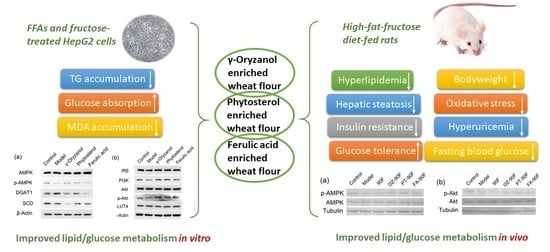
Wheat Flour, Enriched with ?-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. Western Blot Analysis of Protein from Cells
2.4. Animals and Diet
2.5. Measurement of Serum Biochemical Parameters
2.6. Oral Glucose (OGTT) and Insulin (ITT) Tolerance Tests
2.7. Measurement of Hepatic Lipid Accumulation
2.8. Western Blot Analysis of Protein From Liver Tissue
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
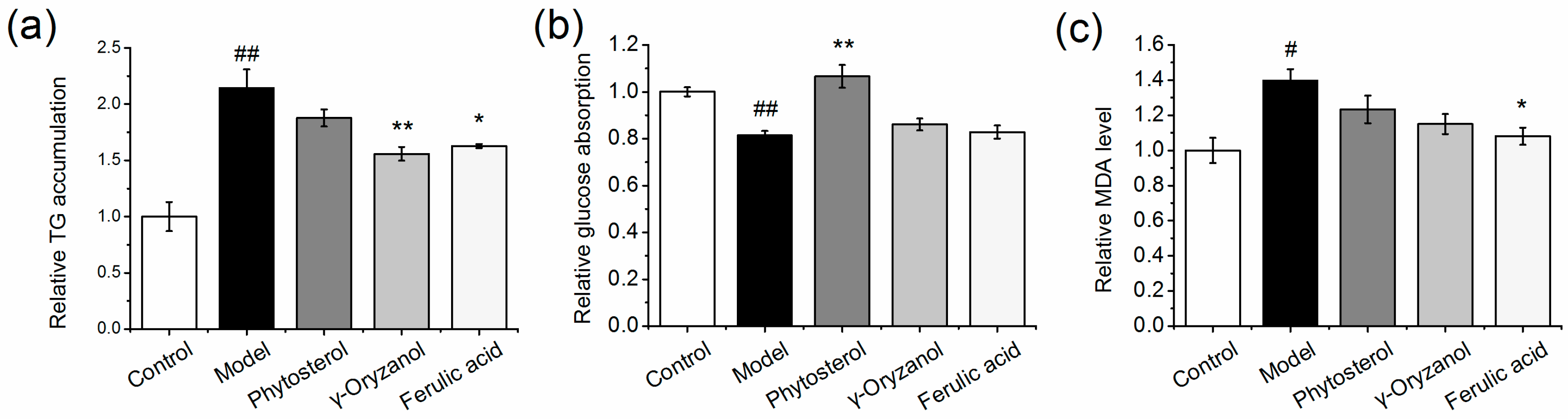
3.1. Effect of Functional Compounds on TG, MDA Levels, and Glucose Absorption in HepG2 Cells
3.2. Expression of Lipid/Glucose Metabolism-Related Proteins in HepG2 Cells
3.3. Effect of Enriched Flour on Body Weight, Food Intake and Organ Indices of Rats
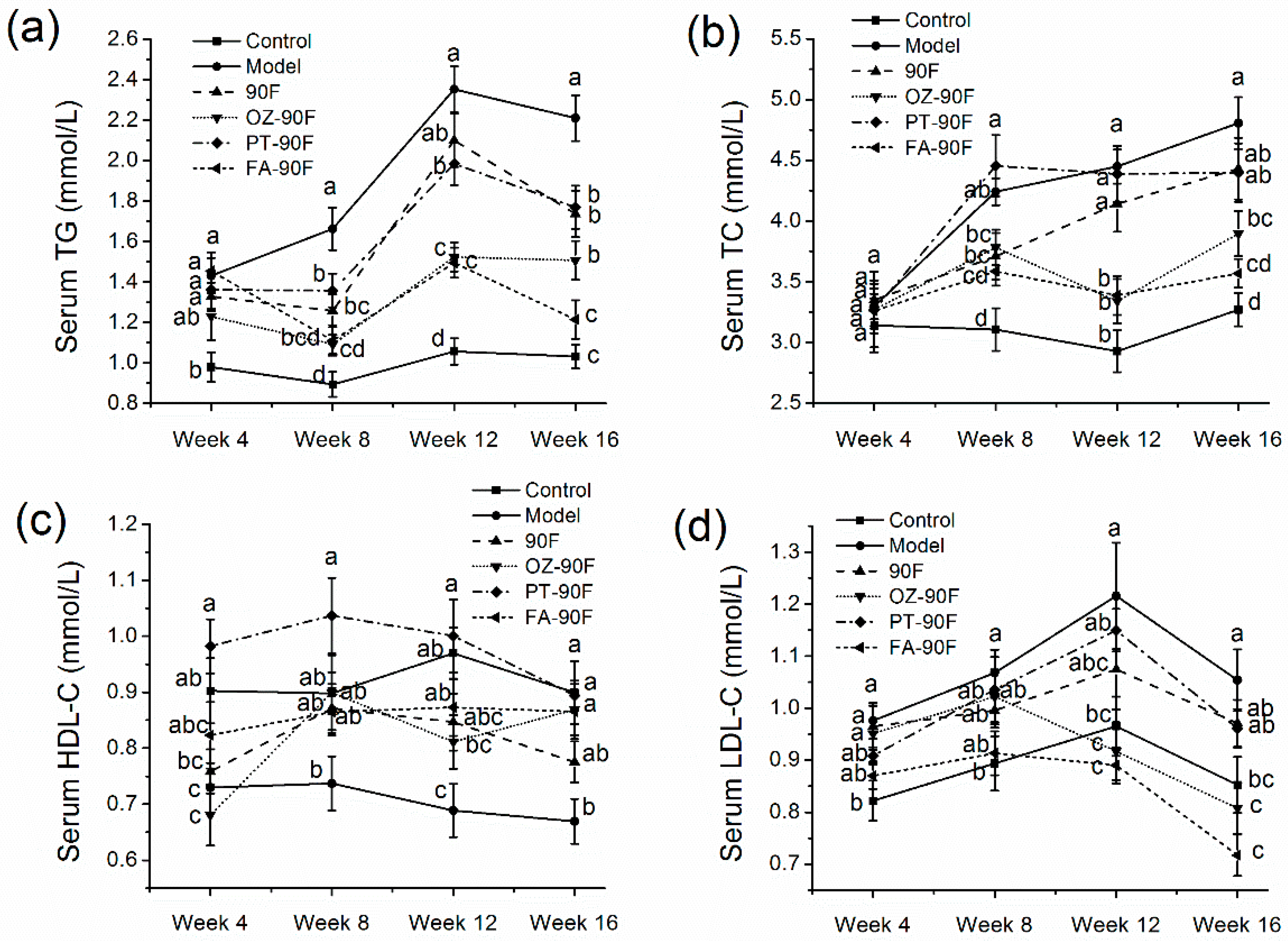
3.4. Effect of Enriched Flour on Blood Lipid Profiles of Rats
3.5. Effect of Enriched Flour on Glucose Metabolism and Insulin Resistance in Rats
3.6. Effect of Enriched Flour on Hepatic Steatosis, MDA Level and Hepatic Function of Rats
3.7. Effect of Enriched Flour on Oxidative Stress and Hyperuricemia in Rats
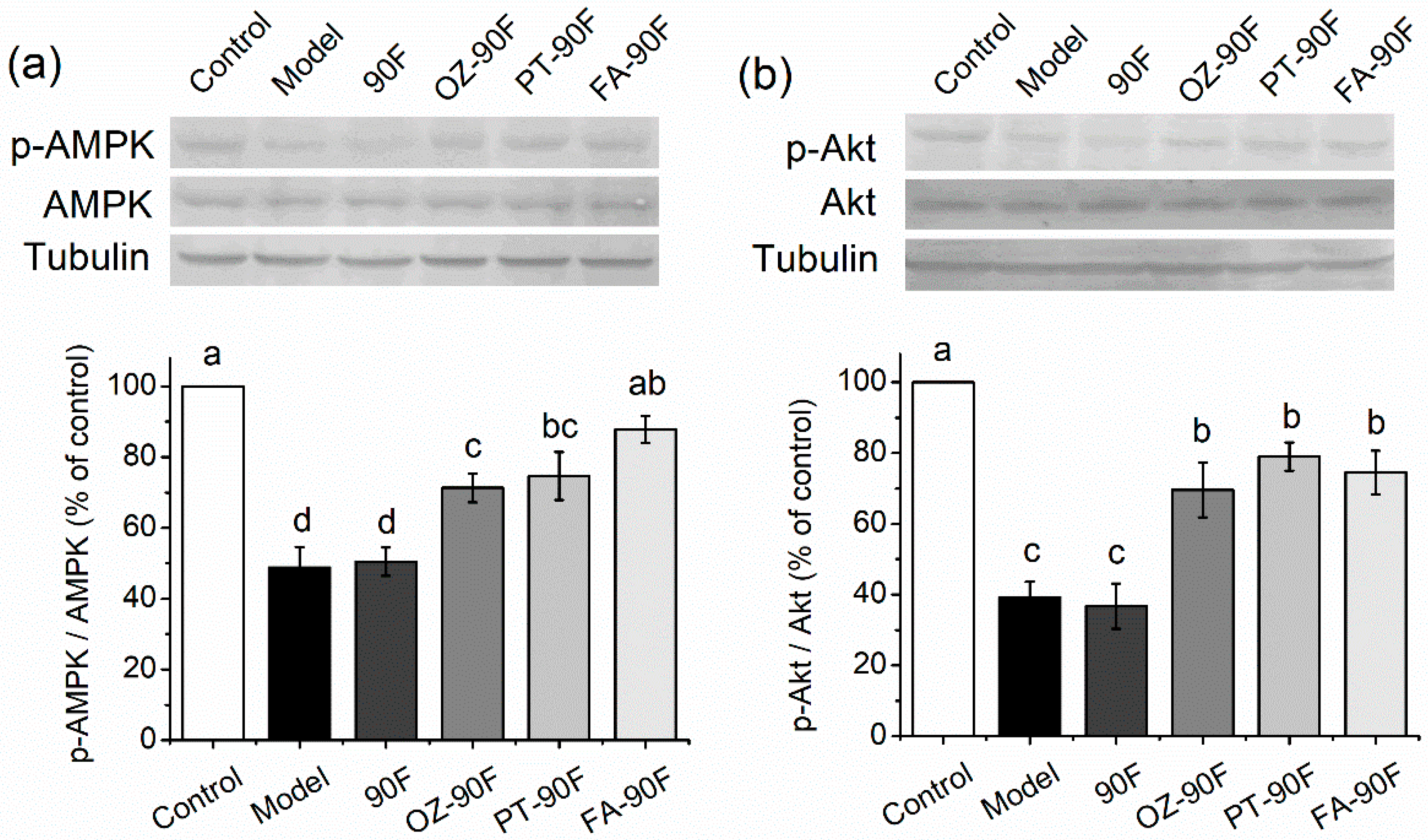
3.8. Expression of Lipid/Glucose Metabolism-Related Proteins in Liver Tissue
3.9. Histological Examination
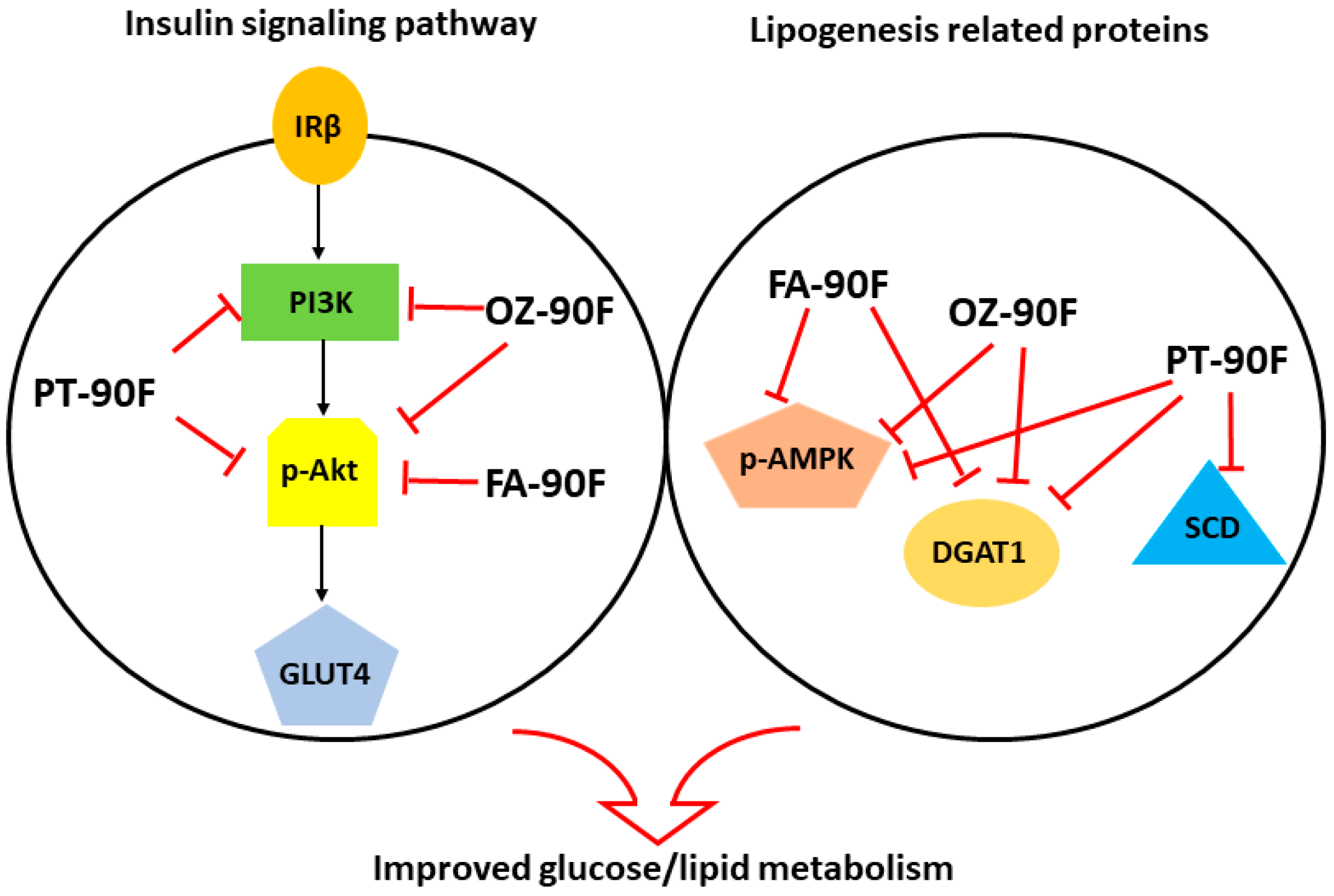
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Formiguera, X.; Canton, A. Obesity: Epidemiology and clinical aspects. Best Prat. Res. Clin. Gastroenterol. 2004, 18, 1125–1146. [Google Scholar] [CrossRef]
- Hill, J.O.; Catenacci, V.; Wyatt, H.R. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Kumar, A. Treatment of overweight and obesity: Lifestyle, pharmacologic, and surgical options. Clin. Cornerstone 2009, 9, 55–71. [Google Scholar] [CrossRef]
- Wyatt, H.R. Update on Treatment Strategies for Obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Radhika, G.; Van Dam, R.M.; Sudha, V.; Ganesan, A.; Mohan, V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 2009, 58, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Clemente, G.; Cipriano, D.; Luongo, D.; Viscovo, D.; Patti, L.; Di Marino, L.; Giacco, A.; Naviglio, D.; Bianchi, M.; et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 186–194. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, W. Phytosterols in cereal by-products. J. Am. Oil Chem. Soc. 2005, 82, 439–444. [Google Scholar] [CrossRef]
- Expert Panel on Detection, E. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Influence of oryzanol and ferulic Acid on the lipid metabolism and antioxidative status in high fat-fed mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef] [PubMed]
- Dills, W.L. Protein fructosylation: Fructose and the Maillard reaction. Am. J. Clin. Nutr. 1993, 58, 779S–787S. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome1–3. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Grudziak, J.; Townsend, R.R.; Dunn, T.N.; Grant, R.W.; Adams, S.H.; Keim, N.L.; Cummings, B.P.; Stanhope, K.L.; Havel, P.J. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J. Clin. Endocrinol. Metab. 2009, 94, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Sakurai, S.; Noumi, K.; Sugimoto, I.; Awata, N. Metabolism of gamma-oryzanol in rabbit (author’s transl). Yakugaku Zasshi 1980, 100, 1011–1018. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Wang, O.; Zhang, H.; Zhou, F.; Liu, J.; Ji, B. Fructose and glucose combined with free fatty acids induce metabolic disorders in HepG2 cell: A new model to study the impacts of high-fructose/sucrose and high-fat diets in vitro. Mol. Nutr. Food Res. 2016, 60, 909–921. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals, 8th ed.; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 495–509. [Google Scholar]
- Das, M.; Das, S.; Lekli, I.; Das, D.K. Caveolin induces cardioprotection through epigenetic regulation. J. Cell. Mol. Med. 2012, 16, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: Positive and negative regulation, and its role in whole-body energy homeostasis. Curr. Opin. Cell Biol. 2015, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2010, 2011, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremer, P.; Nagel, D.; Labrot, B.; Mann, H.; Muche, R.; Elster, H.; Seidel, D. Lipoprotein Lp(a) as predictor of myocardial infarction in comparison to fibrinogen, LDL cholesterol and other risk factors: Results from the prospective Göttingen Risk Incidence and Prevalence Study (GRIPS). Eur. J. Clin. Investig. 1994, 24, 444–453. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, High-fat Diet–induced Metabolic Syndrome and Cardiovascular Remodeling in Rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of Ferulic Acid and γ-Oryzanol on High-Fat and High-Fructose Diet-Induced Metabolic Syndrome in Rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef]
- Heinig, M.; Johnson, R.J. Role of uric acid in hypertension, renal disease, and metabolic syndrome. Clevel. Clin. J. Med. 2006, 73, 1059–1064. [Google Scholar] [CrossRef]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2010, 574, 41–53. [Google Scholar] [CrossRef]
- Song, C.Y.; Shi, J.; Zeng, X.; Zhang, Y.; Xie, W.F.; Chen, Y.X. Sophocarpine alleviates hepatocyte steatosis through activating AMPK signaling pathway. Toxicol. Vitr. 2013, 27, 1065–1071. [Google Scholar] [CrossRef]
- Chen, H.C.; Farese, R.V. Inhibition of Triglyceride Synthesis as a Treatment Strategy for Obesity Lessons from DGAT1-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 482–486. [Google Scholar] [CrossRef]
- Guillory, B.; Jawanmardi, N.; Iakova, P.; Anderson, B.; Zang, P.; Timchenko, N.A.; Garcia, J.M. Ghrelin deletion protects against age-associated hepatic steatosis by downregulating the C/EBPα-p300/DGAT1 pathway. Aging Cell 2018, 17, e12688. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Deng, J.; Guo, Y.; Niu, Y.; Yuan, F.; Yu, J.; Chen, S.; Guo, F. BTG1 ameliorates liver steatosis by decreasing stearoyl-CoA desaturase 1 (SCD1) abundance and altering hepatic lipid metabolism. Sci. Signal. 2016, 9, ra50. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, B. A Phytosterol-Enriched Spread Improves Lipid Profile and Insulin Resistance of Women with Gestational Diabetes Mellitus: A Randomized, Placebo-Controlled Double-Blind Clinical Trial. Diabetes Technol. Ther. 2016, 18, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The Molecular Basis for Oxidative Stress-Induced Insulin Resistance. Antioxid. Redox Signal. 2005, 7, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2010, 91, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Quílez, J. Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 2003, 22, 343–351. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Calpe-Berdiel, L.; Escola-Gil, J.C.; Rotllan, N.; Blanco-Vaca, F. Phytosterols do not change susceptibility to obesity, insulin resistance, and diabetes induced by a high-fat diet in mice. Metabolism 2008, 57, 1497–1501. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.-S.; Lee, M.-K.; Park, Y.B.; Choi, M.-S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Normal Diet | HFFD | |
|---|---|---|
| Corn powder (g/100 g) | 25.0 | |
| Wheat middling (g/100 g) | 20.0 | |
| Refined wheat flour (g/100 g) | 21.9 | 42.0 |
| Soybean meal (g/100 g) | 20.0 | 8.0 |
| Chicken powder (g/100 g) | 8.0 | |
| Fructose (g/100 g) | 18.0 | |
| Lard (g/100 g) | 20.0 | |
| Sodium cholate (g/100 g) | 0.2 | |
| Calcium bicarbonate (g/100 g) | 1.0 | 1.0 |
| Mountain flour (g/100 g) | 1.6 | 0.8 |
| Miscellaneous meal (g/100 g) | 4.5 | |
| Plant oil (g/100 g) | 2.0 | |
| Fish powder (g/100 g) | 2.0 | |
| Vitamin mix (g/100 g) | 2.0 | 2.0 |
| Energy (kJ/g) | 13.9 | 18.9 |
| Initial BW (g) | Final BW (g) | Food Intake (g/day) | Energy Intake (kJ/day) | Liver index (g/100 g BW) | Kidney Index (g/100 g BW) | Spleen Index (g/100 g BW) | Fat index (g/100 g BW) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Perirenal Fat | Epididymal Fat | Retroperitoneal Fat | ||||||||
| Control | 227.3 ± 5.3 a | 531.9 ± 15.8 c | 24.54 ± 0.18 a | 341.28 ± 2.54 b | 2.07 ± 0.04 b | 0.50 ± 0.01 a | 0.113 ± 0.004 a | 0.58 ± 0.03 c | 1.58 ± 0.09 d | 1.63 ± 0.09 d |
| Model | 226.1 ± 5.7 a | 631.0 ± 11.3 a | 20.98 ± 0.44 b | 396.36 ± 8.28 a | 2.41 ± 0.08 a | 0.44 ± 0.02 b | 0.107 ± 0.005 a | 1.12 ± 0.07 a | 3.10 ± 0.10 a | 3.60 ± 0.18 a |
| 90F | 228.4 ± 2.2 a | 607.9 ± 19.6 ab | 20.19 ± 0.55 b | 376.75 ± 10.20 a | 2.28 ± 0.10 ab | 0.47 ± 0.02 ab | 0.101 ± 0.005 a | 0.98 ± 0.06 a | 2.73 ± 0.19 ab | 3.36 ± 0.18 ab |
| OZ-90F | 228.6 ± 5.9 a | 580.0 ± 11.2 b | 20.74 ± 0.51 b | 386.12 ± 9.51 a | 2.22 ± 0.05 ab | 0.47 ± 0.02 ab | 0.110 ± 0.003 a | 0.97 ± 0.05 a | 2.59 ± 0.12 bc | 3.02 ± 0.19 bc |
| PT-90F | 227.9 ± 6.6 a | 606.5 ± 18.5 ab | 21.49 ± 0.25 b | 400.47 ± 4.70 a | 2.27 ± 0.06 ab | 0.45 ± 0.01 b | 0.111 ± 0.006 a | 1.07 ± 0.04 a | 2.95 ± 0.13 ab | 3.45 ± 0.11 ab |
| FA-90F | 229.3 ± 3.1 a | 572.4 ± 17.8 bc | 20.01 ± 0.12 b | 373.17 ± 2.30 a | 2.06 ± 0.08 b | 0.48 ± 0.01 ab | 0.100 ± 0.003 a | 0.76 ± 0.04 b | 2.26 ± 0.14 c | 2.70 ± 0.18 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.-X.; Zeng, Z.; Qian, Y.-Z.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.-P.; Zhou, F. Wheat Flour, Enriched with ?-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats. Nutrients 2019, 11, 1697. https://doi.org/10.3390/nu11071697
Guo X-X, Zeng Z, Qian Y-Z, Qiu J, Wang K, Wang Y, Ji B-P, Zhou F. Wheat Flour, Enriched with ?-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats. Nutrients. 2019; 11(7):1697. https://doi.org/10.3390/nu11071697
Chicago/Turabian StyleGuo, Xiao-Xuan, Zhu Zeng, Yong-Zhong Qian, Jing Qiu, Kai Wang, Yong Wang, Bao-Ping Ji, and Feng Zhou. 2019. "Wheat Flour, Enriched with ?-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats" Nutrients 11, no. 7: 1697. https://doi.org/10.3390/nu11071697
APA StyleGuo, X.-X., Zeng, Z., Qian, Y.-Z., Qiu, J., Wang, K., Wang, Y., Ji, B.-P., & Zhou, F. (2019). Wheat Flour, Enriched with ?-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats. Nutrients, 11(7), 1697. https://doi.org/10.3390/nu11071697