Parabiosis Incompletely Reverses Aging-Induced Metabolic Changes and Oxidant Stress in Mouse Red Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Aging and Parabiotic Mice
2.2. Metabolomics
2.2.1. Sample Extraction
2.2.2. UHPLC–MS Analysis
2.2.3. Proteomics Analyses
2.2.4. Statistical Analysis
3. Results
3.1. RBCs from Aging Mice Are Characterized by Significant Proteome-Wide and Metabolic Changes in Antioxidant Systems
3.2. Focus on the RBC Metabolic Pathways Impacted by Mouse Age
3.2.1. Glutathione, One-Carbon, Glycolysis, and Pentose Phosphate Pathway
3.2.2. Purine Metabolism
3.2.3. Transamination, Carboxylic Acids, and Arginine Metabolism
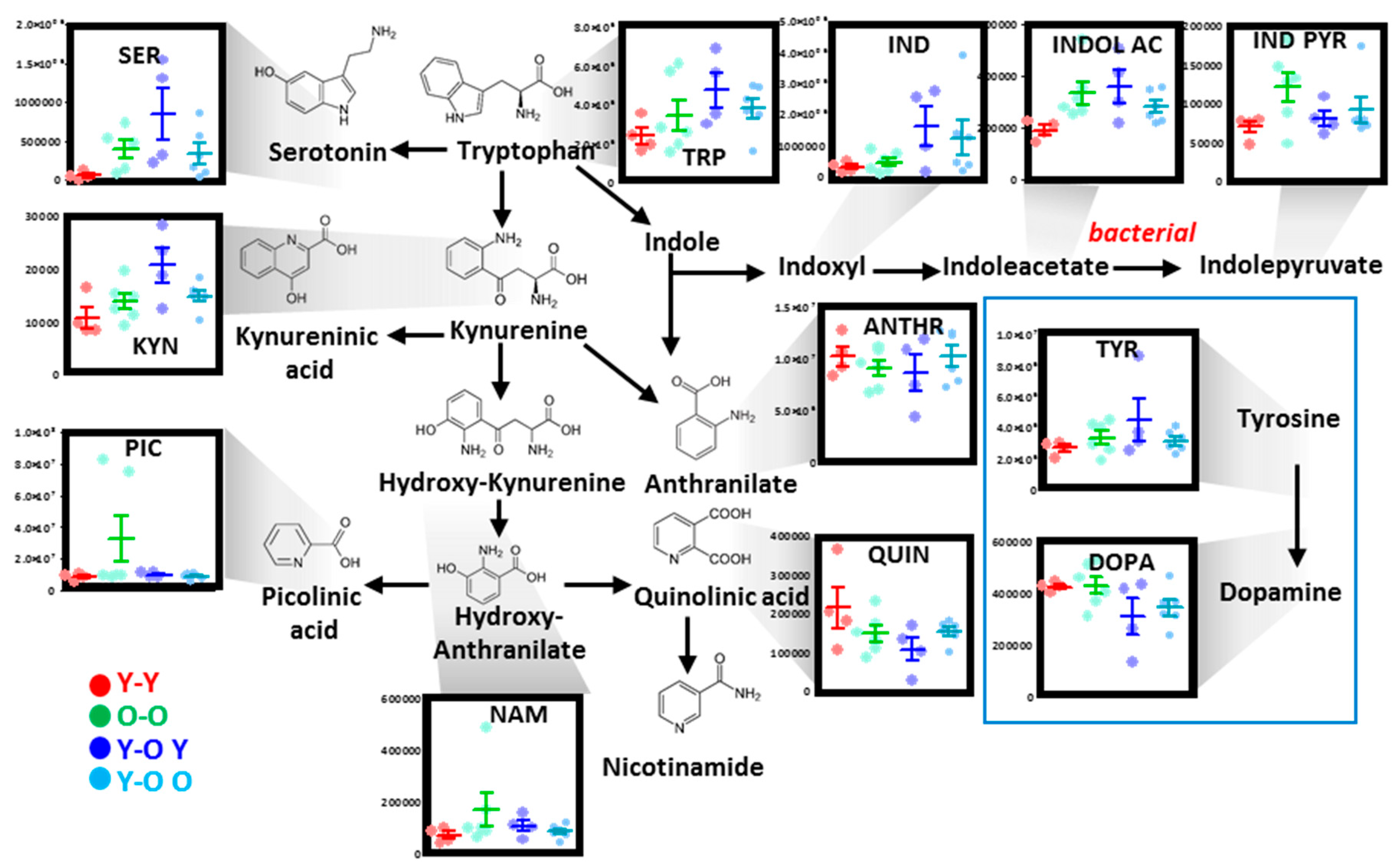
3.2.4. Tryptophan, Tyrosine, and Indole Metabolism
3.2.5. Parabiosis Only Partially Restores Metabolic and Proteome-Wide Defects in RBCs from Aging Mice
3.2.6. Parabiosis Only Partial Rescues the Age-Dependent Changes in RBC Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet (Lond. Engl.) 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Bloom, D.E.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; McKee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet (Lond. Engl.) 2015, 385, 649–657. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Howell, P.R.; Anderson, R.M. Aging and Caloric Restriction Research: A Biological Perspective with Translational Potential. EBioMedicine 2017, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, G.L. Aging, genetics, and the environment: Potential of errors introduced into genetic information transfer by metal ions. Mech. Ageing Dev. 1979, 9, 291–301. [Google Scholar] [CrossRef]
- Rodríguez-Rodero, S.; Fernández-Morera, J.L.; Menéndez-Torre, E.; Calvanese, V.; Fernández, A.F.; Fraga, M.F. Aging Genetics and Aging. Aging Dis. 2011, 2, 186–195. [Google Scholar]
- Piper, M.D.W.; Bartke, A. Diet and aging. Cell Metab. 2008, 8, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef]
- Piperakis, S.M.; Visvardis, E.E.; Sagnou, M.; Tassiou, A.M. Effects of smoking and aging on oxidative DNA damage of human lymphocytes. Carcinogenesis 1998, 19, 695–698. [Google Scholar] [CrossRef][Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef]
- Romano, A.D.; Serviddio, G.; de Matthaeis, A.; Bellanti, F.; Vendemiale, G. Oxidative stress and aging. J. Nephrol. 2010, 23, S29–S36. [Google Scholar]
- Anderson, R.M.; Le Couteur, D.G.; de Cabo, R. Caloric Restriction Research: New Perspectives on the Biology of Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Xia, Y.; Zimring, J.C.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteom. 2018, 15, 855–864. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Dzieciatkowska, M.; Nemkov, T.; Hansen, K.C. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017, 15, 182–187. [Google Scholar]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.B.; Hampton, M.B.; Winterbourn, C.C. Accumulation of oxidized peroxiredoxin 2 in red blood cells and its prevention. Transfusion 2015, 55, 1909–1918. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–Metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 1, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Mirantes-Barbeito, C.; Fong, S.; Loeffler, D.; Kovtonyuk, L.V.; Zhang, S.; Lakshminarasimhan, R.; Chin, C.P.; Techner, J.-M.; Will, B.; et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Schrier, S.L. Evaluation and management of anemia in the elderly. Am. J. Hematol. 2014, 89, 88–96. [Google Scholar] [CrossRef]
- Abraham, E.C.; Taylor, J.F.; Lang, C.A. Influence of mouse age and erythrocyte age on glutathione metabolism. Biochem. J. 1978, 174, 819–825. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.; Chandra, P. Age-dependent detection of erythrocytes glucose-6-phosphate dehydrogenase and its correlation with oxidative stress. Arch. Physiol. Biochem. 2016, 122, 61–66. [Google Scholar] [CrossRef]
- Magnani, M.; Piatti, E.; Serafini, N.; Palma, F.; Dachà, M.; Fornaini, G. The age-dependent metabolic decline of the red blood cell. Mech. Ageing Dev. 1983, 22, 295–308. [Google Scholar] [CrossRef]
- Rodgers, G.P.; Lichtman, H.C.; Sheff, M.F. Red blood cell glucose-6-phosphate dehydrogenase activity in aged humans. J. Am. Geriatr. Soc. 1983, 31, 8–11. [Google Scholar] [CrossRef]
- Rinalducci, S.; D’Amici, G.M.; Blasi, B.; Vaglio, S.; Grazzini, G.; Zolla, L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011, 51, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Paukovich, N.; Xue, M.; Elder, J.R.; Redzic, J.S.; Blue, A.; Pike, H.; Miller, B.G.; Pitts, T.M.; Pollock, D.D.; Hansen, K.; et al. Biliverdin Reductase B Dynamics Are Coupled to Coenzyme Binding. J. Mol. Biol. 2018, 430, 3234–3250. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, V.; Gevi, F.; D’alessandro, A.; Zolla, L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: A metabolomics overview. Blood Transfus. 2014, 12, 376–387. [Google Scholar]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med. 2016, 96, 152–165. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef]
- Culp-Hill, R.; Zheng, C.; Reisz, J.A.; Smith, K.; Rachubinski, A.; Nemkov, T.; Butcher, E.; Granrath, R.; Hansen, K.C.; Espinosa, J.M.; et al. Red blood cell metabolism in Down syndrome: Hints on metabolic derangements in aging. Blood Adv. 2017, 1, 2776–2780. [Google Scholar] [CrossRef]
- Horvath, S.; Garagnani, P.; Bacalini, M.G.; Pirazzini, C.; Salvioli, S.; Gentilini, D.; Di Blasio, A.M.; Giuliani, C.; Tung, S.; Vinters, H.V.; et al. Accelerated epigenetic aging in Down syndrome. Aging Cell 2015, 14, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kusters, M.A.A.; Verstegen, R.H.J.; de Vries, E. Down Syndrome: Is It Really Characterized by Precocious Immunosenescence? Aging Dis. 2011, 2, 538–545. [Google Scholar] [PubMed]
- Galletti, P.; De Bonis, M.L.; Sorrentino, A.; Raimo, M.; D’Angelo, S.; Scala, I.; Andria, G.; D’Aniello, A.; Ingrosso, D.; Zappia, V. Accumulation of altered aspartyl residues in erythrocyte proteins from patients with Down’s syndrome. FEBS J. 2007, 274, 5263–5277. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Bianchi, R.; Pallotta, M.T.; Orabona, C.; Albini, E.; Iacono, A.; Belladonna, M.L.; Vacca, C.; Fallarino, F.; Macchiarulo, A.; et al. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity 2017, 46, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Sullivan, K.D.; Culp-Hill, R.; Ludwig, M.P.; Smith, K.P.; Waugh, K.A.; Minter, R.; Tuttle, K.D.; Rachubinski, A.L.; Granrath, R.E.; et al. Trisomy 21 drives production of neurotoxic tryptophan catabolites via the interferon-inducible kynurenine pathway. BioRxiv 2018. [Google Scholar] [CrossRef]
- Kamran, P.; Sereti, K.-I.; Zhao, P.; Ali, S.R.; Weissman, I.L.; Ardehali, R. Parabiosis in mice: A detailed protocol. J. Vis. Exp. JoVE 2013, 6. [Google Scholar] [CrossRef]
- Gontier, G.; Iyer, M.; Shea, J.M.; Bieri, G.; Wheatley, E.G.; Ramalho-Santos, M.; Villeda, S.A. Tet2 Rescues Age-Related Regenerative Decline and Enhances Cognitive Function in the Adult Mouse Brain. Cell Rep. 2018, 22, 1974–1981. [Google Scholar] [CrossRef]
- Harris, R.B. Loss of body fat in lean parabiotic partners of ob/ob mice. Am. J. Physiol. 1997, 272, R1809–R1815. [Google Scholar] [CrossRef]
- Harris, R.B. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology 1999, 140, 138–145. [Google Scholar] [CrossRef][Green Version]
- Reisz, J.A.; Nemkov, T.; Dzieciatkowska, M.; Culp-Hill, R.; Stefanoni, D.; Hill, R.C.; Yoshida, T.; Dunham, A.; Kanias, T.; Dumont, L.J.; et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018, 58, 2978–2991. [Google Scholar] [CrossRef]
- Nemkov, T.; Hansen, K.C.; D’Alessandro, A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. RCM 2017, 31, 663–673. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Culp-Hill, R.; Reisz, J.A.; Anderson, M.; Fu, X.; Nemkov, T.; Gehrke, S.; Zheng, C.; Kanias, T.; Guo, Y.; et al. Heterogeneity of blood processing and storage additives in different centers impacts stored Red Blood Cell metabolism as much as storage time: Lessons from REDS-III-Omics. Transfusion 2018, 59, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Culp-Hill, R.; Srinivasan, J.; Gehrke, S.; Kamyszek, R.; Ansari, A.; Shah, N.; Welsby, I.; D’Alessandro, A. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion 2018, 58, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Gehrke, S.; Hansen, K.C.; D’Alessandro, A. High-Throughput Metabolomics: Isocratic and Gradient Mass Spectrometry-Based Methods. Methods Mol. Biol. Clifton NJ 2019, 1978, 13–26. [Google Scholar]
- Reisz, J.A.; Zheng, C.; D’Alessandro, A.; Nemkov, T. Untargeted and Semi-targeted Lipid Analysis of Biological Samples Using Mass Spectrometry-Based Metabolomics. Methods Mol. Biol. (Clifton NJ) 2019, 1978, 121–135. [Google Scholar]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic Analysis and Visualization Engine for LC−MS Data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef]
- Reisz, J.A.; Chessler, K.M.; Dzieciatkowska, M.; D’Alessandro, A.; Hansen, K.C. Blood and Plasma Proteomics: Targeted Quantitation and Posttranslational Redox Modifications. Methods Mol. Biol. (Clifton NJ) 2017, 1619, 353–371. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Soukenik, M.; Diehl, A.; Leidert, M.; Sievert, V.; Büssow, K.; Leitner, D.; Labudde, D.; Ball, L.J.; Lechner, A.; Nägler, D.K.; et al. The SEP domain of p47 acts as a reversible competitive inhibitor of cathepsin L. FEBS Lett. 2004, 576, 358–362. [Google Scholar] [CrossRef]
- Nemkov, T.; Hansen, K.C.; Dumont, L.J.; D’Alessandro, A. Metabolomics in transfusion medicine. Transfusion 2016, 56, 980–993. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Nemkov, T.; Yoshida, T.; Bordbar, A.; Palsson, B.O.; Hansen, K.C. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion 2017, 57, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Whillier, S.; Raftos, J.E.; Sparrow, R.L.; Kuchel, P.W. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion 2011, 51, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Andrisse, S.; Koehler, R.M.; Chen, J.E.; Patel, G.D.; Vallurupalli, V.R.; Ratliff, B.A.; Warren, D.E.; Fisher, J.S. Role of GLUT1 in regulation of reactive oxygen species. Redox Biol. 2014, 2, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, J.-H.; Paull, T.T.; Gehrke, S.; D’Alessandro, A.; Dou, Q.; Gladyshev, V.N.; Schroeder, E.A.; Steyl, S.K.; Christian, B.E.; et al. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Ullevig, S.L.; Kim, H.S.; Short, J.D.; Tavakoli, S.; Weintraub, S.T.; Downs, K.; Asmis, R. Protein S-Glutathionylation Mediates Macrophage Responses to Metabolic Cues from the Extracellular Environment. Antioxid. Redox Signal. 2016, 25, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Wither, M.; Dzieciatkowska, M.; Nemkov, T.; Strop, P.; D’Alessandro, A.; Hansen, K.C. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion 2016, 56, 421–426. [Google Scholar] [CrossRef]
- Harper, V.M.; Oh, J.Y.; Stapley, R.; Marques, M.B.; Wilson, L.; Barnes, S.; Sun, C.-W.; Townes, T.; Patel, R.P. Peroxiredoxin-2 Recycling Is Inhibited During Erythrocyte Storage. Antioxid. Redox Signal. 2015, 22, 294–307. [Google Scholar] [CrossRef]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef]
- Aman, Y.; Qiu, Y.; Tao, J.; Fang, E.F. Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl. Med. Aging 2018, 2, 30–37. [Google Scholar] [CrossRef]
- Johnson, S.; Imai, S. NAD+ biosynthesis, aging, and disease. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD metabolism: Implications in aging and longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van der Goot, A.T.; Zhu, W.; Vázquez-Manrique, R.P.; Seinstra, R.I.; Dettmer, K.; Michels, H.; Farina, F.; Krijnen, J.; Melki, R.; Buijsman, R.C.; et al. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc. Natl. Acad. Sci. USA 2012, 109, 14912–14917. [Google Scholar] [CrossRef] [PubMed]
- Van der Goot, A.T.; Nollen, E.A.A. Tryptophan metabolism: Entering the field of aging and age-related pathologies. Trends Mol. Med. 2013, 19, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The Physiological Action of Picolinic Acid in the Human Brain. Int. J. Tryptophan Res. IJTR 2009, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, W. Indole: A signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J. Microbiol. Seoul Korea 2015, 53, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.E. Production and degradation of indole by gram-negative bacteria. Zentralbl. Bakteriol. Mikrobiol. Hyg. 1986, 261, 1–11. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006, 83, 447S–455S. [Google Scholar] [CrossRef]
- Pietras, E.M. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef]
- Henry, C.J.; Nemkov, T.; Casás-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.C.; Serkova, N.J.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J.; et al. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Chadefaux, B.; Rethoré, M.O.; Raoul, O.; Ceballos, I.; Poissonnier, M.; Gilgenkranz, S.; Allard, D. Cystathionine beta synthase: Gene dosage effect in trisomy 21. Biochem. Biophys. Res. Commun. 1985, 128, 40–44. [Google Scholar] [CrossRef]
- McFadden, P.N.; Clarke, S. Methylation at D-aspartyl residues in erythrocytes: Possible step in the repair of aged membrane proteins. Proc. Natl. Acad. Sci. USA 1982, 79, 2460–2464. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.L.; Clarke, S. Enzymatic methylation of band 3 anion transporter in intact human erythrocytes. Biochemistry 1987, 26, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.A.; Clarke, S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J. Biol. Chem. 1980, 255, 11640–11643. [Google Scholar] [PubMed]
- Barber, J.R.; Clarke, S. Membrane protein carboxyl methylation increases with human erythrocyte age. Evidence for an increase in the number of methylatable sites. J. Biol. Chem. 1983, 258, 1189–1196. [Google Scholar] [PubMed]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.A.; Subudhi, A.W.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Slaughter, A.L.; Culp-Hill, R.; Moore, E.E.; Silliman, C.C.; Fragoso, M.; Peltz, E.D.; Hansen, K.C.; Banerjee, A.; D’Alessandro, A. Red blood cells in hemorrhagic shock: A critical role for glutaminolysis in fueling alanine transamination in rats. Blood Adv. 2017, 1, 1296–1305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A.J. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Moore, H.B.; Moore, E.E.; Reisz, J.A.; Wither, M.J.; Ghasasbyan, A.; Chandler, J.; Silliman, C.C.; Hansen, K.C.; Banerjee, A.; et al. Plasma succinate is a predictor of mortality in critically injured patients. J. Trauma Acute Care Surg. 2017, 83, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Yoshida, T.; Dunham, A.; Wen, E.Y.; Wen, A.Q.; Roach, R.C.; Hansen, K.C.; Xia, Y.; et al. Metabolism of Citrate and Other Carboxylic Acids in Erythrocytes As a Function of Oxygen Saturation and Refrigerated Storage. Front. Med. 2017, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Kriebardis, A.G.; Stamoulis, K.E.; Trougakos, I.P.; Papassideri, I.S. Apolipoprotein J/Clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence. PLoS ONE 2011, 6, e26032. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Armenta, J.L.; Mahapatra, G.; Allison Amick, K.; Li, N.; Lu, B.; Molina, A. HETEROCHRONIC PARABIOSIS: OLD BLOOD ATTENUATES MITOCHONDRIAL BIOENERGETICS OF YOUNG MICE. Innov. Aging 2018, 2, 558. [Google Scholar] [CrossRef]
- Hofmann, B. Young Blood Rejuvenates Old Bodies: A Call for Reflection when Moving from Mice to Men. Transfus. Med. Hemother. 2018, 45, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Carbone, A.; Beccia, E.; Angiolillo, A. The Fountain of Youth: A Tale of Parabiosis, Stem Cells, and Rejuvenation. Open Med. 2017, 12, 376–383. [Google Scholar] [CrossRef]
- European Commission of the Press Announcements—Statement from FDA Commissioner Scott Gottlieb, M.D., and Director of FDA’s Center for Biologics Evaluation and Research Peter Marks, M.D., Ph.D., Cautioning Consumers against Receiving Young Donor Plasma Infusions That are Promoted as Unproven Treatment for Varying Conditions. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631568.htm (accessed on 8 April 2019).







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison, E.J.; Champagne, D.P.; Dzieciatkowska, M.; Nemkov, T.; Zimring, J.C.; Hansen, K.C.; Guan, F.; Huffman, D.M.; Santambrogio, L.; D’Alessandro, A. Parabiosis Incompletely Reverses Aging-Induced Metabolic Changes and Oxidant Stress in Mouse Red Blood Cells. Nutrients 2019, 11, 1337. https://doi.org/10.3390/nu11061337
Morrison EJ, Champagne DP, Dzieciatkowska M, Nemkov T, Zimring JC, Hansen KC, Guan F, Huffman DM, Santambrogio L, D’Alessandro A. Parabiosis Incompletely Reverses Aging-Induced Metabolic Changes and Oxidant Stress in Mouse Red Blood Cells. Nutrients. 2019; 11(6):1337. https://doi.org/10.3390/nu11061337
Chicago/Turabian StyleMorrison, Evan J., Devin P. Champagne, Monika Dzieciatkowska, Travis Nemkov, James C. Zimring, Kirk C. Hansen, Fangxia Guan, Derek M. Huffman, Laura Santambrogio, and Angelo D’Alessandro. 2019. "Parabiosis Incompletely Reverses Aging-Induced Metabolic Changes and Oxidant Stress in Mouse Red Blood Cells" Nutrients 11, no. 6: 1337. https://doi.org/10.3390/nu11061337
APA StyleMorrison, E. J., Champagne, D. P., Dzieciatkowska, M., Nemkov, T., Zimring, J. C., Hansen, K. C., Guan, F., Huffman, D. M., Santambrogio, L., & D’Alessandro, A. (2019). Parabiosis Incompletely Reverses Aging-Induced Metabolic Changes and Oxidant Stress in Mouse Red Blood Cells. Nutrients, 11(6), 1337. https://doi.org/10.3390/nu11061337





