Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization
2.4. Interventions
2.5. Outcomes
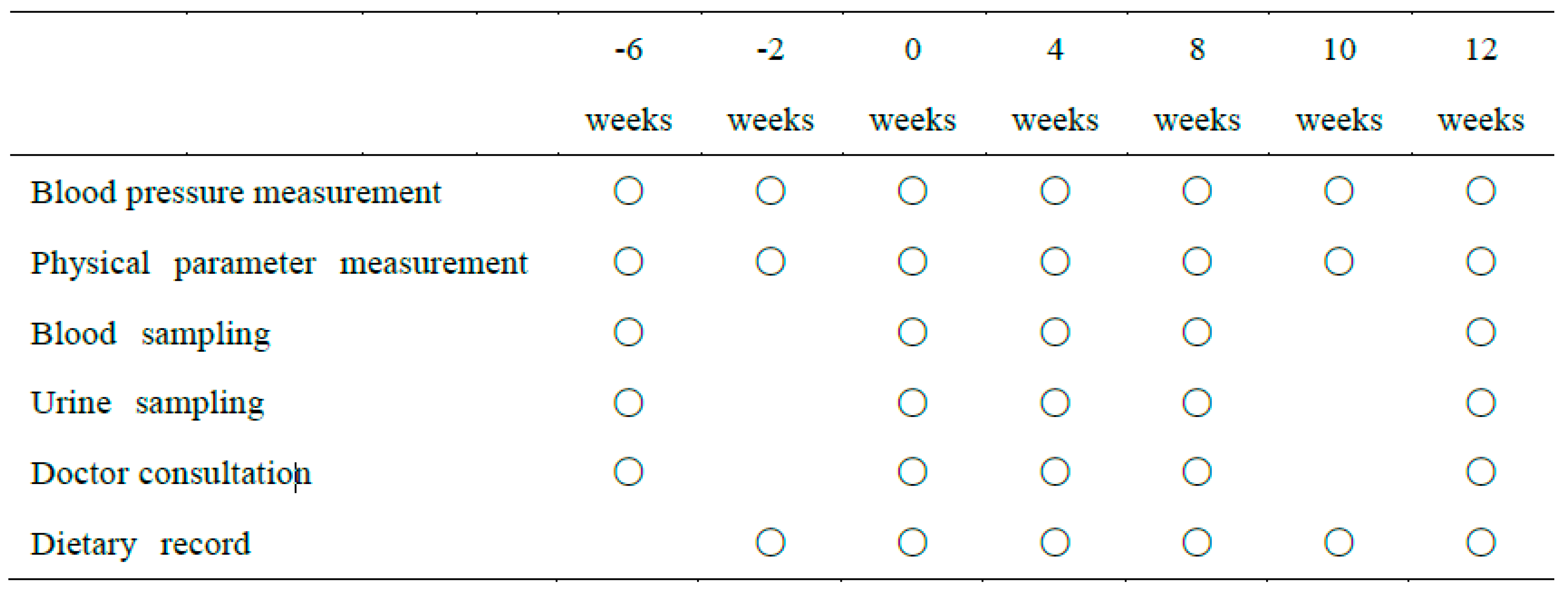
2.6. Procedures
2.7. Statistical Analysis
3. Results
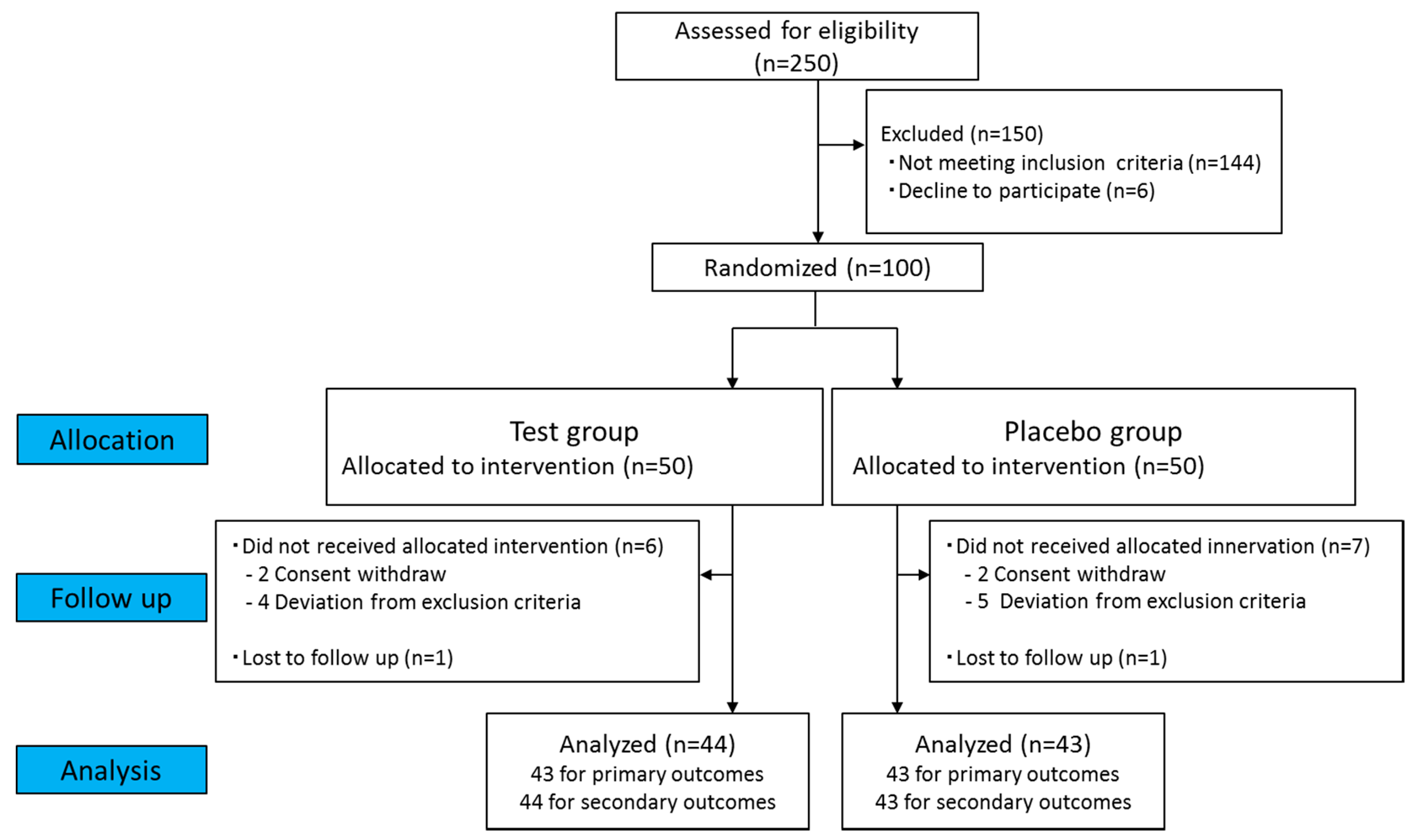
3.1. Participants
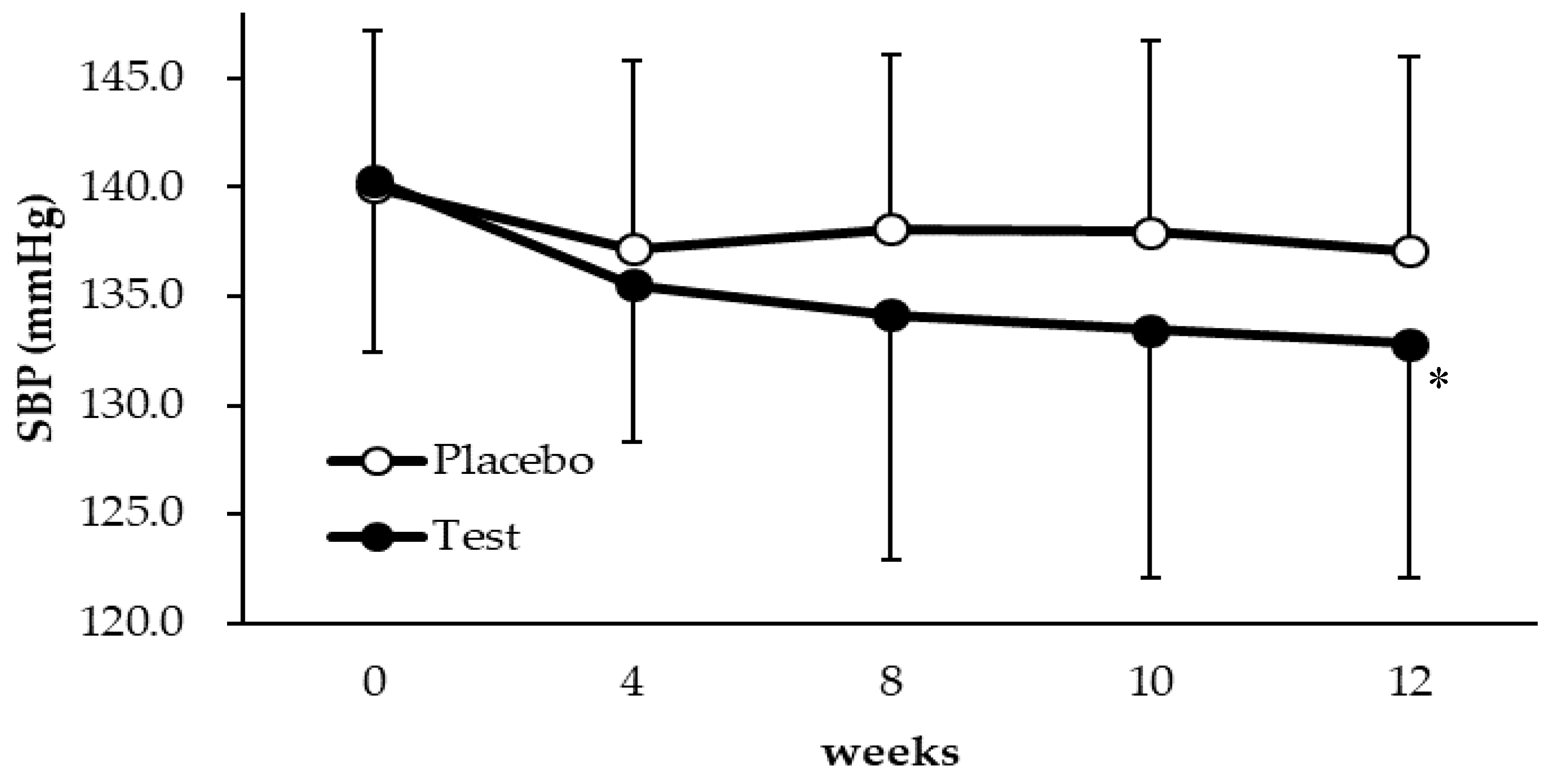
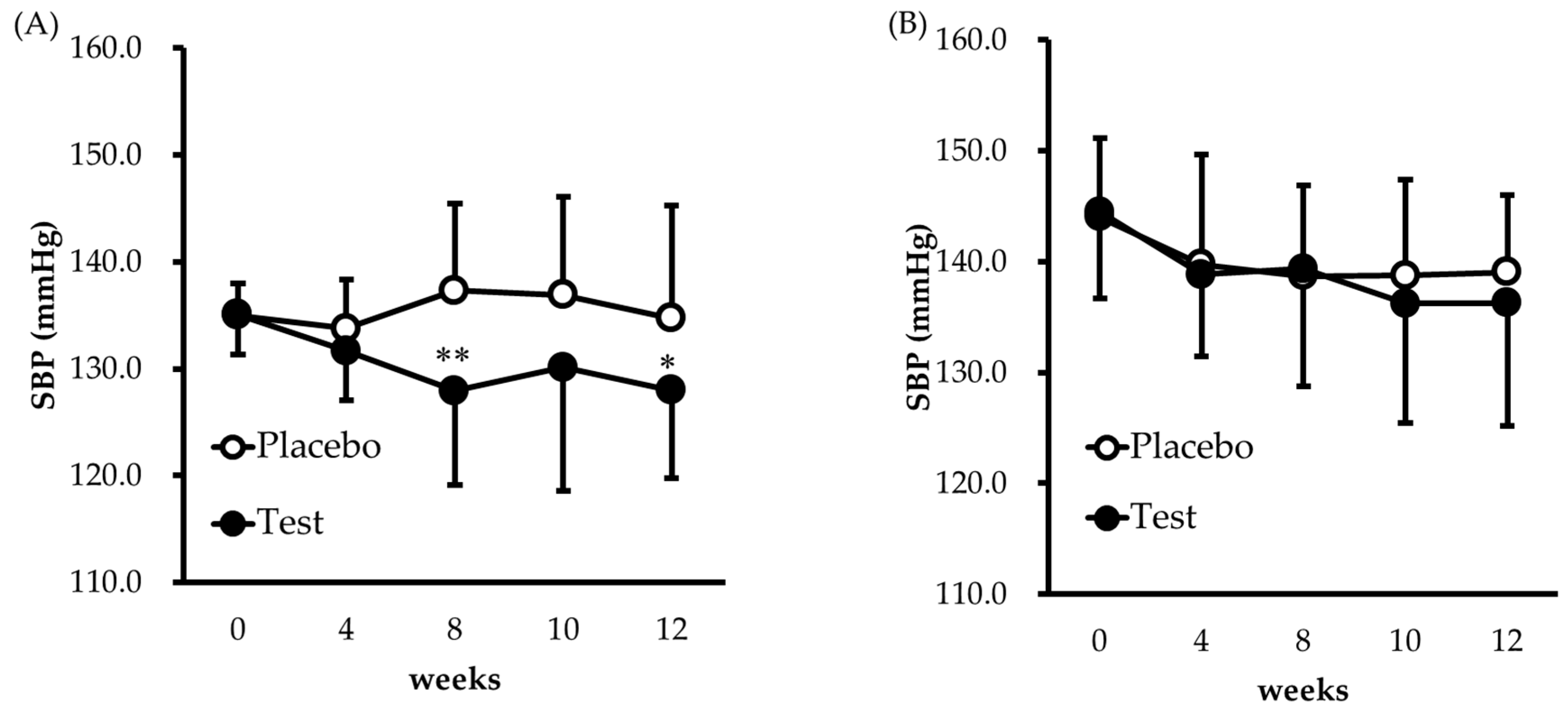
3.2. Blood Pressure Response
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alam, T.; Khan, S.; Gaba, B.; Haider, M.F.; Baboota, S.; Ali, J. Nanocarriers as treatment modalities for hypertension. Drug Deliv. 2017, 24, 358–369. [Google Scholar] [CrossRef]
- Johansson, B.B. Who satellite symposium. Clin. Exp. Pharmacol. Physiol. 1999, 26, 563–565. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Available online: http://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/kekka_shintai_chousa_koumoku.html (accessed on 30 January 2018).
- Kokubo, Y.; Kamide, K.; Okamura, T.; Watanabe, M.; Higashiyama, A.; Kawanishi, K.; Okayama, A.; Kawano, Y. Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: The Suita study. Hypertension 2008, 52, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, K.; Ando, K.; Fujita, T.; Hasebe, N.; Higaki, J.; Horiuchi, M.; Imai, Y.; Imaizumi, T.; Ishimitsu, T.; Ito, M.; et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens. Res. 2014, 37, 253–392. [Google Scholar] [CrossRef]
- Yuda, N.; Tanaka, M.; Yamada, A.; Ochi, D.; Yamauchi, K.; Abe, F.; Sakane, N. Antihypertensive effect of the casein-derived peptide met-lys-pro in individuals with high-normal blood pressure or grade 1 hypertension—A randomized, double-blind, placebo-controlled, parallel-group trial-. Jpn. Pharmacol. Ther. 2018, 46, 529–537. [Google Scholar]
- Tjelle, T.E.; Holtung, L.; Bohn, S.K.; Aaby, K.; Thoresen, M.; Wiik, S.Å.; Paur, I.; Karlsen, A.S.; Retterstol, K.; Iversen, P.O.; et al. Polyphenol-rich juices reduce blood pressure measures in a randomised controlled trial in high normal and hypertensive volunteers. Br. J. Nutr. 2015, 114, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Shida, Y.I.; Hibata, Y.S.; Ukuhara, I.F.; Ano, Y.Y.; Akehara, I.T.; Aneko, K.K. Effect of an excess intake of casein hydrolysate containing val-pro-pro and ile-pro-pro in subjects with normal blood pressure, high-normal blood pressure, or mild hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Shih, F.F. An update on the use of co-products from the milling of rice in value-added food products. J. Am. Oil Chem. Soc. 2012, 89, 1–8. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Adebiyi, A.O.; Hasegawa, Y.; Ogawa, T.; Muramoto, K. Isolation and characterization of protein fractions from deoiled rice bran. Eur. Food Res. Technol. 2009, 228, 391–401. [Google Scholar] [CrossRef]
- Hata, Y.; Yamamoto, M.; Ohni, M.; Nakajima, K.; Nakamura, Y.; Takano, T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996, 64, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Shobako, N.; Ogawa, Y.; Ishikado, A.; Harada, K.; Kobayashi, E.; Suido, H.; Kusakari, T.; Maeda, M.; Suwa, M.; Matsumoto, M.; et al. A novel antihypertensive peptide identified in thermolysin-digested rice bran. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Shobako, N.; Ishikado, A.; Ogawa, Y.; Sono, Y.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ohinata, K. Rice bran-derived tripeptide exhibits vasorelaxant and anti-hypertensive effects dependent on the endothelial NO system. J. Agric. Food Chem. 2019, 67, 1437–1442. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women—A 12-week placebo-controlled and randomized clinical study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.; Raubenheimer, K.; Briskey, D.; Neubauer, O.; Leveritt, M.; Kerr, G.; Ortiz de Zevallos Munoz, J.; Peake, J.; Hickey, D.; Allen, J.; et al. Acute effects of nitrate-rich beetroot juice on blood pressure, hemostasis and vascular inflammation markers in healthy older adults: A randomized, placebo-controlled crossover study. Nutrients 2017, 9, 1270. [Google Scholar] [CrossRef]
- Howe, P.R.C.; Evans, H.M.; Kuszewski, J.C.; Wong, R.H.X. Effects of long chain omega-3 polyunsaturated fatty acids on brain function in mildly hypertensive older adults. Nutrients 2018, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Ueshima, H.; Choudhury, S.R.; Okayama, A.; Hayakawa, T.; Kita, Y.; Kadowaki, T.; Okamura, T.; Minowa, M.; Iimura, O. Cigarette smoking as a risk factor for stroke death in Japan NIPPON DATA80. Stroke 2004, 35, 1836–1841. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Niu, Y.; Song, Q.; Zhao, Q. Comparison of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular outcomes in hypertensive patients with type 2 diabetes mellitus. Medicine 2018, 97, e0256. [Google Scholar] [CrossRef]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef]
- Ishida, Y.; Sagitani, A.; Kaneko, K.; Nakamura, Y.; Mizutani, J.; Watanabe, M.; Sato, S.; Shioya, N.; Masuda, O. Antihypertensive effects of the thblet containing “lactotripeptide (VPP, IPP)” in subjects with high normal blood pressure or mild hypertension. Jpn. Pharmacol. Ther. 2007, 35, 1249–1260. [Google Scholar]
- Cushman, W.C.; Brady, W.E.; Gazdick, L.P.; Zeldin, R.K. The effect of a losartan-based treatment regimen on isolated systolic hypertension. J. Clin. Hypertens. 2002, 4, 101–107. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Hongu, N.; Kitts, D.D.; Zawistowski, J.; Dossett, C.M.; Kopeć, A.; Pope, B.T.; Buchowski, M.S. Pigmented rice bran and plant sterol combination reduces serum lipids in overweight and obese adults. J. Am. Coll. Nutr. 2014, 33, 231–238. [Google Scholar] [CrossRef]
- Devarajan, S.; Singh, R.; Chatterjee, B.; Zhang, B.; Ali, A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J. Clin. Lipidol. 2016, 10, 339–349. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D. Plant food-derived angiotensin i converting enzyme inhibitory peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef]
- He, H.L.; Liu, D.; Ma, C.B. Review on the angiotensin-i-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- Chikama, A.; Yamaguchi, T.; Watanabe, T.; Mori, K.; Katsuragi, Y.; Tokimitsu, I.; Kajimoto, O.; Kitakaze, M. Effects of chlorogenic acids in hydroxyhydroquinone-reduced coffee on blood pressure and vascular endothelial function in humans. Prog. Med. 2006, 26, 1723–1736. [Google Scholar]
- Kouguchi, T.; Ohmori, T.; Shimizu, M.; Takahata, Y.; Maeyama, Y.; Suzuki, T.; Morimatsu, F.; Tanabe, S. Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem. 2013, 77, 691–696. [Google Scholar] [CrossRef]
- Korsager Larsen, M.; Matchkov, V.V. Hypertension and physical exercise: The role of oxidative stress. Medicina 2016, 52, 19–27. [Google Scholar] [CrossRef]
- Umar, S.; Ruffenach, G.; Iorga, A.; Cunningham, C.M.; Eghbali, M.; Moazeni, S. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Disease, C.H.; Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for the framingham heart study. Circulation 1999, 100, 354–360. [Google Scholar]
- Lida, M.; Ueda, K.; Okayama, A.; Kodama, K.; Sawai, K.; Shibata, S.; Tanaka, S.; Keijnkai, T.; Horibe, H.; Minowa, M.; et al. Impact of elevated blood pressure on mortality from all causes, cardiovascular diseases, heart disease and stroke among Japanese: 14 year follow-up of randomly selected population from Japanese—Nippon data 80. J. Hum. Hypertens. 2003, 17, 851–857. [Google Scholar] [CrossRef]
- SPRINT Research Group; Wright, J.T.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef]
- Yuu, I.; Kotaro, A.; Atsuhiro, W.; Kyoko, K.; Jyun, M.; Kayo, N.; Tokuro, S.; Kizaburo, Y.; Yasunori, N. Safety evaluation of excessive intake of the tablet containing “Lactotripeptide (VPP, IPP)” in subjects with normal blood pressure to mild hypertension. Jpn. Pharmacol. Ther. 2006, 34, 1107–1117. [Google Scholar]
| Test | Placebo | |
|---|---|---|
| Calories (kcal/day) | 4.45 | 5.42 |
| Protein (g/day) | 0.26 | 0.00 |
| Fat (g/day) | 0.05 | 0.04 |
| Carbohydrate (g/day) | 0.75 | 1.27 |
| Sodium (mg/day) | 7.39 | 0.00 |
| LRA (μg/day) | 43.00 | 0.00 |
| Placebo | Test | p | |||
|---|---|---|---|---|---|
| Male/Female | 16/27 | 15/29 | |||
| Age (years) | 54.1 | (6.0) | 53.8 | (5.7) | 0.7697 |
| Body weight (kg) | 65.1 | (9.8) | 63.0 | (11.3) | 0.3641 |
| Body mass index (kg/m2) | 24.8 | (2.7) | 24.3 | (3.0) | 0.4534 |
| SBP (mmHg) | 141.9 | (8.5) | 141.0 | (8.5) | 0.6242 |
| DBP (mmHg) | 88.9 | (6.4) | 89.4 | (7.0) | 0.7150 |
| Body temperature (°C) | 36.5 | (0.3) | 36.4 | (0.3) | 0.2501 |
| Pulse rate (beats/min) | 69.9 | (9.6) | 71.0 | (9.8) | 0.6157 |
| Total cholesterol (mg/dL) | 217.3 | (32.1) | 213.6 | (29.4) | 0.5722 |
| HDL-C (mg/dL) | 65.0 | (13.7) | 64.9 | (16.8) | 0.9725 |
| LDL-C (mg/dL) | 130.3 | (25.8) | 126.1 | (29.2) | 0.4804 |
| Triglycerides (mg/dL) | 110.3 | (53.4) | 113.3 | (68.8) | 0.8216 |
| Uric acid (mg/dL) | 5.4 | (1.2) | 5.2 | (1.2) | 0.5373 |
| Fasting blood glucose (mg/dL) | 90.8 | (8.6) | 89.8 | (7.2) | 0.5697 |
| ALT (GPT) (U/L) | 21.1 | (9.8) | 25.6 | (19.1) | 0.1742 |
| Placebo | Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | n | Mean | (SD) | n | Mean | (SD) | ΔBP | p | ||
| SBP | 0 | 43 | 140.0 | (7.2) | 43 | 140.3 | (7.9) | 0.3 (−2.9 to 3.5) | 0.8528 | |
| 12 | 42 | 137.1 | (8.9) | 41 | 132.8 | (10.7) | −4.3 (−8.6 to −0.006) | 0.0497 | ||
| DBP | 0 | 43 | 89.4 | (6.1) | 43 | 90.3 | (5.6) | 0.9 (−1.7 to 3.4) | 0.4985 | |
| 12 | 42 | 87.0 | (5.8) | 41 | 87.0 | (8.0) | −0.1 (−3.1 to 3.0) | 0.9687 | ||
| (A) High-normal BP | Placebo | Test | ||||||||
| Week | n | Mean | (SD) | n | Mean | (SD) | Δ BP | p | ||
| SBP | 0 | 19 | 135.0 | (3.0) | 19 | 135.1 | (3.7) | 0.1 (−2.1 to 2.3) | 0.9426 | |
| 12 | 19 | 134.7 | (10.5) | 17 | 128.0 | (8.2) | −6.8 (−13.2 to −0.3) | 0.0397 | ||
| DBP | 0 | 19 | 84.3 | (4.2) | 19 | 86.0 | (4.0) | 1.7 (−1.0 to 4.4) | 0.2205 | |
| 12 | 19 | 83.6 | (5.3) | 17 | 82.0 | (7.6) | −1.6 (−6.0 to 2.8) | 0.4631 | ||
| (B) Grade 1 HT | Placebo | Test | ||||||||
| Week | n | Mean | (SD) | n | Mean | (SD) | ΔBP | p | ||
| SBP | 0 | 24 | 144.0 | (7.1) | 24 | 144.5 | (7.8) | 0.5 (−3.9 to 4.8) | 0.8247 | |
| 12 | 23 | 139.1 | (6.9) | 24 | 136.3 | (11.1) | −2.8 (−8.3 to 2.6) | 0.2995 | ||
| DBP | 0 | 24 | 93.4 | (4.2) | 24 | 93.7 | (4.2) | 0.2 (−2.2 to 2.7) | 0.8495 | |
| 12 | 23 | 89.8 | (4.7) | 24 | 90.5 | (6.3) | 0.6 (−2.6 to 3.9) | 0.6989 | ||
| Placebo | Test | p | |||
|---|---|---|---|---|---|
| Body weight (kg) | 65.3 | (9.7) | 64.1 | (11.8) | 0.5882 |
| Body mass index (kg/m2) | 24.9 | (2.8) | 24.7 | (3.1) | 0.7985 |
| Total cholesterol (mg/dL) | 211.4 | (33.2) | 215.5 | (31.8) | 0.5642 |
| HDL-C (mg/dL) | 65.8 | (14.5) | 65.3 | (18.5) | 0.9091 |
| LDL-C (mg/dL) | 126.1 | (25.3) | 128.6 | (31.9) | 0.6868 |
| Triglycerides (mg/dL) | 98.0 | (58.5) | 107.7 | (72.0) | 0.4969 |
| Uric acid (mg/dL) | 5.4 | (1.1) | 5.2 | (1.4) | 0.3613 |
| Fasting blood glucose (mg/dL) | 90.7 | (8.2) | 92.2 | (9.3) | 0.4495 |
| ALT (GPT) (U/L) | 23.1 | (9.9) | 29.0 | (29.7) | 0.2184 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, Y.; Shobako, N.; Fukuhara, I.; Satoh, H.; Kobayashi, E.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ishikado, A. Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2019, 11, 726. https://doi.org/10.3390/nu11040726
Ogawa Y, Shobako N, Fukuhara I, Satoh H, Kobayashi E, Kusakari T, Suwa M, Matsumoto M, Ishikado A. Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients. 2019; 11(4):726. https://doi.org/10.3390/nu11040726
Chicago/Turabian StyleOgawa, Yutaro, Naohisa Shobako, Ikuo Fukuhara, Hisao Satoh, Etsuko Kobayashi, Takashi Kusakari, Makoto Suwa, Motonobu Matsumoto, and Atsushi Ishikado. 2019. "Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study" Nutrients 11, no. 4: 726. https://doi.org/10.3390/nu11040726
APA StyleOgawa, Y., Shobako, N., Fukuhara, I., Satoh, H., Kobayashi, E., Kusakari, T., Suwa, M., Matsumoto, M., & Ishikado, A. (2019). Rice Bran Supplement Containing a Functional Substance, the Novel Peptide Leu-Arg-Ala, Has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients, 11(4), 726. https://doi.org/10.3390/nu11040726





