Similarities in Metabolic Flexibility and Hunger Hormone Ghrelin Exist between FTO Gene Variants in Response to an Acute Dietary Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Trial Protocols
Genotyping
2.3. Anthropometric Measurements and Dietary Analysis
2.4. Oral Glucose Load (OGL) Challenge
2.5. Blood Sampling, Treatment and Analysis
2.6. Respiratory Gas Exchange Sampling and Analysis
2.7. Statistical Analysis
3. Results
3.1. Nutritional Intake Analysis
3.2. Substrate Utilisation and Metabolic Flexibility Analysis
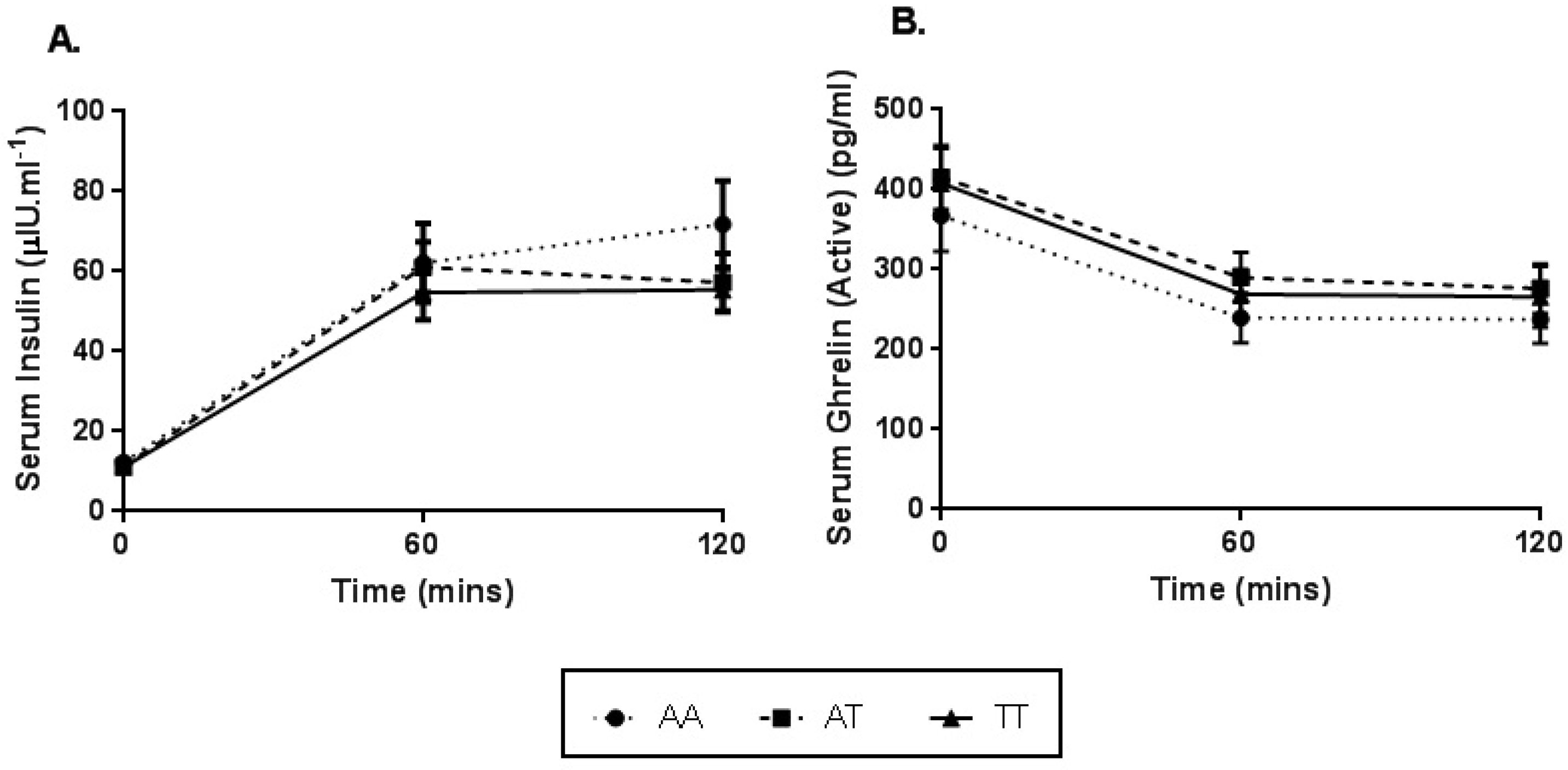
3.3. Plasma Analysis
3.4. Serum Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hainer, V.; Stunkard, A.J.; Kunesová, M.; Parízková, J.; Stich, V.; Allison, D.B. Intrapair resemblance in very low calorie diet-induced weight loss in female obese identical twins. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Maes, H.H.; Neale, M.C.; Eaves, L.J. Genetic and environmental factors in relative body weight and human obesity. Behav. Genet. 1997, 27, 325–351. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- León-Mimila, P.; Villamil-Ramírez, H.; Villalobos-Comparán, M.; Villarreal-Molina, T.; Romero-Hidalgo, S.; López-Contreras, B.; Gutiérrez-Vidal, R.; Vega-Badillo, J.; Jacobo-Albavera, L.; Posadas-Romeros, C.; et al. Contribution of common genetic variants to obesity and obesity-related traits in mexican children and adults. PLoS ONE 2013, 8, e70640. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, J.M.; Papandonatos, G.D.; Peter, I.; Huggins, G.S.; Raynor, H.A.; Delahanty, L.M.; Cheskin, L.J.; Balasubramanyam, A.; Wagenknecht, L.E. Obesity susceptibility loci and dietary intake in the look ahead trial. Am. J. Clin. Nutr. 2012, 95, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Rance, K.A.; Johnstone, A.M. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity 2008, 16, 1961–1965. [Google Scholar] [CrossRef]
- Tanofsky-Kraff, M.; Han, J.C.; Anandalington, K.; Shomaker, L.B.; Columbo, K.M.; Wolkoff, L.E.; Kozlosky, M.; Elliot, C.; Ranzenhofer, L.M.; Roza, C.A.; et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am. J. Clin. Nutr. 2009, 90, 1483–1488. [Google Scholar] [CrossRef]
- Wardle, J.; Carnell, S.; Haworth, C.M.; Farooqi, I.S.; O’Rahilly, S.; Plomin, R. Obesity associated genetic variation in FTO is associated with diminished satiety. J. Clin. Endocrinol. Metab. 2008, 93, 3640–3643. [Google Scholar] [CrossRef]
- Church, C.; Moir, L.; McMurray, F.; Girard, C.; Banks, G.T.; Teboul, L.; Wells, S.; Bruning, J.C.; Nolan, P.M.; Ashcroft, F.M.; et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010, 42, 1086–1092. [Google Scholar] [CrossRef]
- Magno, F.C.C.M.; Guaraná, H.C.; Fonseca, A.C.P.; Cabello, G.M.K.; Carneiro, J.R.I.; Pedrosa, A.P.; Ximenes, A.C.; Rosado, E.L. Influence of FTO rs9939609 polymorphism on appetite, ghrelin, leptin, IL6, TNFα levels, and food intake of women with morbid obesity. Diabetes Metab. Syndr. Obes. 2018, 11, 199–207. [Google Scholar] [CrossRef]
- Karra, E.; O’Daly, O.G.; Choudhury, A.I.; Yousseif, A.; Millership, S.; Neary, M.T.; Scott, W.R.; Chandarana, K.; Manning, S.; Hess, M.E. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Investig. 2013, 123, 3539–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, V.P.; Gao, Y.; Geng, L.; Brimijoin, S. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int. J. Obes. 2017, 41, 1413–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorling, J.L.; Clayton, D.J.; Jones, J.; Carter, W.; Thackray, A.E.; King, J.A.; Pucci, A.; Batterham, R.L.; Stensel, D.J. A randomized cross-over trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations and butyrylcholinesterase activity in normal weight males with variants of the obesity-linked FTO rs9939609 polymorphism. Am. J. Clin. Nutr. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Melhorn, S.J.; Askren, M.K.; Chung, W.K.; Kratz, M.; Bosch, T.A.; Tyagi, V.; Webb, M.F.; De Leon, M.R.B.; Grabowski, T.J.; Leibel, R.L.; et al. FTO genotype impacts food intake and corticolimbic activation. Am. J. Clin. Nutr. 2018, 107, 145–154. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Nilsson, E.; Ling, C.; Hansen, T.; Pedersen, O.; Groop, L.; Vaag, A.; Poulsen, P. Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes 2009, 58, 2402–2408. [Google Scholar] [CrossRef]
- Bravard, A.; Lefai, E.; Meugnier, E.; Pesenti, S.; Disse, E.; Vouillarmet, J.; Peretti, N.; Rabasa-Lhoret, R.; Laville, M.; Vidal, H.; et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 2011, 60, 258–268. [Google Scholar] [CrossRef]
- Wu, W.; Feng, J.; Jiang, D.; Zhou, X.; Jiang, Q.; Cai, M.; Wang, X.; Shan, T.; Wang, Y. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine. Sci. Rep. 2017, 4, e41606. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Serrenho, D.; Santos, S.D.; Carvalho, A.L. The role of ghrelin in regulating synaptic function and plasticity of feeding-associated circuits. Front. Cell Neurosci. 2019, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Goltz, F.R.; Thackray, A.E.; Atkinson, G.; Lolli, L.; King, J.A.; Dorling, J.L.; Dowejko, M.; Mastana, S.; Stensel, D.J. True interindividual variability exists in postprandial appetite responses in healthy men but is not moderated by the FTO genotype. J. Nutr. 2019, 149, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr. Res. 2013, 57, 20028. [Google Scholar] [CrossRef] [PubMed]
- Sonestedt, E.; Roos, C.; Gullberg, B.; Ericson, U.; Wirfält, E.; Orho-Melander, M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 2009, 90, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Hakanen, M.; Raitakari, O.T.; Lehtimäki, T.; Peltonen, N.; Pahkala, K.; Sillanmäki, L.; Lagström, H.; Viikari, J.; Simell, O.; Rönnemaa, T. FTO genotype is associated with body mass index after the age of seven years but not with energy intake of leisure-time physical activity. J. Clin. Endocrinol. Metab. 2009, 94, 1281–1287. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, H.; Lagou, V.; Gutin, B.; Stallmann-Jorgensen, I.S.; Treiber, F.A.; Dong, Y.; Snieder, H. FTO variant rs9939609 is associated with body mass index and waist circumference, but not with energy intake or physical activity in European- and African-American youth. BMC Med. Genet. 2010, 11, 57. [Google Scholar] [CrossRef]
- Eelderink, C.; Rietsema, S.; van Vliet, I.M.Y.; Loef, L.C.; Boer, T.; Koehorst, M.; Nolte, I.M.; Westerhuis, R.; Singh-Povel, C.M.; Geurts, J.M.W.; et al. The effect of high compared with low dairy consumption on glucose metabolism, insulin sensitivity, and metabolic flexibility in overweight adults: A randomized crossover trial. Am. J. Clin. Nutr. 2019, 109, 1555–1568. [Google Scholar] [CrossRef]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging. Prostaglandins Leukot Essent Fat. Acids 2016, 114, 28–34. [Google Scholar] [CrossRef]
- Pitman, R.T.; Fong, J.T.; Billman, P.; Puri, N. Knockdown of the fat mass and obesity gene disrupts cellular energy balance in a cell-type specific manner. PLoS ONE 2012, 7, e38444. [Google Scholar] [CrossRef] [PubMed]
- Berentzen, T.; Kring, S.I.; Holst, C.; Zimmermann, E.; Jess, T.; Hansen, T.; Pedersen, O.; Toubro, S.; Astrup, A.; Sørensen, T.I.A. Lack of association of fatness-related FTO gene variants with energy expenditure or physical activity. J. Clin. Endocrinol. Metab. 2008, 93, 2904–2908. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.E.; Tavendale, R.; Watt, P.; Hetherington, M.M.; Palmer, C.N.D. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 2008, 359, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Mensink, M.; Kooi, M.E.; Roekaerts, P.M.; Saris, W.H.; Blaak, E.E. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity 2008, 16, 1025–1032. [Google Scholar] [CrossRef]
- Thiebaud, D.; Jacot, E.; Defronzo, R.A.; Maeder, E.; Jequier, E.; Felber, J. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 1982, 31, 957–963. [Google Scholar] [CrossRef]
- Janseen, P.; Vanden Berghe, P.; Verschueren, S.; Lehmann, A.; Depoortere, I.; Tack, J. Review article: The role of gastric motility in the control of food intake. Aliment. Pharm. Ther. 2011, 33, 880–894. [Google Scholar] [CrossRef]
- Kelley, D.E. Skeletal muscle fat oxidation: Timing and flexibility are everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef]


| Genotype | AA | AT | TT | P Value |
|---|---|---|---|---|
| n | 32 | 62 | 53 | |
| Age (year) | 29.8 ± 1.7 | 28.8 ± 1.0 | 28.9 ± 1.2 | 0.839 |
| Total Body Mass (kg) | 70.7 ± 2.3 | 70.3 ± 1.5 | 69.9 ± 2.1 | 0.971 |
| Height (cm) | 169.0 ± 1.8 | 169.7 ± 1.2 | 168.3 ± 1.3 | 0.713 |
| BMI (kg/m2) | 24.6 ± 0.6 | 24.5 ± 0.5 | 24.5 ± 0.6 | 0.981 |
| Hip Circumference (cm) | 102.1 ± 1.6 | 101.1 ± 0.9 | 100.9 ± 1.2 | 0.804 |
| Waist Circumference (cm) | 77.6 ± 1.7 | 77.0 ± 1.1 | 77.1 ± 1.4 | 0.958 |
| Fat Mass (%) | 27.5 ± 1.6 | 26.3 ± 1.2 | 27.4 ± 1.2 | 0.769 |
| Lean Body Mass (kg) | 48.1 ± 1.8 | 49.0 ± 1.3 | 47.9 ± 1.5 | 0.830 |
| Systolic BP (mmHg) | 121.9 ± 2.3 | 122.8 ± 1.1 | 122.8 ± 2.1 | 0.945 |
| Diastolic BP (mmHg) | 76.0 ± 1.6 | 72.7 ± 1.2 | 74.7 ± 1.2 | 0.190 |
| Genotype | AA | AT | TT | P Value |
|---|---|---|---|---|
| Total Energy (kcal) | 1779.4 ± 79.7 | 1850.2 ± 63.0 | 1808.4 ± 61.2 | 0.768 |
| Protein (%) | 19.9 ± 0.9 | 22.1 ± 0.7 | 20.7 ± 0.6 | 0.089 |
| Total Fat (%) | 34.0 ± 2.4 | 31.8 ± 0.8 | 32.8 ± 0.9 | 0.460 |
| Saturated Fat (%) | 43.9 ± 1.4 | 37.0 ± 1.0 | 37.9 ± 1.4 | 0.002 |
| Monounsaturated Fat (%) | 41.0 ± 0.8 | 44.8 ± 0.7 | 44.2 ± 1.0 | 0.026 |
| Polyunsaturated Fat (%) | 15.1 ± 0.9 | 18.2 ± 0.8 | 17.83 ± 1.0 | 0.065 |
| Carbohydrate (%) | 44.0 ± 1.3 | 40.9 ± 0.9 | 41.6 ± 1.0 | 0.017 |
| Alcohol (%) | 0.8 ± 0.3 | 1.8 ± 0.4 | 1.7 ± 0.4 | 0.237 |
| Fibre (%) | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | 0.811 |
| Genotype | AA | AT | TT | P Value |
|---|---|---|---|---|
| Alpha-Linolenic Acid (ALA, 18:3 n-3) (g) | 1.29 ± 0.19 | 1.41 ± 0.11 | 1.53 ± 0.17 | 0.604 |
| Eicosapentaenoic Acid (EPA, 20:5 n-3) (g) | 0.05 ± 0.01 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.262 |
| Docosapentaenoic Acid (DPA, 22:5 n-3) (g) | 0.07 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.733 |
| Docosahexaenoic Acid (DHA, 22:6 n-3) (g) | 0.20 ± 0.05 | 0.16 ± 0.03 | 0.16 ± 0.03 | 0.805 |
| Linoleic Acid (18:2 n-6) (g) | 8.68 ± 0.66 | 11.25 ± 0.80 | 10.18 ± 0.83 | 0.149 |
| Genotype | AA | AT | TT | P Value | |||
|---|---|---|---|---|---|---|---|
| Pre OGL | Post OGL | Pre OGL | Post OGL | Pre OGL | Post OGL | ||
| RER | 0.82 ± 0.01 | 0.93 ± 0.01 | 0.80 ± 0.02 | 0.92 ± 0.01 | 0.81 ± 0.01 | 0.92 ± 0.01 | 0.251 |
| Energy Expenditure (kJ·kgLBM−1·min−1) | 0.102 ± 0.002 | 0.114 ± 0.003 | 0.104 ± 0.002 | 0.117 ± 0.002 | 0.104 ± 0.002 | 0.115 ± 0.002 | 0.679 |
| Glucose Oxidation (g·kgLBM−1·min−1) | 2.47 ± 0.28 × 10−3 | 4.08 ± 0.22 × 10−3 | 2.16 ± 0.18 × 10−3 | 4.16 ± 0.15 × 10−3 | 2.31 ± 0.21 × 10−3 | 4.13 ± 0.13 × 10−3 | 0.630 |
| Fat Oxidation (g·kgLBM−1·min−1) | 1.54 ± 0.10 × 10−3 | 1.15 ± 0.06 × 10−3 | 1.71 ± 0.08 × 10−3 | 1.19 ± 0.06 × 10−3 | 1.64 ± 0.10 × 10−3 | 1.15 ± 0.05 × 10−3 | 0.696 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danaher, J.; Stathis, C.G.; Cooke, M.B. Similarities in Metabolic Flexibility and Hunger Hormone Ghrelin Exist between FTO Gene Variants in Response to an Acute Dietary Challenge. Nutrients 2019, 11, 2518. https://doi.org/10.3390/nu11102518
Danaher J, Stathis CG, Cooke MB. Similarities in Metabolic Flexibility and Hunger Hormone Ghrelin Exist between FTO Gene Variants in Response to an Acute Dietary Challenge. Nutrients. 2019; 11(10):2518. https://doi.org/10.3390/nu11102518
Chicago/Turabian StyleDanaher, Jessica, Christos G. Stathis, and Matthew B. Cooke. 2019. "Similarities in Metabolic Flexibility and Hunger Hormone Ghrelin Exist between FTO Gene Variants in Response to an Acute Dietary Challenge" Nutrients 11, no. 10: 2518. https://doi.org/10.3390/nu11102518
APA StyleDanaher, J., Stathis, C. G., & Cooke, M. B. (2019). Similarities in Metabolic Flexibility and Hunger Hormone Ghrelin Exist between FTO Gene Variants in Response to an Acute Dietary Challenge. Nutrients, 11(10), 2518. https://doi.org/10.3390/nu11102518






