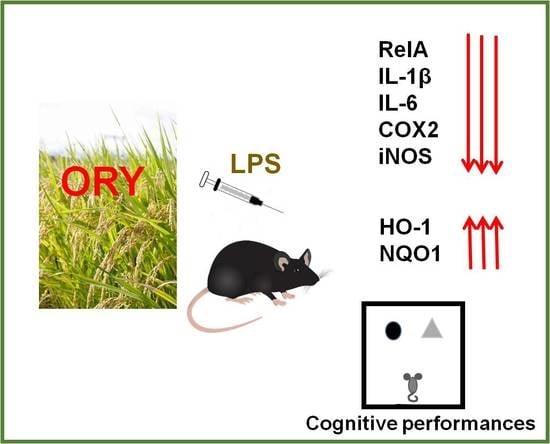
Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Treatment
2.3. Rotarod Test
2.4. Novel Object Recognition
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. γ-oryzanol has No Effect on Mouse Body Weight or Food Intake
3.2. γ-oryzanol Prevents LPS-induced Cognitive Impairment
3.3. γ-oryzanol is Able to Counteract the Increase of LPS-dependent Proinflammatory Markers Increase in the Hippocampus
3.4. γ-oryzanol Upregulates the Expression of Second-generation Antioxidant Enzymes in the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Menguer, P.K.; Sperotto, R.A.; Ricachenevsky, F.K. A walk on the wild side: Oryza species as source for rice abiotic stress tolerance. Genet. Mol. Biol. 2017, 40 (Suppl. 1), 238–252. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Morishima, H.; Kadowaki, K. Diversity in the Oryza genus. Curr. Opin. Plant Biol. 2003, 6, 139–146. [Google Scholar] [CrossRef]
- Choi, J.Y.; Platts, A.E.; Fuller, D.Q.; Hsing, Y.I.; Wing, R.A.; Purugganan, M.D. The Rice Paradox: Multiple Origins but Single Domestication in Asian Rice. Mol. Biol. Evol. 2017, 34, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Nuijten, E.; Shewfelt, R.L.; Kays, S.J. Aroma chemistry of African Oryza glaberrima and Oryza sativa rice and their interspecific hybrids. J. Sci. Food Agric. 2014, 94, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.; Zafar, T.A.; Sidhu, J. Parboiled rice metabolism differs in healthy and diabetic individuals with similar improvement in glycemic response. Nutrition 2018, 47, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.E.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Britz, S.J.; Prasad, P.V.; Moreau, R.A.; Allen, L.H., Jr.; Kremer, D.F.; Boote, K.J. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and gamma-oryzanol in brown rice. J. Agric. Food Chem. 2007, 55, 7559–7565. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Anwar, F.; Ashraf, M.; Uddin, M.K. Characterization of high-value bioactives in some selected varieties of Pakistani Rice (Oryza sativa L.). Int. J. Mol. Sci. 2012, 13, 4608–4622. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R.; Rudzinska, M.; Klensporf-Pawlik, D. gamma-Oryzanols of North American Wild Rice (Zizania palustris). J. Am. Oil Chem. Soc. 2013, 90, 1101–1109. [Google Scholar] [CrossRef]
- Lin, Y.T.; Pao, C.C.; Wu, S.T.; Chang, C.Y. Effect of different germination conditions on antioxidative properties and bioactive compounds of germinated brown rice. Biomed Res. Int. 2015, 2015, 608761. [Google Scholar] [CrossRef]
- Huang, S.H.; Ng, L.T. An improved high-performance liquid chromatographic method for simultaneous determination of tocopherols, tocotrienols and gamma-oryzanol in rice. J. Chromatogr. A 2011, 1218, 4709–4713. [Google Scholar] [CrossRef]
- Panchal, S.S.; Patidar, R.K.; Jha, A.B.; Allam, A.A.; Ajarem, J.; Butani, S.B. Anti-Inflammatory and Antioxidative Stress Effects of Oryzanol in Glaucomatous Rabbits. J. Ophthalmol. 2017, 2017, 1468716. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.S.; Garcia, J.L.; de Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Abate, G.; Serafini, M.M.; Guarienti, M.; Catanzaro, M.; Marziano, M.; Memo, M.; Lanni, C.; Uberti, D. Characterization of the Antioxidant Effects of gamma-Oryzanol: Involvement of the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 2987249. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Butler, J.P.; Sasaki, H. Gamma-oryzanol for behavioural and psychological symptoms of dementia. Psychogeriatrics 2018, 18, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, C.; Kaname, T.; Shimizu-Okabe, C.; Takayama, C.; Tsutsui, M.; Matsushita, M.; Abe, K.; Masuzaki, H. Impact of brown rice-specific gamma-oryzanol on epigenetic modulation of dopamine D2 receptors in brain striatum in high-fat-diet-induced obesity in mice. Diabetologia 2017, 60, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Q.; Yang, T.; Liang, Y.; Nie, Y.; Luo, Y.; Shen, J.; Fu, X.; Tang, Y.; Luo, F. Oryzanol Modifies High Fat Diet-Induced Obesity, Liver Gene Expression Profile, and Inflammation Response in Mice. J. Agric. Food Chem. 2017, 65, 8374–8385. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Gibb, J.; Hayley, S.; Poulter, M.O.; Anisman, H. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav. Immun. 2011, 25, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A. Smoke Signals: A Social History of Marijuana: Medical, Recreational, and Scientific, 1st ed.; Scribner: New York, NY, USA, 2012; p. vii. 519p. [Google Scholar]
- Turrin, N.P.; Gayle, D.; Ilyin, S.E.; Flynn, M.C.; Langhans, W.; Schwartz, G.J.; Plata-Salaman, C.R. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 2001, 54, 443–453. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Redus, L.; O’Connor, J.C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflamm. 2016, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Donzis, E.J.; Tronson, N.C. Modulation of learning and memory by cytokines: Signaling mechanisms and long term consequences. Neurobiol. Learn. Mem. 2014, 115, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.M.; Stasko, M.R.; Matousek, S.B.; Scott-McKean, J.J.; Maier, S.F.; Olschowka, J.A.; Costa, A.C.; O’Banion, M.K. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav. Immun. 2010, 24, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Hennessy, E.; Dansereau, M.A.; Cunningham, C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Custodio, C.S.; Mello, B.S.; Cordeiro, R.C.; de Araujo, F.Y.; Chaves, J.H.; Vasconcelos, S.M.; Nobre Junior, H.V.; de Sousa, F.C.; Vale, M.L.; Carvalho, A.F.; et al. Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur. J. Pharmacol. 2013, 713, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, T.; Kise, M.; Morikawa, K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci. Lett. 2008, 430, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Grilli, M.; Memo, M. Nuclear factor-kappaB/Rel proteins: A point of convergence of signalling pathways relevant in neuronal function and dysfunction. Biochem. Pharmacol. 1999, 57, 1–7. [Google Scholar] [CrossRef]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa, L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb. Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Goffi, F.; Memo, M.; Spano, P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 1996, 271, 15002–15007. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Memo, M. Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ. 1999, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Denis-Donini, S.; Dellarole, A.; Crociara, P.; Francese, M.T.; Bortolotto, V.; Quadrato, G.; Canonico, P.L.; Orsetti, M.; Ghi, P.; Memo, M.; et al. Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J. Neurosci. 2008, 28, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.M.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619. [Google Scholar] [CrossRef]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- McPherson, C.A.; Aoyama, M.; Harry, G.J. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav. Immun. 2011, 25, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.N.; Parry-Jones, A.R.; Allan, S.M. Interleukin-1 and acute brain injury. Front. Cell. Neurosci. 2015, 9, 18. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Buchanan, J.B.; Sparkman, N.L.; Godbout, J.P.; Freund, G.G.; Johnson, R.W. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav. Immun. 2008, 22, 301–311. [Google Scholar] [CrossRef]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1beta in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar]
- Sparkman, N.L.; Buchanan, J.B.; Heyen, J.R.; Chen, J.; Beverly, J.L.; Johnson, R.W. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J. Neurosci. 2006, 26, 10709–10716. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Dugravot, A.; Brunner, E.; Kumari, M.; Shipley, M.; Elbaz, A.; Kivimaki, M. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 2014, 83, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Grinan-Ferre, C.; Sarroca, S.; Ivanova, A.; Puigoriol-Illamola, D.; Aguado, F.; Camins, A.; Sanfeliu, C.; Pallas, M. Epigenetic mechanisms underlying cognitive impairment and Alzheimer disease hallmarks in 5XFAD mice. Aging 2016, 8, 664–684. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Machida, K.; Kalra, V.K. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J. Biol. Chem. 2010, 285, 35359–35373. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J.; O’Connell, M.A. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.U.; Huang, F.; Wang, D.; Weng, Z.; Deng, Z. Upregulation of heme oxygenase-1 expression may facilitate memory and learning in mice. Exp. Ther. Med. 2013, 5, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Kurucz, A.; Bombicz, M.; Kiss, R.; Priksz, D.; Varga, B.; Hortobágyi, T.; Trencsényi, G.; Szabó, R.; Pósa, A.; Gesztelyi, R.; et al. Heme Oxygenase-1 Activity as a Correlate to Exercise-Mediated Amelioration of Cognitive Decline and Neuropathological Alterations in an Aging Rat Model of Dementia. Biomed Res. Int. 2018, 2018, 7212861. [Google Scholar] [CrossRef] [PubMed]
- Mhillaj, E.; Catino, S.; Miceli, F.M.; Santangelo, R.; Trabace, L.; Cuomo, V.; Mancuso, C. Ferulic Acid Improves Cognitive Skills Through the Activation of the Heme Oxygenase System in the Rat. Mol. Neurobiol. 2017, 55, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.; King, A.E.; Gueven, N. Alzheimer’s Disease and NQO1: Is there a Link? Curr. Alzheimer Res. 2018, 15, 56–66. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription Factors NRF2 and NF-κB Are Coordinated Effectors of the Rho Family, GTP-binding Protein RAC1 during Inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Karkkainen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 regulates neurogenesis and protects neural progenitor cells against Abeta toxicity. Stem Cells 2014, 32, 1904–1916. [Google Scholar] [CrossRef]
- Mendez-David, I.; Tritschler, L.; Ali, Z.E.; Damiens, M.H.; Pallardy, M.; David, D.J.; Kerdine-Romer, S.; Gardier, A.M. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Anton, N.; Rojo, A.I.; Ferreiro, E.; Nunez, A.; Krause, K.H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]




| Genes | Primer Sequences |
|---|---|
| Rel-A (p65) | f-5′-TTCCTGGCGAGAGAAGCAC-3′; r-5′-AAGCTATGGATACTGCGGTCT-3′ |
| Inducible nitric oxide synthase (iNOS) | f-5′-CAGCTGGGCTGTACAAAC-3′; r-5′-CATTGGAAGTGAAGCGTTTCG-3′ |
| Cyclooxygenase 2 (COX-2) | f-5′-GCAAATCCTTGCTGTTCCAACCCA-3′; r-5′-TTGGGGATCCGGGATGAACTCTCT-3′ |
| Interleukin 1 beta (IL-1β) | f-5′-GCTTCAGGCAGGCAGTATC-3′; r-5′-TAATGGGAACGTCACACACC-3′ |
| Interleukin 6 (IL-6) | f-5′-CCTACCCCAATTTCCAATGCT-3′; r-5′-TATTTTCTGACCACAGTGAGGAAT-3′ |
| Heme oxygenase 1 (HO-1) | f-5′-TGAAGGAGGCCACCAAGGAGG-3′; r-5′-AGAGGTCACCCAGGTAGCGGG-3′ |
| NAD(P)H dehydrogenase (quinone) 1 (NQO1) | f-5′-AGGATGGGAGGTACTCGAATC-3′; r-5′-TGCTAGAGATGACTCGGAAGG-3′ |
| Nuclear factor (erythroid-derived 2)-like 2 (NRF2) | f-5′-AGCCCCATTCACAAAAGACA-3′; r-5′-GAAGTCATCAACAGGGAGGTTA-3′ |
| Actin (β-act) | f-5′-AGCCATGTACGTAGCCATCC-3′; r-5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Body Weight (g) | Food Intake (g) | |||
|---|---|---|---|---|
| Days of Treatment | VH | ORY | VH | ORY |
| 0 | 41.9 ± 1.9 | 44.7 ± 1.3 | / | / |
| 7 | 40.4 ± 1.8 | 41.7 ± 1.2 | 19.3 ± 0.7 | 17.4 ± 0.5 |
| 14 | 40.0 ± 1.8 | 41.7 ± 1.1 | 19.8 ± 0.5 | 20.0 ± 0.4 |
| 21 | 41.3 ± 1.9 | 42.5 ± 1.2 | 22.9 ± 1.0 | 22.6 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. https://doi.org/10.3390/nu11040728
Mastinu A, Bonini SA, Rungratanawanich W, Aria F, Marziano M, Maccarinelli G, Abate G, Premoli M, Memo M, Uberti D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients. 2019; 11(4):728. https://doi.org/10.3390/nu11040728
Chicago/Turabian StyleMastinu, Andrea, Sara Anna Bonini, Wiramon Rungratanawanich, Francesca Aria, Mariagrazia Marziano, Giuseppina Maccarinelli, Giulia Abate, Marika Premoli, Maurizio Memo, and Daniela Uberti. 2019. "Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice" Nutrients 11, no. 4: 728. https://doi.org/10.3390/nu11040728
APA StyleMastinu, A., Bonini, S. A., Rungratanawanich, W., Aria, F., Marziano, M., Maccarinelli, G., Abate, G., Premoli, M., Memo, M., & Uberti, D. (2019). Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients, 11(4), 728. https://doi.org/10.3390/nu11040728