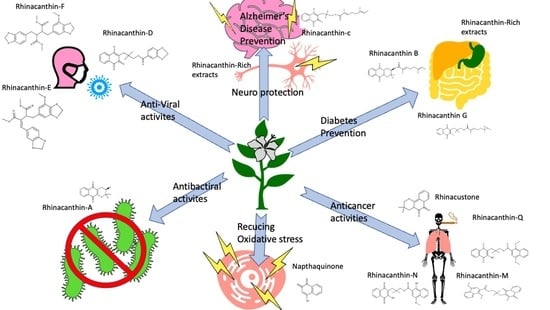
Rhinacanthus nasutus “Tea” Infusions and the Medicinal Benefits of the Constituent Phytochemicals
Abstract
1. Introduction
2. Phytochemicals Found in Rhinacanthus nasutus
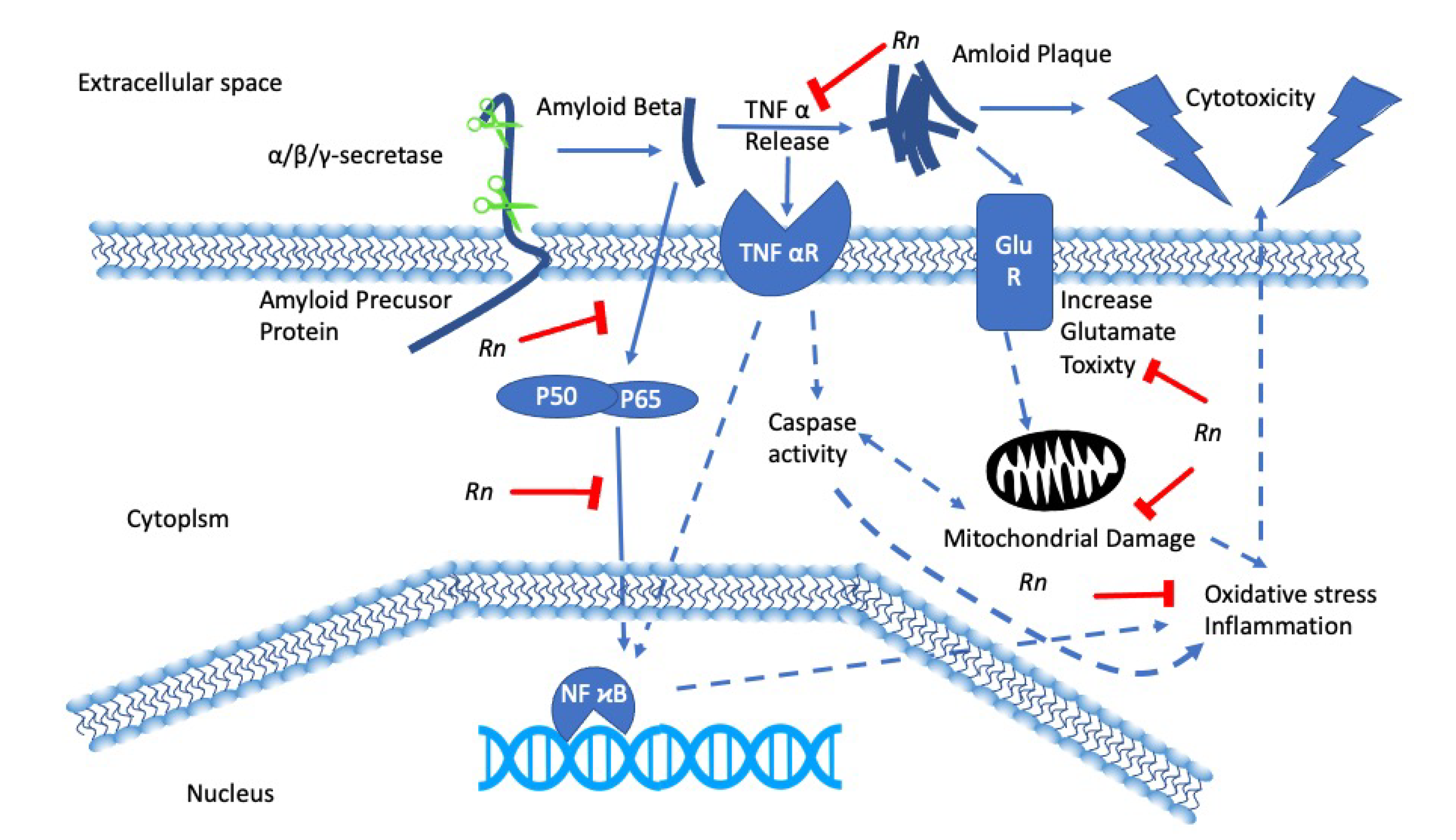
3. Rhinacanthus nasutus and Neurodegenerative Diseases
4. Rhinacanthus nasutus and Cancer
5. Rhinacanthus nasutus and Diabetes
6. Antibacterial, Anti-Fungal, and Anti-Viral Activities of Rhinacanthus nasutus
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bukke, S.; Raghu, P.S.; Sailaja, G.; Kedam, T.R. The study on morphological, phytochemical and pharmacological aspects of Rhinacanthus nasutus. (L) kurz (A review). J. Appl. Pharm. Sci. 2011, 1, 26–32. [Google Scholar]
- Devanarayana, A.; ERHSS, E.; SSSBDP, S.; Karunarathna, N. Therapeutic usages of Rhinacanthus nasutus (L) kurz (aniththa) in Sri Lankan traditional medicine. Unique J. Ayurvedic Herb. Med. 2015, 3, 10–15. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Puttarak, P.; Charoonratana, T.; Panichayupakaranant, P. Antimicrobial activity and stability of rhinacanthins-rich Rhinacanthus nasutus extract. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 323–327. [Google Scholar] [CrossRef]
- Darah, I.; Jain, K. Efficacy of the Rhinacanthus nasutus Nees leaf extract on dermatophytes with special reference to Trichophyton mentagrophytes var. Mentagrophytes and Microsporum canis. Nat. Prod. Sci. 2001, 7, 114–119. [Google Scholar]
- Pushpalatha, E.; Muthukrishnan, J. Efficacy of two tropical plant extracts for the control of mosquitoes. J. Appl. Entomol. 1999, 123, 369–373. [Google Scholar] [CrossRef]
- Komalamisra, N.; Trongtokit, Y.; Rongsriyam, Y.; Apiwathnasorn, C. Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J. Trop. Med. Public Health 2005, 36, 1412. [Google Scholar]
- Rongsriyam, Y.; Trongtokit, Y.; Komalamisra, N.; Sinchaipanich, N.; Apiwathnasorn, C.; Mitrejet, A. Formulation of tablets from the crude extract of Rhinacanthus nasutus (Thai local plant) against Aedes aegypti and Culex quinquefasciatus larvae: A preliminary study. Southeast Asian J. Trop. Med. Public Health 2006, 37, 265–271. [Google Scholar]
- Rao, P.V.; Naidu, M.D. Anti diabetic effect of Rhinacanthus nasutus leaf extract in streptozotocin induced diabetic rats. Libyan Agric. Res. Center J. 2010, 1, 310–312. [Google Scholar]
- Rao, P.V.; Sujana, P.; Vijayakanth, T.; Naidu, M.D. Rhinacanthus nasutus—Its protective role in oxidative stress and antioxidant status in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Dis. 2012, 2, 327–330. [Google Scholar] [CrossRef]
- Horii, H.; Ueda, J.-Y.; Tamura, M.; Sakagami, H.; Tomomura, M.; Tomomura, A.; Shirataki, Y. New biological activity of Rhinacanthus nasutus extracts. In Vivo 2011, 25, 367–373. [Google Scholar]
- Shyamal, S.; Latha, P.G.; Suja, S.R.; Shine, V.J.; Anuja, G.I.; Sini, S.; Pradeep, S.; Shikha, P.; Rajasekharan, S. Hepatoprotective effect of three herbal extracts on aflatoxin B1-intoxicated rat liver. Singap. Med. J. 2010, 51, 326–331. [Google Scholar]
- Brimson, J.M.; Tencomnao, A.P.T.; Tencomnao, T. Rhinacanthus nasutus protects cultured neuronal cells against hypoxia induced cell death. Molecules 2011, 16, 6322–6338. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Brimson, S.J.; Brimson, C.A.; Rakkhitawatthana, V.; Tencomnao, T. Rhinacanthus nasutus Extracts Prevent Glutamate and Amyloid-β Neurotoxicity in HT-22 Mouse Hippocampal Cells: Possible Active Compounds Include Lupeol, Stigmasterol and β-Sitosterol. Int. J. Mol. Sci. 2012, 13, 5074–5097. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org (accessed on 1 October 2020).
- Kodama, O.; Ichikawa, H.; Akatsuka, T.; Santisopasri, V.; Kato, A.; Hayashi, Y. Isolation and identification of an antifungal naphthopyran derivative from Rhinacanthus nasutus. J. Nat. Prod. 1993, 56, 292–294. [Google Scholar] [CrossRef]
- Wu, T.S.; Hsu, H.C.; Wu, P.L.; Leu, Y.L.; Chan, Y.Y.; Chern, C.Y.; Yeh, M.Y.; Tien, H.J. Naphthoquinone esters from the root of Rhinacanthus nasutus. Chem. Pharm. Bull. 1998, 46, 413–418. [Google Scholar] [CrossRef]
- Wu, T.S.; Hsu, H.C.; Wu, P.L.; Teng, C.M.; Wu, Y.C. Rhinacanthin-Q, a naphthoquinone from Rhinacanthus nasutus and its biological activity. Phytochemistry 1998, 49, 2001–2003. [Google Scholar] [CrossRef]
- Su, T.-P.; Wu, X.Z.; Cone, E.J.; Shukla, K.; Gund, T.M.; Dodge, A.L.; Parish, D.W. Sigma compounds derived from phencyclidine: Identification of PRE-084, a new, selective sigma ligand. J. Pharmacol. Exp. Ther. 1991, 259, 543–550. [Google Scholar]
- Boonyaketgoson, S.; Rukachaisirikul, V.; Phongpaichit, S.; Trisuwan, K. Naphthoquinones from the leaves of Rhinacanthus nasutus having acetylcholinesterase inhibitory and cytotoxic activities. Fitoterapia 2018, 124, 206–210. [Google Scholar] [CrossRef]
- Zhao, L.L.; Makinde, E.A.; Shah, M.A.; Olatunji, O.J.; Panichayupakaranant, P. Rhinacanthins-rich extract and rhinacanthin C ameliorate oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic nephropathy. J. Food Biochem. 2019, 43, e12812. [Google Scholar] [CrossRef]
- Sendl, A.; Chen, J.L.; Jolad, S.D.; Stoddart, C.; Rozhon, E.; Kernan, M.; Nanakorn, W.; Balick, M. Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J. Nat. Prod. 1996, 59, 808–811. [Google Scholar] [CrossRef]
- Kernan, M.R.; Sendl, A.; Chen, J.L.; Jolad, S.D.; Blanc, P.; Murphy, J.T.; Stoddart, C.A.; Nanakorn, W.; Balick, M.J.; Rozhon, E.J. Two new lignans with activity against influenza virus from the medicinal plant Rhinacanthus nasutus. J. Nat. Prod. 1997, 60, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Phuong, N.T.T.; Khoi, N.M.; Park, S.J.; Kwak, H.J.; Nhiem, N.X.; Trang, B.T.T.; Tai, B.H.; Song, J.H.; Ko, H.J.; et al. A new naphthoquinone analogue and antiviral constituents from the root of Rhinacanthus nasutus. Nat. Prod. Res. 2019, 33, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.J.; Park, S.J.; Kim, N.; Yoo, G.; Park, J.H.; Oh, Y.; Nhiem, N.X.; Kim, S.H. Neuraminidase inhibitory activity by compounds isolated from aerial parts of Rhinacanthus nasutus. Nat. Prod. Res. 2018, 32, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M.; Stubberfield, C.R. Pyridine nucleotide changes in hepatocytes exposed to quinones. Free Radic. Res. Commun. 1990, 8, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kongkathip, N.; Kongkathip, B.; Siripong, P.; Sangma, C.; Luangkamin, S.; Niyomdecha, M.; Pattanapa, S.; Piyaviriyagul, S.; Kongsaeree, P. Potent antitumor activity of synthetic 1,2-naphthoquinones and 1,4-naphthoquinones. Bioorg. Med. Chem. 2003, 11, 3179–3191. [Google Scholar] [CrossRef]
- Maarisit, W.; Yamazaki, H.; Abdjul, D.B.; Takahashi, O.; Kirikoshi, R.; Namikoshi, M. A new pyranonaphtoquinone derivative, 4-oxo-rhinacanthin a, from roots of Indonesian Rhinacanthus nasutus. Chem. Pharm. Bull. 2017, 65, 586–588. [Google Scholar] [CrossRef]
- Pethuan, S.; Duangkaew, P.; Sarapusit, S.; Srisook, E.; Rongnoparut, P. Inhibition against mosquito cytochrome P450 enzymes by rhinacanthin-A, -B, and -C elicits synergism on cypermethrin cytotoxicity in Spodoptera frugiperda cells. J. Med. Entomol. 2012, 49, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.A.; Li, M.H.; Lin, N.H.; Chang, C.H.; Lu, I.H.; Pan, I.H.; Takahashi, T.; Perng, M.D.; Wen, S.F. Rhinacanthin C Alleviates Amyloid-β Fibrils’ Toxicity on Neurons and Attenuates Neuroinflammation Triggered by LPS, Amyloid-β, and Interferon-γ in Glial Cells. Oxid. Med. Cell Longev. 2017, 2017, 5414297. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Z.; Wu, S.C.; Kwan, A.L.; Lin, C.L. Rhinacanthin-C, A Fat-Soluble Extract from Rhinacanthus nasutus, Modulates High-Mobility Group Box 1-Related Neuro-Inflammation and Subarachnoid Hemorrhage-Induced Brain Apoptosis in a Rat Model. World Neurosurg. 2016, 86, 349–360. [Google Scholar] [CrossRef]
- Boueroy, P.; Saensa-Ard, S.; Siripong, P.; Kanthawong, S.; Hahnvajanawong, C. Rhinacanthin-C extracted from Rhinacanthus nasutus (L.) inhibits cholangiocarcinoma cell migration and invasion by decreasing MMP-2, uPA, FAK and MAPK pathways. Asian Pac. J. Cancer Prev. 2018, 19, 3605. [Google Scholar] [CrossRef][Green Version]
- Kongkathip, N.; Luangkamin, S.; Kongkathip, B.; Sangma, C.; Grigg, R.; Kongsaeree, P.; Prabpai, S.; Pradidphol, N.; Piyaviriyagul, S.; Siripong, P. Synthesis of novel rhinacanthins and related anticancer naphthoquinone esters. J. Med. Chem. 2004, 47, 4427–4438. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Yahuafai, J.; Shimizu, K. Induction of apoptosis in tumor cells by three naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus KURZ. Biol. Pharm. Bull. 2006, 29, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Yahuafai, J.; Piyaviriyakul, S.; Kanokmedhakul, K.; Koide, H.; Ishii, T.; Shimizu, K.; Ruchirawat, S.; Oku, N. Inhibitory effect of liposomal rhinacanthin-N isolated from Rhinacanthus nasutus on pulmonary metastasis in mice. Biol. Pharm. Bull. 2012, 35, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Yahuafai, J.; Shimizu, K.; Ichikawa, K.; Yonezawa, S.; Asai, T.; Kanokmedakul, K.; Ruchirawat, S.; Oku, N. Antitumor activity of liposomal naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus KURZ. Biol. Pharm. Bull. 2006, 29, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Ramalho, R.M.; Rodrigues, C.M.P.; Ferreira, M.-J.U. Dibenzylbutane- and butyrolactone-type lignans as apoptosis inducers in human hepatoma HuH-7 cells. Phytother. Res. 2012, 26, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.; Lage, H.; Abrantes, M.; Ferreira, M.J. Phenolic compounds as selective antineoplasic agents against multidrug-resistant human cancer cells. Planta Med. 2010, 76, 975–980. [Google Scholar] [CrossRef]
- Siripong, P.; Hahnvajanawong, C.; Yahuafai, J.; Piyaviriyakul, S.; Kanokmedhakul, K.; Kongkathip, N.; Ruchirawat, S.; Oku, N. Induction of apoptosis by rhinacanthone isolated from Rhinacanthus nasutus roots in human cervical carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1251–1260. [Google Scholar] [CrossRef]
- Thirumurugan, R.S.; Kavimani, S.; Srivastava, R.S. Antitumour activity of rhinacanthone against Dalton’s ascitic lymphoma. Biol. Pharm. Bull. 2000, 23, 1438–1440. [Google Scholar] [CrossRef]
- Prince, M.J.; Prina, M.; Guerchet, M. World Alzheimer Report 2013—Journey of Caring: An Analysis of Long-Term Care for Dementia; Alzheimer’s Disease International: London, UK, 2013; pp. 1–8. [Google Scholar]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Dorsey, R.E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Felsenstein, K.M.; Candelario, K.M.; Steindler, D.A.; Borchelt, D.R. Regenerative medicine in Alzheimer’s disease. Transl. Res. 2014, 163, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Browne, A.; Child, D.; Tanzi, R.E. Curcumin decreases amyloid-β peptide levels by attenuating the maturation of amyloid-β precursor protein. J. Biol. Chem. 2010, 285, 28472–28480. [Google Scholar] [CrossRef] [PubMed]
- Wansawat, S.; Tencomnao, T.; Prasanth, M.I.; Isidoro, C. Mucuna pruriens seed extract promotes neurite outgrowth via ten-4 dependent and independent mechanisms in Neuro2a cells. Sains Malays. 2018, 47, 3009–3015. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J. Tradit. Complement. Med. 2020, 10, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Tencomnao, T. Medicinal herbs and antioxidants: Potential of Rhinacanthus nasutus for disease treatment? Phytochem. Rev. 2013, 13, 643–651. [Google Scholar] [CrossRef]
- Brimson, J.M.; Tencomnao, T. Rhinacanthus nasutus Extract as a Neuroprotectant; Elsevier: London, UK, 2015; pp. 77–84. [Google Scholar]
- Maheshu, V.; Mahalingam, J.; Teepica, D.; Darsini, P.; Bell, G.S. In Vitro Antioxidant Activity and Polyphenolic Contents of Rauvolfia tetraphylla L., Rhinacanthus nasutus Kurz. and Solena amplexicaulis (Lam.). Int. J. Biomed. Pharm. Sci. 2010, 4, 81–86. [Google Scholar]
- Chonthida, T.; Kanjana, P.; Maitree, S.; Somdet, S. Anti-oxidant properties and anti-hemolytic activity of Psidium guajava, Pandanous odorus and Rhinacanthus nasutus. J. Med. Plant Res. 2013, 7, 2001–2009. [Google Scholar] [CrossRef][Green Version]
- Shah, M.A.; Muhammad, H.; Mehmood, Y.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. Superoxide scavenging and antiglycation activity of rhinacanthins-rich extract obtained from the leaves of Rhinacanthus nasutus. Pharmacogn. Mag. 2017, 13, 652. [Google Scholar]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 210–212. [Google Scholar] [CrossRef]
- Ellwardt, E.; Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp. Neurol. 2014, 262, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hooten, K.G.; Beers, D.R.; Zhao, W.; Appel, S.H. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Yan, M.; Chan, A.M. A thirty-year quest for a role of R-Ras in cancer: From an oncogene to a multitasking GTPase. Cancer Lett. 2017, 403, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shostak, K.; Chariot, A. EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; O’Donnell, K.A.; Zeller, K.I.; Nguyen, T.; Osthus, R.C.; Li, F. The c-Myc target gene network. Semin. Cancer Biol. 2006, 16, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef]
- Siripong, P.; Kanokmedakul, K.; Piyaviriyagul, S.; Yahuafai, J.; Chanpai, R.; Ruchirawat, S.; Oku, N. Antiproliferative naphthoquinone esters from Rhinacanthus nasutus Kurz. roots on various cancer cells. J. Tradit. Med. 2006, 23, 166–172. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Rao, P.V.; Madhavi, K.; Naidu, M.D. Hypolipidemic properties of Rhinacanthus nasutus in streptozotocin induced diabetic rats. J. Pharmacol. Toxicol. 2011, 6, 589–595. [Google Scholar] [CrossRef][Green Version]
- Van De Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van De Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. α-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef]
- Shah, M.A.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. α-Glucosidase inhibitory effect of rhinacanthins-rich extract from Rhinacanthus nasutus leaf and synergistic effect in combination with acarbose. J. Funct. Foods 2017, 36, 325–331. [Google Scholar] [CrossRef]
- Shah, M.A.; Jakkawanpitak, C.; Sermwittayawong, D.; Panichayupakaranant, P. Rhinacanthins-rich extract enhances glucose uptake and inhibits adipogenesis in 3T3-L1 adipocytes and L6 myotubes. Pharmacogn. Mag. 2017, 13, S817. [Google Scholar]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Sompong, W.; Adisakwattana, S. Inhibitory effect of herbal medicines and their trapping abilities against methylglyoxal-derived advanced glycation end-products. BMC Complement. Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Krauss, R.M. Atherogenicity of triglyceride-rich lipoproteins. Am. J. Cardiol. 1998, 81, 13B–17B. [Google Scholar] [CrossRef]
- Shah, M.A.; Reanmongkol, W.; Radenahmad, N.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. Anti-hyperglycemic and anti-hyperlipidemic effects of rhinacanthins-rich extract from Rhinacanthus nasutus leaves in nicotinamide-streptozotocin induced diabetic rats. Biomed. Pharmacother. 2019, 113, 108702. [Google Scholar] [CrossRef]
- Rao, P.; Madhavi, K.; Dhananjaya Naidu, M.; Gan, S.H. Rhinacanthus nasutus Ameliorates Cytosolic and Mitochondrial Enzyme Levels in Streptozotocin-Induced Diabetic Rats. Evid. Based Complementary Altern. Med. 2013, 2013, 486047. [Google Scholar] [CrossRef]
- Adam, S.H.; Giribabu, N.; Rao, P.V.; Sayem, A.S.M.; Arya, A.; Panichayupakaranant, P.; Korla, P.K.; Salleh, N. Rhinacanthin C ameliorates hyperglycaemia, hyperlipidemia and pancreatic destruction in streptozotocin–nicotinamide induced adult male diabetic rats. Eur. J. Pharmacol. 2016, 771, 173–190. [Google Scholar] [CrossRef]
- Frederiks, W.M.; Bosch, K.S.; De Jong, J.S.; Van Noorden, C.J. Post-translational regulation of glucose-6-phosphate dehydrogenase activity in (pre) neoplastic lesions in rat liver. J. Histochem. Cytochem. 2003, 51, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.; Malaisse-Lagae, F.; Malaisse, W.J. Stimulation of pancreatic islet metabolism and insulin release by a nonmetabolizable amino acid. Proc. Natl. Acad. Sci. USA 1981, 78, 5460–5464. [Google Scholar] [CrossRef] [PubMed]
- Edalat, A.; Schulte-Mecklenbeck, P.; Bauer, C.; Undank, S.; Krippeit-Drews, P.; Drews, G.; Düfer, M. Mitochondrial succinate dehydrogenase is involved in stimulus-secretion coupling and endogenous ROS formation in murine beta cells. Diabetologia 2015, 58, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Favelyukis, S.; Nguyen, A.-K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179. [Google Scholar] [CrossRef]
- Wannasiri, S.; Piyabhan, P.; Naowaboot, J. Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice. Asian Pac. J. Trop. Biomed. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Bradford, B.L. PPARγ: An essential regulator of adipogenesis and modulator of fat cell function. Cell 1999, 99, 239–242. [Google Scholar]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.A.; Abdullah, N.; Khan, A.H.; Noor, A. Evaluation of anti-fungal and anti-bacterial activity of a local plant Rhinacanthus nasutus (L.). J. Biol. Sci. 2004, 4, 498–500. [Google Scholar]
- Siripong, P.; Wongseri, V.; Piyaviriyakul, S.; Yahaufai, J.; Chanpai, R.; Kanakmedakul, K. Antibacterial potential of Rhinacanthus nasutus against clinically isolated bacteria from Thai cancer patients. MU J. Pharm. 2006, 33, 15–22. [Google Scholar]
- Ponglux, D. Medicinal Plants; International Congress on Natural Products; Committee: Bangkok, Thailand, 1987. [Google Scholar]
- Chaliewchalad, P.; Thongwai, N.; Tragoolpua, Y. Inhibitory effect of Rhinacanthus nasutus (Linn.) Kurz. and Stemona tuberosa (Lour.) extracts on herpes simplex virus infection. J. Med. Plant Res. 2013, 7, 76–84. [Google Scholar]
- Thongchuai, B.; Trisuwan, K.; Roytrakul, S.; Tragoolpua, Y.; Sangthong, P. Anti-herpes simplex virus type 2 activity from Rhinacanthus nasutus (Linn.) Kurz. extracts as affected by different extraction solvents. CMUJ. Nat. Sci. 2019, 18, 14–26. [Google Scholar] [CrossRef]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef]

| Synonyms | |
|---|---|
| Rhinacanthus nasutus (L.) Kurz | |
| Rhinacanthus communis Nees | |
| Rhinacanthus osmospermus Bojer ex Nees | |
| Justicia dichotoma Rottler * | |
| Justicia nasuta L. | |
| Justicia macilenta E. Mey. * | |
| Justicia rottleriana Wall. * | |
| Justicia silvatica Nees * | |
| Justicia sylvatica Vahl * | |
| Pseuderanthemum connatum Lindau | |
| Dianthera paniculata Lour. | |
| Common Names | Common Name Origin |
| জূঈপান Juipana | Bengali |
| Dainty Spurs, Snake jasmine White Crane flower | English |
| पालकजूही Palakjuhi, जूहीपानी Juhipani | Hindi |
| ನಾಗಮಲ್ಲಿಗೆ Nagamallige, Doddapatike | Kannada |
| Dadmari | Konkani |
| Puzhukkolli Nagamulla Purukolli Orukaalmudanthi Vellakkurunji Nagamulla (നാഗമുല്ല) Pushpakedal | Malayalam |
| गजकर्णी Gajkarni | Marathi |
| Yudhikaparni, Yoodhikaparni | Sanskrit |
| Uragamalli Nagamalli (நாகமல்லீ) | Tamil |
| Nagamalle (నాగమల్లె) | Telugu |
| Palakjuhi | Urdu |
| Thong Phan chang (ทองพันชั่ง), Yaa man kai (หญ้ามันไก่) (ตะ ซิ ชี ซี) Ta si chi si (กะเหรี่ยงสะกอ) | Thai |
| Name | Structure | Reported Activities |
|---|---|---|
| 2-Hydroxy-1,4-naphthoquinone | 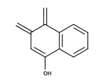 | An interconversion of pyridine nucleotides to combat the effects of oxidative stress [26] |
| 3,4-dihydro-3,3-dimethyl-2H-naphtho[2,3-b] pyran-5,10-dione | 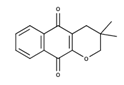 | Antitumor activity [27] |
| Rhinacanthin A | 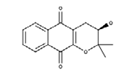 | Inhibited the growth of S. aureus with an inhibition zone of 16 mm (25 mm disk) [28] |
| Rhinacanthin B | 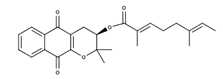 | Inhibition of cytochrome enzymes CYP6AA3 and CYP6P7 in vitro [29] |
| Rhinacanthin C | 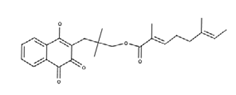 | Prevented Aβ-induced toxicity in rat hippocampal neurons and attenuated lipopolysaccharides (LPS)-activated nitric oxide (NO) production, inducible NO synthase (iNOS) expression, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling in rat glia [30] ;Exhibited neuroprotective effect by reducing cleaved caspase-3- and caspase-9a-related apoptosis and anti-inflammatory effect by decreasing High mobility group box 1 (HMGB-1) mRNA and protein expression [31]; Inhibits cholangiocarcinoma cell migration and invasion by decreasing matrix metalloproteinase-2 (MMP-2), Urokinase-Type Plasminogen Activator (uPA), focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) pathways, along with inhibition of cell migration and antiproliferative effects [32] |
| Rhinacanthin D |  | Inhibitory activity against cytomegalovirus [22]; antiviral activities against influenza virus A/PR/8/34 (PR8), Anti-Human Rhinovirus 1B (HRV1B), and Coxsackievirus B3 (CVB3)-infected Vero cells [24] |
| Rhinacanthin E |  | Antiviral activity against influenza virus type A [23] |
| Rhinacanthin F |  | Antiviral activity against influenza virus type A [30] |
| Rhinacanthin G |  | Inhibition of cytochrome enzyme CYP6P7 in vitro [29] |
| Rhinacanthin M | 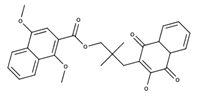 | Inhibition of cancer cell lines KB, HeLa, and HepG2 with IC50 values of 1.5, 3.0 and 4.6 µM, respectively [33] |
| Rhinacanthin N |  | Rhinacanthin-N caused G2/M arrest of HeLaS3 cells after 24 h incubation and increased the proportion of sub-G1 hypodiploid cells, apoptotic cells [34]; Antimetastatic activity as it inhibited the metastatic pulmonary colonization of the melanoma cells in C57BL/6 male mice [35]; Antiproliferative activity against HeLaS3 cells and suppressed tumor growth in vivo [36] |
| Rhinacanthin O |  | Unknown activities |
| Rhinacanthin Q | 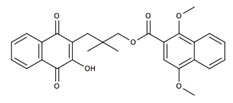 | Induction of apoptosis in tumor cells that may be associated with the activation of the caspase-3 pathway [34]; Antiviral activities against PR8, HRV1B, and CVB3-infected Vero cells [24] |
| Rhinacasutone | 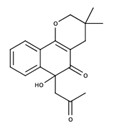 | Unknown activities |
| Heliobuphthalmin | 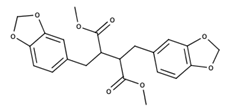 | Strong inducer of apoptosis in HuH-7 cells [37]; High antineoplasic activities against the classical (multi-drug-resistant) MDR subline derived from gastric carcinoma [38] |
| Rhinacanthone |  | Rhinacanthone-induced apoptosis in HeLa cells is mediated primarily through the mitochondrial-dependent signaling pathway as it inhibited proliferation of HeLa cells along with chromatin condensation, internucleosomal DNA fragmentation, increase in the proportion of sub G(1) apoptotic cells, and externalization of annexin-V. Increase in the level of Bax and a decrease in the level of Bcl-2 and activation of caspase 3 and 9 [39]; Inhibited tumor cell growth in Dalton’s ascitic lymphoma (DAL) in Swiss albino mice [40] |
| Cell Line | Chloroform Extract µg/mL | Methanol Extract µg/mL | RnC µM | RnD µM | RnG µM | RnO µM | RnM µM | RnN µM | RnQ µM | Rhinacanthone µM |
|---|---|---|---|---|---|---|---|---|---|---|
| KB | 0.55 | 3.9 | 0.46 | 0.47 | 4.7 | 5.5 | 2.6 | 0.33 | 1.4 | 3.8 |
| Hep-2 | 0.3 | 4.0 | 0.8 | 7.6 | 3.3 | 3.7 | 6.1 | 1.2 | 3.6 | 4.4 |
| MCF-7 | 0.8 | 20.0 | 0.88 | 14.7 | 8.7 | 8.1 | 8.9 | 2.6 | 10.6 | 4.9 |
| HepG2 | 0.95 | 8.5 | 0.41 | 1.9 | 1.2 | 6.5 | 3.8 | 0.37 | 3.0 | 4.9 |
| HeLa | 0.39 | 4.4 | 0.29 | 0.49 | 4.7 | 6.1 | 4.3 | 0.87 | 3.8 | 4.2 |
| SiHa | 1.5 | 21.0 | 0.49 | 6.6 | 18.8 | 7.4 | 39.1 | 3.9 | 6.1 | 2.9 |
| C-32 | 5.0 | 30.0 | 9.8 | 14.7 | 16.4 | 30.7 | 37.0 | 39.1 | 8.4 | 2.1 |
| LLC | 1.8 | 40 | 0.98 | 6.1 | 6.1 | 8.2 | 54.4 | 5.4 | 8.3 | 2.9 |
| Colon-26 | 0.3 | 5.0 | 0.44 | 2.3 | 1.5 | 6.6 | 5.4 | 1.1 | 1.1 | 3.4 |
| P-388 | 0.3 | 7 | 1.5 | 9.3 | 3.3 | 8.9 | 8.1 | 3.7 | 10.3 | 4.4 |
| Vero | 0.2 | 2.5 | 11.0 | 34.3 | 16.4 | 12.3 | 36.1 | 12.7 | 41.1 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Brimson, S.; Tencomnao, T. Rhinacanthus nasutus “Tea” Infusions and the Medicinal Benefits of the Constituent Phytochemicals. Nutrients 2020, 12, 3776. https://doi.org/10.3390/nu12123776
Brimson JM, Prasanth MI, Malar DS, Brimson S, Tencomnao T. Rhinacanthus nasutus “Tea” Infusions and the Medicinal Benefits of the Constituent Phytochemicals. Nutrients. 2020; 12(12):3776. https://doi.org/10.3390/nu12123776
Chicago/Turabian StyleBrimson, James Michael, Mani Iyer Prasanth, Dicson Sheeja Malar, Sirikalaya Brimson, and Tewin Tencomnao. 2020. "Rhinacanthus nasutus “Tea” Infusions and the Medicinal Benefits of the Constituent Phytochemicals" Nutrients 12, no. 12: 3776. https://doi.org/10.3390/nu12123776
APA StyleBrimson, J. M., Prasanth, M. I., Malar, D. S., Brimson, S., & Tencomnao, T. (2020). Rhinacanthus nasutus “Tea” Infusions and the Medicinal Benefits of the Constituent Phytochemicals. Nutrients, 12(12), 3776. https://doi.org/10.3390/nu12123776