Lunar Surface Resource Exploration: Tracing Lithium, 7 Li and Black Ice Using Spectral Libraries and Apollo Mission Samples
Abstract
1. Introduction
Mapping the Mineral Composition of the Moon
2. Materials and Methods
2.1. Lunar Samples
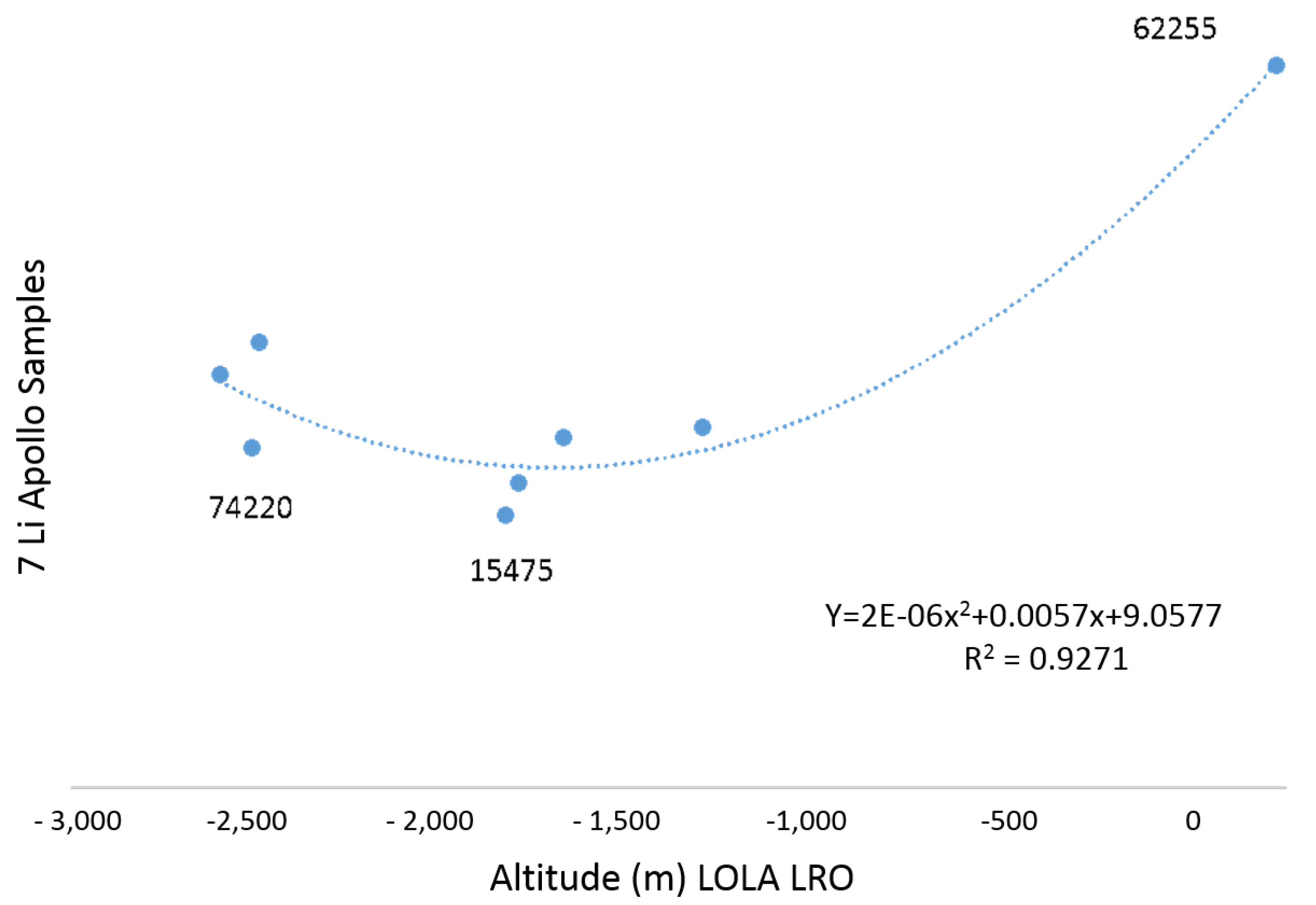
- Sample 62255 (1239 g) was collected during the Apollo 16 mission at the south rim of Buster Crater. The sample is composed of approximately 65% ferroan anorthosite and 35% dark, finely crystalline melt that has been crushed and granulated by shock. It is also penetrated with patches and veins of mafic melt. The anorthosite is chemically pristine but enriched in some volatiles. The total K-Ar age is about 3.66 ± 0.08 b.y. [46];
- Sample 12045 (63 g) was collected during the Apollo 12 mission and contains glomerophyric aggregates of olivine phenocrysts, spinel grains and rare metal droplets. These phenocryst assemblages are set in a groundmass of acicular pyroxene, plagioclase, silica, ilmenite and troilite. The age of the sample could not be determined;
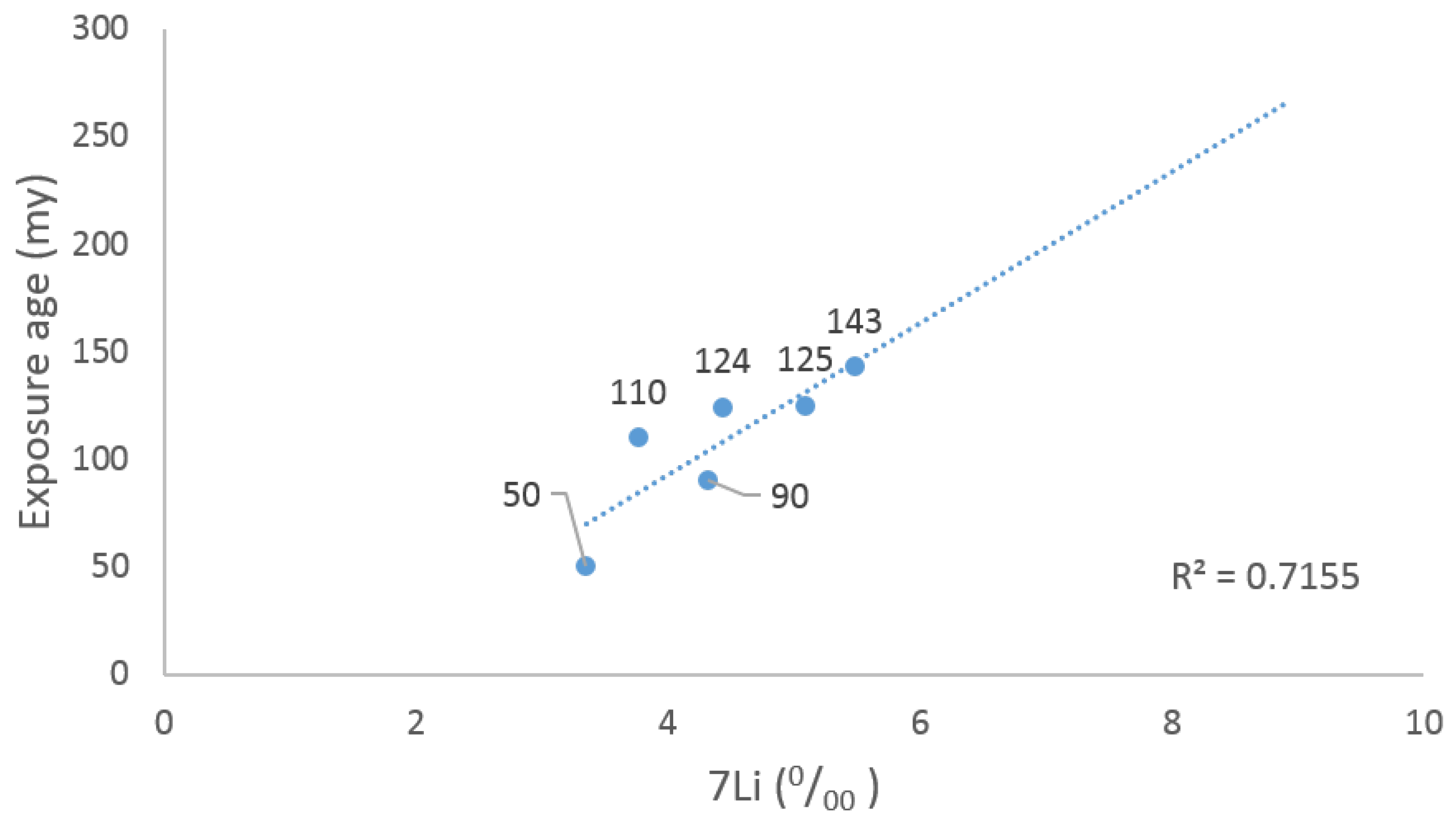
- Sample 15058 (2672 g) was collected during the Apollo 15 mission. It is a quartz-normative mare basalt with elongate pyroxene crystals. The sample has been dated to 3.4 billion years, with an exposure age of approximately 135 million years;
- Sample 70035 (5765 g) was collected during the Apollo 17 mission. It is a vesicular, medium-grained, high-Ti basalt taken from a boulder on the rim of a subdued crater. The bottom surface of this sample is coated with glass. Sample 70035 is 3.7 b.y. old and [46] has been exposed on the lunar surface 122 ± 3 m.y. by the 81Kr dating method [46];
- Sample 15475 (406.8 g) was collected from the rim of Dune Crater during the Apollo 15 mission. It is a porphyritic pigeonite basalt that was dated to be approximately 3.4 billion years old and has been exposed to cosmic rays for about 200 million years;
- Sample 75075 (1008 g) was discovered during the Apollo 17 mission. It was found lying loose on top of a large boulder in Camelot Crater. The sample was dated to 3.74 billion years old and consists of a vuggy ilmenite basalt with an apparent old exposure age of 143 million years [47]. The sample, classified as a Type S1 Apollo 17 high-Ti basalt using the scheme of Rhodes [48], shows few micrometeorite craters despite its old exposure age and being found on top of a boulder;
- Sample 15555 (9614 g), also known as “Great Scott”, is the largest and most extensively studied rock from the Apollo 15 mission. It is a medium-grained olivine basalt with a few small vugs and has been dated to 3.3 billion years old. It has been exposed for 90 million years;
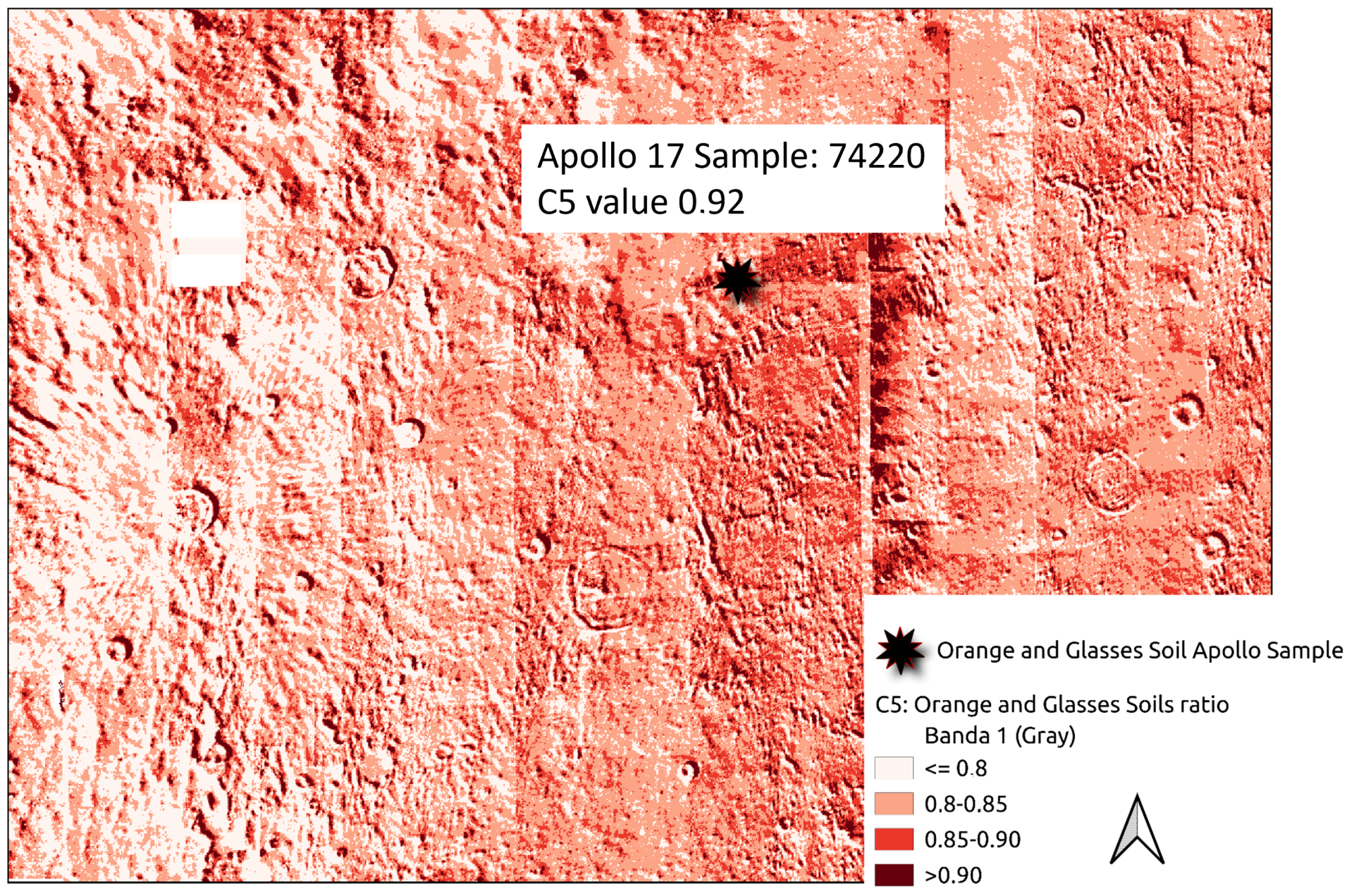
- Sample 74220 (1180 g) was taken during the Apollo 17 mission. It is considered a clod of friable material taken from a depth of 6–8 cm in a trench from Station 4 on the rim of the 120-meter Shorty crater. The orange and black glass samples were identified as a pyroclastic deposit [49]. Ref. [50] reported the exposure age as 27 m.y. The Orange and Glasses soils represent lunar regolith that has been weathered and originated from materials impacted by meteorites. Sample 74220 exhibits the highest concentration of lithium at 13.3 ppm, but the lowest concentration of the 7 Li isotope (4.19‰) (Table 1).
2.2. Lithium Exploration
Numerical Methods
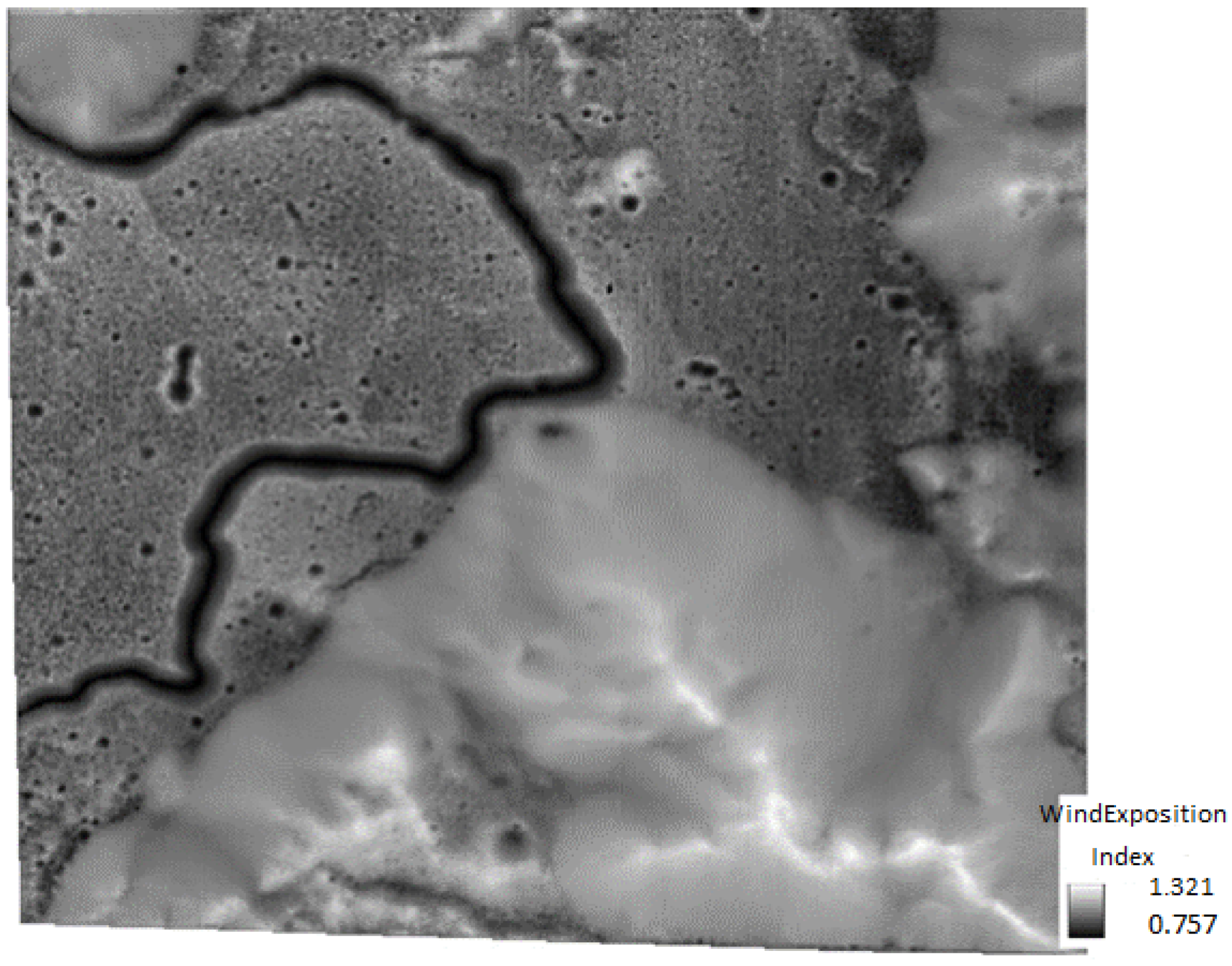
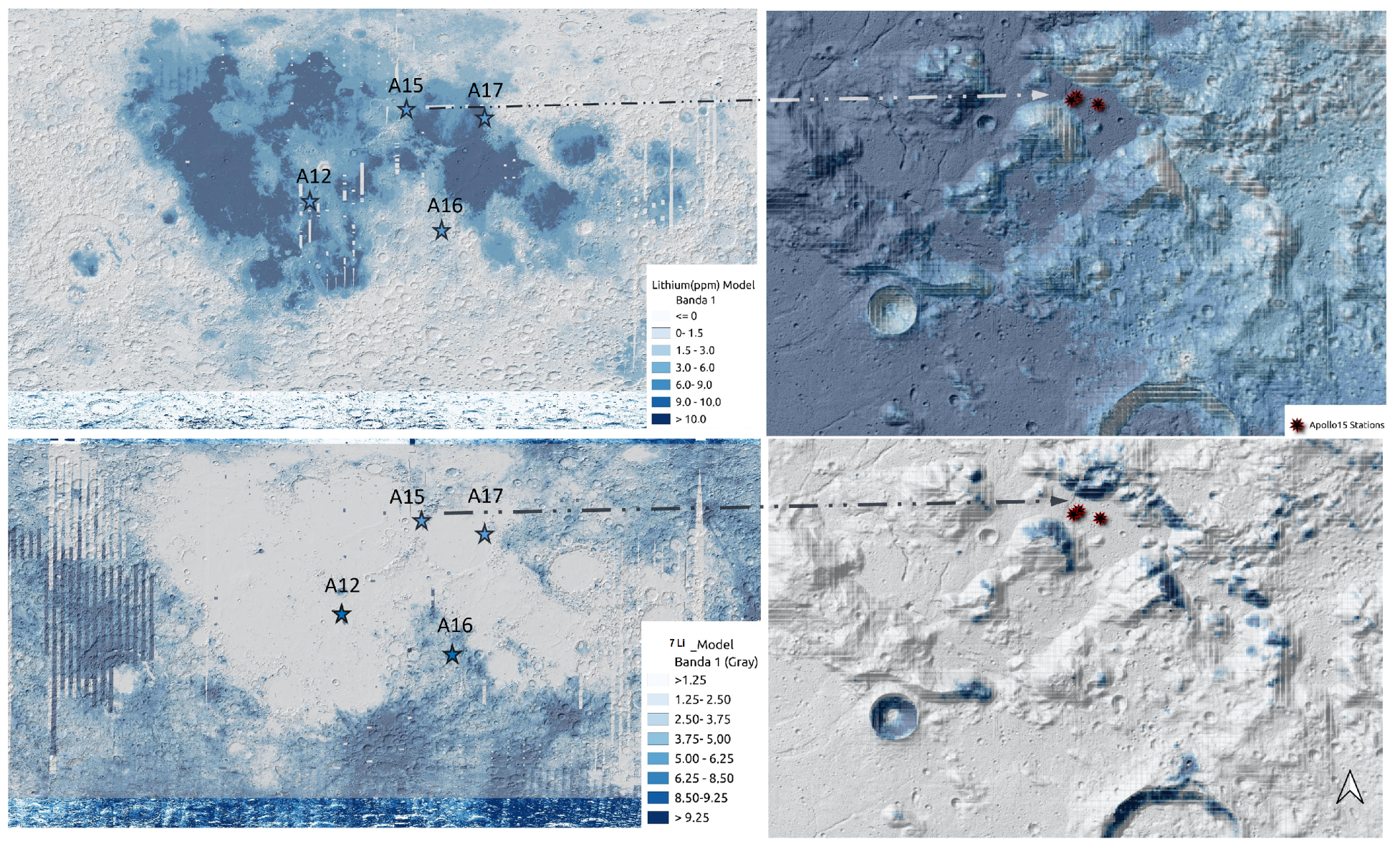
2.3. Lithium Distribution and Relief Patterns
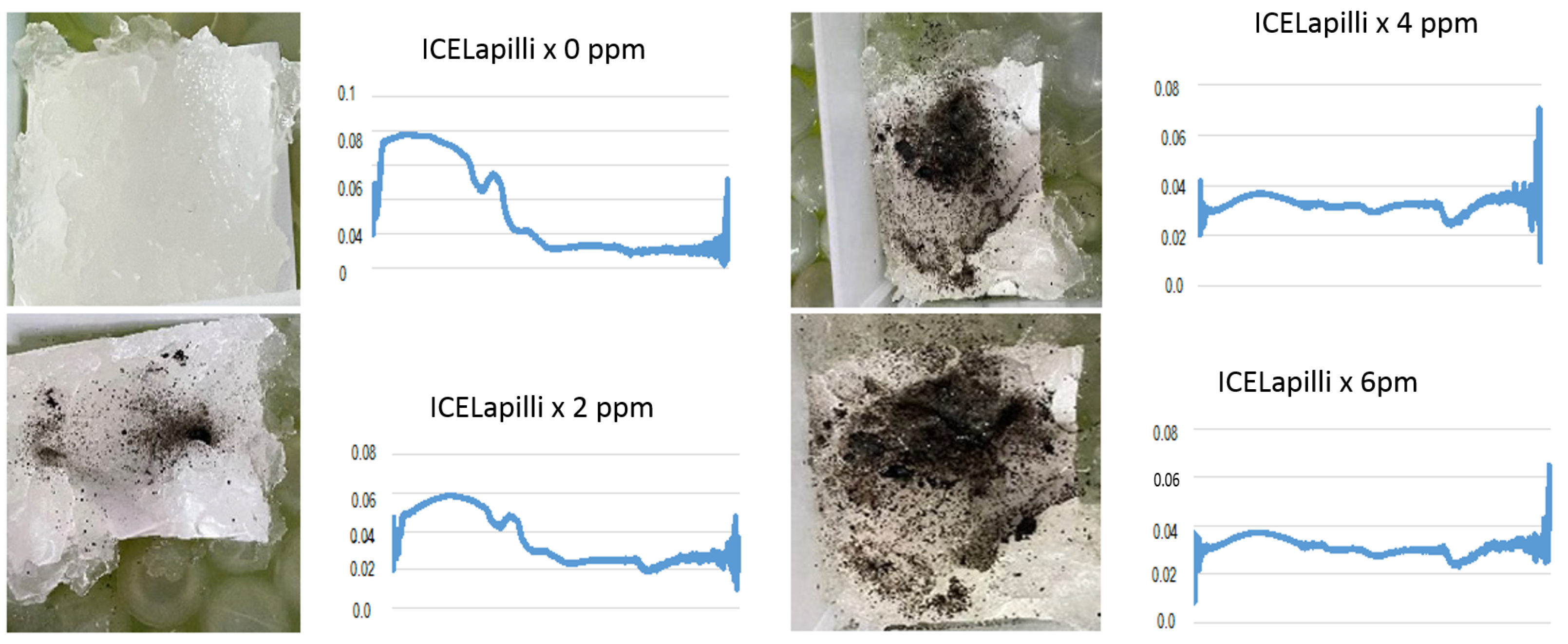
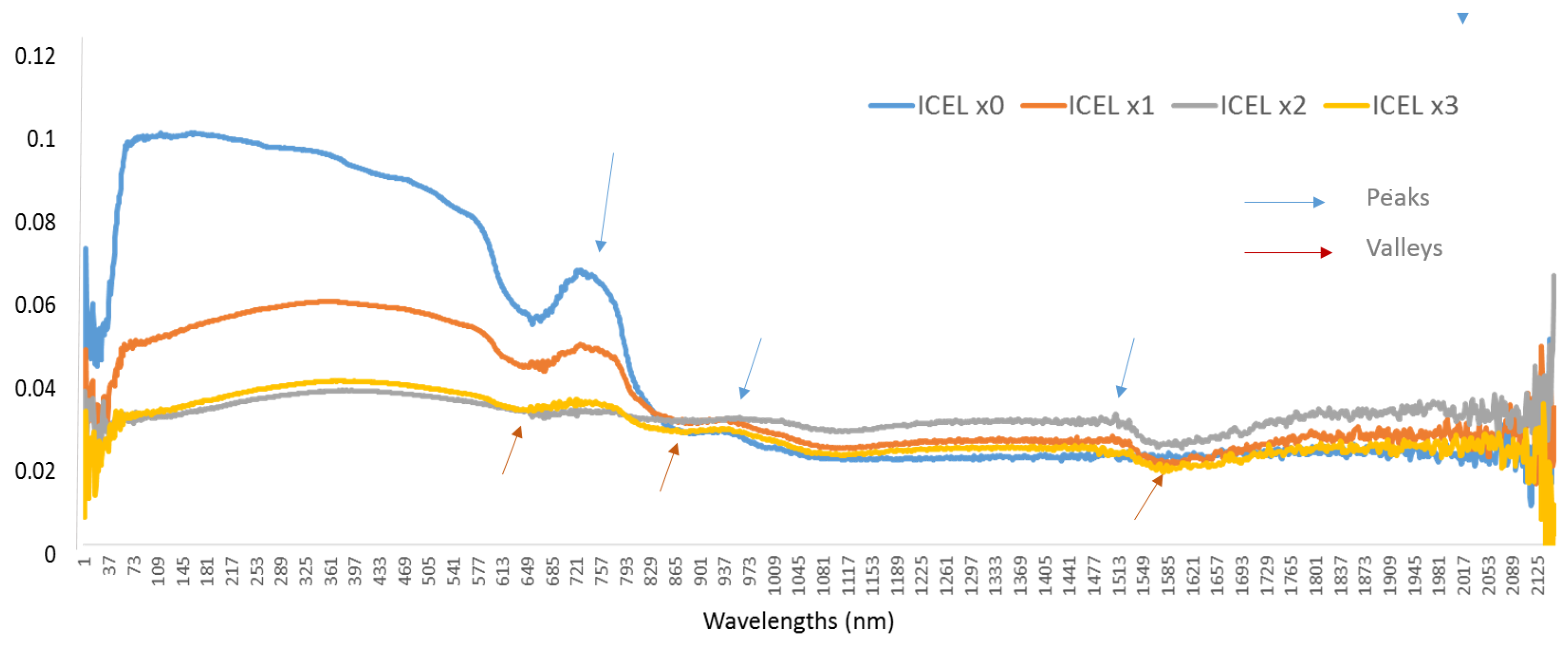
2.4. Dirty Ice Samples
3. Results and Discussion
3.1. Lunar Samples and Exposure Ages
3.2. Types of Materials and Geological Samples
3.3. Linear Correlations between Clementine Bands, Spectral Ratios and Lithium Concentration
3.4. LRMs
3.5. Maps of Lithium and 7 Li
3.6. Relations with the DTMs
3.7. Dirty Ice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, E.A.; Lucey, P.G.; Lemelin, M.; Greenhagen, B.T.; Siegler, M.A.; Mazarico, E.; Aharonson, O.; Williams, J.P.; Hayne, P.O.; Neumann, G.A.; et al. Evidence for surface water ice in the lunar polar regions using reflectance measurements from the Lunar Orbiter Laser Altimeter and temperature measurements from the Diviner Lunar Radiometer Experiment. Icarus 2017, 292, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hayne, P.O.; Hendrix, A.; Sefton-Nash, E.; Siegler, M.A.; Lucey, P.G.; Retherford, K.D.; Williams, J.P.; Greenhagen, B.T.; Paige, D.A. Evidence for exposed water ice in the Moon’s south polar regions from Lunar Reconnaissance Orbiter ultraviolet albedo and temperature measurements. Icarus 2015, 255, 58–69. [Google Scholar] [CrossRef]
- Magna, T.; Wiechert, U.; Halliday, A.N. New constraints on the lithium isotope compositions of the Moon and terrestrial planets. Earth Planet. Sci. Lett. 2006, 243, 336–353. [Google Scholar] [CrossRef]
- Ohtake, M.; Matsunaga, T.; Haruyama, J.; Yokota, Y.; Morota, T.; Honda, C.; Ogawa, Y.; Torii, M.; Miyamoto, H.; Arai, T.; et al. The global distribution of pure anorthosite on the Moon. Nature 2009, 461, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lucey, P.G. Mineral maps of the Moon. Geophys. Res. Lett. 2004, 31, 8. [Google Scholar] [CrossRef]
- Colaprete, A.; Schultz, P.; Heldmann, J.; Wooden, D.; Shirley, M.; Ennico, K.; Hermalyn, B.; Marshall, W.; Ricco, A.; Elphic, R.C.; et al. Detection of water in the LCROSS ejecta plume. Science 2010, 330, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Charette, M.P.; McCord, T.B.; Pieters, C.M.; Adams, J.B. Application of Remote Spectral Reflectance Measurements to Lunar Geology Classification and Determination of Titanium Content of Lunar Soils. J. Geophys. Res. 1974, 79, 1605–1613. [Google Scholar] [CrossRef]
- Adler, I.; Trombka, J.; Gerard, J.; Lowman, P.; Schmadebeck, R.; Blodget, H.; Eller, E.; Yin, L.; Lamothe, R.; Osswald, G.; et al. Apollo 16 geochemical X-ray fluorescence experiment: Preliminary report. Science 1972, 177, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Swinyard, B.; Joy, K.; Kellett, B.; Crawford, I.; Grande, M.; Howe, C.; Fernandes, V.; Gasnault, O.; Lawrence, D.; Russell, S.; et al. X-ray fluorescence observations of the Moon by SMART-1/D-CIXS and the first detection of Ti Kα from the lunar surface. Planet. Space Sci. 2009, 57, 744–750. [Google Scholar] [CrossRef]
- Athiray, P.; Narendranath, S.; Sreekumar, P.; Dash, S.; Babu, B. Validation of methodology to derive elemental abundances from X-ray observations on Chandrayaan-1. Planet. Space Sci. 2013, 75, 188–194. [Google Scholar] [CrossRef]
- Fa, W.; Zhu, M.H.; Liu, T.; Plescia, J.B. Regolith stratigraphy at the Chang’E-3 landing site as seen by lunar penetrating radar. Geophys. Res. Lett. 2015, 42, 10–179. [Google Scholar] [CrossRef]
- Li, C.; Zuo, W.; Wen, W.; Zeng, X.; Gao, X.; Liu, Y.; Fu, Q.; Zhang, Z.; Su, Y.; Ren, X.; et al. Overview of the Chang’e-4 mission: Opening the frontier of scientific exploration of the lunar far side. Space Sci. Rev. 2021, 217, 35. [Google Scholar] [CrossRef]
- Metzger, A. Composition of the Moon as Determined from Orbit by Gamma-Ray Spectroscopy, in Remote Geochemical Analysis: Elemental and Mineralogical Composition. Ph.D. Thesis, Cambridge University Press, New York, NY, USA, 1993. Available online: https://www.semanticscholar.org/paper/Composition-of-the-Moon-as-Determined-from-Orbit-by-Metzger/ea880d11ccf05dee2c2109f4e1fc394eeccf41e4 (accessed on 1 February 2024).
- Lawrence, D.; Feldman, W.; Barraclough, B.; Binder, A.; Elphic, R.; Maurice, S.; Thomsen, D. Global elemental maps of the Moon: The Lunar Prospector gamma-ray spectrometer. Science 1998, 281, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Feldman, W.; Barraclough, B.; Binder, A.; Elphic, R.; Maurice, S.; Miller, M.; Prettyman, T. Thorium abundances on the lunar surface. J. Geophys. Res. Planets 2000, 105, 20307–20331. [Google Scholar] [CrossRef]
- Prettyman, T.H.; Hagerty, J.; Elphic, R.; Feldman, W.; Lawrence, D.; McKinney, G.; Vaniman, D. Elemental composition of the lunar surface: Analysis of gamma ray spectroscopy data from Lunar Prospector. J. Geophys. Res. Planets 2006, 111. [Google Scholar] [CrossRef]
- Naito, M.; Hasebe, N.; Nagaoka, H.; Shibamura, E.; Ohtake, M.; Kim, K.; Wöhler, C.; Berezhnoy, A. Iron distribution of the Moon observed by the Kaguya gamma-ray spectrometer: Geological implications for the South Pole-Aitken basin, the Orientale basin, and the Tycho crater. Icarus 2018, 310, 21–31. [Google Scholar] [CrossRef]
- Elphic, R.; Lawrence, D.; Feldman, W.; Barraclough, B.; Maurice, S.; Binder, A.; Lucey, P. Lunar Fe and Ti abundances: Comparison of Lunar Prospector and Clementine data. Science 1998, 281, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Elphic, R.; Lawrence, D.; Feldman, W.; Barraclough, B.; Maurice, S.; Binder, A.; Lucey, P. Lunar rare earth element distribution and ramifications for FeO and TiO2: Lunar Prospector neutron spectrometer observations. J. Geophys. Res. Planets 2000, 105, 20333–20345. [Google Scholar] [CrossRef]
- Elphic, R.; Lawrence, D.; Feldman, W.; Barraclough, B.; Gasnault, O.; Maurice, S.; Lucey, P.; Blewett, D.; Binder, A. Lunar Prospector neutron spectrometer constraints on TiO2. J. Geophys. Res. Planets 2002, 107, 8-1–8-9. [Google Scholar] [CrossRef]
- Feldman, W.; Barraclough, B.; Maurice, S.; Elphic, R.; Lawrence, D.; Thomsen, D.; Binder, A. Major compositional units of the Moon: Lunar Prospector thermal and fast neutrons. Science 1998, 281, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Feldman, W.; Lawrence, D.; Elphic, R.; Barraclough, B.; Maurice, S.; Genetay, I.; Binder, A. Polar hydrogen deposits on the Moon. J. Geophys. Res. Planets 2000, 105, 4175–4195. [Google Scholar] [CrossRef]
- Lucey, P.G.; Taylor, G.J.; Malaret, E. Abundance and distribution of iron on the Moon. Science 1995, 268, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Lucey, P.G.; Blewett, D.T.; Hawke, B.R. Mapping the FeO and TiO2 content of the lunar surface with multispectral imagery. J. Geophys. Res. Planets 1998, 103, 3679–3699. [Google Scholar] [CrossRef]
- Lucey, P.G.; Blewett, D.T.; Jolliff, B.L. Lunar iron and titanium abundance algorithms based on final processing of Clementine ultraviolet-visible images. J. Geophys. Res. Planets 2000, 105, 20297–20305. [Google Scholar] [CrossRef]
- Lucey, P.G. Radiative transfer modeling of the effect of mineralogy on some empirical methods for estimating iron concentration from multispectral imaging of the Moon. J. Geophys. Res. Planets 2006, 111. [Google Scholar] [CrossRef]
- Bandfield, J.L.; Hayne, P.O.; Williams, J.P.; Greenhagen, B.T.; Paige, D.A. Lunar surface roughness derived from LRO Diviner Radiometer observations. Icarus 2015, 248, 357–372. [Google Scholar] [CrossRef]
- Sato, H.; Robinson, M.S.; Lawrence, S.J.; Denevi, B.W.; Hapke, B.; Jolliff, B.L.; Hiesinger, H. Lunar mare TiO2 abundances estimated from UV/Vis reflectance. Icarus 2017, 296, 216–238. [Google Scholar] [CrossRef]
- Ma, M.; Li, B.; Chen, S.; Lu, T.; Lu, P.; Lu, Y.; Jin, Q. Global estimates of lunar surface chemistry derived from LRO diviner data. Icarus 2022, 371, 114697. [Google Scholar] [CrossRef]
- Ling, Z.; Zhang, J.; Liu, J.; Zhang, W.; Bian, W.; Ren, X.; Mu, L.; Liu, J.; Li, C. Preliminary results of FeO mapping using Imaging Interferometer data from Chang’E-1. Chin. Sci. Bull. 2011, 56, 376–379. [Google Scholar] [CrossRef][Green Version]
- Yan, B.; Xiong, S.Q.; Wu, Y.; Wang, Z.; Dong, L.; Gan, F.; Yang, S.; Wang, R. Mapping Lunar global chemical composition from Chang’E-1 IIM data. Planet. Space Sci. 2012, 67, 119–129. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, B.; Zhao, B.; Lucey, P.; Chen, J.; Xu, X.; Li, C.; Ouyang, Z. Global estimates of lunar iron and titanium contents from the Chang’E-1 IIM data. J. Geophys. Res. Planets 2012, 117. [Google Scholar] [CrossRef]
- Sun, L.; Ling, Z.; Zhang, J.; Li, B.; Chen, J.; Wu, Z.; Liu, J. Lunar iron and optical maturity mapping: Results from partial least squares modeling of Chang’E-1 IIM data. Icarus 2016, 280, 183–198. [Google Scholar] [CrossRef]
- Xia, W.; Wang, X.; Zhao, S.; Jin, H.; Chen, X.; Yang, M.; Wu, X.; Hu, C.; Zhang, Y.; Shi, Y.; et al. New maps of lunar surface chemistry. Icarus 2019, 321, 200–215. [Google Scholar] [CrossRef]
- Logan, L.M.; Hunt, G.R.; Salisbury, J.W.; Balsamo, S.R. Compositional implications of Christiansen frequency maximums for infrared remote sensing applications. J. Geophys. Res. 1973, 78, 4983–5003. [Google Scholar] [CrossRef]
- Salisbury, J.W.; Basu, A.; Fischer, E.M. Thermal infrared spectra of lunar soils. Icarus 1997, 130, 125–139. [Google Scholar] [CrossRef]
- Paige, D.; Foote, M.; Greenhagen, B.; Schofield, J.; Calcutt, S.; Vasavada, A.; Preston, D.; Taylor, F.; Allen, C.; Snook, K.; et al. The lunar reconnaissance orbiter diviner lunar radiometer experiment. Space Sci. Rev. 2010, 150, 125–160. [Google Scholar] [CrossRef]
- Greenhagen, B.T.; Lucey, P.G.; Wyatt, M.B.; Glotch, T.D.; Allen, C.C.; Arnold, J.A.; Bandfield, J.L.; Bowles, N.E.; Hanna, K.L.D.; Hayne, P.O.; et al. Global Silicate Mineralogy of the Moon from the Diviner Lunar Radiometer. Science 2010, 329, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.D.; Greenhagen, B.; Patterson Iii, W.; Pieters, C.; Mustard, J.; Bowles, N.; Paige, D.; Glotch, T.; Thompson, C. Effects of varying environmental conditions on emissivity spectra of bulk lunar soils: Application to Diviner thermal infrared observations of the Moon. Icarus 2017, 283, 326–342. [Google Scholar] [CrossRef]
- Lucey, P.G.; Greenhagen, B.T.; Song, E.; Arnold, J.A.; Lemelin, M.; Hanna, K.D.; Bowles, N.E.; Glotch, T.D.; Paige, D.A. Space weathering effects in Diviner Lunar Radiometer multispectral infrared measurements of the lunar Christiansen Feature: Characteristics and mitigation. Icarus 2017, 283, 343–351. [Google Scholar] [CrossRef]
- Lucey, P.G.; Hayne, P.O.; Costello, E.; Green, R.; Hibbitts, C.; Goldberg, A.; Mazarico, E.; Li, S.; Honniball, C. The spectral radiance of indirectly illuminated surfaces in regions of permanent shadow on the Moon. Acta Astronaut. 2021, 180, 25–34. [Google Scholar] [CrossRef]
- Nozette, S.; Rustan, P.; Pleasance, L.; Kordas, J.; Lewis, I.; Park, H.; Priest, R.; Horan, D.; Regeon, P.; Lichtenberg, C.; et al. The Clementine mission to the Moon: Scientific overview. Science 1994, 266, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- McEwen, A.; Robinson, M. Mapping of the Moon by Clementine. Adv. Space Res. 1997, 19, 1523–1533. [Google Scholar] [CrossRef]
- Pieters, C.; Shkuratov, Y.; Kaydash, V.; Stankevich, D.; Taylor, L. Lunar soil characterization consortium analyses: Pyroxene and maturity estimates derived from Clementine image data. Icarus 2006, 184, 83–101. [Google Scholar] [CrossRef]
- Pieters, C.M.; Hiroi, T. RELAB (Reflectance Experiment Laboratory): A NASA multiuser spectroscopy facility. In Proceedings of the Lunar and Planetary Science Conference, League City, TX, USA, 15–19 March 2004; p. 1720. [Google Scholar]
- Drozd, R.; Hohenberg, C.; Morgan, C.; Ralston, C. Cosmic-ray exposure history at the Apollo 16 and other lunar sites: Lunar surface dynamics. Geochim. Cosmochim. Acta 1974, 38, 1625–1642. [Google Scholar] [CrossRef]
- Wolfe, E.W.; Lucchitta, B.K.; Reed, V.S.; Ulrich, G.E.; Sánchez, A.G. Geology of the Taurus-Littrow Valley Floor; NASA: Washington, DC, USA, 1975. [Google Scholar]
- Butler, J. Origins of Mare Basalts and Their Implications for Lunar Evolution; NASA: Washington, DC, USA, 1975. [Google Scholar]
- Heiken, G.H.; McKay, D.S.; Brown, R. Lunar deposits of possible pyroclastic origin. Geochim. Cosmochim. Acta 1974, 38, 1703–1718. [Google Scholar] [CrossRef]
- Hintenberger, H.; Weber, H.W.; Schultz, L. Solar, Spallogenic, and Radiogenic Rare Gases in Apollo 17 Soils and Breccias; NASA: Washington, DC, USA, 1974. [Google Scholar]
- Dubayah, R.; Rich, P.M. Topographic solar radiation models for GIS. Int. J. Geogr. Inf. Syst. 1995, 9, 405–419. [Google Scholar] [CrossRef]
- Wilson, J.P.; Gallant, J.C. Digital terrain analysis. Terrain Anal. Princ. Appl. 2000, 6, 1–27. [Google Scholar]
- Olaya, V.; Conrad, O. Geomorphometry in SAGA. Dev. Soil Sci. 2009, 33, 293–308. [Google Scholar]
- Böhner, J.; Antonić, O. Land-surface parameters specific to topo-climatology. Dev. Soil Sci. 2009, 33, 195–226. [Google Scholar]
- Blewett, D.T.; Hawke, B.R.; Lucey, P.G. Lunar pure anorthosite as a spectral analog for Mercury. Meteorit. Planet. Sci. 2002, 37, 1245–1254. [Google Scholar] [CrossRef]
- Papike, J.; Taylor, L.; Simon, S. Lunar minerals. In Lunar Sourcebook: A User’s Guide to the Moon; Lunar and Planetary Institute: Houston, TX, USA, 1991; pp. 121–181. [Google Scholar]
- Warren, P.H.; Shirley, D.N.; Kallemeyn, G.W. A potpourri of pristine Moon rocks, including a VHK mare basalt and a unique, augite-rich Apollo 17 anorthosite. J. Geophys. Res. Solid Earth 1986, 91, 319–330. [Google Scholar] [CrossRef]
- Pieters, C.; Pratt, S. Earth-based near-infrared collection of spectra for the Moon: A new PDS data set. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 13–17 March 2000; p. 2059. [Google Scholar]
- Pieters, C.M.; Ammannito, E.; Blewett, D.T.; Denevi, B.W.; De Sanctis, M.C.; Gaffey, M.J.; Le Corre, L.; Li, J.Y.; Marchi, S.; McCord, T.B.; et al. Distinctive space weathering on Vesta from regolith mixing processes. Nature 2012, 491, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Hapke, B. Space weathering from Mercury to the asteroid belt. J. Geophys. Res. Planets 2001, 106, 10039–10073. [Google Scholar] [CrossRef]
- Vernazza, P.; Binzel, R.; Rossi, A.; Fulchignoni, M.; Birlan, M. Solar wind as the origin of rapid reddening of asteroid surfaces. Nature 2009, 458, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Domingue, D.L.; Chapman, C.R.; Killen, R.M.; Zurbuchen, T.H.; Gilbert, J.A.; Sarantos, M.; Benna, M.; Slavin, J.A.; Schriver, D.; Trávníček, P.M.; et al. Mercury’s weather-beaten surface: Understanding Mercury in the context of lunar and asteroidal space weathering studies. Space Sci. Rev. 2014, 181, 121–214. [Google Scholar] [CrossRef]
- Adams, J.B.; McCord, T.B. Optical properties of mineral separates, glass, and anorthositic fragments from Apollo mare samples. In Proceedings of the Lunar Science Conference, Houston, TX, USA, 11–14 January 1971; Volume 2, p. 2183. [Google Scholar]
- Pieters, C.M.; Head, J.; Sunshine, J.; Fischer, E.; Murchie, S.; Belton, M.; McEwen, A.; Gaddis, L.; Greeley, R.; Neukum, G.; et al. Crustal diversity of the Moon: Compositional analyses of Galileo solid state imaging data. J. Geophys. Res. Planets 1993, 98, 17127–17148. [Google Scholar] [CrossRef]
- Sasaki, S.; Nakamura, K.; Hamabe, Y.; Kurahashi, E.; Hiroi, T. Production of iron nanoparticles by laser irradiation in a simulation of lunar-like space weathering. Nature 2001, 410, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.K.; Pieters, C.M.; Keller, L.P. An experimental approach to understanding the optical effects of space weathering. Icarus 2007, 192, 629–642. [Google Scholar] [CrossRef]
- Meyer, C., Jr.; McKay, D.; Anderson, D.; Butler, P., Jr. The source of sublimates on the Apollo 15 green and Apollo 17 orange glass samples. In Proceedings of the Lunar Science Conference, Houston, TX, USA, 17–21 March 1975; Volume 6, pp. 1673–1699. [Google Scholar]
- Butler, J., Jr.; Meyer, C., Jr. Sulfur prevails in coatings on glass droplets-Apollo 15 green and brown glasses and Apollo 17 orange and black/devitrified/glasses. In Proceedings of the Lunar and Planetary Science Conference Proceedings, Houston, TX, USA, 15–19 March 1976; Volume 7, pp. 1561–1581. [Google Scholar]
- Day, J.M.; Walker, R.J.; James, O.B.; Puchtel, I.S. Osmium isotope and highly siderophile element systematics of the lunar crust. Earth Planet. Sci. Lett. 2010, 289, 595–605. [Google Scholar] [CrossRef]
- Tompkins, S.; Pieters, C.M. Mineralogy of the lunar crust: Results from Clementine. Meteorit. Planet. Sci. 1999, 34, 25–41. [Google Scholar] [CrossRef]
- Blewett, D.T.; Lucey, P.G.; Hawke, B.R.; Jolliff, B.L. Clementine images of the lunar sample-return stations: Refinement of FeO and TiO2 mapping techniques. J. Geophys. Res. Planets 1997, 102, 16319–16325. [Google Scholar] [CrossRef]
- Giguere, T.A.; Taylor, G.J.; HAWKE, B.R.; Lucey, P.G. The titanium contents of lunar mare basalts. Meteorit. Planet. Sci. 2000, 35, 193–200. [Google Scholar] [CrossRef]
- Escadafal, R.; Girard, M.C.; Courault, D. Munsell soil color and soil reflectance in the visible spectral bands of Landsat MSS and TM data. Remote. Sens. Environ. 1989, 27, 37–46. [Google Scholar] [CrossRef]
- Bhatt, M.; Wöhler, C.; Dhingra, D.; Thangjam, G.; Rommel, D.; Mall, U.; Bhardwaj, A.; Grumpe, A. Compositional studies of Mare Moscoviense: New perspectives from Chandrayaan-1 VIS-NIR data. Icarus 2018, 303, 149–165. [Google Scholar] [CrossRef]







| Sample # | Substrata | Li (ppm) | 7 Li (0/00) | Ratio (7 Li/Li) | Altitude (m) | m.y. (38Ar) | Spectral Index |
|---|---|---|---|---|---|---|---|
| 62255 | Anortosite | 0.8 | 8.89 | 11.11 | −23 | 3 | C4 |
| 1205 | Basalt | 9.5 | 4.43 | 0.47 | −1441 | 124 | C2 |
| 15058 | Basalt | 7.7 | 3.76 | 0.49 | −1895 | 135 | C2 |
| 15475 | Basalt | 7.2 | 3.35 | 0.47 | −1928 | 200 | C2 |
| 70035 | High-Ti Mare Basalt | 8.6 | 5.09 | 0.59 | −2633 | 125 | C3 |
| 75075 | High-Ti Mare Basalt | 10.3 | 5.48 | 0.53 | −2534 | 143 | C3 |
| 15555 | Olivine Basalt | 6 | 4.32 | 0.72 | −1786 | 90 | C1 |
| 74220 | Orange Glasses Soil | 13.3 | 4.19 | 0.32 | −2552 | 27 | C5 |
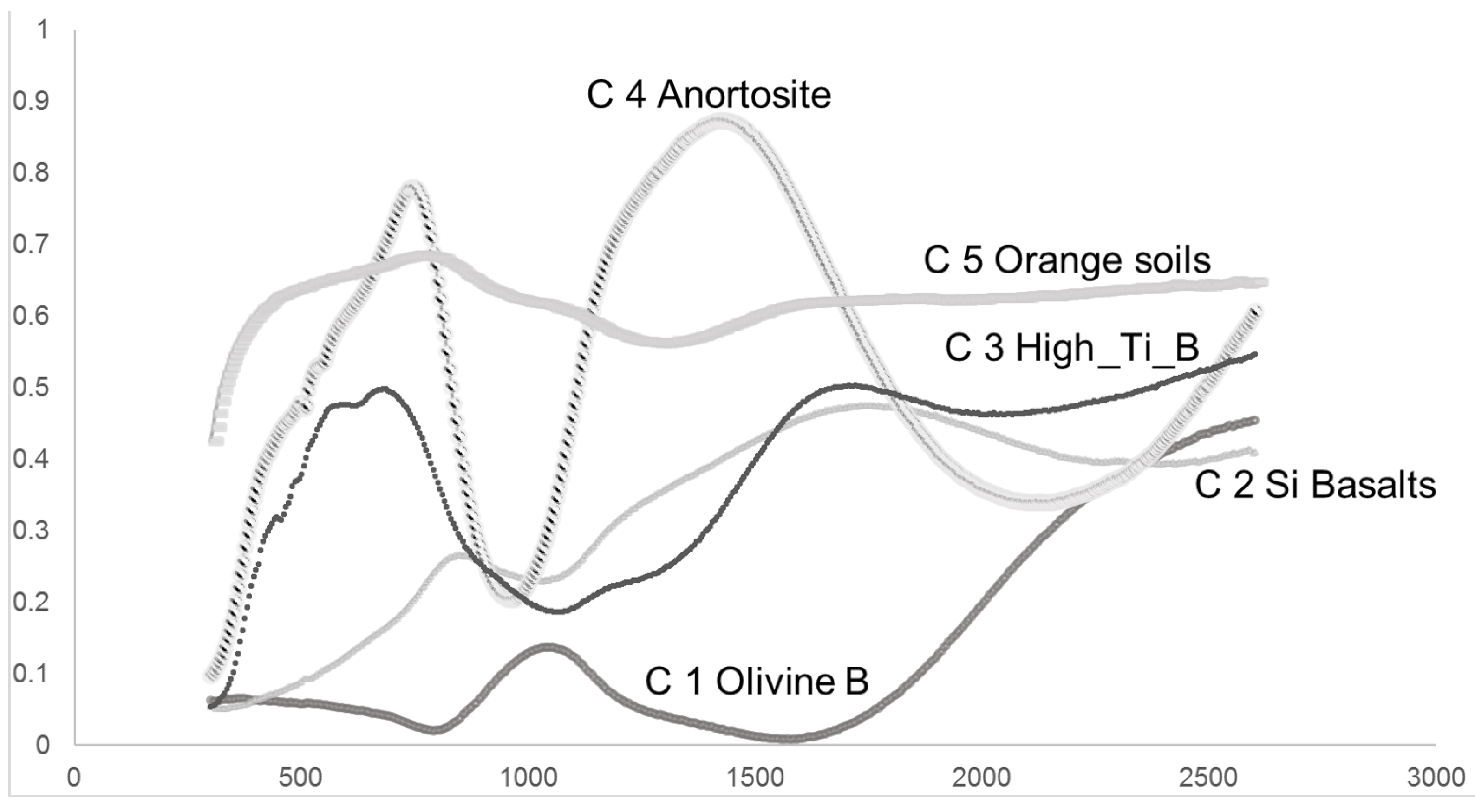
| Types of Spectra | Sustrata | (Peak/Valley) Coefficients | nm | Clementine Bands |
|---|---|---|---|---|
| C1 | Olivine Basalts | 15.641 | 1044/1575 | Band5/Nir3 |
| C2 | Basalts with Silica | 2.0639 | 1755/1030 | Nir4/Nir5 |
| C3 | Hight_Ti Mare Basalts | 2.7029 | 1705/1060 | Nir3/Band5 |
| C4 | Anorthosite | 4.2786 | 1420/965 | Nir3/Band4 |
| C5 | Orange and Glasses Soils | 1.2198 | 778/1300 | Band2/Nir2 |
| R Pearson | Lithium (ppm) | 7 Li (‰) |
|---|---|---|
| C1 | 0.47 | 0.23 |
| C2 | −0.41 | −0.27 |
| C3 | −0.44 | −0.24 |
| C4 | 0.21 | 0.57 |
| C5 | 0.45 | 0.18 |
| Band1 | −0.64 | 0.92 |
| Band2 | −0.73 | 0.87 |
| Band3 | −0.69 | 0.88 |
| Band4 | −0.68 | 0.87 |
| Band5 | −0.69 | 0.87 |
| Nir1 | −0.89 | 0.57 |
| Nir2 | −0.89 | 0.59 |
| Nir3 | −0.87 | 0.49 |
| Nir4 | −0.80 | 0.27 |
| Nir5 | −0.60 | −0.11 |
| Nir6 | −0.79 | 0.25 |
| Beta | Std.Err. | B | Std.Err. | p-Level | |
|---|---|---|---|---|---|
| Intercept | (7 Li) | 2.615 | 2.010 | 0.263 | |
| C4 | 0.623 | 0.226 | 8.924 | 3.243 | 0.051 |
| C5 | −0.495 | 0.204 | −7.468 | 3.082 | 0.073 |
| Band1 | 0.736 | 0.137 | 60.845 | 11.337 | 0.006 |
| Intercept | (Lithium) | 64.760 | 20.821 | 0.036 | |
| Nir1 | −7.233 | 3.581 | −672.392 | 332.918 | 0.114 |
| C5 | −0.539 | 0.251 | −74.410 | 34.623 | 0.098 |
| Nir2 | 6.106 | 3.501 | 536.480 | 307.561 | 0.156 |
| Types of Spectra | (Peak/Valley) Coefficients | nm | Clementine Bands |
|---|---|---|---|
| ICELx1 | 3.530 | 1076/2383 | NIR1/Nir4 |
| ICELx2 | 2.496 | 1076/1954 | Nir1/Nir3 |
| ICELx3 | 1.686 | 2397/1940 | Nir4/NIR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, S.d.C.; Alberquilla, F.; Fernández, J.M.; Díez, E.; Rodríguez, J.; Muñiz, R.; Calleja, J.F.; de Cos, F.J.; Martínez-Frías, J. Lunar Surface Resource Exploration: Tracing Lithium, 7 Li and Black Ice Using Spectral Libraries and Apollo Mission Samples. Remote Sens. 2024, 16, 1306. https://doi.org/10.3390/rs16071306
Fernández SdC, Alberquilla F, Fernández JM, Díez E, Rodríguez J, Muñiz R, Calleja JF, de Cos FJ, Martínez-Frías J. Lunar Surface Resource Exploration: Tracing Lithium, 7 Li and Black Ice Using Spectral Libraries and Apollo Mission Samples. Remote Sensing. 2024; 16(7):1306. https://doi.org/10.3390/rs16071306
Chicago/Turabian StyleFernández, Susana del Carmen, Fernando Alberquilla, Julia María Fernández, Enrique Díez, Javier Rodríguez, Rubén Muñiz, Javier F. Calleja, Francisco Javier de Cos, and Jesús Martínez-Frías. 2024. "Lunar Surface Resource Exploration: Tracing Lithium, 7 Li and Black Ice Using Spectral Libraries and Apollo Mission Samples" Remote Sensing 16, no. 7: 1306. https://doi.org/10.3390/rs16071306
APA StyleFernández, S. d. C., Alberquilla, F., Fernández, J. M., Díez, E., Rodríguez, J., Muñiz, R., Calleja, J. F., de Cos, F. J., & Martínez-Frías, J. (2024). Lunar Surface Resource Exploration: Tracing Lithium, 7 Li and Black Ice Using Spectral Libraries and Apollo Mission Samples. Remote Sensing, 16(7), 1306. https://doi.org/10.3390/rs16071306










