Honey Supplementation and Exercise: A Systematic Review
Abstract
:1. Introduction
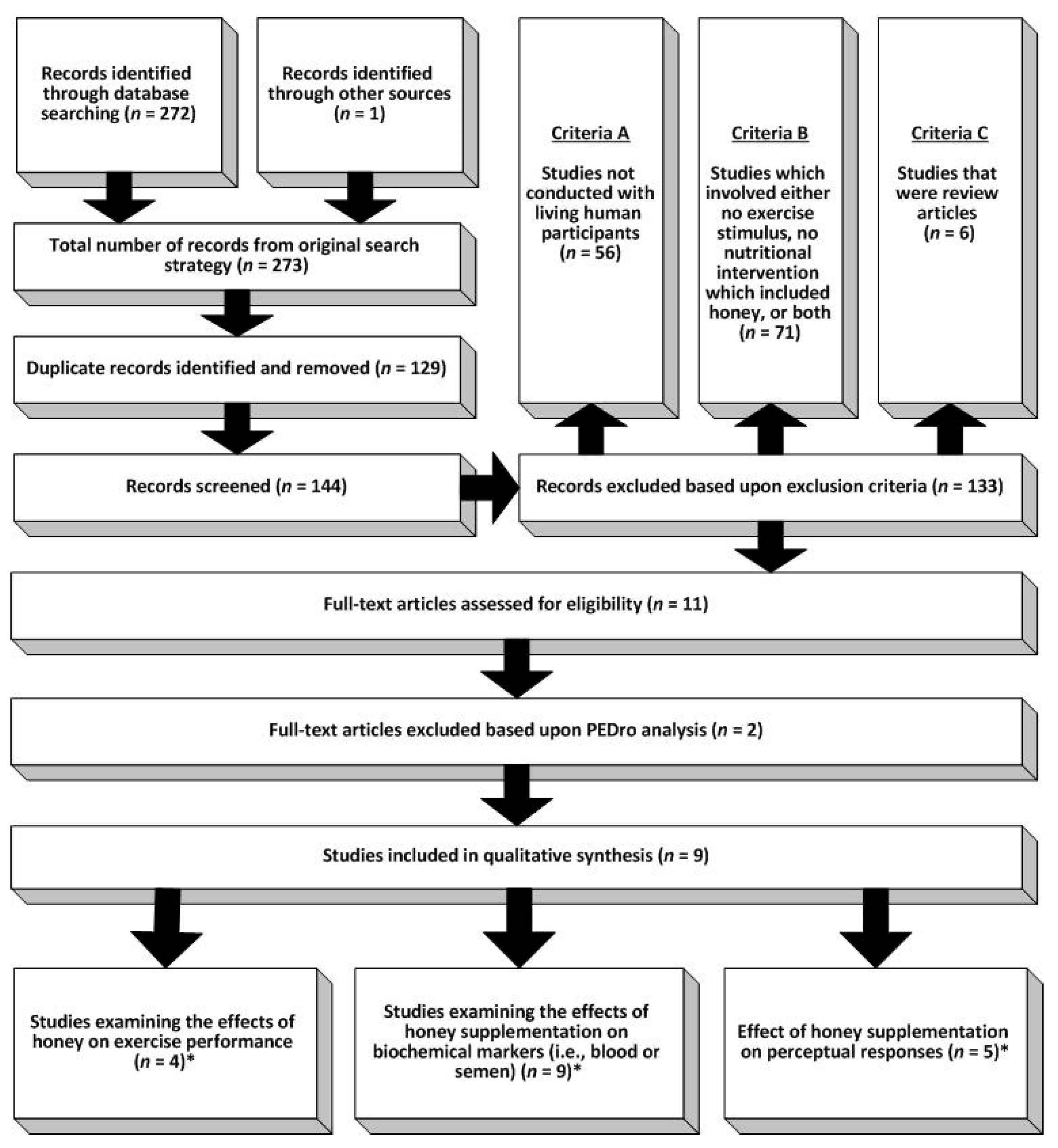
2. Materials and Methods
2.1. Study Selection
2.2. Quality Assessment
3. Results
3.1. Effect of Honey Supplementation on Biochemical Markers (i.e., Blood or Semen)
3.1.1. Acute Honey Consumption around a Single Exercise Session
3.1.2. Honey Supplementation over Multiple Weeks
3.2. Effect of Honey Supplementation on Physical or Skilled Performance
3.3. Effect of Honey Supplementation on Perceptual Responses
4. Discussion
4.1. Effect of Honey Supplementation on Biochemical Markers (i.e., Blood or Semen)
4.1.1. Acute Honey Consumption around a Single Exercise Session
4.1.2. Honey Supplementation over Multiple Weeks
4.2. Effect of Honey Supplementation on Physical or Skilled Performance
4.3. Effect of Honey Supplementation on Perceptual Responses
5. Conclusions and Future Research Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Council, E. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Commun. L 2002, 10, 47–52. [Google Scholar]
- Schneider, M.; Coyle, S.; Warnock, M.; Gow, I.; Fyfe, L. Anti-microbial activity and composition of manuka and portobello honey. Phytother. Res. 2013, 27, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121. [Google Scholar]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Conti, M.E.; Stripeikis, J.; Campanella, L.; Cucina, D.; Tudino, M.B. Characterization of Italian honeys (Marche Region) on the basis of their mineral content and some typical quality parameters. Chem. Cent. J. 2007, 1, 14. [Google Scholar] [CrossRef]
- Batista, B.; Da Silva, L.; Rocha, B.; Rodrigues, J.; Berretta-Silva, A.; Bonates, T.; Gomes, V.; Barbosa, R.; Barbosa, F. Multi-element determination in Brazilian honey samples by inductively coupled plasma mass spectrometry and estimation of geographic origin with data mining techniques. Food Res. Int. 2012, 49, 209–215. [Google Scholar] [CrossRef]
- Deibert, P.; König, D.; Kloock, B.; Groenefeld, M.; Berg, A. Glycaemic and insulinaemic properties of some German honey varieties. Eur. J. Clin. Nutr. 2010, 64, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.D.; Ismail, A. Two varieties of honey that are available in Malaysia gave intermediate glycemic index values when tested among healthy individuals. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2009, 153, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New strategies in sport nutrition to increase exercise performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, E.F.; Coggan, A.R.; Hemmert, M.; Ivy, J.L. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 1986, 61, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.; Hawley, J.A. Postexercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2016, 122, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Schabort, E.J.; Noakes, T.D.; Dennis, S.C. Carbohydrate-loading and exercise performance. Sports Med. 1997, 24, 73–81. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate feeding during exercise. Eur. J. Sports Sci. 2008, 8, 77–86. [Google Scholar] [CrossRef]
- Nicholas, C.W.; Williams, C.; Lakomy, H.K.; Phillips, G.; Nowitz, A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J. Sports Sci. 1995, 13, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.D.; Stevenson, E.J.; Rollo, I.; Russell, M. The influence of a 12% carbohydrate-electrolyte beverage on self-paced soccer-specific exercise performance. J. Sci. Med. Sport 2017, 20, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.M.; Maughan, R.J. The Governor has a sweet tooth–mouth sensing of nutrients to enhance sports performance. Eur. J. Sports Sci. 2015, 15, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; Di, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sport Exerc. 2009, 41, 709–731. [Google Scholar]
- Currell, K.; Jeukendrup, A. Superior endurance performance with ingestion of multiple transportable carbohydrates. Med. Sci. Sport Exerc. 2008, 40, 275. [Google Scholar] [CrossRef] [PubMed]
- Triplett, D.; Doyle, J.A.; Rupp, J.C.; Benardot, D. An isocaloric glucose-fructose beverage’s effect on simulated 100-km cycling performance compared with a glucose-only beverage. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, M.; Penas-Ruiz, C.; Terry, C.; Russell, M. Effects of carbohydrate-hydration strategies on glucose metabolism, sprint performance and hydration during a soccer match simulation in recreational players. J. Sci. Med. Sport 2014, 17, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.; Russell, M. Carbohydrates for soccer: A focus on skilled actions and half-time practices. Nutrients 2017, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Benton, D.; Kingsley, M. Influence of carbohydrate supplementation on skill performance during a soccer match simulation. J. Sci. Med. Sport 2012, 15, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.J.; Watson, A.; Theis, S.; Holz, A.; Harper, L.D.; Russell, M. A comparison of isomaltulose versus maltodextrin ingestion during soccer-specific exercise. Eur. J. Appl. Physiol. 2017, 117, 2321–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goete, L.; et al. Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2016, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 1990, 30, 316–328. [Google Scholar] [PubMed]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar] [PubMed]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Henson, D.A.; Utter, A.; Davis, J.M.; Williams, F.; Butterworth, D.E. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med. Sci. Sport Exerc. 1998, 30, 671–678. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Karatza, M.-H.; Katsaounou, P.; Kollintza, A.; Zakynthinos, S.; Roussos, C. Antioxidants attenuate the plasma cytokine response to exercise in humans. J. Appl. Physiol. 2003, 94, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [PubMed]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Russell, M.; Kingsley, M. The efficacy of acute nutritional interventions on soccer skill performance. Sports Med. 2014, 44, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Łagowska, K.; Podgórski, T.; Celińska, E.; Wiertel, Ł.; Kryściak, J. A comparison of the effectiveness of commercial and natural carbohydrate–electrolyte drinks. Sci. Sports 2017, 32, 160–164. [Google Scholar] [CrossRef]
- Earnest, C.P.; Lancaster, S.L.; Rasmussen, C.J.; Kerksick, C.M.; Lucia, A.; Greenwood, M.C.; Almada, A.L.; Cowan, P.A.; Kreider, R.B. Low vs. high glycemic index carbohydrate gel ingestion during simulated 64-km cycling time trial performance. J. Strength Cond. Res. 2004, 18, 466–472. [Google Scholar] [PubMed]
- Abbey, E.L.; Rankin, J.W. Effect of ingesting a honey-sweetened beverage on soccer performance and exercise-induced cytokine response. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 659–672. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Foong Kiew, O.; Ismail, M.S.; Mohamed, M. Effects of post-exercise honey drink ingestion on blood glucose and subsequent running performance in the heat. Asian J. Sports Med. 2015, 6, e24044. [Google Scholar] [CrossRef]
- Kreider, R.B.; Earnest, C.P.; Lundberg, J.; Rasmussen, C.; Greenwood, M.; Cowan, P.; Almada, A.L. Effects of ingesting protein with various forms of carbohydrate following resistance-exercise on substrate availability and markers of anabolism, catabolism, and immunity. J. Int. Soc. Sports Nutr. 2007, 4, 18. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycling training. J. Androl. 2012, 33, 449–461. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B.; Mooren, F.C.; Kruger, K.; FitzGerald, L.Z.; Chehrazi, M. A randomized controlled trial examining the effects of 16 weeks of moderate-to-intensive cycling and honey supplementation on lymphocyte oxidative DNA damage and cytokine changes in male road cyclists. Cytokine 2016, 88, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gmünder, F.K.; Joller, P.W.; Joller-Jemelka, H.I.; Bechler, B.; Cogoli, M.; Ziegler, W.H.; Müller, J.; Aeppli, R.E.; Cogoli, A. Effect of a herbal yeast food supplement and long-distance running on immunological parameters. Br. J. Sport Med. 1990, 24, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ooi, F.K. Effects of combined aerobic dance exercise and honey supplementation on bone turnover markers in young females. Asian J. Exerc. Sports Sci. 2011, 8, 53–63. [Google Scholar]
- Schönfeld, P.; Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy?-Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.J.; Nagy, R.J.; O’Connor, A.M.; Kempers, S.F.; Yeo, R.A.; Qualls, C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc. Natl. Acad. Sci. USA 1994, 91, 9352–9356. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, S.; Maughan, R.; Shirreffs, S.; Ozgünen, K.; Kurdak, S.; Ersöz, G.; Binnet, M.; Dvorak, J. The effects of exercise, heat, cooling and rehydration strategies on cognitive function in football players. Scand. J. Med. Sci. Sports 2010, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Kiens, B.; Ivy, J.L. Carbohydrates and fat for training and recovery. J. Sports Sci. 2004, 22, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Östman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef]
- Bishop, N.C.; Gleeson, M.; Nicholas, C.W.; Ali, A. Influence of carbohydrate supplementation on plasma cytokine and neutrophil degranulation responses to high intensity intermittent exercise. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 145–156. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Othman, N.H.; Soelaiman, I.-N.; Shuid, A.N.; Mohamad, N.; Muhamad, N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics 2012, 67, 779–784. [Google Scholar] [CrossRef]
- Smith, J.W.; Zachwieja, J.J.; Péronnet, F.; Passe, D.H.; Massicotte, D.; Lavoie, C.; Pascoe, D.D. Fuel selection and cycling endurance performance with ingestion of [13C] glucose: Evidence for a carbohydrate dose response. J. Appl. Physiol. 2010, 108, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Pascoe, D.D.; Passe, D.H.; Ruby, B.C.; Stewart, L.K.; Baker, L.B.; Zachwieja, J.J. Curvilinear dose-response relationship of carbohydrate (0–120 gh −1) and performance. Med. Sci. Sports Exerc. 2013, 45, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Moseley, L.; Mainwaring, G.I.; Samuels, S.; Perry, S.; Mann, C.H. Exogenous carbohydrate oxidation during ultraendurance exercise. J. Appl. Physiol. 2006, 100, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Passe, D.; Horn, M.; Murray, R. Impact of beverage acceptability on fluid intake during exercise. Appetite 2000, 35, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Carstens, M.; Millen, A.M. Ingesting isomaltulose versus fructose-maltodextrin during prolonged moderate-heavy exercise increases fat oxidation but impairs gastrointestinal comfort and cycling performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 427–438. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants | Design | Exercise Stimulus | Nutritional Intervention | Data Collection Method and Time-Points | Outcome Variables | Main Results |
|---|---|---|---|---|---|---|---|
| Abbey and Rankin [47] | Male soccer players of NCAA Division I, post collegiate, and club standard (n = 10). | Randomised, single blind, crossover. | Soccer specific exercise (5 × 15 min blocks, plus 10 min half-time), followed by progressive 20 m shuttle run to fatigue. | 6% CHO-electrolyte solution (honey, commercial sports drink, or placebo). 8.8 mL∙kg−1 (0.5 g∙kg∙body mass−1) CHO consumed 30 min pre-exercise and at half-time. | Blood samples at 30 min pre-exercise (T1), immediately post-exercise (T2), 60 min post-exercise (T3). | Blood glucose, insulin, cortisol, plasma volume, IL-1ra, IL-6 and IL-10, total ORAC, and plasma ORAC. | Plasma IL-1ra was ↓ at T2 for honey vs. sports drink, and ↓ at T3 for honey vs. placebo. ↔ between trials for glucose, insulin, cortisol, plasma volume, IL-6, IL-10, total or plasma ORAC. |
| Ahmad et al. [48] | Male runners of recreational standard (n = 10). | Randomised, single blind, crossover. | 60 min run at ~65% V̇O2 max, followed by 2 h ‘rehydration phase’ and 20 min treadmill running test. | 6.8% CHO solution (honey) or water to recover 150% of body mass lost during run one. Fluid consumed 0 min (60% of mass loss), 30 min (50%), and 60 min (40%) after run one. | Blood samples at pre (T1), immediately post (T2), 30 min post (T3), 60 min post (T4), 90 min post (T5), and 120 min post (T6) 60 min run, and immediately post 20 min run (T7). | Blood glucose, serum insulin, haematocrit, and serum osmolality. | Serum insulin was ↑ at T3-T6 for honey vs. water. Serum osmolality was ↑ at T4 for honey vs. water. ↔ between trials for glucose or haematocrit. |
| Earnest et al. [46] | Amateur male cyclists (n = 9). | Randomised, double blind, counterbalanced, crossover. | 64 km time trial on cycling ergometer. | 15 g of gel (honey, dextrose, or placebo) with 250 mL H2O consumed every 16 km (5 × 15 g total) plus an additional 250 mL of water every 3.2 km. | Blood samples at pre-exercise (T1), 16 km (T2), 32 km (T3), 48 km (T4), and 64 km (T5). | Blood glucose and insulin concentrations. | ↔ between trials for glucose or insulin. In dextrose, glucose at T4 was ↓ vs. T1 (not the case for honey or placebo). |
| Kreider et al. [49] | Resistance trained individuals (n = 40; males: n = 19, females: n = 21). | Randomised, four independent groups. | 3 sets of 10 repetitions at approximately 70% of 1RM on chest press, seated row, shoulder press, lat pulldown, leg extension, leg curl, biceps curl, triceps extension, and leg press. | 40 g of whey protein with 120 g of either sucrose, powdered honey, or maltodextrin consumed within 5 min post-exercise. Other group consumed no supplement. | Blood samples at pre-exercise (T1), post exercise (T2), and 30 min (T3), 60 min (T4), 90 min (T5), and 120 min (T6) post-feeding. | Glucose, insulin, testosterone, cortisol, testosterone: cortisol ratio, WBC, neutrophils, total neutrophils: total lymphocytes ratio, creatinine, BUN, BUN: creatinine ratio, CK, LDH, AST, and ALT. | Glucose at T4 was ↑ for honey vs sucrose, and at T3 was ↑ for honey vs. sucrose, maltodextrin, and no CHO. Insulin at T3-T6 was ↑ for honey, sucrose, and maltodextrin vs. no CHO. ↔ between groups for testosterone, cortisol, testosterone:cortisol, WBC, neutrophils, total neutrophils, total lymphocytes, LDH, AST, or ALT. BUN: creatinine at T5, was ↑ for honey and maltodextrin, and at T6 was ↑ for honey and sucrose vs. no CHO. |
| Łagowska et al. [45] | Trained male rowers (n = 11). | Randomised, crossover. | Rowing ergometer: 2 × 40 min with a 5 min break, performed at an intensity corresponding to ~75% of the onset of blood lactate accumulation. | 150 mL of CHO solution (either commercial sports drink; 7.8% CHO, or ‘natural’ drink containing banana, fruit juice, and honey; 6.7% CHO), consumed immediately pre-exercise and every 15 min during exercise (6 × 150 mL total). | Blood samples at pre-exercise (T1), and 3 min post-exercise (T2). | Blood glucose, lactate, chemical antioxidants, urea, CK, haematocrit, leukocytes, WBC, lymphocytes, monocytes, and granulocytes. | Glucose was ↓ at T2, for natural vs. commercial drink. Glucose at T2, was ↓ for natural, but ↑ for commercial drink vs. T1. Chemical antioxidant level at T2, was ↓ for natural vs. commercial drink. ↔ between trials for lactate, urea, CK, haematocrit, leukocytes, WBC, lymphocytes, monocytes, or granulocytes. |
| Study | Participants | Design | Exercise Stimulus | Nutritional Intervention | Data Collection Method and Time-Points | Outcome Variables | Main Results |
|---|---|---|---|---|---|---|---|
| Gmunder et al. [52] | Long distance runners (n = 16: male: n = 13, female: n = 3). Standard not specified. | Randomised, double blind, two independent groups. | 27 day training period, followed by 21 km running time trial. | 3 x 10 mL per day (30 mL per day for 31 days) of either a supplement comprised of herbal yeast, malt, honey, and orange juice, or a supplement comprised of sucrose and caramel. | Blood samples at day 0 (T1), pre 21 km run at day 27 (T2), immediately post run (T3), two days post run (T4). | WBC, leukocyte counts, lymphocyte counts, Con A, CD3, CD4, CD16, CD16/8, CD19, IgA, IgE, IgM, IgG subclasses 1-4, β2M, IL-2 receptors, neopterin, plasma proteins, cortisol, adrenaline, noradrenaline. | ↔ between groups for any blood marker. In honey, natural killer/suppressor cells (CD8, CD16, and CD16/8), and IgG subclass 1 at T4 were ↓, whilst Con A, and IgG subclass 2 at T4 were ↑ vs. T3 (not the case for sucrose). In sucrose, neopterin and β2M at T4 were ↓ vs. T3 (not the case for honey). |
| Hajizadeh et al. [51] | Amateur male cyclists (n = 24). | Randomised, two independent groups. | 16 week training period. | 70 g honey dissolved in 250 mL distilled water. Consumed 90 min prior to each training session for 16 weeks. Other group consumed no supplement. | Blood samples at week 0 (T1), immediately (T2), 12 h (T3), and 24 h (T4) after the last training session in week 8, and immediately (T5), 12 h (T6), 24 h (T7), 7 days (T8), and 30 days (T9) after the last training session in week 16. | Lymphocyte counts, DNA damage, IL-1β, IL-6, IL-8, IL-10, TNF-a, glutathione, TAS, hydrogen peroxide, and lipid peroxide. | Hydrogen peroxide, and lipid peroxide, IL-1β, IL-6, IL-10, and TNF-a at T2-T8, were ↓ for honey vs. no supplement. Glutathione at T4-T7 was ↑ for honey vs. no supplement. T2-T7 was ↑ for honey vs. no supplement. |
| Ooi et al. [53] | Females (n = 37). | Four (matched) independent groups. | Two groups: 3 aerobic dance sessions per week for 6 weeks. Other two groups: no exercise. | Two groups: 20 g honey with 300 mL water consumed daily for 6 weeks. Other two groups: no supplementation. | Blood samples at week 0 (T1) and after week 6 (T2). | ALP and 1CTP. | ALP at T2 for honey, and honey plus exercise was ↑ vs. T1 (not the case with no honey). ↔ between groups for ALP or 1CTP. ↔ between T1 and T2 for 1CTP. |
| Tartiban and Maleki [50] | Male amateur road cyclists (n = 39). | Randomised, double blind, two independent groups. | 8 weeks intensified training period. | 70 g of honey or 70 g CHO-free sweetener, with 250 mL water. Consumed 90 min prior to each training session for 8 weeks. | Semen samples at week 0 (T1), immediately (T2), 12 h (T3), and 24 h (T4) after the last training session in week 4, and immediately (T5), 12 h (T6), and 24 h (T7) after the last training session in week 8. | Semen volume, motility, morphology, concentration, and number of spermatozoa, plus IL-1β, IL-6, IL-8, TNF-α, ROS, malondialdehyde, and antioxidants superoxide dismutase, catalase, and TAC. | Semen volume at T6 was ↑ for honey vs. placebo. Semen motility, morphology and TAC at T6-T7, semen concentration at T4-T5, number of spermatozoa at T2-T3 and T5-T7, catalase at T4 and T5-T7, and superoxide dismutase at T2-T3 and T5-T7 were all ↑ for honey vs. placebo. IL-1β at T2 and T5-T7, IL-6 at It2-T7, IL-8 at T2-T3, TNF-α at T4, ROS at T4-T7, and malondialdehyde at T5-T7 were all ↓ for honey vs. placebo. |
| Study | Participants | Design | Exercise Stimulus | Nutritional Intervention | Data Collection Method and Time-Points | Outcome Variables | Main results |
|---|---|---|---|---|---|---|---|
| Abbey and Rankin [47] | Male soccer players of NCAA Division I, post collegiate, and club standard (n = 10). | Randomised, single blind, crossover. | Soccer specific exercise (5 × 15 min blocks, plus 10 min half-time), followed by progressive 20 m shuttle run to fatigue. | 6% CHO-electrolyte solution (either honey, commercial sports drink, or placebo). 8.8 mL∙kg−1 (0.5 g∙kg∙body mass−1) CHO consumed 30 min pre-exercise and at half-time. | 220 m running time trial, dribbling/agility assessment, and soccer shooting test all performed every 15 min during exercise: 220 m running time trial Progressive 20 m shuttle run to fatigue performed following 75 min of exercise. | Time taken to complete (time trial, and dribbling/ agility test), number of targets hit (shooting assessment). Time to exhaustion. | ↔ between trials for any performance measure. |
| Ahmad et al. [48] | Male runners of recreational standard (n = 10). | Randomised, single blind, crossover. | 60 min run at ~65% V̇O2 max, followed by 2 h ‘rehydration phase’ and 20 min treadmill running test. | Either 6.8% CHO solution (honey) or water, to recover 150% of body mass lost during run one consumed at 0 min (60% of mass loss), 30 min (50%), and 60 min (40%) after run one. | 20 min treadmill running test performed 120 min following completion of 60 min run | Total distance covered. | 20 min running performance was ↑ for honey vs. water. |
| Earnest et al. [46] | Amateur male cyclists (n = 9). | Randomised, double blind, counterbalanced, crossover. | 64 km time trial on cycling ergometer. | 15 g of gel (honey, dextrose, or placebo) with 250 mL water consumed every 16 km (5 × 15 g total). Plus an additional 250 mL of water every 3.2 km. | 64 km cycling ergometer test. | Time taken to complete 64 km, and per 16 km. Mean power over 64 km, and per 16 km. | ↔ between trials for time taken to complete, or mean power over 64 km. In honey and dextrose trials, mean power over 48-64 km was ↑ vs. 0-16 km, 16-32 km, and 32-48 km (not the case for placebo). In placebo, time taken for 48-64 km and 32-48 km was ↑ vs. 0-16 km, (not the case for honey or dextrose). |
| Hajizadeh et al. [51] | Amateur male road cyclists (n = 24). | Randomised, two independent groups. | 16 week training period. | 70 g honey dissolved in 250 mL distilled water. Consumed 90 min prior to each training session for 16 weeks. Other group consumed no supplement. | 5 km, and 40 km cycling ergometer tests, and power assessment at week 0 (T1) and week 16 (T2). | Time taken to complete (5 km and 40 km tests), peak power output (power assessment). | ↔ between groups for time taken to complete 5 km or 40 km, or peak power output at T2. For all measures, performance in both groups was ↑ at T2 vs. T1 |
| Study | Participants | Design | Exercise Stimulus | Nutritional Intervention | Data collection Method and Time-Points | Outcome Variables | Main Results |
|---|---|---|---|---|---|---|---|
| Abbey and Rankin [47] | Male soccer players of NCAA Division I, post-collegiate, and club standard (n = 10). | Randomised, single blind crossover. | Soccer-specific exercise (5 × 15 min blocks, plus 10 min half-time), followed by progressive 20 m shuttle run to fatigue. | 6% CHO-electrolyte solution (honey, commercial sports drink, or placebo). 8.8 mL∙kg−1 (0.5 g∙kg∙body mass−1) CHO consumed 30 min pre-exercise and at half-time. | Every 15 min during exercise, scale not reported. | RPE. | ↔ between trials for RPE. |
| Ahmad et al. [48] | Male runners of recreational standard (n = 10). | Randomised, single blind crossover. | 60 min run at ~65% V̇O2 max, followed by 2 h ‘rehydration phase’ and 20 min treadmill running test on. | 6.8% CHO solution (honey) or H2O to recover 150% of body mass lost during run one. Fluid consumed 0 min (60% of mass loss), 30 min (50%), and 60 min (40%) after run one. | Likert scale (1–5), 0 min (T1), 30 min (T2), and 60 min (T3) after run one. | Perceptions of thirst, sweetness, nausea, fullness and stomach upset. | Perceptions of sweetness at T1-T3 were ↑ for honey vs. for honey vs. water. ↔ between trials for perceptions of thirst nausea, fullness, or stomach upset between trials. |
| Earnest et al. [46] | Amateur male cyclists (n = 9). | Randomised, double blind, counterbalanced, crossover. | 64 km time trial on cycling ergometer. | 15 g of gel (honey, dextrose, or placebo) with 250 mL water consumed every 16 km. Plus an additional 250 mL of water every 3.2 km. | Likert scale (6–20) at pre-exercise, 16 km, 32 km, 48 km, and 64 km. | RPE. | ↔ between trials for RPE. |
| Kreider et al. [49] | Resistance trained individuals (n = 40; males: n = 19, females: n = 21). | Randomised, four independent groups. | 3 sets of 10 repetitions at approximately 70% of 1 RM on chest press, seated row, shoulder press, lat pulldown, leg extension, leg curl, biceps curl, triceps extension, and leg press. | 40 g of whey protein with 120 g of either sucrose, powdered honey, or maltodextrin. Consumed within 5 min post-exercise. Other group consumed no supplement. | Likert scale (1–10) at pre-exercise (T1), post exercise (T2), and 30 min (T3), 60 min (t4), 90 min (T5), and 120 min (T6) post-feeding. | Severity of perceived hypoglycaemia, dizziness, fatigue, headache, and stomach upset. | ↔ between groups for perceptions of hypoglycaemia, dizziness, fatigue, headache, or stomach upset. |
| Łagowska et al. [45] | Trained male rowers (n = 11). | Randomised, crossover. | Rowing ergometer: 2 × 40 min with a 5 min break, performed at an intensity corresponding to ~75% of the onset of blood lactate accumulation. | 150 mL of CHO solution (either commercial sports drink; 7.8% CHO, or ‘natural’ drink containing banana, fruit juice, and honey; 6.7% CHO), consumed immediately pre-exercise and every 15 min during exercise (6 × 150 mL total). | Likert scale (1–5) at post-exercise: | Perceptions of taste, smell, thirst quenching ability, beverage consistency, and refreshment. | Satisfaction with beverage consistency was ↓ for natural vs. commercial drink. ↔ between trials for perceptions of taste, smell, thirst quenching ability, or refreshment. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hills, S.P.; Mitchell, P.; Wells, C.; Russell, M. Honey Supplementation and Exercise: A Systematic Review. Nutrients 2019, 11, 1586. https://doi.org/10.3390/nu11071586
Hills SP, Mitchell P, Wells C, Russell M. Honey Supplementation and Exercise: A Systematic Review. Nutrients. 2019; 11(7):1586. https://doi.org/10.3390/nu11071586
Chicago/Turabian StyleHills, Samuel P., Peter Mitchell, Christine Wells, and Mark Russell. 2019. "Honey Supplementation and Exercise: A Systematic Review" Nutrients 11, no. 7: 1586. https://doi.org/10.3390/nu11071586
APA StyleHills, S. P., Mitchell, P., Wells, C., & Russell, M. (2019). Honey Supplementation and Exercise: A Systematic Review. Nutrients, 11(7), 1586. https://doi.org/10.3390/nu11071586




