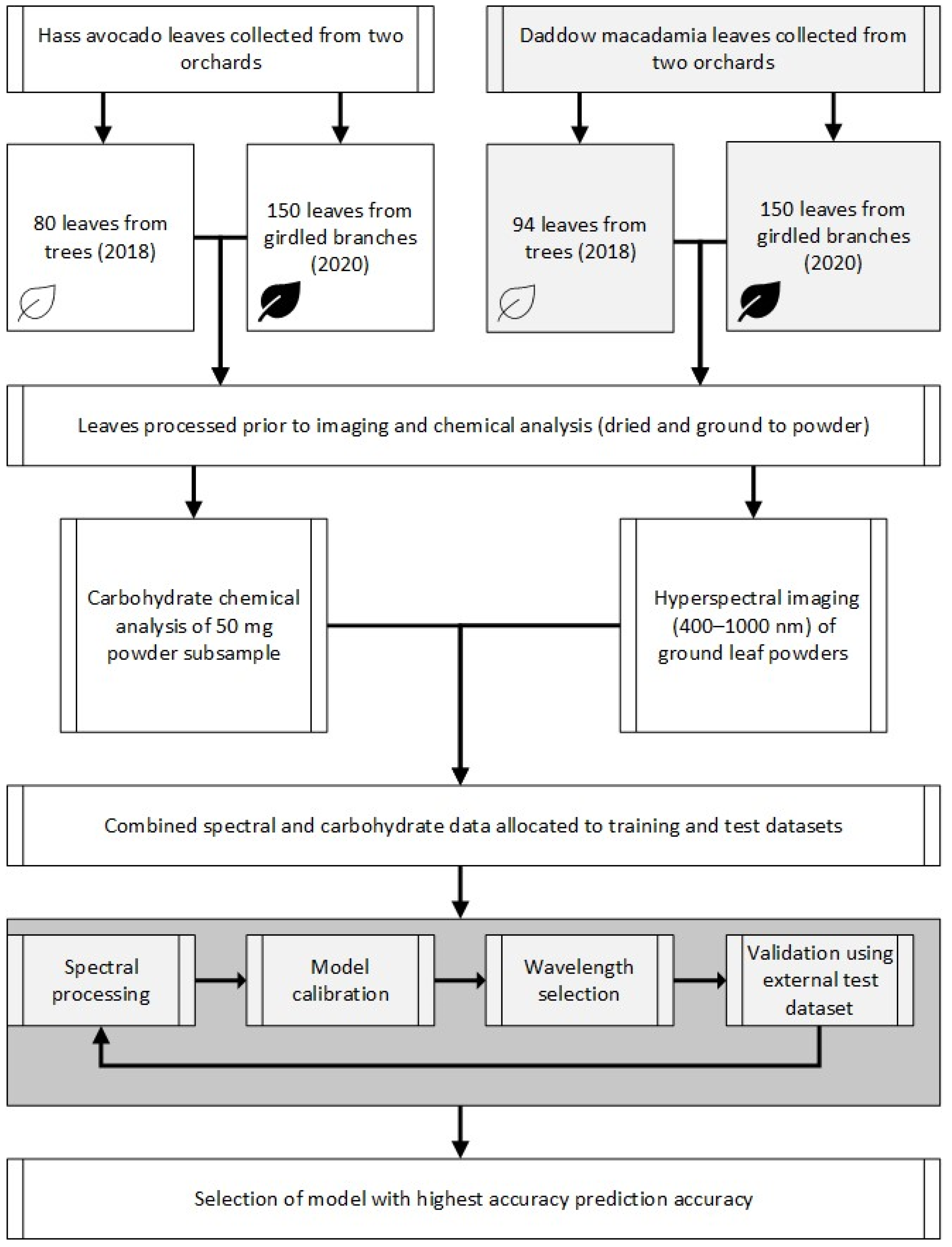
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Carbohydrate Analysis
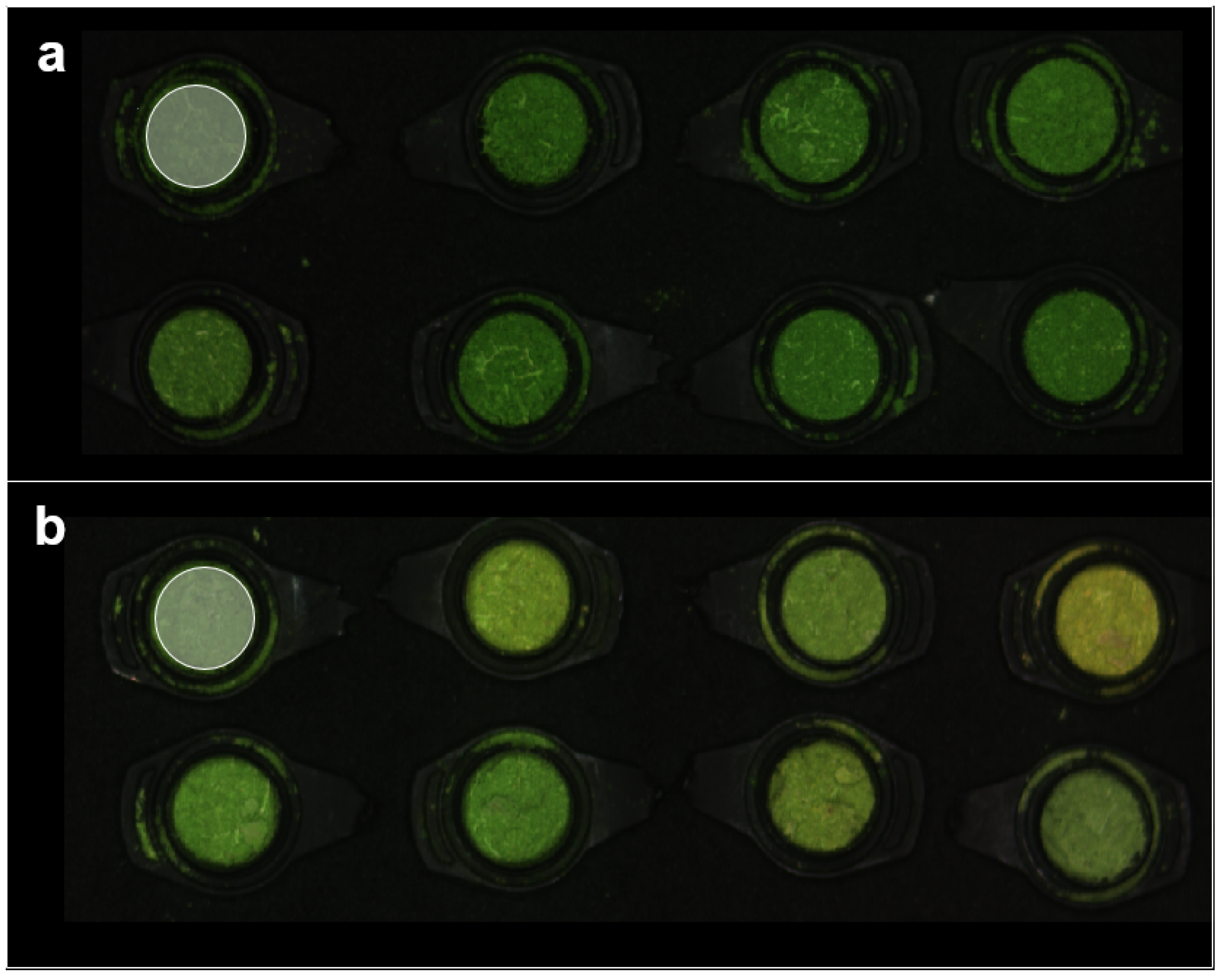
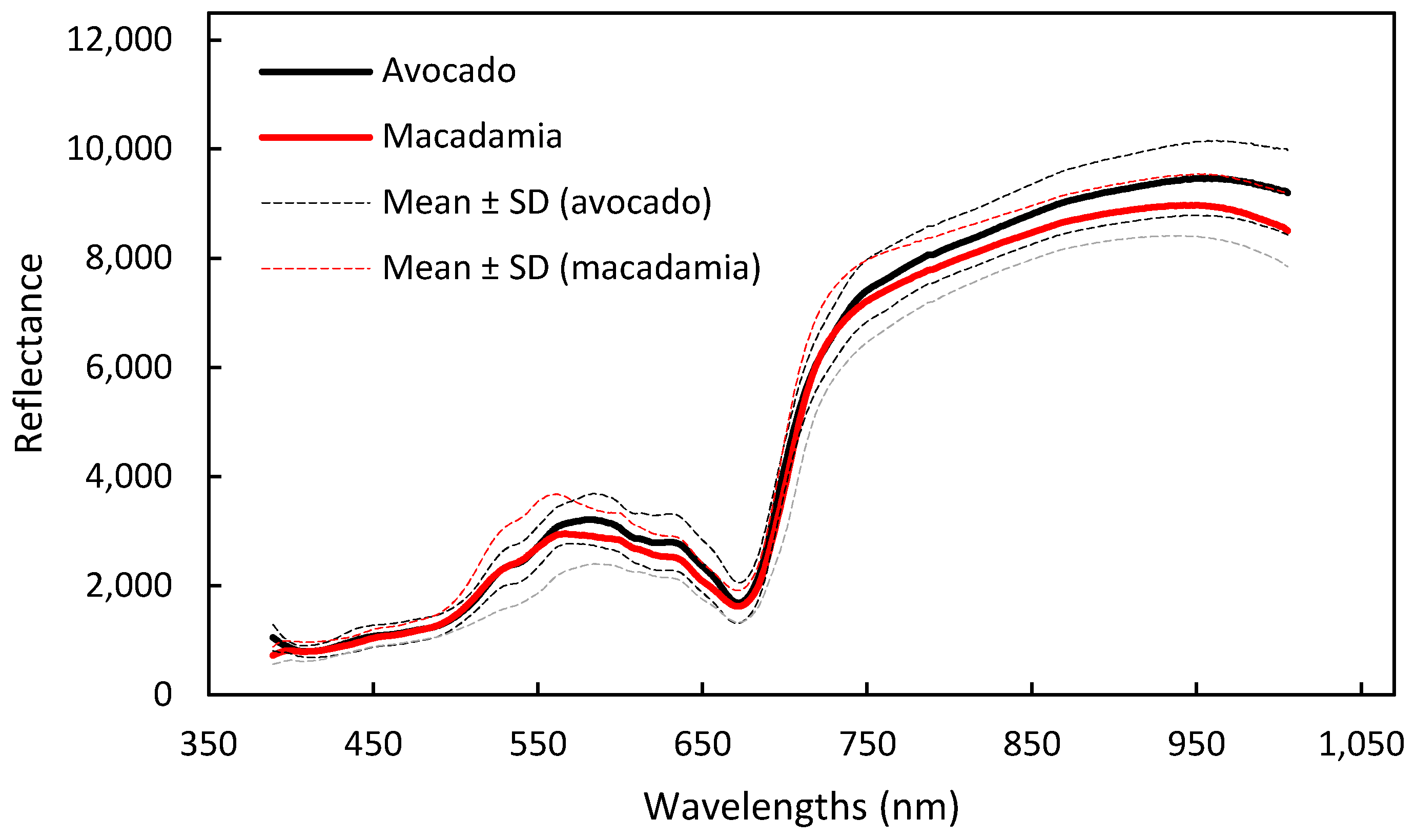
2.3. Hyperspectral Imaging System, Image Acquisition, and Spectral Data Extraction
2.4. Model Development, Selection, and Evaluation
3. Results
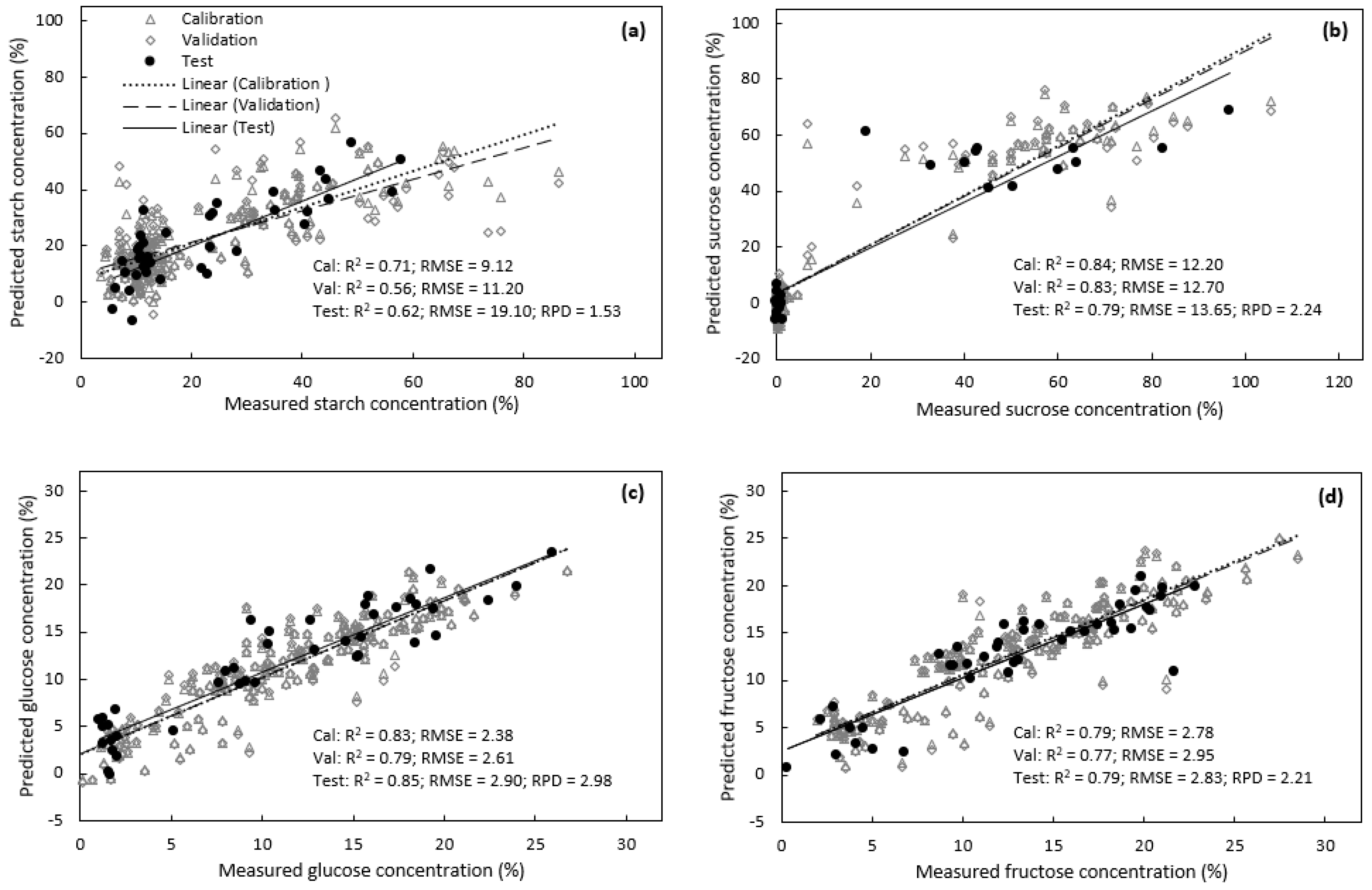
3.1. Prediction of Carbohydrate Concentrations in Avocado Leaves
3.2. Prediction of Carbohydrate Concentrations in Macadamia Leaves
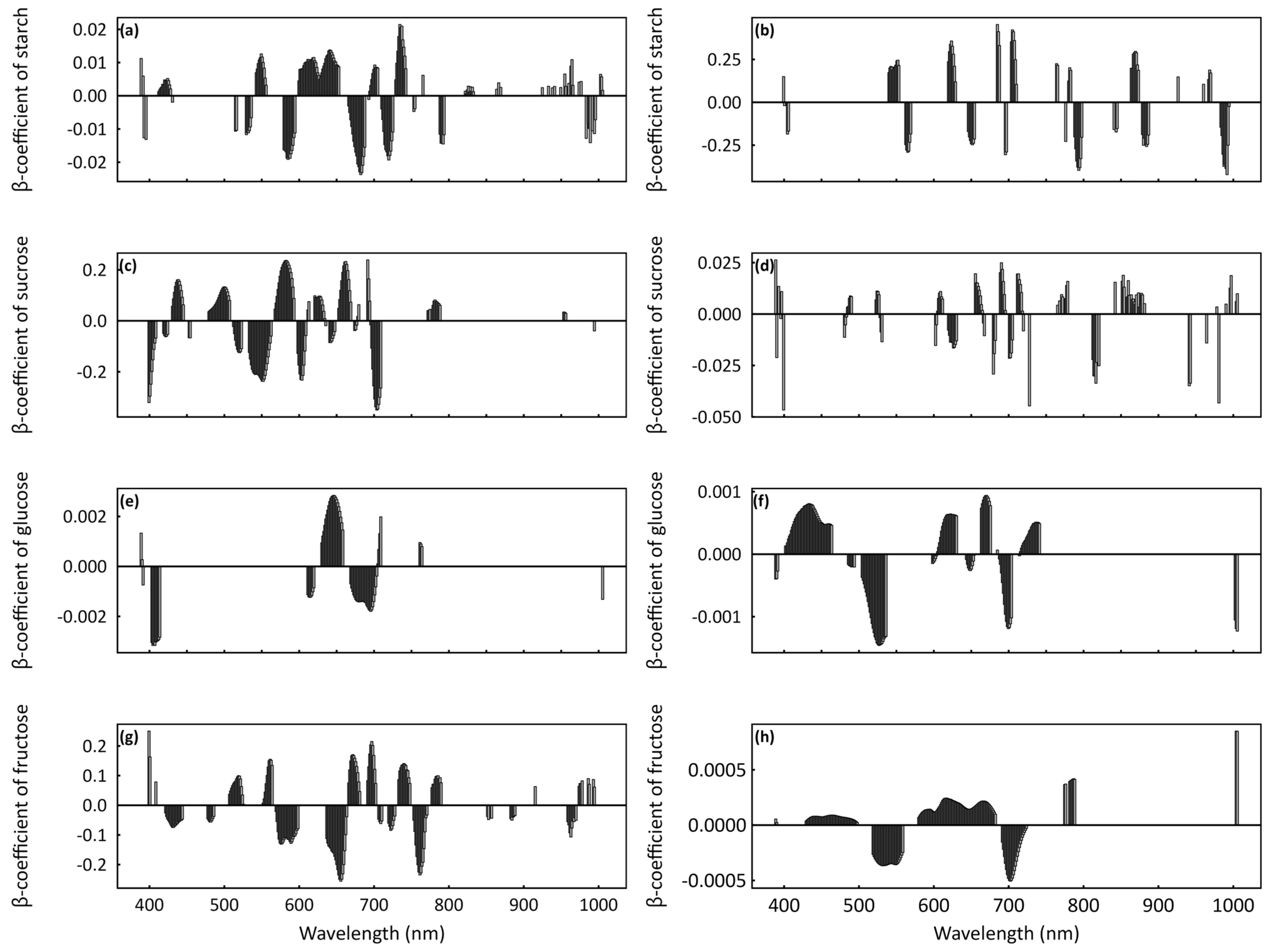
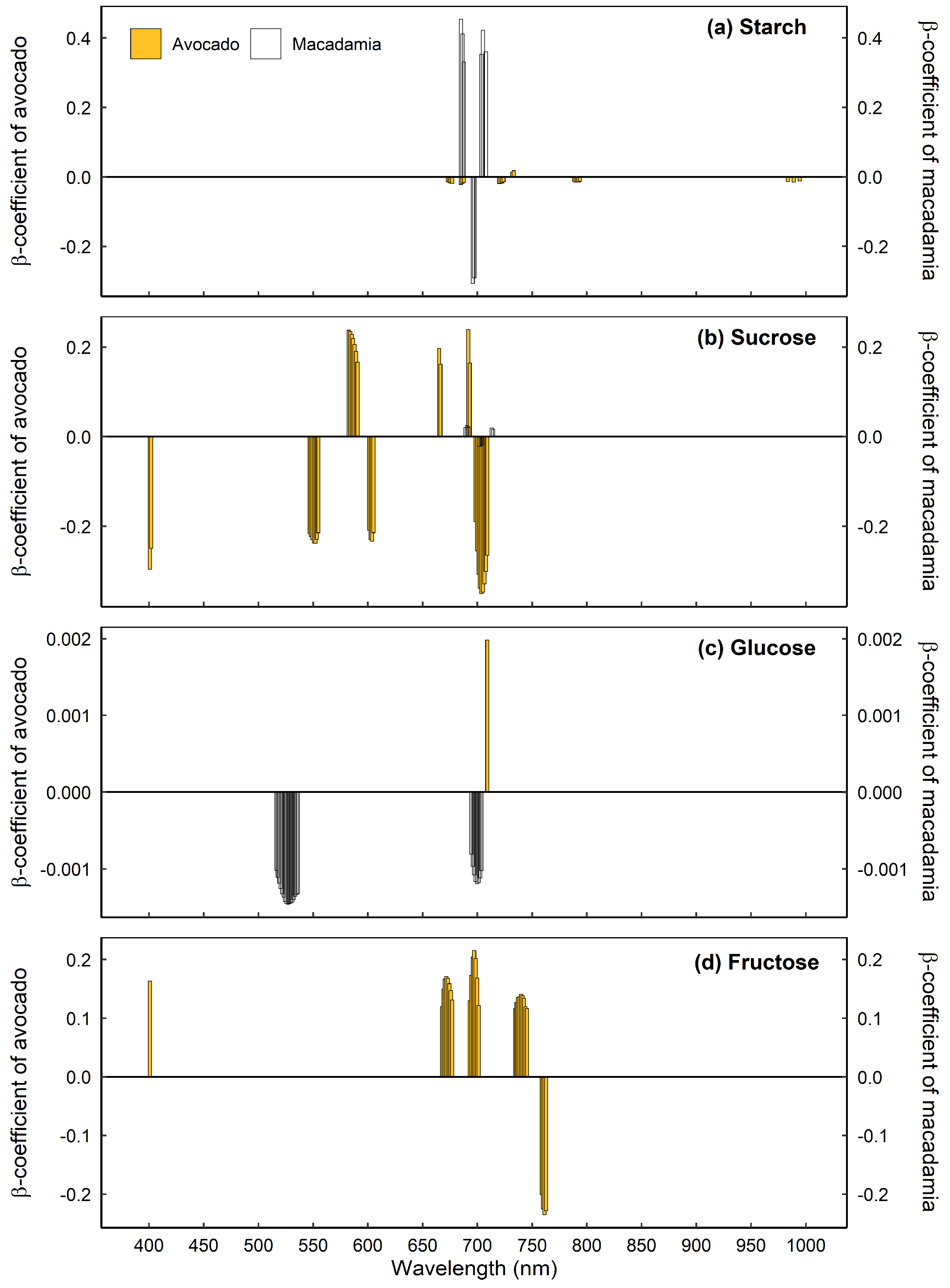
3.3. Important and Overlapping Principal Wavelengths
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture–Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef] [PubMed]
- Huett, D.; Dirou, J. An evaluation of the rationale for fertiliser management of tropical fruit crops. Aust. J. Exp. Agric. 2000, 40, 1137–1143. [Google Scholar] [CrossRef]
- Matsuoka, K. Methods for nutrient diagnosis of fruit trees early in the growing season by using simultaneous multi-element analysis. Hortic. J. 2020, 89, 197–207. [Google Scholar] [CrossRef]
- Boldingh, H.; Alcaraz, M.; Thorp, T.; Minchin, P.; Gould, N.; Hormaza, J. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Herbert, S.W.; Walton, D.A.; Wallace, H.M. The influence of pollen-parent and carbohydrate availability on macadamia yield and nut size. Sci. Hortic. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Huett, D. Macadamia physiology review: A canopy light response study and literature review. Aust. J. Agric. Res. 2004, 55, 609–624. [Google Scholar] [CrossRef]
- McFadyen, L.M.; Robertson, D.; Sedgley, M.; Kristiansen, P.; Olesen, T. Post-pruning shoot growth increases fruit abscission and reduces stem carbohydrates and yield in macadamia. Ann. Bot. 2011, 107, 993–1001. [Google Scholar] [CrossRef]
- Olesen, T.; Robertson, D.; Muldoon, S.; Meyer, R. The role of carbohydrate reserves in evergreen tree development, with particular reference to macadamia. Sci. Hortic. 2008, 117, 73–77. [Google Scholar] [CrossRef]
- Smith, G.; Clark, C.; Boldingh, H. Seasonal accumulation of starch by components of the kiwifruit vine. Ann. Bot. 1992, 70, 19–25. [Google Scholar] [CrossRef]
- Rivas, F.; Gravina, A.; Agustí, M. Girdling effects on fruit set and quantum yield efficiency of PSII in two Citrus cultivars. Tree Physiol. 2007, 27, 527–535. [Google Scholar] [CrossRef]
- Boldingh, H.; Pereira, T.; Shepherd, S.; Hewitt, K.; Cooney, J.; Rowan, D.; Hedderley, D.; Jensen, D.; Trower, T.; Richardson, A. Diurnal fluctuations of metabolites in leaves of ‘Zesy002’ kiwifruit. Acta Hortic. 2022, 1322, 155–162. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Trueman, S.J.; Kämper, W.; Farrar, M.B.; Wallace, H.M.; Nichols, J.; Bai, S.H. Hyperspectral imaging of adaxial and abaxial leaf surfaces for rapid assessment of foliar nutrient concentrations in Hass avocado. Remote Sens. 2023, 15, 3100. [Google Scholar] [CrossRef]
- Watt, M.S.; Buddenbaum, H.; Leonardo, E.M.C.; Estarija, H.J.C.; Bown, H.E.; Gomez-Gallego, M.; Hartley, R.; Massam, P.; Wright, L.; Zarco-Tejada, P.J. Using hyperspectral plant traits linked to photosynthetic efficiency to assess N and P partition. ISPRS J. Photogramm. Remote Sens. 2020, 169, 406–420. [Google Scholar] [CrossRef]
- Ye, X.; Abe, S.; Zhang, S. Estimation and mapping of nitrogen content in apple trees at leaf and canopy levels using hyperspectral imaging. Precis. Agric. 2020, 21, 198–225. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef]
- Farrar, M.B.; Wallace, H.M.; Tahmasbian, I.; Yule, C.M.; Dunn, P.K.; Hosseini Bai, S. Rapid assessment of soil carbon and nutrients following application of organic amendments. Catena 2023, 223, 106928. [Google Scholar] [CrossRef]
- Malmir, M.; Tahmasbian, I.; Xu, Z.; Farrar, M.B.; Bai, S.H. Prediction of macronutrients in plant leaves using chemometric analysis and wavelength selection. J. Soils Sediments 2020, 20, 249–259. [Google Scholar] [CrossRef]
- Sun, D.-W. Computer Vision Technology for Food Quality Evaluation; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Tahmasbian, I.; Hosseini Bai, S.; Wang, Y.; Boyd, S.; Zhou, J.; Esmaeilani, R.; Xu, Z. Using laboratory-based hyperspectral imaging method to determine carbon functional group distributions in decomposing forest litterfall. Catena 2018, 167, 18–27. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Xu, Z.; Boyd, S.; Zhou, J.; Esmaeilani, R.; Che, R.; Hosseini Bai, S. Laboratory-based hyperspectral image analysis for predicting soil carbon, nitrogen and their isotopic compositions. Geoderma 2018, 330, 254–263. [Google Scholar] [CrossRef]
- Farrar, M.B.; Omidvar, R.; Nichols, J.; Pelliccia, D.; Lateef Al-Khafaji, S.; Tahmasbian, I.; Hapuarachchi, N.; Hosseini Bai, S. Hyperspectral imaging predicts macadamia nut-in-shell and kernel moisture using machine vision and learning tools. Comput. Electron. Agric. 2024, 224, 109209. [Google Scholar] [CrossRef]
- Chu, B.; Li, C.; Wang, S.; Jin, W.; Li, X.; He, G.; Xiao, G. Nondestructive determination and visualization of protein and carbohydrate concentration of Chlorella pyrenoidosa in situ using hyperspectral imaging technique. Comput. Electron. Agric. 2023, 206, 107684. [Google Scholar] [CrossRef]
- Ely, K.S.; Burnett, A.C.; Lieberman-Cribbin, W.; Serbin, S.P.; Rogers, A. Spectroscopy can predict key leaf traits associated with source–sink balance and carbon–nitrogen status. J. Exp. Bot. 2019, 70, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Posada, J.M.; Handa, I.T.; Hoch, G.; Vohland, M.; Messier, C.; Reu, B. Near-infrared spectroscopy (NIRS) predicts non-structural carbohydrate concentrations in different tissue types of a broad range of tree species. Methods Ecol. Evol. 2015, 6, 1018–1025. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Song, S.; Xie, S.; Kang, F. Study on starch content detection and visualization of potato based on hyperspectral imaging. Food Sci. Nutr. 2021, 9, 4420–4430. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.L.; Trueman, S.J.; Kämper, W.; Wallace, H.M.; Nichols, J.; Hosseini Bai, S. Hyperspectral Imaging of Adaxial and Abaxial Leaf Surfaces as a Predictor of Macadamia Crop Nutrition. Plants 2023, 12, 558. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Q.; He, S.; Yi, S.; Liu, X.; Xie, R.; Zheng, Y.; Deng, L. Prediction of nitrogen and phosphorus contents in citrus leaves based on hyperspectral imaging. Int. J. Agric. Biol. Eng. 2015, 8, 80–88. [Google Scholar]
- Farrar, M.B.; Wallace, H.M.; Brooks, P.; Yule, C.M.; Tahmasbian, I.; Dunn, P.K.; Hosseini Bai, S. A performance evaluation of Vis/NIR hyperspectral imaging to predict curcumin concentration in fresh turmeric rhizomes. Remote Sens. 2021, 13, 1807. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Khoshelham, K.; Bai, S.H. Quality estimation of nuts using deep learning classification of hyperspectral imagery. Comput. Electron. Agric. 2021, 180, 105868. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Morgan, N.K.; Hosseini Bai, S.; Dunlop, M.W.; Moss, A.F. Comparison of Hyperspectral Imaging and Near-Infrared Spectroscopy to Determine Nitrogen and Carbon Concentrations in Wheat. Remote Sens. 2021, 13, 1128. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Wallace, H.M.; Gama, T.; Bai, S.H. An automated non-destructive prediction of peroxide value and free fatty acid level in mixed nut samples. LWT 2021, 143, 110893. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Tahmasbian, I.; Bai, S.H. Rapid determination of nutrient concentrations in Hass avocado fruit by Vis/NIR hyperspectral imaging of flesh or skin. Remote Sens. 2020, 12, 3409. [Google Scholar] [CrossRef]
- Mayr, S.; Beć, K.B.; Grabska, J.; Wiedemair, V.; Pürgy, V.; Popp, M.A.; Bonn, G.K.; Huck, C.W. Challenging handheld NIR spectrometers with moisture analysis in plant matrices: Performance of PLSR vs. GPR vs. ANN modelling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119342. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Bai, S.H.; Trueman, S.J.; Khoshelham, K.; Kämper, W. Predicting the ripening time of ‘Hass’ and ‘Shepard’avocado fruit by hyperspectral imaging. Precis. Agric. 2023, 24, 1889–1905. [Google Scholar] [CrossRef]
- Larose, D.T.; Larose, C.D. Discovering Knowledge in Data: An Introduction to Data Mining, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Araújo, S.R.; Wetterlind, J.; Demattê, J.A.M.; Stenberg, B. Improving the prediction performance of a large tropical vis-NIR spectroscopic soil library from Brazil by clustering into smaller subsets or use of data mining calibration techniques. Eur. J. Soil Sci. 2014, 65, 718–729. [Google Scholar] [CrossRef]
- Lucà, F.; Conforti, M.; Castrignanò, A.; Matteucci, G.; Buttafuoco, G. Effect of calibration set size on prediction at local scale of soil carbon by Vis-NIR spectroscopy. Geoderma 2017, 288, 175–183. [Google Scholar] [CrossRef]
- Boldingh, H.; Richardson, A.; Minchin, P.; MacRae, E. Planteose is a major sugar translocated in Actinidia arguta ‘Hortgem Tahi’. Sci. Hortic. 2015, 193, 261–268. [Google Scholar] [CrossRef]
- Rajput, D.; Wang, W.-J.; Chen, C.-C. Evaluation of a decided sample size in machine learning applications. BMC Bioinform. 2023, 24, 48. [Google Scholar] [CrossRef]
- Gama, T.; Farrar, M.B.; Tootoonchy, M.; Wallace, H.M.; Trueman, S.J.; Tahmasbian, I.; Bai, S.H. Hyperspectral imaging predicts free fatty acid levels, peroxide values, and linoleic acid and oleic acid concentrations in tree nut kernels. LWT 2024, 199, 116068. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Anderson, N.; Walsh, K.; Subedi, P.; Hayes, C. Achieving robustness across season, location and cultivar for a NIRS model for intact mango fruit dry matter content. Postharvest Biol. Technol. 2020, 168, 111202. [Google Scholar] [CrossRef]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Davie, S.J.; Stassen, P.J.C.; van der Walt, M.; Snijder, B. Girdling Avocado Trees for Improved Production. In South African Avocado Growers’ Association Yearbook 1995; Institute for Tropical and Subtropical Crops: Nelspruit, South Africa, 1995; pp. 51–53. [Google Scholar]
- Bai, S.H.; Tahmasbian, I.; Zhou, J.; Nevenimo, T.; Hannet, G.; Walton, D.; Randall, B.; Gama, T.; Wallace, H.M. A non-destructive determination of peroxide values, total nitrogen and mineral nutrients in an edible tree nut using hyperspectral imaging. Comput. Electron. Agric. 2018, 151, 492–500. [Google Scholar] [CrossRef]
- Malmir, M.; Tahmasbian, I.; Xu, Z.; Farrar, M.B.; Bai, S.H. Prediction of soil macro- and micro-elements in sieved and ground air-dried soils using laboratory-based hyperspectral imaging technique. Geoderma 2019, 340, 70–80. [Google Scholar] [CrossRef]
- Li, B.; Liew, O.W.; Asundi, A.K. Pre-visual detection of iron and phosphorus deficiency by transformed reflectance spectra. J. Photochem. Photobiol. B Biol. 2006, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wold, S. PLS for Multivariate Linear Modeling. In Chemometric Methods in Molecular Design, 1st ed.; Waterbeemd, H.v.d., Ed.; Wiley VCH: Weinheim, Germany, 1995; Volume 2, pp. 195–218. [Google Scholar]
- Huang, H.-H.; He, Q. Nonlinear regression analysis. In International Encyclopedia of Education, 4th ed.; Tierney, R.J., Rizvi, F., Ercikan, K., Eds.; Elsevier: Oxford, UK, 2023; pp. 558–567. [Google Scholar]
- Huang, C.; Chang, Y.; Han, L.; Chen, F.; Li, S.; Hong, J. Bandwidth correction of spectral measurement based on Levenberg–Marquardt algorithm with improved Tikhonov regularization. Appl. Opt. 2019, 58, 2166–2173. [Google Scholar] [CrossRef]
- Weiss, S.M.; Kulikowski, C.A. Computer Systems That Learn: Classification and Prediction Methods from Statistics, Neural Nets, Machine Learning, and Expert Systems; Morgan Kaufmann Publishers Inc.: Burlington, MA, USA, 1991. [Google Scholar]
- Kamruzzaman, M.; Elmasry, G.; Sun, D.W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. Technol. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of Levene’s test of equality of variances on statistical theory and practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosys. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Williams, P.; Antoniszyn, J.; Manley, M. Near-Infrared Technology: Getting the Best out of Light; African Sun Media: Stellenbosch, South Africa, 2019. [Google Scholar] [CrossRef]
- Peirs, A.; Tirry, J.; Verlinden, B.; Darius, P.; Nicolaï, B.M. Effect of biological variability on the robustness of NIR models for soluble solids content of apples. Postharvest Biol. Technol. 2003, 28, 269–280. [Google Scholar] [CrossRef]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FT-IR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-wavelength near-infrared spectra of sucrose, glucose, and fructose with respect to sugar concentration and temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Miao, Y.; Zhang, H.; Matsunaga, R. Non-destructive determination of carbohydrate content in potatoes using near infrared spectroscopy. J. Near Infrared Spectrosc. 2004, 12, 311–314. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Guthrie, J.; Walsh, K. Non-invasive assessment of pineapple and mango fruit quality using near infra-red spectroscopy. Aust. J. Exp. Agric. 1997, 37, 253–263. [Google Scholar] [CrossRef]
- Kawano, S.; Watanabe, H.; Iwamoto, M. Determination of sugar content in intact peaches by near infrared spectroscopy with fiber optics in interactance mode. J. Jpn. Soc. Hort. Sci. 1992, 61, 445–451. [Google Scholar] [CrossRef]
- Esbensen, K.H.; Swarbrick, B.; Westad, F.; Whitcombe, P.; Anderson, M. Multivariate Data Analysis: An Introduciton to Multivariate Data Analysis, Process Analytical Technology and Quality by Design, 6th ed.; CAMO Software: Oslo, Norway, 2018. [Google Scholar]
- Rossel, R.A.V. ParLeS: Software for chemometric analysis of spectroscopic data. Chemom. Intellig. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Habibi, V.; Ahmadi, H.; Jafari, M.; Moeini, A. Machine learning and multispectral data-based detection of soil salinity in an arid region, Central Iran. Environ. Monit. Assess. 2020, 192, 759. [Google Scholar] [CrossRef]
- Bian, M.; Skidmore, A.; Schlerf, M.; Liu, Y.; Wang, T. Estimating biochemical parameters of tea (Camellia sinensis (L.)) using hyperspectral techniques. Int. Arch. Photogram. Remote. Sens. Spatial Inf. Sci. 2012, XXXIX-B8, 237–241. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]


| Kernels | Set | Average | SD | Min | Max | CV |
|---|---|---|---|---|---|---|
| Avocado | ||||||
| Starch | Calibration | 24.36 | 21.71 | 3.74 | 139.92 | 89.12 |
| Test | 25.69 | 29.35 | 5.92 | 183.29 | 114.39 | |
| Sucrose | Calibration | 26.10 | 30.94 | 0.02 | 105.32 | 118.54 |
| Test | 25.93 | 30.62 | 0.03 | 96.70 | 118.08 | |
| Sucrose 1 * | Calibration | 58.15 | 15.14 | 33.08 | 96.7 | 26.04 |
| Test | 66.49 | 20.21 | 31.13 | 105.33 | 0.3 | |
| Sucrose 2 * | Calibration | 0.49 | 0.74 | 0.02 | 4.37 | 153 |
| Test | 6.06 | 8.17 | 0.02 | 27.24 | 1.34 | |
| Glucose | Calibration | 11.22 | 5.81 | 0.10 | 26.77 | 51.78 |
| Test | 10.84 | 7.45 | 1.03 | 25.98 | 68.72 | |
| Fructose | Calibration | 12.82 | 6.17 | 1.96 | 28.47 | 48.12 |
| Test | 12.82 | 6.26 | 0.30 | 22.87 | 48.82 | |
| Macadamia | ||||||
| Starch | Calibration | 4.66 | 4.10 | 0.14 | 21.81 | 87.98 |
| Test | 4.59 | 3.08 | 0.50 | 12.20 | 67.10 | |
| Sucrose | Calibration | 6.88 | 5.55 | 0.17 | 22.03 | 80.66 |
| Test | 7.96 | 6.56 | 0.42 | 22.20 | 82.41 | |
| Glucose | Calibration | 27.46 | 14.91 | 2.84 | 47.57 | 54.29 |
| Test | 29.10 | 14.79 | 2.92 | 47.23 | 50.82 | |
| Glucose 1 * | Calibration | 5.00 | 2.68 | 2.84 | 20.17 | 53.62 |
| Test | 6.12 | 4.26 | 3.59 | 19.85 | 68.67 | |
| Glucose 2 * | Calibration | 37.25 | 5.42 | 21.87 | 47.57 | 14.55 |
| Test | 35.48 | 3.32 | 28.43 | 41.94 | 9.30 | |
| Fructose | Calibration | 17.87 | 11.39 | 0.64 | 34.01 | 63.73 |
| Test | 20.27 | 11.10 | 0.59 | 33.86 | 54.76 | |
| Fructose 1 * | Calibration | 2.48 | 2.35 | 0.59 | 8.91 | 94.81 |
| Test | 2.70 | 3.10 | 0.83 | 13.7 | 114.9 | |
| Fructose 2 * | Calibration | 26.98 | 3.26 | 15.13 | 34.01 | 12.08 |
| Test | 24.47 | 2.62 | 18.99 | 29.27 | 10.68 |
| RMSE (%) | RMSE (%) | RMSE (%) | R2 | R2 | R2 | RPD | ||
|---|---|---|---|---|---|---|---|---|
| Calibration | Validation | Test | Calibration | Validation | Test | Test | ||
| Avocado | ||||||||
| Starch | PLSR (284) | 9.12 | 11.20 | 19.10 | 0.71 | 0.56 | 0.62 | 1.53 |
| PLSR (462) | 10.58 | 12.85 | 21.01 | 0.64 | 0.47 | 0.51 | 1.39 | |
| ANN (462) | 9.82 | 14.74 | 15.10 | 0.90 | 0.79 | 0.83 | 1.98 | |
| Sucrose | PLSR (179) | 12.20 | 12.70 | 13.65 | 0.84 | 0.83 | 0.79 | 2.24 |
| PLSR (462) | 10.53 | 11.99 | 12.69 | 0.88 | 0.85 | 0.82 | 2.41 | |
| ANN (462) | 10.45 | 7.58 | 14.72 | 0.95 | 0.95 | 0.83 | 1.29 | |
| Sucrose 1 | PLSR (462) | 15.15 | 11.78 | 20.21 | 0.89 | 0.71 | 0.69 | 1.00 |
| ANN (462) | 5.15 | 13.94 | 19.90 | 94.00 | 0.74 | 0.55 | 1.02 | |
| Sucrose 2 | PLSR (462) | 6.08 | 6.85 | 7.28 | 0.95 | 0.96 | 0.98 | 1.12 |
| ANN (462) | 6.03 | 6.60 | 4.06 | 0.99 | 0.98 | 0.99 | 2.01 | |
| Glucose | PLSR (76) | 2.38 | 2.61 | 2.90 | 0.83 | 0.79 | 0.85 | 2.98 |
| PLSR (462) | 2.30 | 2.62 | 2.50 | 0.84 | 0.79 | 0.86 | 2.98 | |
| ANN (462) | 1.21 | 2.06 | 3.66 | 0.98 | 0.90 | 0.78 | 0.89 | |
| Fructose | PLSR (192) | 2.78 | 2.96 | 2.83 | 0.79 | 0.77 | 0.79 | 2.21 |
| PLSR (462) | 2.58 | 2.95 | 2.46 | 0.82 | 0.77 | 0.85 | 2.54 | |
| ANN (462) | 1.15 | 2.37 | 3.39 | 0.98 | 0.84 | 0.86 | 1.11 | |
| Macadamia | ||||||||
| Starch | PLSR (93) | 2.01 | 2.16 | 2.15 | 0.75 | 0.72 | 0.52 | 1.45 |
| PLSR (462) | 1.90 | 2.26 | 2.20 | 0.78 | 0.69 | 0.60 | 1.57 | |
| ANN (462) | 1.89 | 2.16 | 3.59 | 0.89 | 0.61 | 0.67 | 1.17 | |
| Sucrose | PLSR (111) | 2.83 | 3.40 | 3.81 | 0.73 | 0.62 | 0.65 | 1.44 |
| PLSR (462) | 2.67 | 3.35 | 3.89 | 0.76 | 0.63 | 0.64 | 1.36 | |
| ANN (462) | 2.58 | 2.56 | 3.61 | 0.89 | 0.92 | 0.82 | 1.47 | |
| Glucose | PLSR (166) | 4.56 | 4.87 | 4.37 | 0.90 | 0.89 | 0.92 | 3.53 |
| PLSR (462) | 4.32 | 4.63 | 4.40 | 0.91 | 0.90 | 0.91 | 3.51 | |
| ANN (462) | 1.04 | 1.62 | 2.54 | 0.99 | 0.99 | 0.98 | 1.30 | |
| Glucose 1 | PLSR (462) | 3.59 | 1.24 | 1.17 | 0.96 | 0.97 | 0.86 | 3.64 |
| ANN (462) | 3.92 | 2.45 | 3.51 | 0.66 | 0.97 | 0.18 | 1.21 | |
| Glucose 2 | PLSR (462) | 5.42 | 4.84 | 3.33 | 0.81 | 0.82 | 0.87 | 1.00 |
| ANN (462) | 1.89 | 3.07 | 4.00 | 0.91 | 0.82 | 0.71 | 0.83 | |
| Fructose | PLSR (200) | 2.97 | 3.09 | 2.37 | 0.93 | 0.92 | 0.95 | 4.68 |
| PLSR (462) | 2.93 | 3.05 | 2.60 | 0.93 | 0.92 | 0.95 | 4.25 | |
| ANN (462) | 0.65 | 2.50 | 2.26 | 0.99 | 0.97 | 0.98 | 1.22 | |
| Fructose 1 | PLSR (462) | 2.36 | 3.68 | 3.10 | 0.94 | 0.98 | 0.99 | 1.00 |
| ANN (462) | 1.21 | 0.41 | 3.50 | 0.91 | 0.48 | 0.53 | 0.89 | |
| Fructose 2 | PLSR (462) | 3.26 | 3.06 | 2.61 | 0.94 | 0.81 | 0.86 | 1.00 |
| ANN (462) | 2.91 | 1.92 | 3.37 | 0.67 | 0.04 | 0.49 | 0.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.H.; Tootoonchy, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Boldingh, H.; Pereira, T.; Jonson, H.; Nichols, J.; Wallace, H.M.; et al. Predicting Carbohydrate Concentrations in Avocado and Macadamia Leaves Using Hyperspectral Imaging with Partial Least Squares Regressions and Artificial Neural Networks. Remote Sens. 2024, 16, 3389. https://doi.org/10.3390/rs16183389
Bai SH, Tootoonchy M, Kämper W, Tahmasbian I, Farrar MB, Boldingh H, Pereira T, Jonson H, Nichols J, Wallace HM, et al. Predicting Carbohydrate Concentrations in Avocado and Macadamia Leaves Using Hyperspectral Imaging with Partial Least Squares Regressions and Artificial Neural Networks. Remote Sensing. 2024; 16(18):3389. https://doi.org/10.3390/rs16183389
Chicago/Turabian StyleBai, Shahla Hosseini, Mahshid Tootoonchy, Wiebke Kämper, Iman Tahmasbian, Michael B. Farrar, Helen Boldingh, Trisha Pereira, Hannah Jonson, Joel Nichols, Helen M. Wallace, and et al. 2024. "Predicting Carbohydrate Concentrations in Avocado and Macadamia Leaves Using Hyperspectral Imaging with Partial Least Squares Regressions and Artificial Neural Networks" Remote Sensing 16, no. 18: 3389. https://doi.org/10.3390/rs16183389







