Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients
Abstract
:1. Introduction
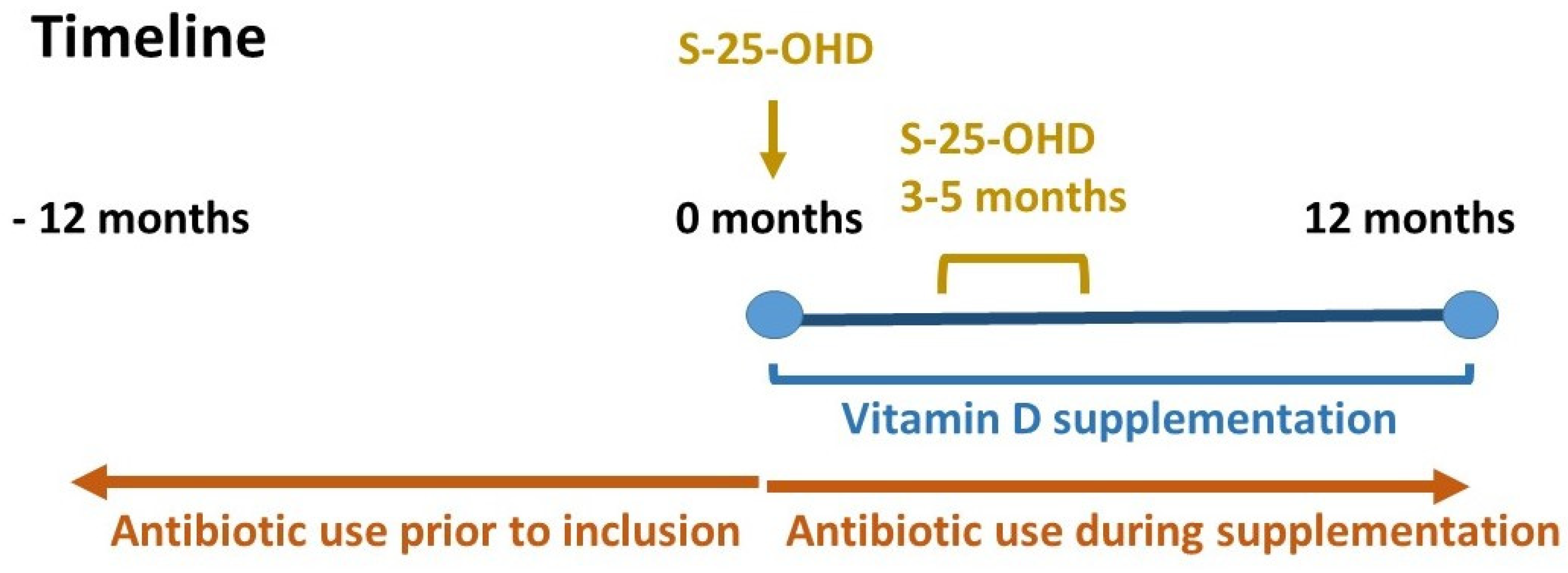
2. Materials and Methods
3. Results
3.1. Baseline Demography
3.2. S-25-OHD Concentrations
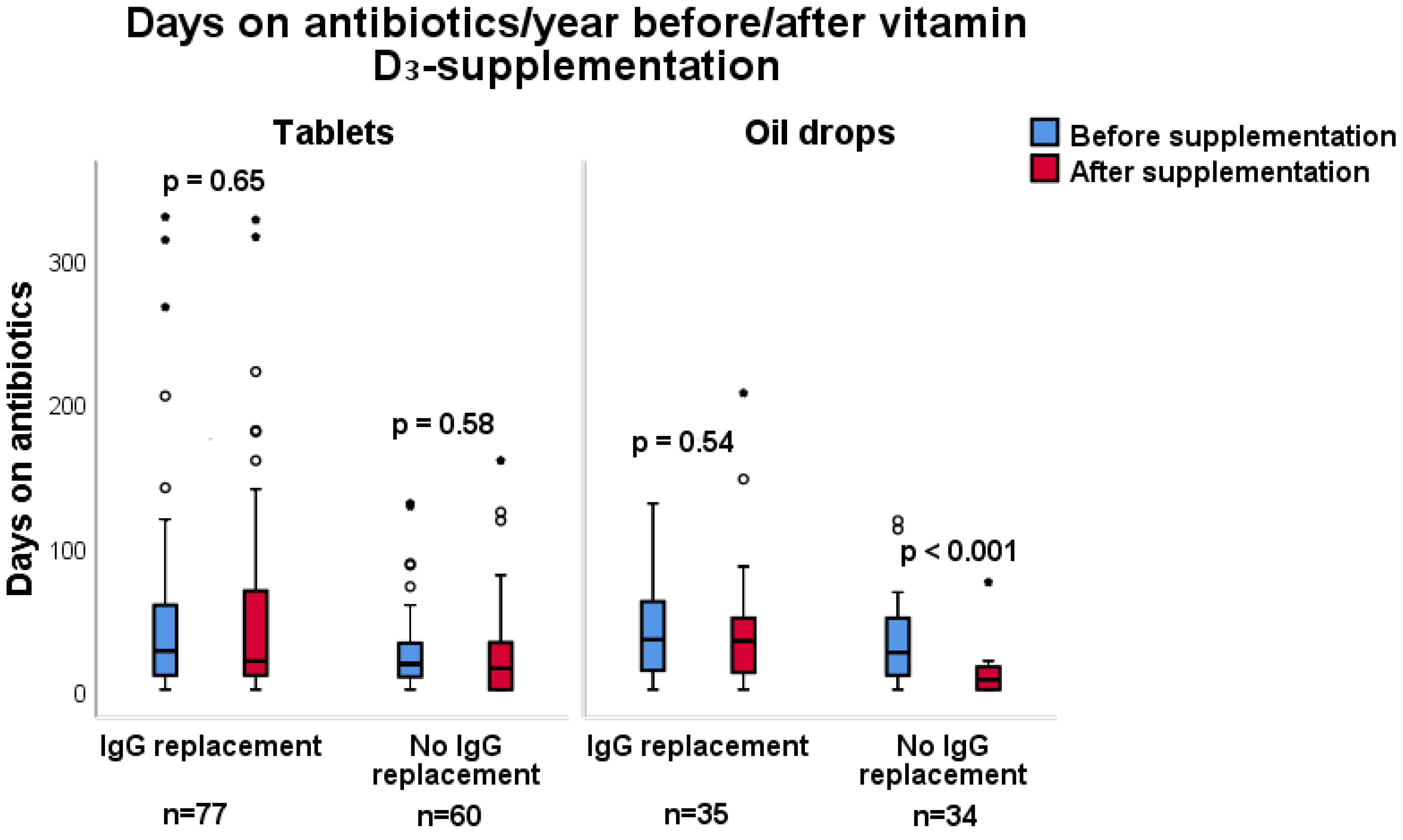
3.3. Antibiotic Consumption
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Fighting infections with vitamin D. Nat. Med. 2006, 12, 388–390. [Google Scholar] [CrossRef]
- Hollis, B.W. Measuring 25-hydroxyvitamin D in a clinical environment: Challenges and needs. Am. J. Clin. Nutr. 2008, 88, 507s–510s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Monlezun, D.J.; Bittner, E.A.; Christopher, K.B.; Camargo, C.A.; Quraishi, S.A. Vitamin D status and acute respiratory infection: Cross sectional results from the United States National Health and Nutrition Examination Survey, 2001–2006. Nutrients 2015, 7, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Bjorkhem-Bergman, L.; Ekstrom, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open 2012, 2, e001663. [Google Scholar] [CrossRef] [Green Version]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Bjorkhem-Bergman, L. Vitamin D supplementation to patients with frequent respiratory tract infections: A post hoc analysis of a randomized and placebo-controlled trial. BMC Res. Notes 2015, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Norlin, A.C.; Hansen, S.; Wahren-Borgstrom, E.; Granert, C.; Bjorkhem-Bergman, L.; Bergman, P. Vitamin D3 Supplementation and Antibiotic Consumption—Results from a Prospective, Observational Study at an Immune-Deficiency Unit in Sweden. PLoS ONE 2016, 11, e0163451. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.R.S.; Silva, R.; Andrade, I.G.A.; Fonseca, F.L.A.; Costa-Carvalho, B.T.; Sarni, R.O.S. Assessment of vitamin D status in common variable immunodeficiency or ataxia-telangiectasia patients. Allergologia et Immunopathologia 2019, 47, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska-Leonik, N.; Bernatowska, E.; Pac, M.; Filipiuk, W.; Mulawka, J.; Pietrucha, B.; Heropolitanska-Pliszka, E.; Bernat-Sitarz, K.; Wolska-Kusnierz, B.; Mikoluc, B. Vitamin D deficiency in children with recurrent respiratory infections, with or without immunoglobulin deficiency. Adv. Med. Sci. 2018, 63, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Brodlie, M.; Orchard, W.A.; Reeks, G.A.; Pattman, S.; McCabe, H.; O’Brien, C.J.; Thomas, M.F.; Spencer, D.A. Vitamin D in children with cystic fibrosis. Arch. Dis. Child. 2012, 97, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Hermes, W.A.; Alvarez, J.A.; Lee, M.J.; Chesdachai, S.; Lodin, D.; Horst, R.; Tangpricha, V. Prospective, Randomized, Double-Blind, Parallel-Group, Comparative Effectiveness Clinical Trial Comparing a Powder Vehicle Compound of Vitamin D With an Oil Vehicle Compound in Adults With Cystic Fibrosis. JPEN J. Parenter. Enter. Nutr. 2017, 41, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Holvik, K.; Madar, A.A.; Meyer, H.E.; Lofthus, C.M.; Stene, L.C. A randomised comparison of increase in serum 25-hydroxyvitamin D concentration after 4 weeks of daily oral intake of 10 microg cholecalciferol from multivitamin tablets or fish oil capsules in healthy young adults. Br. J. Nutr. 2007, 98, 620–625. [Google Scholar] [CrossRef]
- Holvik, K.; Madar, A.A.; Meyer, H.E.; Lofthus, C.M.; Stene, L.C. Changes in the vitamin D endocrine system and bone turnover after oral vitamin D3 supplementation in healthy adults: Results of a randomised trial. BMC Endocr. Disord. 2012, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Tun, R.R.L.C.; Porhownik, N.; Taback, S.; Oleschuk, C. Effect of high dose vitamin D3 therapy on serum vitamin D3 levels in vitamin D insufficient adults with cystic fibrosis. Clin. Nutr. ESPEN 2018, 23, 84–88. [Google Scholar]
- Grossmann, R.E.; Tangpricha, V. Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Mol. Nutr. Food Res. 2010, 54, 1055–1061. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2019, 10, 910. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S.; Luu, S.; Glowacki, J. Megalin mediates 25-hydroxyvitamin D3 actions in human mesenchymal stem cells. FASEB J. 2019, 33, 7684–7693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Invest. Suppl. 2012, 243, 92–102. [Google Scholar] [PubMed]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef]
- Xiaoyu, Z.; Payal, B.; Melissa, O.; Zanello, L.P. 1alpha,25(OH)2-vitamin D3 membrane-initiated calcium signaling modulates exocytosis and cell survival. J. Steroid Biochem. Mol. Biol. 2007, 103, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazahery, H.; von Hurst, P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef] [Green Version]
- Kull, M., Jr.; Kallikorm, R.; Tamm, A.; Lember, M. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health 2009, 9, 22. [Google Scholar] [CrossRef] [Green Version]



| Variable | Oil-Drops n = 69 | Tablets n = 137 | p-Value |
|---|---|---|---|
| Gender, n (%) | |||
| Men | 28 (41) | 49 (36) | 0.5 a |
| Women | 41 (59) | 88 (64) | |
| Age, years Median (IQR) Mean–max | 54 (36–63) 22–83 | 57 (40–69) 19–88 | 0.62 b |
| S-25-OHD, nmol/L Median (IQR) Min–max | 55 (42–63) 12–75 | 53 (42–66) 11–74 | 0.90 a |
| IgG-replacement, n (%) | 35 (51) | 77 (56) | 0.46 a |
| No antibiotic use, n (%) | 10 (15) | 22 (16) | 0.77 a |
| Number of prescriptions Median (IQR) Min–max | 3 (1–5) 0–12 | 2 (1–5) 0–12 | 0.40 b |
| Days of antibiotics /year Median (IQR) Min–max | 27 (10–52) 0–130 | 20 (10–48) 0–330 | 0.30 b |
| Characteristic | Before Vitamin D3 Supplementation | After Vitamin D3 Supplementation | p-Value |
|---|---|---|---|
| S-25-OHD, ng/L | |||
| Oil drops (n = 69) Median (IQR) Min–max | 55 (42–63) 12–75 | 38–139 86 (68–104) 38–139 | <0.001a |
| Tablets (n = 137) Median (IQR) Min–max | 11–74 53 (42–66) 11–74 | 87 (73–98) 14–169 | 0.001a |
| Antibiotic free n (%) | |||
| Oil drops (n = 69) | 10 (14) | 21 (30) | 0.03b |
| Tablets (n = 137) | 22 (16) | 32 (23) | 0.13 b |
| Number of prescriptions | |||
| Oil drops (n = 69) Median (IQR) Min–max | 3 (1–5) 0–12 | 2 (0–4) 0–10 | 0.003a |
| Tablets (n = 137) Median (IQR) Min–max | 2 (0–12) 0–12 | 2 (0–17) 0–17 | 0.35 a |
| Days of antibiotics/year | |||
| Oil drops (n = 69) Median (IQR) Min–max | 27 (10–52) 0–130 | 15 (7–46) 0–207 | 0.003a |
| Tablets (n = 137) Median (IQR) Min–max | 20 (1–48) 0–330 | 19 (0–328) 0–328 | 0.43 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helde Frankling, M.; Norlin, A.-C.; Hansen, S.; Wahren Borgström, E.; Bergman, P.; Björkhem-Bergman, L. Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients. Nutrients 2020, 12, 1230. https://doi.org/10.3390/nu12051230
Helde Frankling M, Norlin A-C, Hansen S, Wahren Borgström E, Bergman P, Björkhem-Bergman L. Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients. Nutrients. 2020; 12(5):1230. https://doi.org/10.3390/nu12051230
Chicago/Turabian StyleHelde Frankling, Maria, Anna-Carin Norlin, Susanne Hansen, Emilie Wahren Borgström, Peter Bergman, and Linda Björkhem-Bergman. 2020. "Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients" Nutrients 12, no. 5: 1230. https://doi.org/10.3390/nu12051230
APA StyleHelde Frankling, M., Norlin, A.-C., Hansen, S., Wahren Borgström, E., Bergman, P., & Björkhem-Bergman, L. (2020). Are Vitamin D3 Tablets and Oil Drops Equally Effective in Raising S-25-Hydroxyvitamin D Concentrations? A Post-Hoc Analysis of an Observational Study on Immunodeficient Patients. Nutrients, 12(5), 1230. https://doi.org/10.3390/nu12051230






